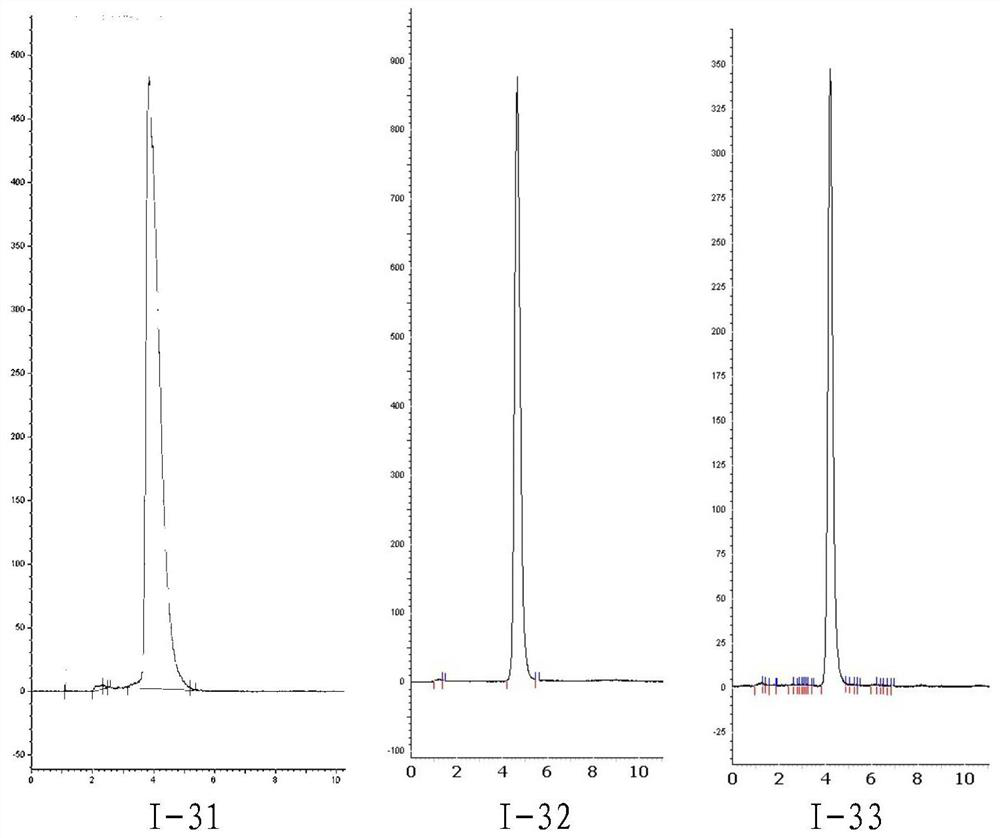
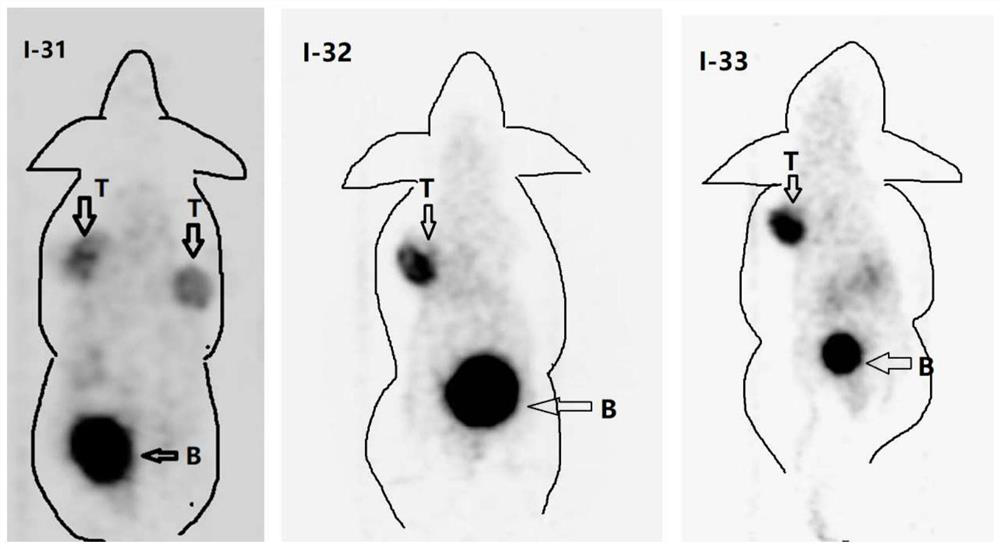
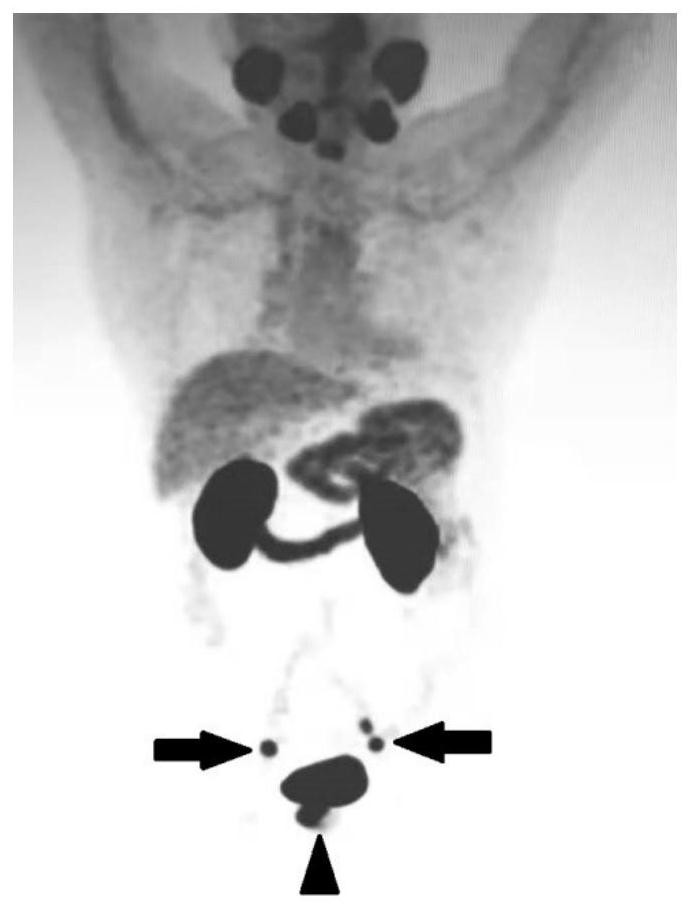
Diagnosis and treatment integrated PSMA inhibitor, compound and preparation method and application thereof
A compound and inhibitor technology, applied in the field of radiopharmaceuticals and their preparation, can solve the problems of inability to treat the imaging agent, decreased sensitivity, low sensitivity, etc., and achieve the effects of reducing non-specific uptake and accelerating radioactive excretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the synthesis of I-11 (3-quinoline-cyclohexylamine-DOTA-PSMA)
[0057] Take 100 mg of Fmoc-Glu-urea-Lys-resin (tert-butyl protected glutamic acid urea-lysine resin) (0.22 mmol / g) in a solid-phase synthesis tube. DCM washed (3×5min×2mL), DMF washed (3×5min×2mL). De-Fmoc using 20% piperidine in DMF (1 x 2 min x 2 mL, 2 x 10 min x 2 mL), followed by washing with DMF (6 x 1 min x 2 mL).
[0058] Take Fmoc-3-(3-quinoline)-D-alanine (3M, 0.06mmol, 26.2mg), HBTU (0.072mmol, 27mg), HOBt (0.072mmol, 10mg), DIPEA (0.15mmol, 25μL) in 3 mL of DMF at room temperature for 15 minutes. The above activated 2-pyridine-alanine was added to the washed resin and reacted for 1 hour under nitrogen. Wash with DMF (6×1 min×2 mL). De-Fmoc using 20% piperidine in DMF (1 x 2 min x 2 mL, 2 x 10 min x 2 mL), followed by washing with DMF (6 x 1 min x 2 mL).
[0059] Take trans-4-(Fmoc-aminomethyl)cyclohexanecarboxylic acid (3M, 0.06mmol, 23mg), HBTU (0.072mmol, 27mg), HOBt (0.0...
Embodiment 2
[0062] Embodiment 2: the synthesis of I-12 (3-quinoline-benzylamine-DOTA-PSMA)
[0063] The trans-4-(Fmoc-aminomethyl)cyclohexanecarboxylic acid in Example 1 was replaced by trans-4-(Fmoc-aminomethyl)benzoic acid, and finally synthesized and purified by HPLC to obtain a white final product: 4 Pyridine-Benzylamine-NODA-PSMA. MS: 1022.4[M+H]
Embodiment 3
[0064] Embodiment 3: the synthesis of I-13 (3-quinoline-butylamine-DOTA-PSMA)
[0065] The trans-4-(Fmoc-aminomethyl)cyclohexanecarboxylic acid in Example 1 was replaced by trans-4-(Fmoc-aminomethyl)butyric acid, and finally synthesized and purified by HPLC to obtain a white final product: 4 Pyridine-Benzylamine-NODA-PSMA. MS: 1003.4[M+H]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com