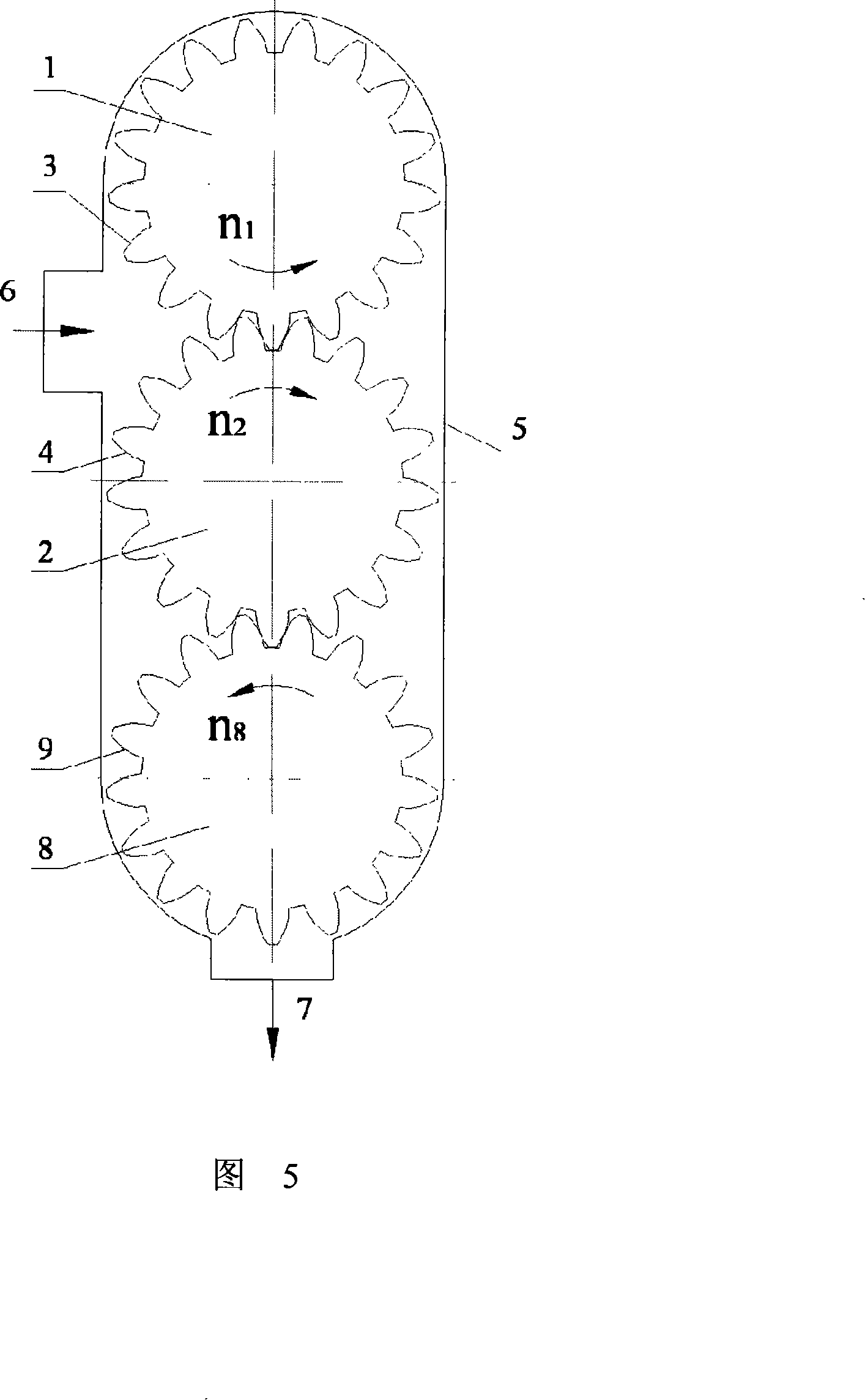Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Drug industry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for comprehensively preventing and controlling plant diseases and insect pests in artificial gastrodia cultivation under wild-simulated condition
InactiveCN101695247AImprove the level of prevention and controlIncrease economic incomeHorticultureThree levelGastrodia
The invention discloses a method for comprehensively preventing and controlling plant diseases and insect pests in artificial gastrodia cultivation under wild-simulated condition. The method comprises the following steps: paving fallen leaves and branches in a pit; scattering three-level strains on the branches; after covering the pit with soil, cultivating the strains for 40 to 60 days; chopping out a scale gap in sticks; paving the fallen leaves in the cultivation pit; putting a layer of sticks; adding strain branches among the sticks; covering the pit by sandy soil; and obtaining the strains for later use through cultivation. During planting, the strains are alternately put among the sticks so that gastrodia seeds are close to the strains as possible; then, the pit is covered by the soil and all the gaps are filled. The growing process only needs routine technology for management. By the technology, the incidence rate of the plant diseases is reduced from 8 percent to below 4 percent; the incidence rate of the insect pests is reduced from 10 percent to below 5 percent, thereby remarkably improving the level of preventing and controlling the plant diseases and insect pests in the artificial gastrodia cultivation, improving the output of gastrodia, providing raw materials for pharmacy and improving the economic income of farmers in mountainous areas.
Owner:XISHUANGBANNA TROPICAL BOTANICAL GARDEN CHINESE ACAD OF SCI
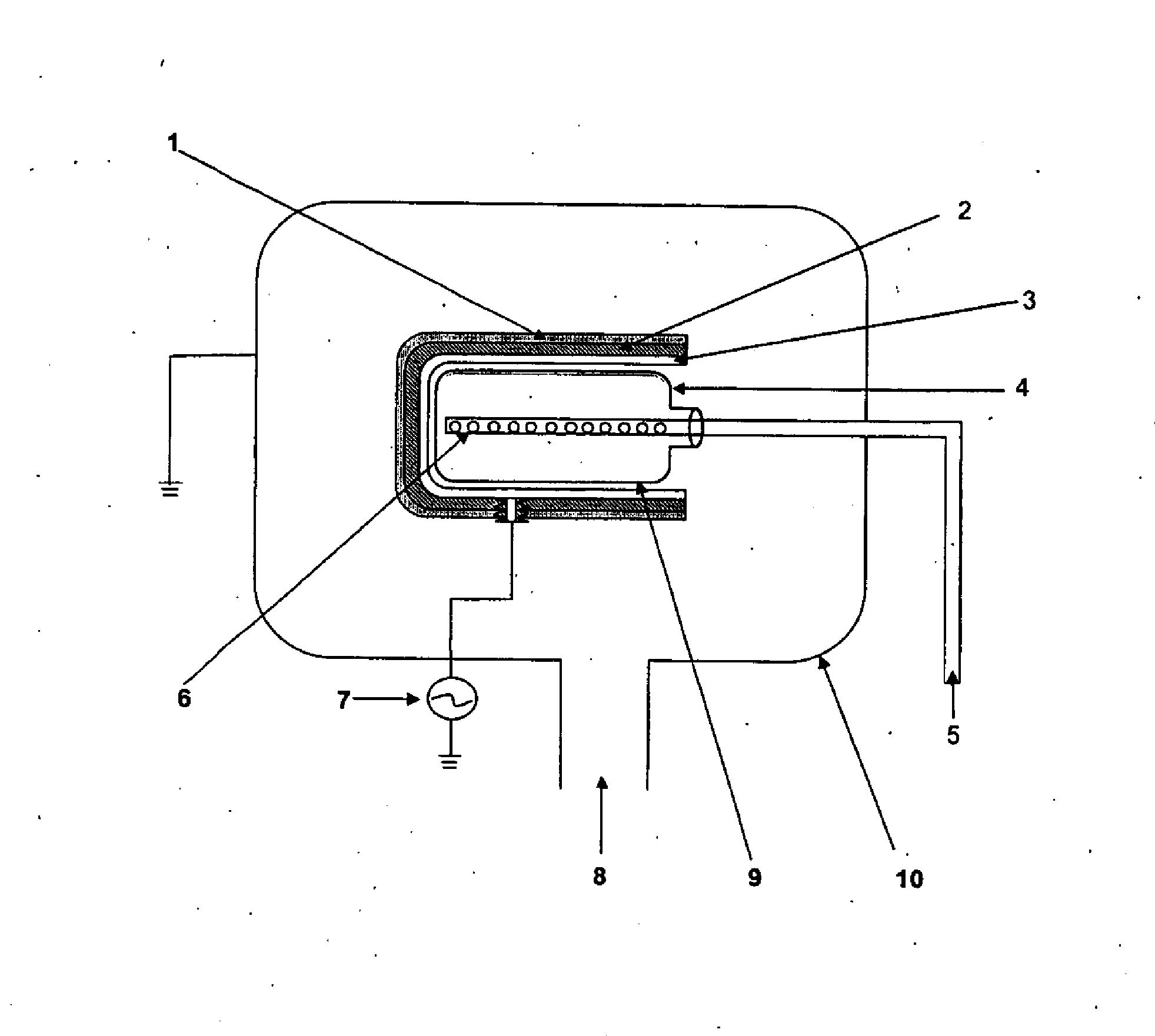
Resonance driven changes in chain molecule structure
InactiveUS6060293AEfficient inductionPeptide preparation methodsElectrical/wave energy microorganism treatmentChemical industryDisease
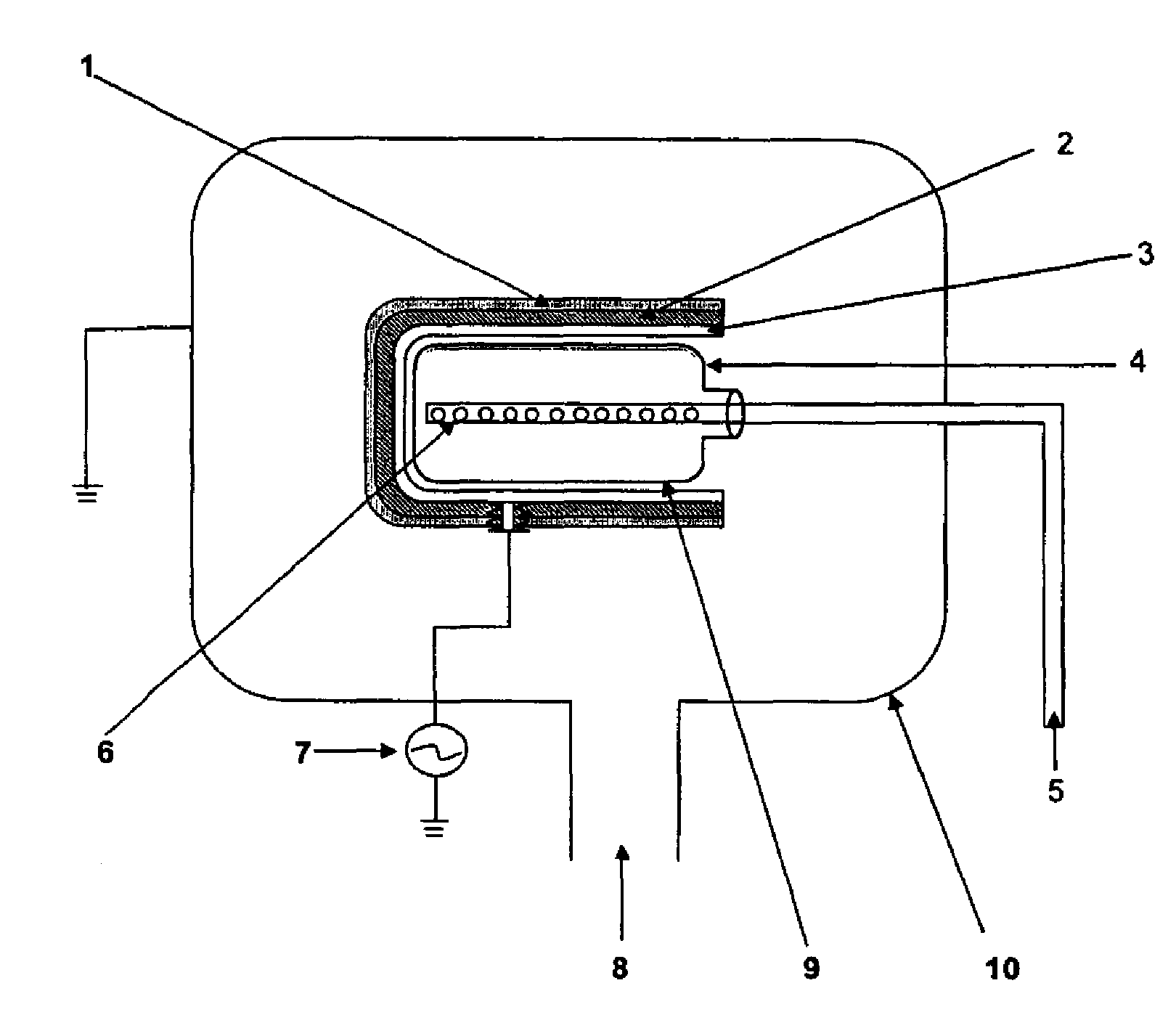
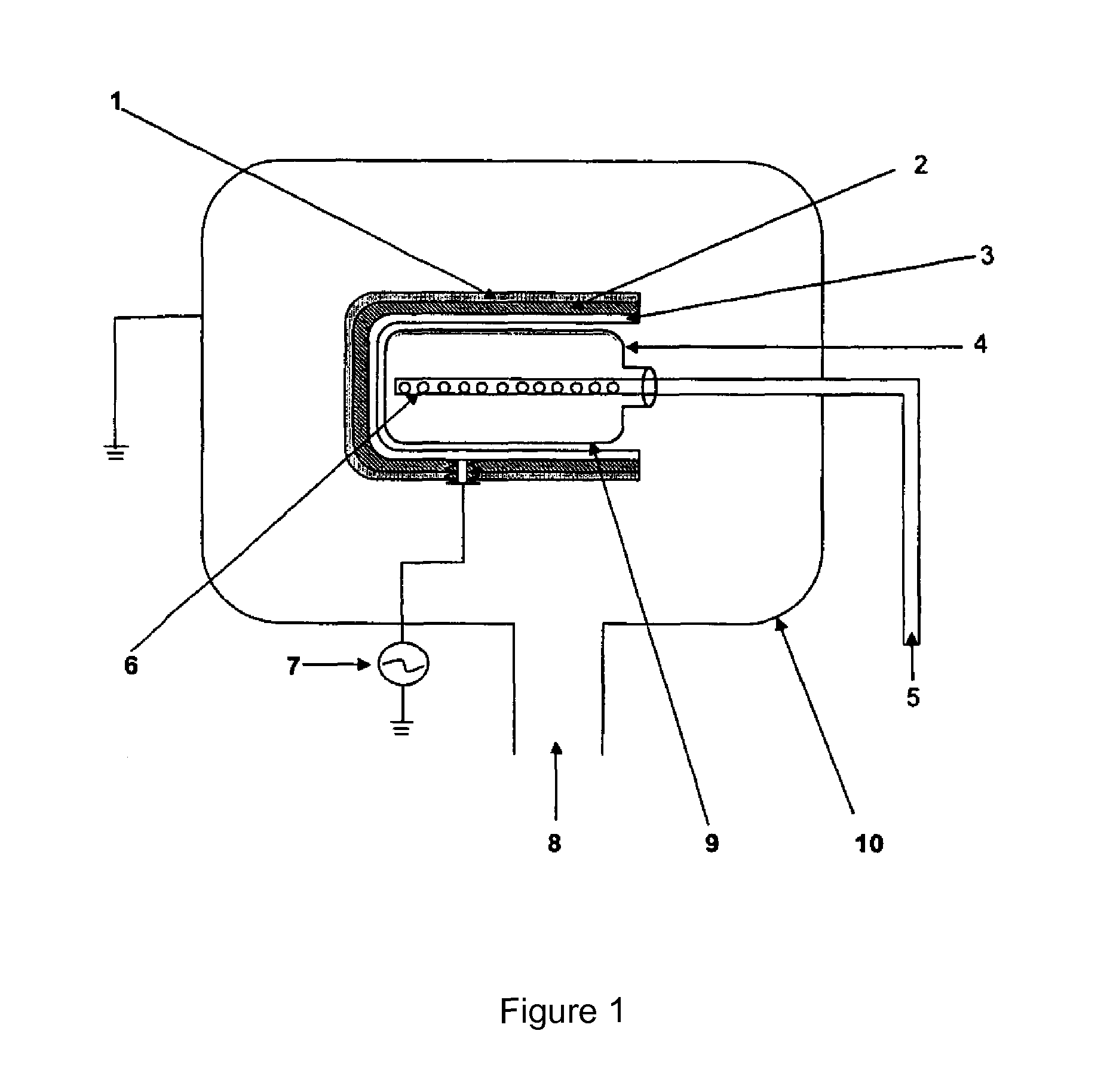
PCT No. PCT / DK96 / 00158 Sec. 371 Date Nov. 26, 1997 Sec. 102(e) Date Nov. 26, 1997 PCT Filed Apr. 1, 1996 PCT Pub. No. WO96 / 30394 PCT Pub. Date Oct. 3, 1996The invention relates to the technical application of electromagnetic radiation such as microwaves and radiowaves and application of ultra sound to chain molecules. In particular, the present invention relates to the utilization of topological excitations such as wring, twist and torsional modes, e.g., for generating structure, such as in folding, refolding or renaturation, and denaturation or unfolding of peptides, polypeptides, proteins, and enzymes; for generating changes in molecular affinity; for stimulating drug receptor interactions; and for changing molecular communication, is described. The technique is based on a new understanding of the underlying physical phenomenon and can also be applied to other chain molecules and biologically active biomolecules and tailored polymers such as glucoproteins, antibodies, genomic chain molecules such as DNA and RNA as well as PNA, carbohydrates, and synthetic and natural organic polymers. The invention is especially applicable for solving problems related to inclusion bodies and aggregation when using recombinant DNA and protein engineering techniques. Furthermore, the invention can be utilized in therapeutic treatment and in development and production of pharmaceuticals. The area of applicability ranges from biotechnological industry, food industry, drug industry, pharmacological industry, chemical industry, and concerns, e.g., the treatment of conditions and diseases related to influenza, hepatitis, polio, malaria, borrelia, diabetes, Alzheimer's disease, Creutzfeldt Jakob disease, other prion related diseases, multiple sclerosis, cataract, heart diseases, cancer, and aging.
Owner:PROKYON
Process to Deposit Diamond Like Carbon as Surface of a Shaped Object
InactiveUS20120045592A1Accurate frequencyMinimize and completely avoid damageElectric discharge tubesLinings/internal coatingsDiamond-like carbonCompanion animal
A plasma based deposition process to deposit thin film on the inner surfaces of the shaped objects such as plastic or metallic object like bottles, hollow tubes etc. at room temperature has been developed. In present invention uniform hydrogenated amorphous carbon (also called Diamond-Like Carbon, DLC) films on inner surfaces of plastic bottles is successfully deposited. Applications of such product include entire food and drug industries. There is a huge demand of polyethylene terephthalate (PET) or polyethylene naphthalate (PEN)) bottles, meant for the storage of potable water, carbonated soft drinks, wines, medicines etc. However, the higher cost prohibits their wide, spread use. The cheaper alternative is to use plastic bottles inside coated with chemically inert material such as Diamond-Like Carbon (DLC) will be commercially viable. Inventor process can be scaled up for mass production. This process can also be used for coating on inner surface of metallic cane or tube with a carbide forming interlayer (like hydrogenated amorphous silicon) to get the DLC films with better adhesion to inner surface of metals.
Owner:COUNCIL OF SCI & IND RES
System and method for correlating market research data based on attitude information
InactiveUS20050114171A1Maximizing frequencyMaximizing timelinessMedical data miningDrug and medicationsPhysician perceptionPharmaceutical industry
The invention relates to an integrated system and method for collecting information for the pharmaceutical industry to assess opinions concerning sales and marketing forces, prescribing patterns and attitudinal physician perceptions regarding specific pharmaceutical brands. These three areas are evaluated through three modules, each consisting of a separate survey administered via the internet, and capable of producing reports integrating all three areas. The surveys are entitled: 1) Continuous Promotion Tracking Study (CPT); 2) Rx Intentions and Treatment Study (RxIT); and 3) Therapeutic Class Attitude and Perception Study (TCAP).
Owner:GFK US HLDG
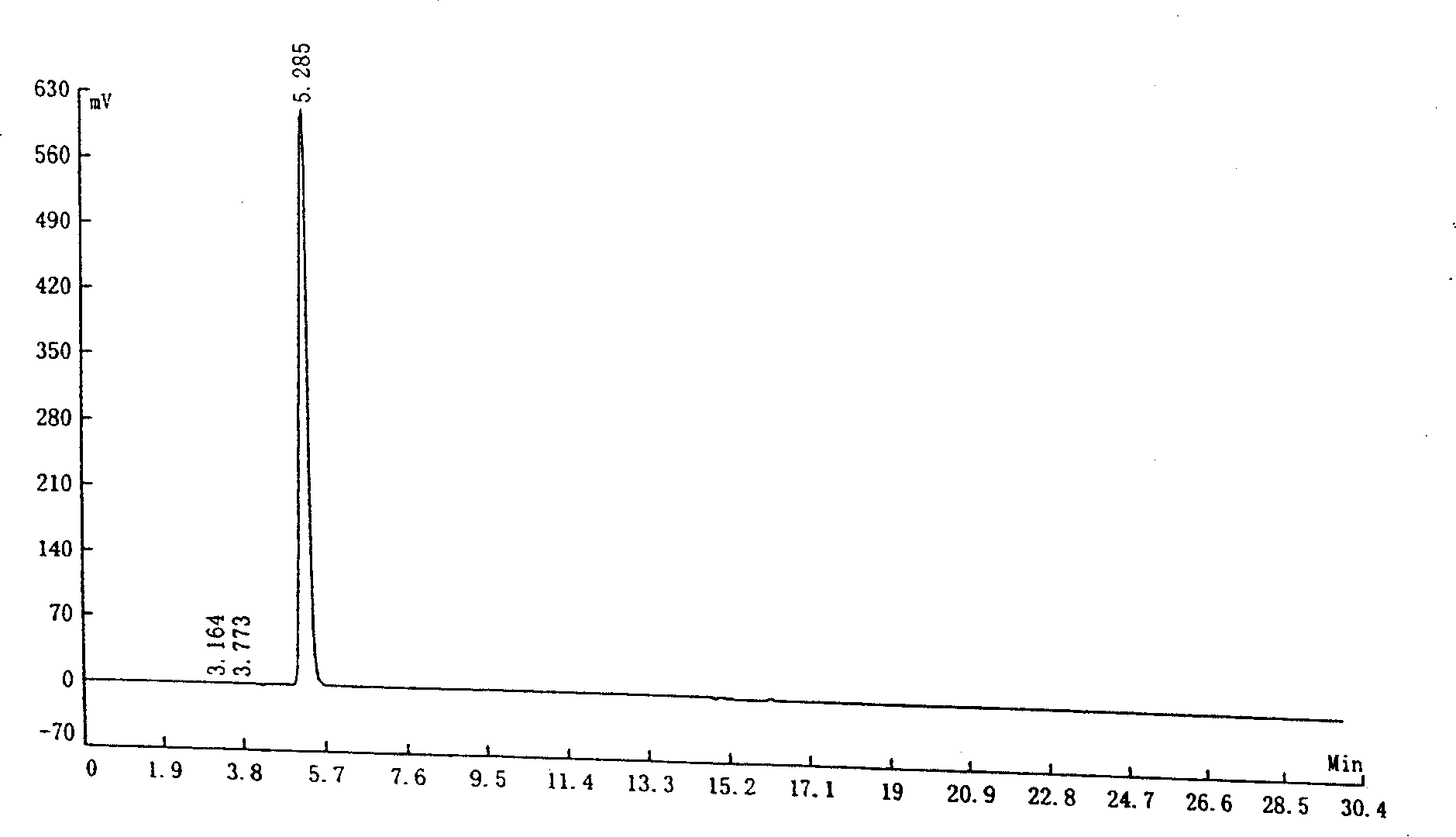
Solvent recovery method in pharmacy and solvent recovery system for implementing same
ActiveCN101249309AReduce lossReduce pollutionVapor condensationBoiling apparatus with condenserRecovery methodCondensation process
A solvent recovery device in pharmacy which is mainly characterized in that a liquid ring pump is used for extracting the solvent steam which is separated from a gas-liquid separator, the liquid ring pump increases the pressure of the solvent steam, and then a condenser and the gas-liquid separator carries out the secondary condensation and the gas-liquid separation of the solvent steam after the pressure increases. A solvent recovery system for achieving the method is mainly characterized in that a steam exhaust port of the gas-liquid separator is connected with the liquid ring pump, an output of the liquid ring pump is sequentially connected with a secondary condenser and a secondary gas-liquid separator in series, a liquid discharge port of the secondary gas-liquid separator is connected with a working liquid input port of the liquid ring pump and a recovery tank, and the liquid ring pump takes the solvent as the working liquid. The solvent recovery device can not only increase the times of the condensation and the gas-liquid separation of the solvent stream, but can also increase the condensation pressure during the secondary condensation process, thus significantly increasing the solvent recovery rate, reducing the solvent loss and the production cost of the pharmaceutical industry and reducing the environmental pollution.
Owner:GUANGDONG KENFLO PUMP CO LTD
Micro grinding gear mill
The invention discloses a micro-grinding gear grinder, belonging to micro-grinding machinery. The technical scheme includes that a grinding roller (1) and a conjugate tooth profile (3) uniformly distributed on the surface of an outer cylinder of the roller compose a gear grinding roller, and a grinding roller (2) and a conjugate tooth profile (4) uniformly distributed on the surface of the outer cylinder of the roller compose one another gear grinding roller. Two gear grinding rollers with parallel axes and definite central distances are placed in a grinding body (5), each gear grinding roller is in toothed rotation around each axial cord relative to the grinding body (5) via fixed velocity ratio, and a feed inlet (6) and a feed outlet (7) are respectively arranged on the grinding body (5), forming the micro-grinding gear grinder. The working principle of the invention is that the material is disintegrated through the squeezing, grinding and shearing of the two tooth surfaces of the rollers, the invention can be adaptable to micro-grinding of powder or liquid material of the fields of chemical industry, building material, drug industry and the like.
Owner:XINGTAI POLYTECHNIC COLLEGE
Method for preparing low-chlorine bleach lac
InactiveCN101157828AMeet the requirements of low chlorine bleached shellacNatural resin purificationFood preparationFiltrationBleach
A preparation method of less chlorine bleaching lac: first, lac grains are put into sodium carbonate solution under the temperature of 60 DEG C to 100 DEG C and are completely dissolved. And then the solution is cooled below 70 DEG C and waxiness and insoluble content in the lac are filtrated; the filtrated solution under the temperature of 20 DEG C to 40 DEG C is added with sodium hypochlorite to blanch, then the temperature is increased to 70 DEG C to 100 DEG C. Next, sodium ethoxide is put into the solution to react. After the solution being cooled to room temperature or below 30 DEG C, sodium hypochlorite solution is dropped into the solution to blanch. Then the solution is filtrated with filtration aids such as white mud, diatomite and so on; the filtrated solution is added with dilute sulphuric acid or dilute hydrochloric acid to precipitate. The precipitation is dried by heat air after being washed and solid / liquid separation. In the end, the less chlorine bleaching lac which is white or slight yellow powder can be gotten. The product can satisfy the demand for food industry and pharmacy. The chlorine content in the product is less than 0.3 percent (wt percent). The time for thermal polymerization under the temperature of 170 DEG C plus or minus 1 DEG C amounts to above 5 minutes. The storage period is more than 2 years.
Owner:KUNMING UNIV OF SCI & TECH
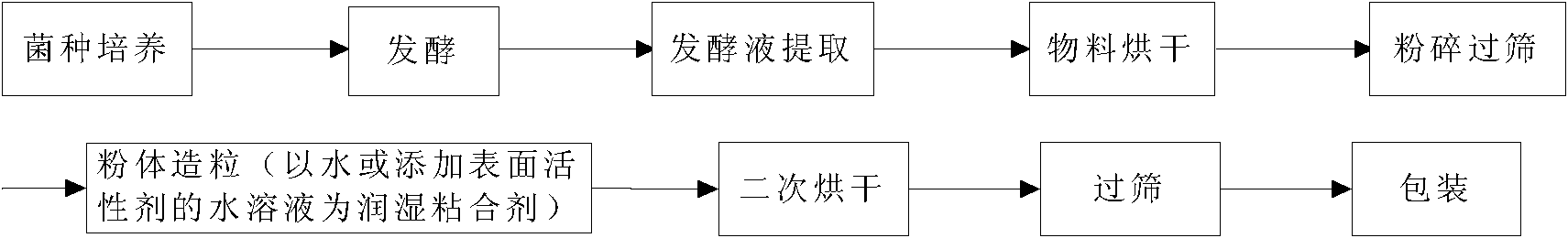
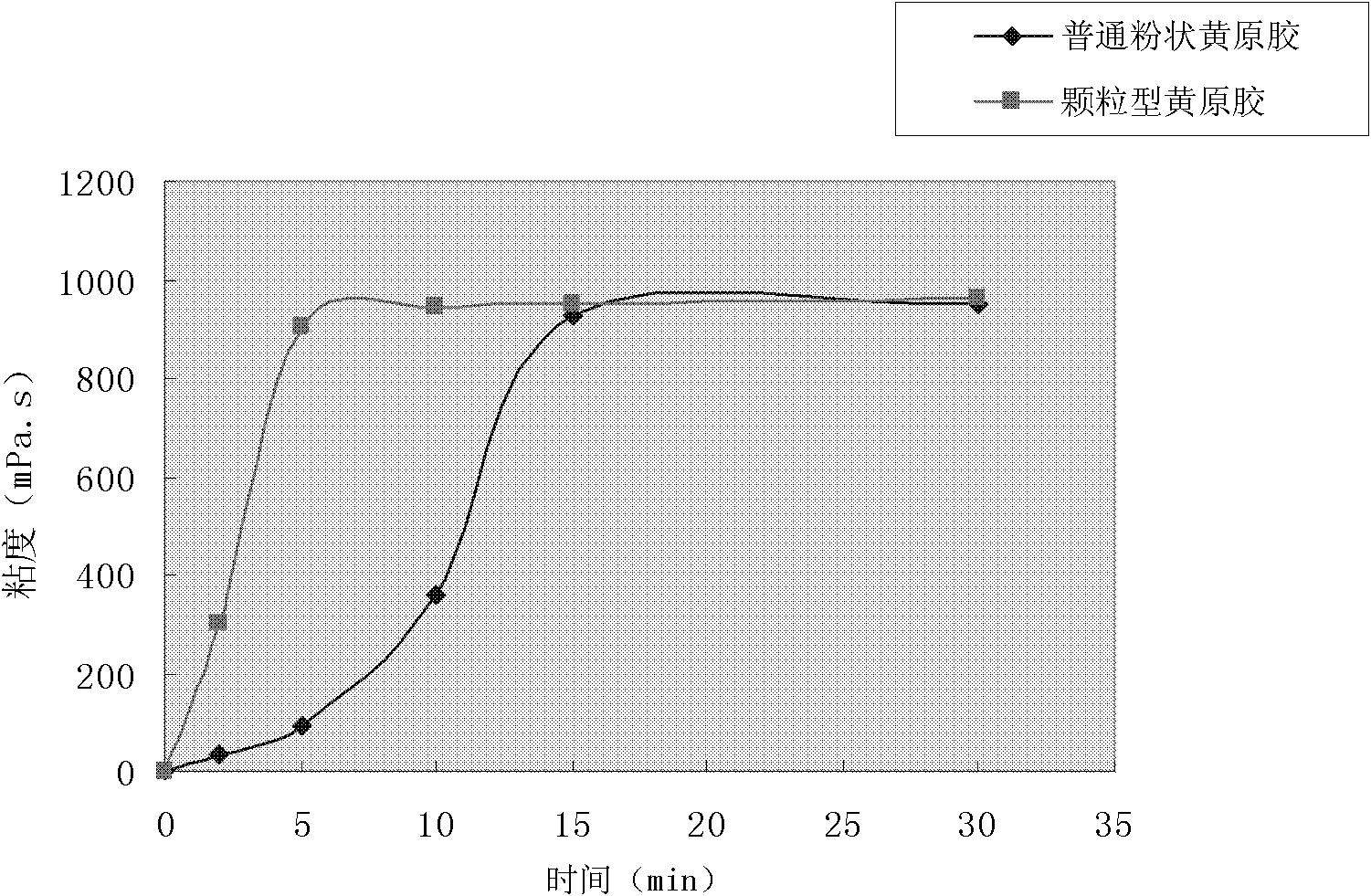
Preparation method for granular type xanthan gum
ActiveCN102219912AImprove dispersion hydration speedSolve the technical problems of agglomerationPharmaceutical non-active ingredientsFood preparationPorosityD-Glucose
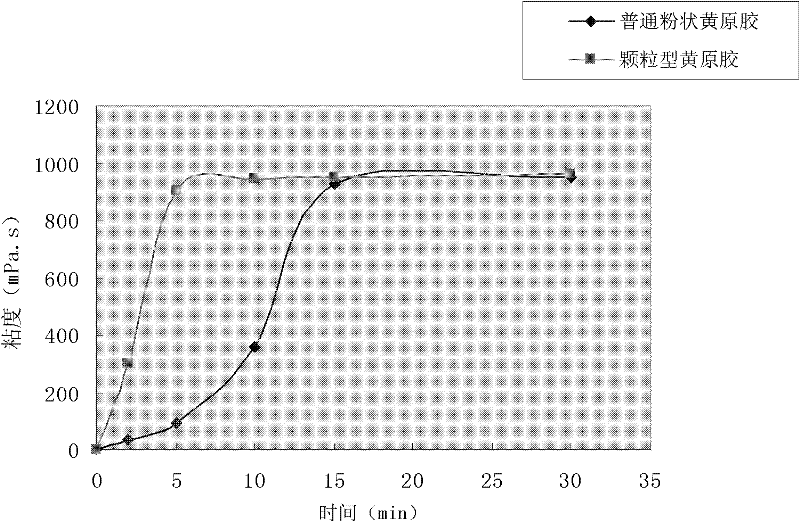
The invention relates to a preparation method for granular type xanthan gum, belonging to the field of preparing glucose residue-containing compound. The preparation method is characterized by taking xanthan gum powder as starting raw materials, adopting a boiling granulation drying device to prepare the granular type xanthan gum, and comprises the following steps of 1.discharging of xanthan gum powder; 2. introduction of hot air; 3. spraying of atomized water; 4. boiling granulation; 5. secondary drying; 6. screening; and 7. quality inspection, and finally packaging for storage after qualification. The invention provides a preparation method for granular type xanthan gum with large specific area and high porosity. The prepared granular type xanthan gum substitutes powder products selling on the market, and solves the problems that the powder xanthan gum in the prior art is slowly dissolved, and blocked during dissolving process and has dust pollution. The preparation method is applicable for granulation and processing of unpackaged powder after crushing and screening during production process of xanthan gum powder production devices, as well as granulation and processing xanthan gum powder used in market sauce seasoning, soup bases, drinks, food, drug industry and petroleum industry.
Owner:ORDOS ZHONGXUAN BIOCHEM
Cefuroxime axetil pharmaceutical composition and preparation method thereof
ActiveCN104586854AHigh dissolution rateGood dissolution effectAntibacterial agentsOrganic active ingredientsPharmaceutical industryFluidized bed
The invention provides a cefuroxime axetil pharmaceutical composition and a preparation method thereof. The method comprises the following steps: fully fluidizing cefuroxime axetil with specific particle size distribution in a fluidized bed; slowly spraying molten refractory materials on the surface of cefuroxime axetil through a spraying device, and fully enveloping the surface of the cefuroxime axetil; and adding other auxiliary materials, mixing evenly, and preparing a cefuroxime axetil dry suspension or granule. The preparation process can be finished in the fluidized bed; a hot melt pelletizer or a spray dryer is not used; and industrialized mass production of the modern pharmaceutical industry is facilitated.
Owner:SHIJIAZHUANG NO 4 PHARMA
Gold nanometer particle grain size control method based on glutathione
Disclosed is a method for controlling the grain diameter of gold nanometer particles with glutathion, belonging to the field of nanometer technology. The specific steps are as following: a. mixing the citric acid trisodium solution with glutathion solution; b. heating separately the solution prepared by step-a and chlorauric acid solution, then mixing; c. heating the solution to boiling to make the reaction complete after the solution prepared by step-b off-color, then cooling the liquid to prepare gold nanometer particle sol solution. The method is characterized in that: it is simple and the efficiency is high, the particle dimension is easy to adjust, and the creature compatibility is good, and the prepared nanometer particles has a good dispersibility and a uniform grain diameter which can be controlled by a range of 8-40nm. The gold nanometer particles can apply in the field of DNA detection, biology, drug industry, and so on.
Owner:SHANGHAI JIAO TONG UNIV
Candida utilis expression vector and construction method thereof
InactiveCN101487019ASave manpower, material and financial resourcesFermentationVector-based foreign material introductionGenomic DNAPBR322
The invention discloses an expression carrier of an industrial microorganism that is candida utili and simultaneously provides a construction method of the candida utili expression carrier. The invention uses a basic skeleton of a pBR322 carrier and a template of candida utili genomic DNA, obtains a cycloheximide resistance gene with the site-directed mutagenesis method, obtains a rDNA segment and a GAP promoter-terminator segment of the candida utili genomic DNA by adopting the PCR method and the template of the candida utili genomic DNA, and constructs the integrated candida utili expression carrier that is mediated by 18S rDNA and has cycloheximide resistance. The integrated candida utili expression carrier can be used for preparing a foreign candida utili expression protein and can be widely applied into the fields of feedstuff addition agents, food industry, and pharmacy, and the like.
Owner:THE INST OF MICROBIOLOGY XINJIANG ACADEMY OF AGRI SCI
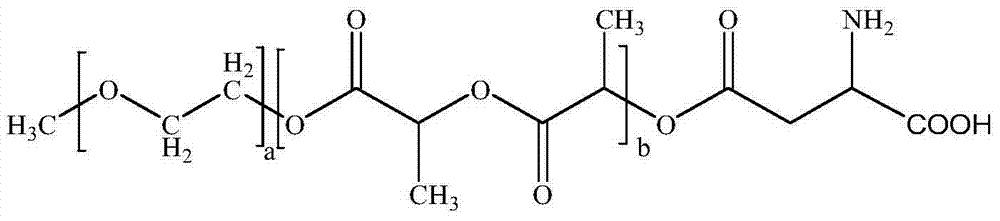
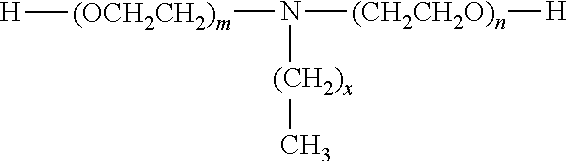
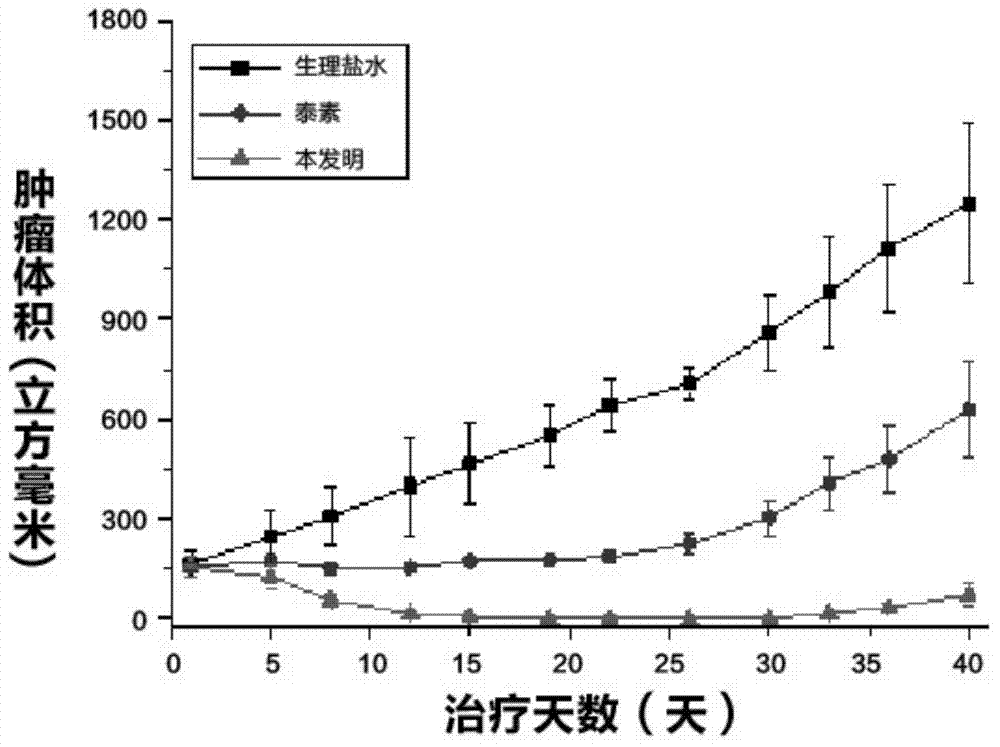
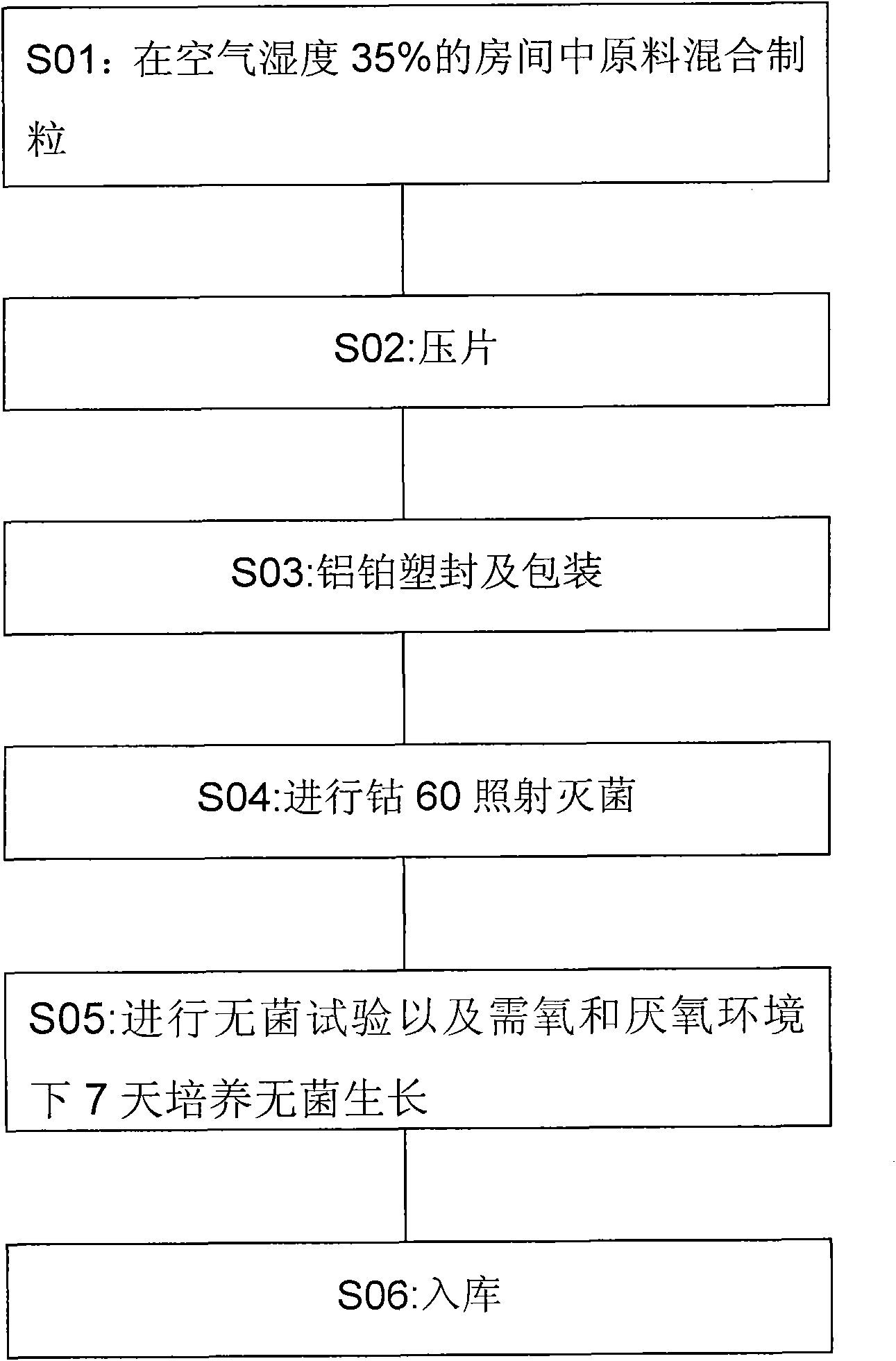
Medicinal composition of paclitaxel
InactiveCN104511022AMeet treatment requirementsHigh stability in vitroPowder deliveryOrganic active ingredientsBenproperineFreeze-drying
The invention belongs to the technical field of medicinal preparations, and concretely relates to a medicinal composition of paclitaxel. The medicinal composition comprises, by weight, 2.87-27.66 parts of paclitaxel and 100 parts of mPEG-PLA-phenylalanine. The medicinal composition has the following advantages: 1, the in vitro stability is high, and a re-dissolved paclitaxel solution keeps stable at room temperature for above 12h to meet medicinal treatment requirements; 2, the medicinal composition has high stability in blood and small particle size, and can easily perform the ERP effect; 3, a novel micelle carrier is adopted, so the toxicity is low, and the safety is high; and 4, a pharmacy common freeze-drying technology is directly adopted to prepare freeze-dried powder, so the medicinal composition is simple to prepare, and is convenient to transport and store, and untoward effects brought by the addition of an excipient are avoided The medicinal composition can be instantly re-dissolved by using common normal saline or glucose injection, so the administration process is greatly simplified.
Owner:POLYMERCHEM
Method for preparing high purity alpha corn protein
The present invention provides a kind of high purity alpha-corn protein preparing process. The preparation process includes the following steps: extracting alpha-corn protein from corn protein powder with 90 % concentration ethanol solution in a countercurrent extraction process; the subsequent regulating the temperature and pH value of ethanol solution of alpha-corn protein, and adding active carbon through stirring to decolorize; and the final closed-path spray drying under the protection of nitrogen to obtain high quality alpha-corn protein. The decolorized and deodorized alpha-corn protein may be used safely in food and medicine industry.
Owner:JIANGSU UNIV
Medicinal composition of paclitaxel
InactiveCN104511020AMeet treatment requirementsHigh stability in vitroOrganic active ingredientsPowder deliveryFreeze-dryingExcipient
The invention belongs to the technical field of medicinal preparations, and concretely relates to a medicinal composition of paclitaxel. The medicinal composition comprises, by weight, 1.12-27.66 parts of paclitaxel and 100 parts of mPEG-PLA-aspartic acid. The medicinal composition has the following advantages: 1, the in vitro stability is high, and a re-dissolved paclitaxel solution keeps stable at room temperature for above 12h to meet medicinal treatment requirements; 2, the medicinal composition has high stability in blood and small particle size, and can easily perform the ERP effect; 3, a novel micelle carrier is adopted, so the toxicity is low, and the safety is high; and 4, a pharmacy common freeze-drying technology is directly adopted to prepare freeze-dried powder, so the medicinal composition is simple to prepare, and is convenient to transport and store, and untoward effects brought by the addition of an excipient are avoided. The medicinal composition can be instantly re-dissolved by using common normal saline or glucose injection, so the administration process is greatly simplified.
Owner:POLYMERCHEM
Cosmetic composition comprising peba
The present invention relates to a composition comprising from 0.1 to 30% by weight of copolymer comprising polyether blocks and polyamide blocks (PEBA), and from 70 to 99.9% by weight of a medium which is acceptable in the cosmetics industry, in the perfumery industry and / or in the pharmaceutical industry. The present invention also relates in particular to a process for incorporating a copolymer comprising polyether blocks and polyamide blocks into a cosmetic, perfumery and / or pharmaceutical medium. The subject matter of the present invention is also the use of a copolymer comprising polyether blocks and polyamide blocks (PEBA) for producing a cosmetic, pharmaceutical or perfumery product, said PEBA being incorporated in the form of a composition in accordance with the invention.
Owner:ARKEMA FRANCE SA
Preparation method of phosphatidic acid
The invention discloses a preparation method of phosphatidic acid. The preparation method includes the following steps that rough lecithin is taken and dissolved in an organic solvent to be prepared intro a rough lecithin solution, phospholipase D is taken and prepared into a phospholipase D aqueous solution, the rough lecithin solution and the phospholipase D aqueous solution are mixed to perform an enzyme catalysis reaction, an organic phase is separated out after the reaction ends, the organic phase is dried in a concentration mode, concentrated matter is obtained and extracted through an extracting agent and precipitates at low temperature, undissolved matter in the extracting agent is taken after heat preservation, the undissolved matter in the extracting agent passes a chromatography column after being dried in a concentration mode, obtained products are dried to remove eluting liquid after an eluting solvent is used for elution, and then high-purity phosphatidic acid is obtained. The soybean or sunflower seed rough lecithin serves as raw materials, and therefore the price is low as the phosphatidylcholine content is low. According to the method, the overall technology is simple, energy consumption and cost are low, large-scale industrialization can be achieved easily, the content of prepared phosphatidylcholine is 60% or above, purity is high, and the method has great significance in the phospholipids industry, health-care food and the pharmaceutical industry in China.
Owner:WENGYUAN GUANGYE QINGYI FOOD TECH
Medicinal composition of paclitaxel
InactiveCN104511023AMeet treatment requirementsHigh stability in vitroOrganic active ingredientsPowder deliveryFreeze-dryingExcipient
The invention belongs to the technical field of medicinal preparations, and concretely relates to a medicinal composition of paclitaxel. The medicinal composition comprises, by weight, 1.04-28.37 parts of paclitaxel and 100 parts of mPEG-PLA-lysine. The medicinal composition has the following advantages: 1, the in vitro stability is high, and a re-dissolved paclitaxel solution keeps stable at room temperature for above 12h to meet medicinal treatment requirements; 2, the medicinal composition has high stability in blood and small particle size, and can easily perform the ERP effect; 3, a novel micelle carrier is adopted, so the toxicity is low, and the safety is high; and 4, a pharmacy common freeze-drying technology is directly adopted to prepare freeze-dried powder, so the medicinal composition is simple to prepare, and is convenient to transport and store, and untoward effects brought by the addition of an excipient are avoided. The medicinal composition can be instantly re-dissolved by using common normal saline or glucose injection, so the administration process is greatly simplified.
Owner:POLYMERCHEM
Preparation method of phosphatidyl serine
The invention discloses a preparation method of phosphatidyl serine, which comprises the following steps: taking coarse soybean lecithin as a raw material, adding water-saturated butyl acetate, stirring, and performing reflux degradation; evaporating the butyl acetate under reduced pressure, adding methanol into the concentrate, and performing reflux dissolution; recovering the methanol from the methanol layer under reduced pressure, thus obtaining a methanol solvend; preparing the methanol solvend and organic solvent into a solution, then preparing the organic phase and an enzyme-containing water solution into a water phase, mixing the organic phase and the water phase, and reacting; after the reaction is finished, separating out the organic phase, washing the organic phase with water, and removing the organic solvent in vacuum; and dissolving the concentrate with anhydrous ethanol, stirring, separating out an anhydrous ethanol insoluble substance, and performing vacuum drying to obtain the phosphatidyl serine product. Compared with the prior art, the preparation method has the following advantages: (1) the used raw material is the coarse soybean lecithin, so that the phosphatidylcholine content is low, and the price is low; and (2) the whole process is simple and easy to industrialize, the content of the prepared phosphatidyl serine is 40% or above, and the purity is high. Thus, the preparation method disclosed by the invention has important meanings for phospholipid industry, health food and medicine industry in China.
Owner:WENGYUAN GUANGYE QINGYI FOOD TECH +1
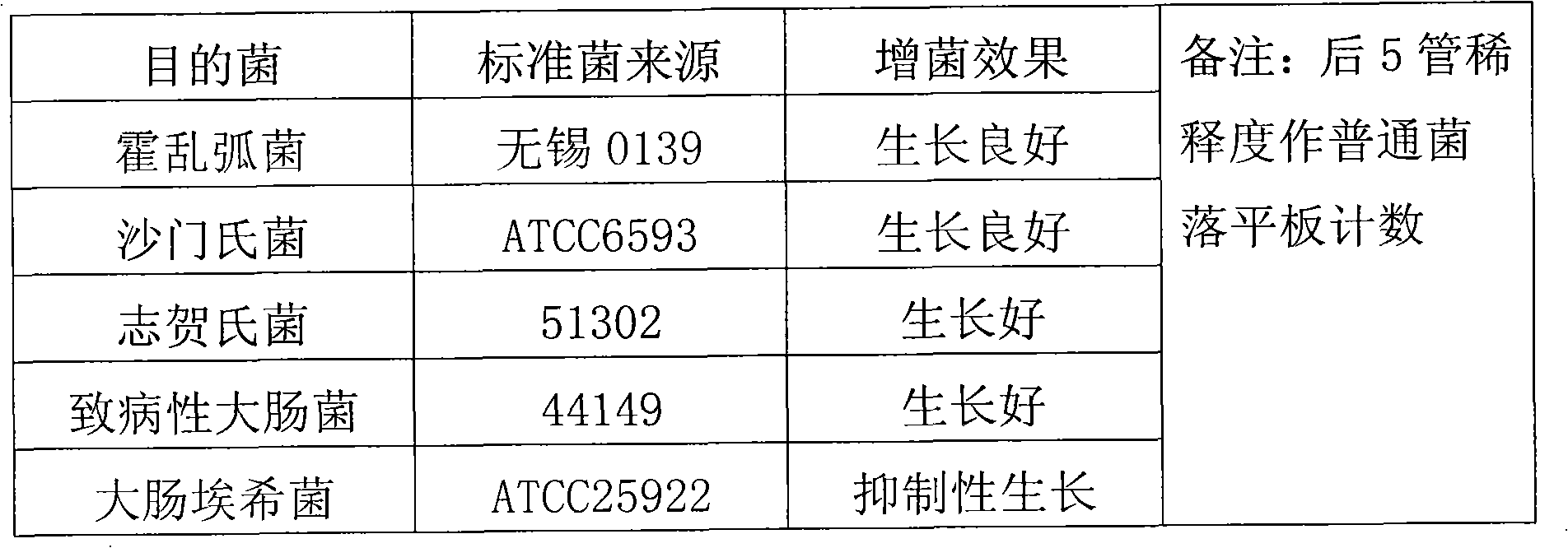
Intestinal enriched tablet culture medium and process method thereof
InactiveCN102212604AWide detection rangeGood effect of bacteria enrichmentMicrobiological testing/measurementAgainst vector-borne diseasesHigh concentrationEscherichia coli
The invention discloses an intestinal enriched tablet culture medium. An enriched tablet of the intestinal enriched tablet culture medium mainly comprises peptone, trypsin, beef powder, bovine bile salt, sodium deoxycholate, a special matrix, an additive, sodium citrate, sodium thiosulfate, brilliant green and the like, wherein the high-concentration bovine bile salt and sodium deoxycholate in the culture medium can inhibit the growth of gram-positive bacteria in excrement, have certain synergistic inhibitory effects on Escherichia coli and have good enriching effects on cholera bacteria, salmonella and shigella. The invention also provides a process method of the intestinal enriched tablet culture medium. The intestinal enriched tablet culture medium has the advantages of wide detection range, good stability and high sensitivity, can realize energy conservation and emission reduction, and is a low-carbon product with great popularization prospects. At present, the technology has passed various tests and detections of the State Food and Drug Administration and has been approved for production, and the detection results and data of the technology have become new standards in the drug industry.
Owner:WUXI HUSNG BIOLOGICAL REAGENT
Process for Determining the particle size distribution of an aerosol and apparatus for carrying out such a process
InactiveUS20060246010A1Less-time-consuming and costlyPowder deliveryInvestigating moving fluids/granular solidsCascade impactorDrug industry
The present invention provides, for the first time, a process that meets the needs of the drug industry for measuring the particle size of nebulised aerosols simultaneously or one after another by the laser diffraction method and the cascade impactor method which is known in the art. In this way it is possible to bring the reliability of the results of the laser diffraction process according to the invention into conformity with that of the cascade impactor, and thereby obtain a process which combines the advantages of the rapid laser diffraction process with the accuracy of the otherwise time-consuming cascade impactor method. In addition to the process, apparatus for carrying out the process are also disclosed.
Owner:BOEHRINGER INGELHEIM INT GMBH
Food and drug industry protective boots and boots producing method and boots technical criterion
The invention discloses safety shoes for food and medicinal industries and the production method and the technical standard of the shoes. The sides and the soles of the shoes respectively contain PVC-S-1000 51.0%, 51.2%; DOP 30.6%, 26.1%; polyester plasticizer GLOBINEX-2340-S 15.3%, 19.1%; compound rare earth stabilizer 2.1%, 2.5%; and inorganic silver antibacterial agent 1.0%, 1.2%. The safety shoes are manufactured by following steps: weighting materials of the sides and the soles of the shoes according to the weight percentage, granulating by a granulator to obtain plastic particles, respectively adding the plastic particles into an injection molding machine, and molding twice to obtain the safety shoes. The safety shoes produced by the method meet a certain technical standard, have resistance to chemical corrosion, animal and vegetal oil, impact and bacteria, and obviate hazardness existing in the food and the medicinal industries.
Owner:朱国侯
Process for determining the particle size distribution of an aerosol and apparatus for carrying out such a process
InactiveUS20050238588A1Less-time-consuming and costlyPowder deliveryMedical devicesCascade impactorDrug industry
The present invention provides, for the first time, a process that meets the needs of the drug industry for measuring the particle size of nebulised aerosols simultaneously or one after another by the laser diffraction method and the cascade impactor method which is known in the art. In this way it is possible to bring the reliability of the results of the laser diffraction process according to the invention into conformity with that of the cascade impactor, and thereby obtain a process which combines the advantages of the rapid laser diffraction process with the accuracy of the otherwise time-consuming cascade impactor method. In addition to the process, apparatus for carrying out the process are also disclosed.
Owner:BOEHRINGER INGELHEIM INT GMBH
Indole-3-formic acid purification process
InactiveCN1807412ASuitable for purity requirementsSuitable for high efficiency requirementsOrganic chemistryInorganic saltsOrganic solvent
The invention discloses a purifying process of indole-3-aminic acid, this process can accomplish by procedures as follows: 1)adding methyl ketones organic solvent into alkali metal salt solution of indole-3-aminic acid which is made by indole-3-formaldehyde; 2)adding reduction inorganic salt into alkali metal salt solution of indole-3-aminic acid which is made by indole-3-formaldehyde to reduce it; 3)acidizing the products of 1)and 2)by inorganic acid; 4)conductting recrystallization of the products uupwords to have high-purity indole-3-aminic acid. This invention is simple to operate, easy to find the material and low in cost. It is propitious to industrial production and adapt to the request of chemical industry, drug industry. ect.
Owner:北京成宇化工有限公司 +1
Process to deposit diamond like carbon as surface of a shaped object
InactiveUS9260781B2Minimize and completely avoid damageSuitable for mass productionElectric discharge tubesLinings/internal coatingsDiamond-like carbonCompanion animal
A plasma based deposition process to deposit thin film on the inner surfaces of the shaped objects such as plastic or metallic object like bottles, hollow tubes etc. at room temperature has been developed. In present invention uniform hydrogenated amorphous carbon (also called Diamond-Like Carbon, DLC) films on inner surfaces of plastic bottles is successfully deposited. Applications of such product include entire food and drug industries. There is a huge demand of polyethylene terephthalate (PET) or polyethylene naphthalate (PEN)) bottles, meant for the storage of potable water, carbonated soft drinks, wines, medicines etc. However, the higher cost prohibits their wide, spread use. The cheaper alternative is to use plastic bottles inside coated with chemically inert material such as Diamond-Like Carbon (DLC) will be commercially viable. Inventor process can be scaled up for mass production. This process can also be used for coating on inner surface of metallic cane or tube with a carbide forming interlayer (like hydrogenated amorphous silicon) to get the DLC films with better adhesion to inner surface of metals.
Owner:COUNCIL OF SCI & IND RES
Preparation method for granular type xanthan gum
ActiveCN102219912BLarge specific surface areaGood dispersionPharmaceutical non-active ingredientsFood preparationProcess engineeringXanthan gum
The invention relates to a preparation method for granular type xanthan gum, belonging to the field of preparing glucose residue-containing compound. The preparation method is characterized by taking xanthan gum powder as starting raw materials, adopting a boiling granulation drying device to prepare the granular type xanthan gum, and comprises the following steps of 1.discharging of xanthan gum powder; 2. introduction of hot air; 3. spraying of atomized water; 4. boiling granulation; 5. secondary drying; 6. screening; and 7. quality inspection, and finally packaging for storage after qualification. The invention provides a preparation method for granular type xanthan gum with large specific area and high porosity. The prepared granular type xanthan gum substitutes powder products sellingon the market, and solves the problems that the powder xanthan gum in the prior art is slowly dissolved, and blocked during dissolving process and has dust pollution. The preparation method is applicable for granulation and processing of unpackaged powder after crushing and screening during production process of xanthan gum powder production devices, as well as granulation and processing xanthan gum powder used in market sauce seasoning, soup bases, drinks, food, drug industry and petroleum industry.
Owner:ORDOS ZHONGXUAN BIOCHEM
Royal jelly enzymolysis product with moisture-absorbing moisture-keeping function
InactiveCN101129410AIncrease added valueGood moisture absorptionCosmetic preparationsAnthropod material medical ingredientsBee productsDrug industry
The invention provides a bee milk enzymolysis product with hygroscopic and humectant property, which enzymolyzes bee milk with parenzyme in the constant temperature and freezes and dries the product after filtering. The bee milk of the invention improves the bound water ability of the product, increases more hygroscopic and humectant property of the product than the raw material by enzymolysising and increasing amino and carboxy group or the like hydrophilic group. The invention explores the bee milk product deeply, which improves the added value of the bee product, develops the application range, has the good hygroscopic and humectant property, the nutrient function, can reinforces the effect by inside and outside association, and can apply to drug industry, food and cosmetics field as the nutrient extender and humectant.
Owner:ZHEJIANG UNIV
The synthetic method of carboxyamidotriazole
ActiveCN112358451BMeet market requirementsImprove processing stabilityOrganic chemistryPotassium carbonateDrug industry
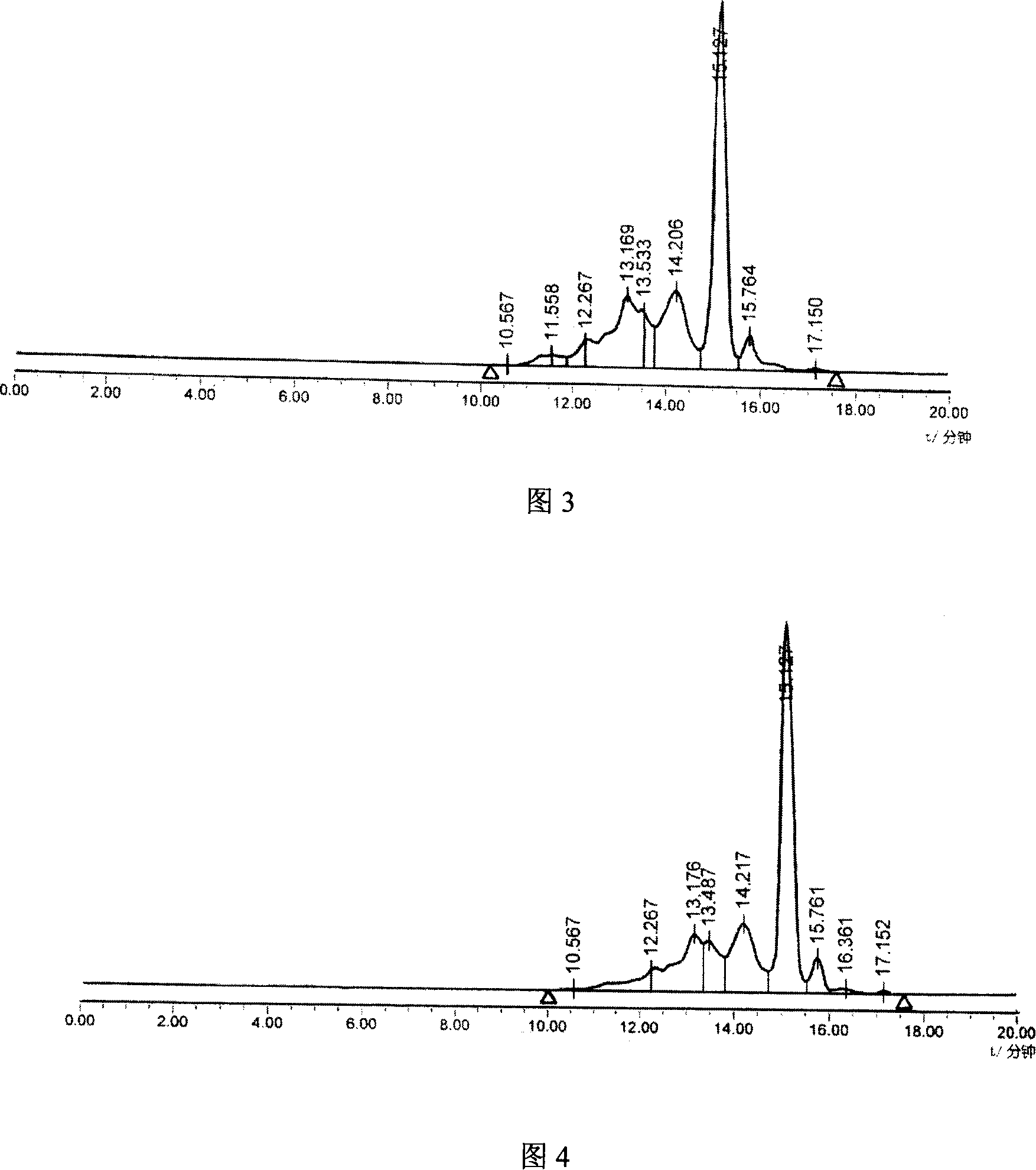
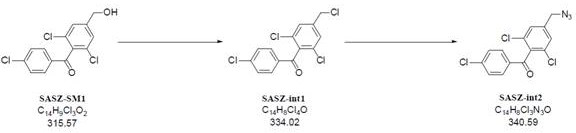
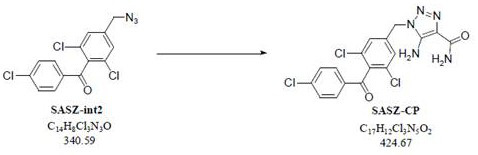
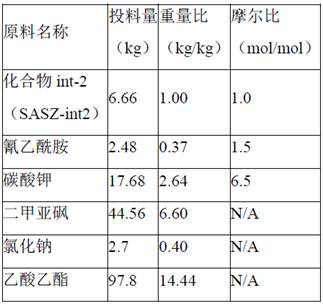
The present invention relates to a kind of synthetic method of carboxyamine triazole. The synthetic method of carboxyaminotriazole comprises the following steps: step (1) adopts " one pot method " to carry out halogenation reaction and azide substitution reaction successively; Step (2) uses DMSO as solvent, in potassium carbonate (K 2 CO 3 ) under the action of cyanoacetamide and compound int‑2 to undergo a Click ring-forming reaction; step (3) refines the product of step (2), and the raw material ratio, feeding sequence, feeding temperature, post-treatment and other multiple nodes for optimal control, so that the related substances in the prepared carboxyaminotriazole product can meet the requirements of drug quality and industrial production of drugs, and the synthesis method has high yield, low process cost, good stability, and is easy to use. Carry out industrialized scale-up production.
Owner:GUANGDONG YINZHU PHARMACEUTICAL TECHNOLOGY CO LTD
Indole-3-formic acid purification process
The invention discloses a purifying process of indole-3-aminic acid, this process can accomplish by procedures as follows: 1)adding methyl ketones organic solvent into alkali metal salt solution of indole-3-aminic acid which is made by indole-3-formaldehyde; 2)adding reduction inorganic salt into alkali metal salt solution of indole-3-aminic acid which is made by indole-3-formaldehyde to reduce it; 3)acidizing the products of 1)and 2)by inorganic acid; 4)conductting recrystallization of the products uupwords to have high-purity indole-3-aminic acid. This invention is simple to operate, easy to find the material and low in cost. It is propitious to industrial production and adapt to the request of chemical industry, drug industry. ect.
Owner:北京成宇化工有限公司 +1
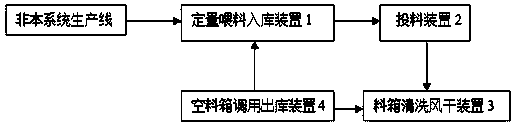
Storage-type full-automatic feeding and charging conveying system
InactiveCN108516328AAddress the degree of automationSolve efficiency problemsPharmaceutical product form changeConveyor partsControl systemChain type
The invention relates to the technical field of material conveying equipment, in particular to a storage-type full-automatic feeding and charging conveying system. The storage-type full-automatic feeding and charging conveying system comprises a quantitative feeding warehouse-in device, a charging device, a material box cleaning and air-drying device and an empty material box calling warehouse-outdevice. Chain-type conveying belts are arranged among the four devices and provided with a rotating charging shuttle vehicle for material conveying. The quantitative feeding warehouse-in device, thecharging device, the material box cleaning and air-drying device, the empty material box calling warehouse-out device and the rotating charging shuttle vehicle are connected with an external control system. The problems of low automation degree, low efficiency and poor effect in the production process in current food and drug industries can be solved.
Owner:云南迦南飞奇科技有限公司
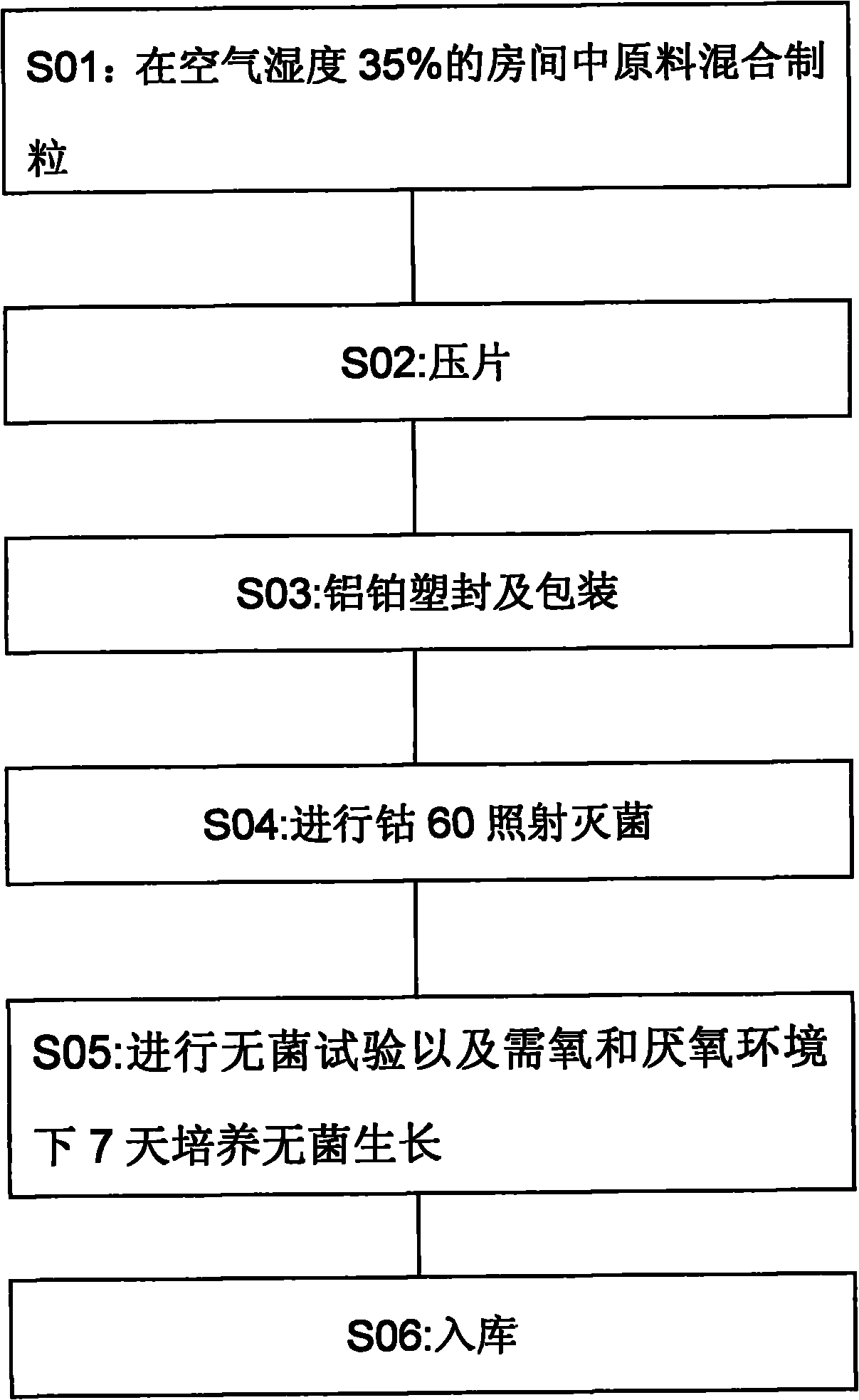
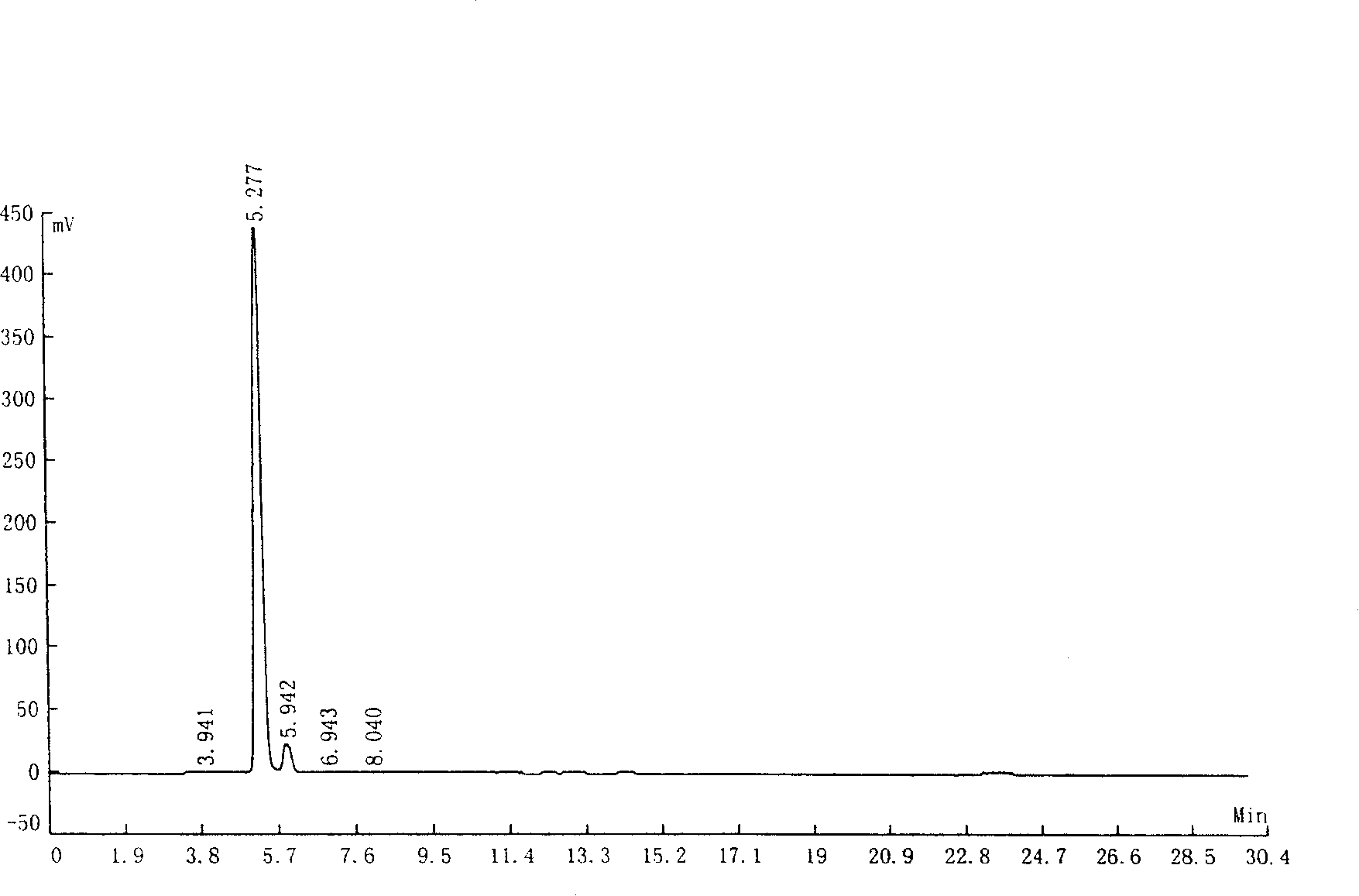
Solvent recovery method in pharmacy and solvent recovery system for implementing same
ActiveCN100574838CReduce lossReduce pollutionVapor condensationBoiling apparatus with condenserCondensation processRecovery method
A solvent recovery device in pharmacy which is mainly characterized in that a liquid ring pump is used for extracting the solvent steam which is separated from a gas-liquid separator, the liquid ring pump increases the pressure of the solvent steam, and then a condenser and the gas-liquid separator carries out the secondary condensation and the gas-liquid separation of the solvent steam after the pressure increases. A solvent recovery system for achieving the method is mainly characterized in that a steam exhaust port of the gas-liquid separator is connected with the liquid ring pump, an output of the liquid ring pump is sequentially connected with a secondary condenser and a secondary gas-liquid separator in series, a liquid discharge port of the secondary gas-liquid separator is connected with a working liquid input port of the liquid ring pump and a recovery tank, and the liquid ring pump takes the solvent as the working liquid. The solvent recovery device can not only increase the times of the condensation and the gas-liquid separation of the solvent stream, but can also increase the condensation pressure during the secondary condensation process, thus significantly increasing the solvent recovery rate, reducing the solvent loss and the production cost of the pharmaceutical industry and reducing the environmental pollution.
Owner:GUANGDONG KENFLO PUMP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com