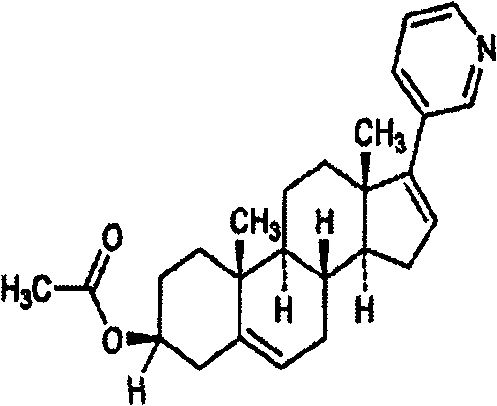
Medicinal composition containing abiraterone acetate and preparation technology thereof
A technology of solid composition and adhesive, applied in the field of medicine, can solve the problems of affecting the quality of preparations, uneven mixing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
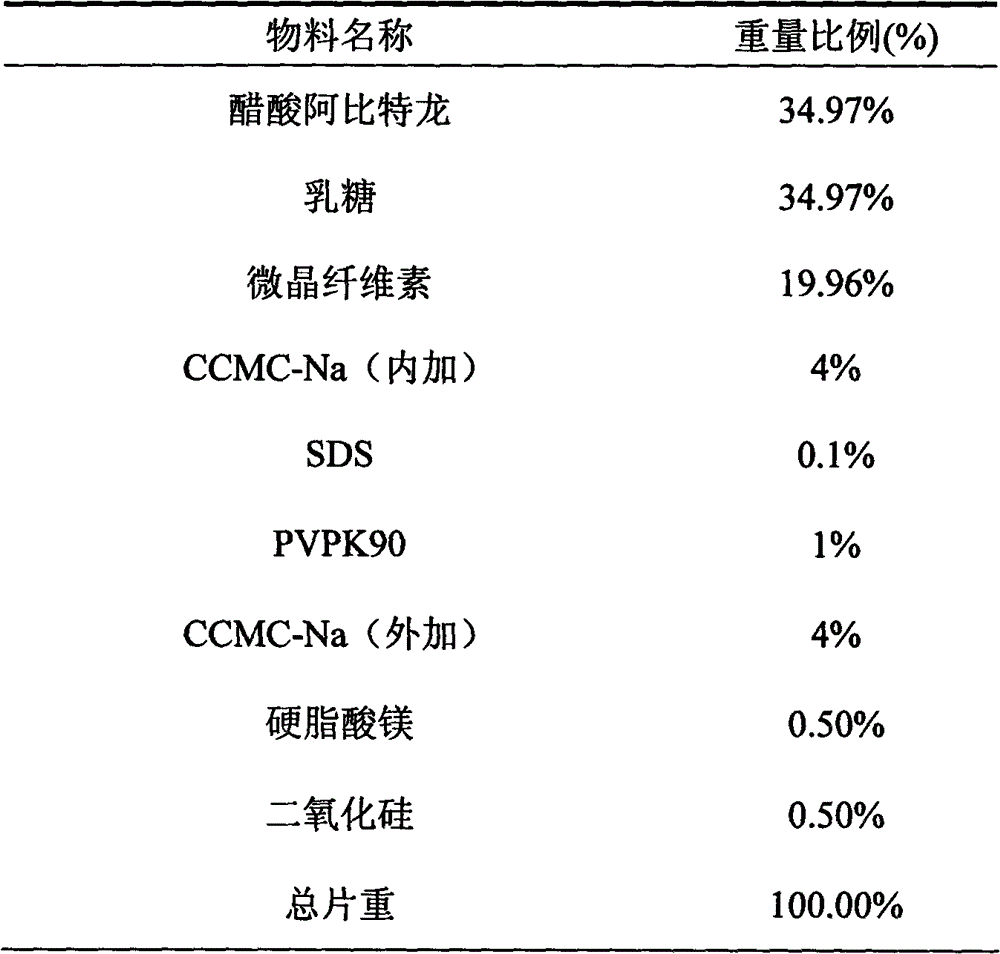
[0017] Embodiment 1 Abiraterone acetate sheet preparation
[0018]
[0019] Preparation process: Weigh the prescribed amount of abiraterone acetate: lactose (1:1), pulverize to obtain a co-powder, add the prescribed amount of microcrystalline cellulose, croscarmellose sodium (internal addition) and pass through an 80-mesh sieve Mix well. Put the above mixed powder in a dry grinding cup, add an appropriate amount of soft material made of 5% povidone K90 aqueous solution containing 0.1% SDS in the prescription amount, granulate with a 18-mesh sieve, dry until the moisture is lower than 5%, 24-mesh Sieve for granulation, add croscarmellose sodium, magnesium stearate and silicon dioxide, mix well; press into tablets to obtain.
Embodiment 2
[0038] The preparation of embodiment 2 Abiraterone Acetate Tablets
[0039]
[0040]
[0041] Preparation process: Weigh the prescribed amount of abiraterone acetate: lactose (1:1), pulverize to obtain a co-powder, add the remaining amount of lactose, microcrystalline cellulose, croscarmellose sodium (internal addition) over 80 Mesh sieve to mix. Put the above mixed powder in a dry grinding cup, add an appropriate amount of soft material made of 5% povidone K90 aqueous solution containing 0.1% SDS in the prescription amount, granulate with a 18-mesh sieve, dry until the moisture is lower than 5%, 24-mesh Sieve for granulation, add croscarmellose sodium, magnesium stearate and silicon dioxide, mix well; press into tablets to obtain.
Embodiment 2-1
[0042] The preparation of embodiment 2-1 Abiraterone acetate sheet
[0043]
[0044] Preparation process: Weigh the prescribed amount of abiraterone acetate: lactose (1:1), pulverize to obtain a co-powder, add the remaining amount of lactose, microcrystalline cellulose, croscarmellose sodium (internal addition) over 80 Mesh sieve to mix. Put the above mixed powder in a dry grinding cup, add an appropriate amount of soft material made of 5% povidone K90 aqueous solution containing 0.1% SDS in the prescription amount, granulate with a 18-mesh sieve, dry until the moisture is lower than 5%, 24-mesh Sieve for granulation, add croscarmellose sodium, magnesium stearate and silicon dioxide, mix well; press into tablets to obtain.
[0045] Embodiment 2-2 Dissolution comparative study of embodiment 2 and comparative example 2-1
[0046]Measure the abiraterone acetate sheet prepared by the embodiment of the present invention 2 and its comparative example, take 900ml, 0.25% SDSpH4.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com