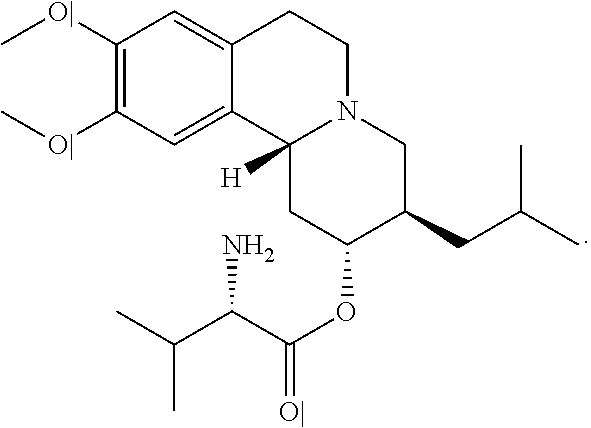
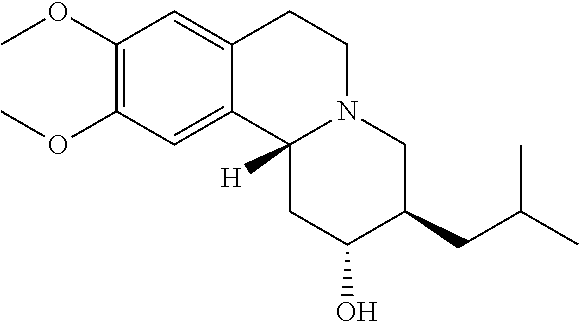
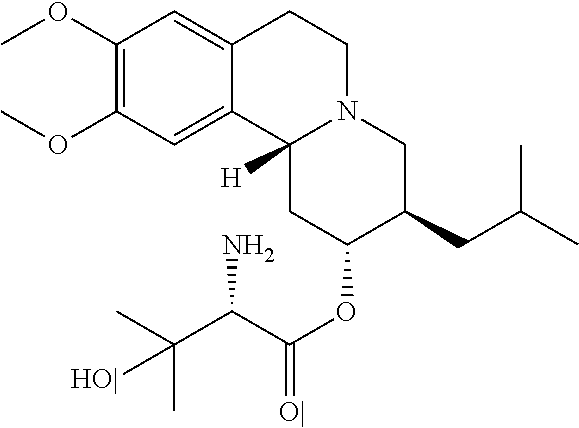
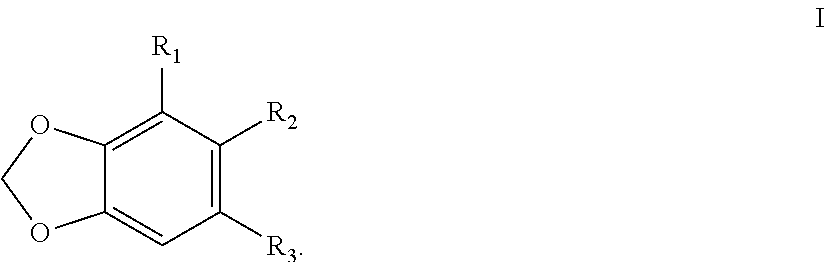Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69 results about "CYP3A4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
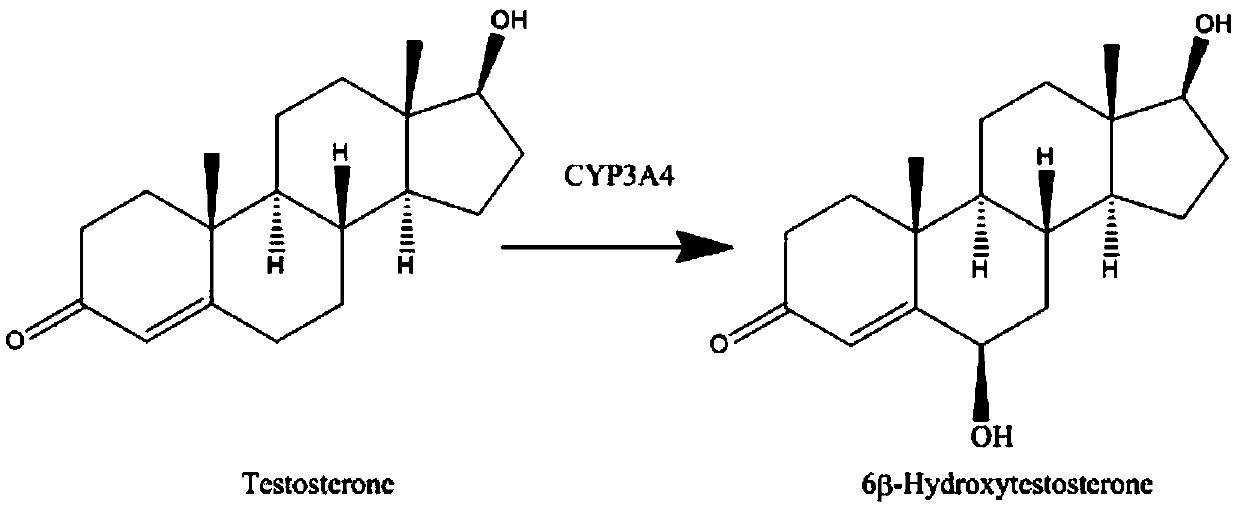
Cytochrome P450 3A4 (abbreviated CYP3A4) (EC 1.14.13.97) is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body.
Methods of treatment with cyp3a4 substrate drugs
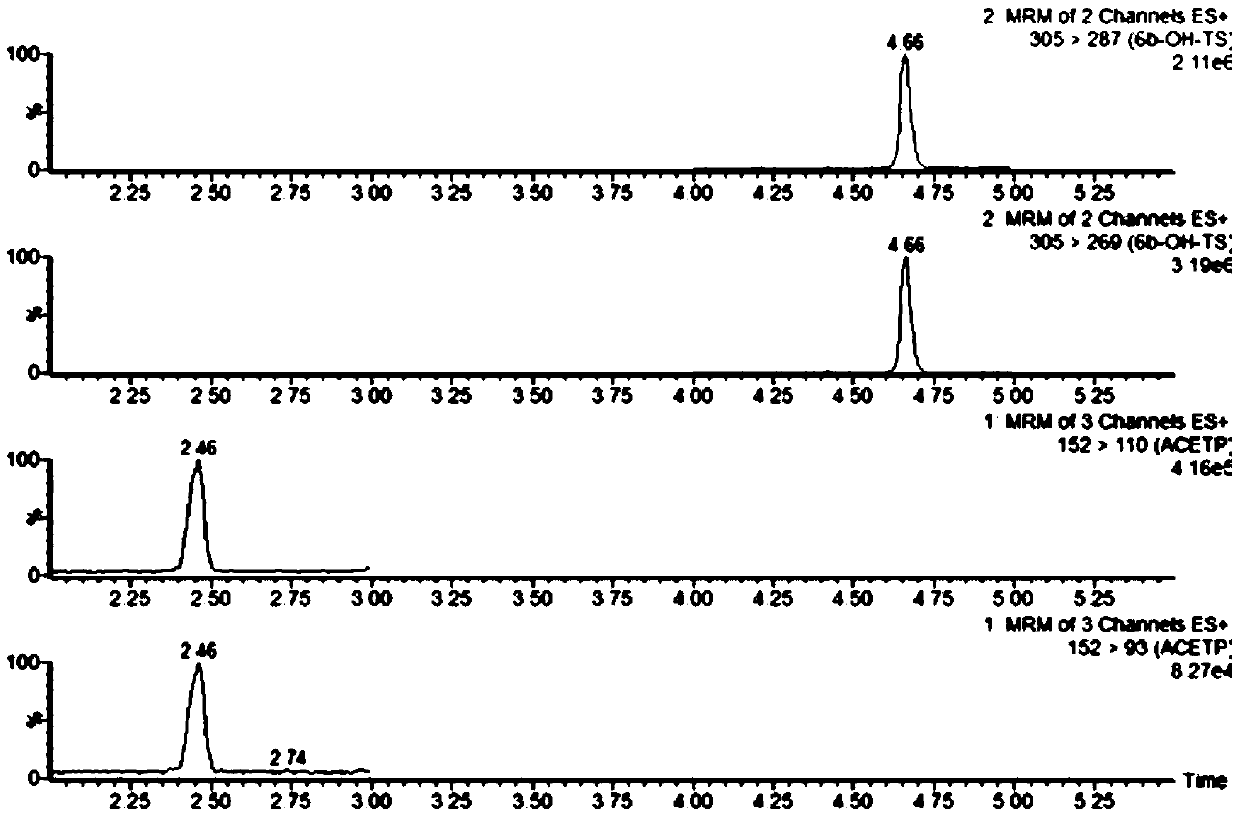
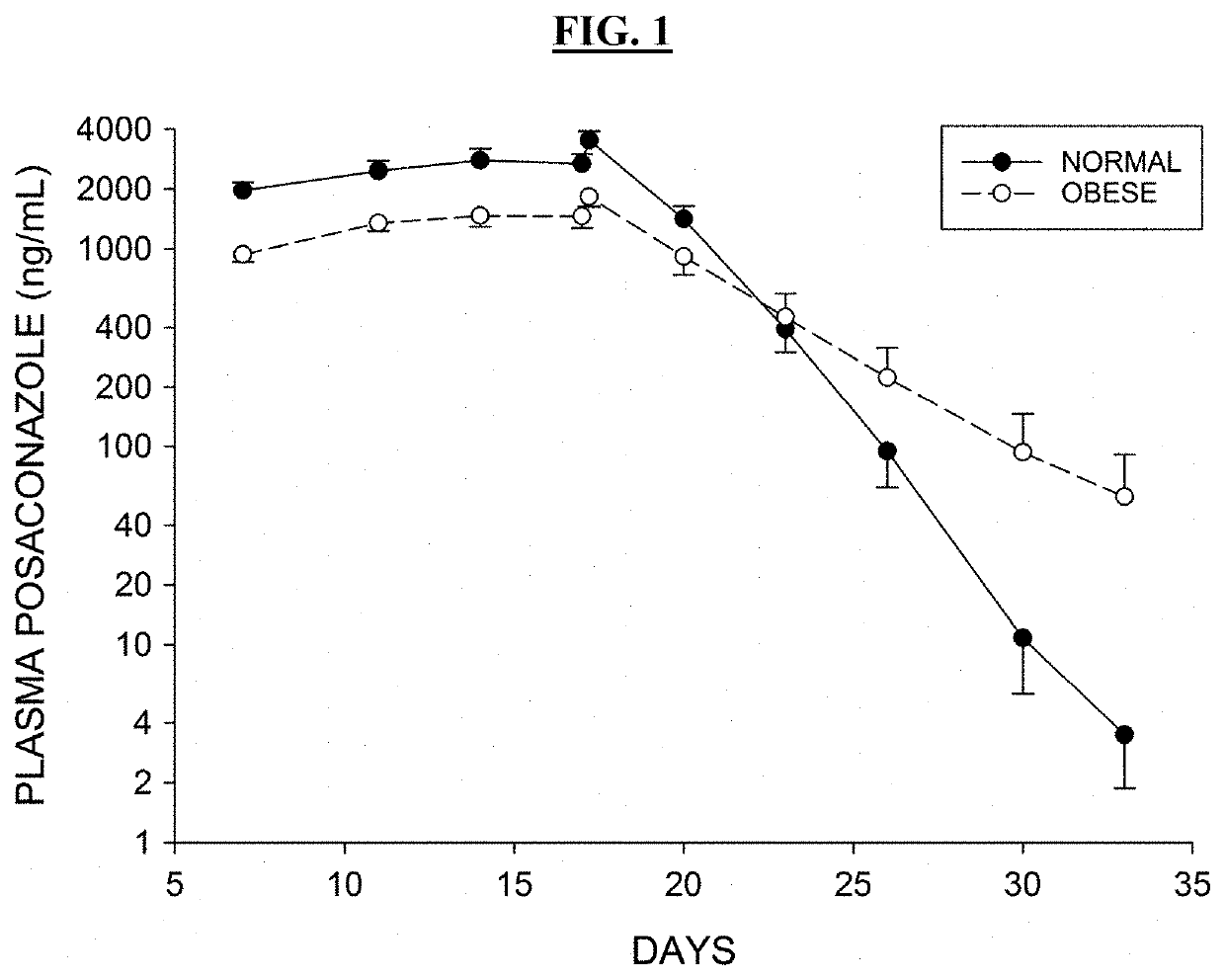
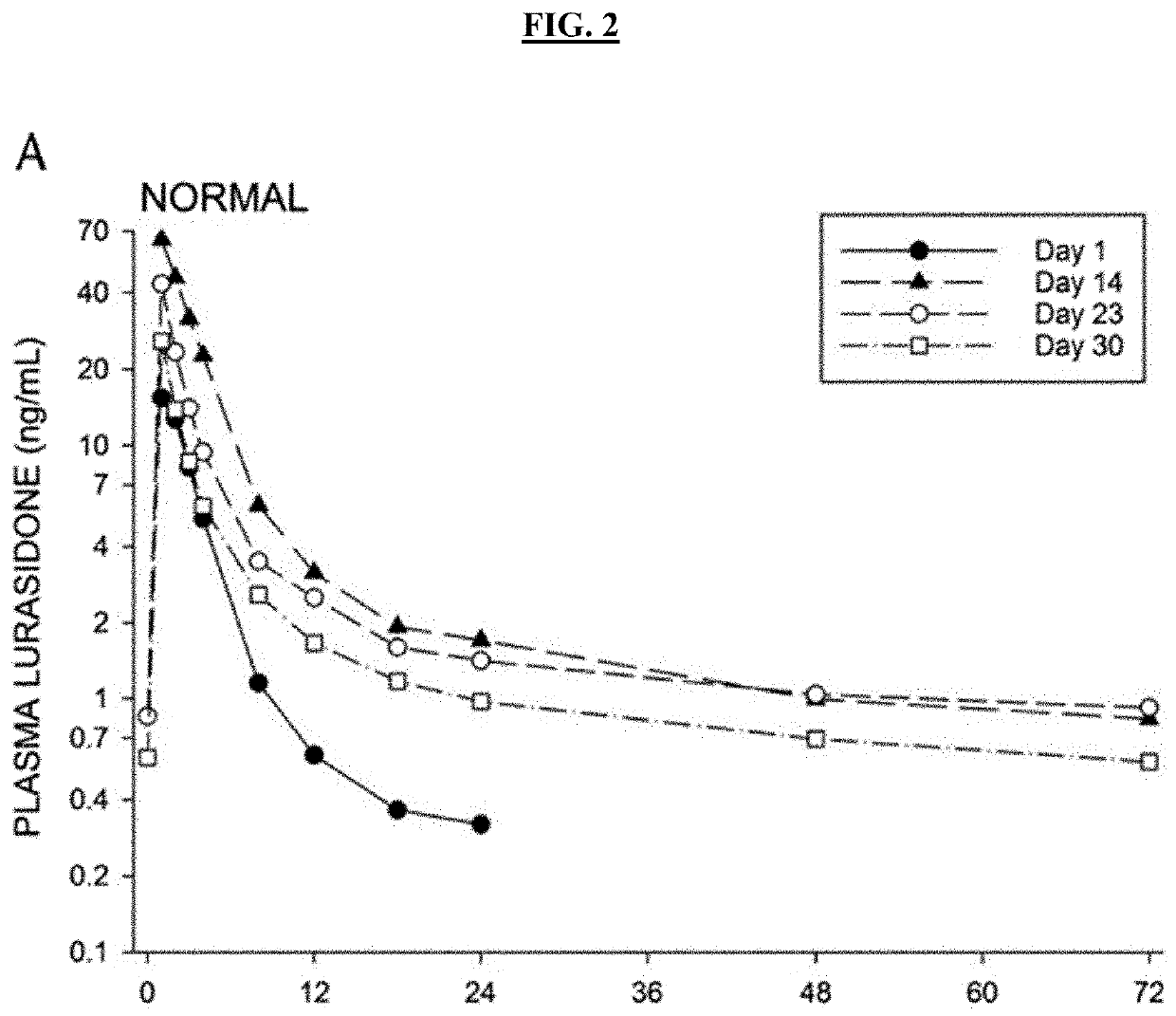
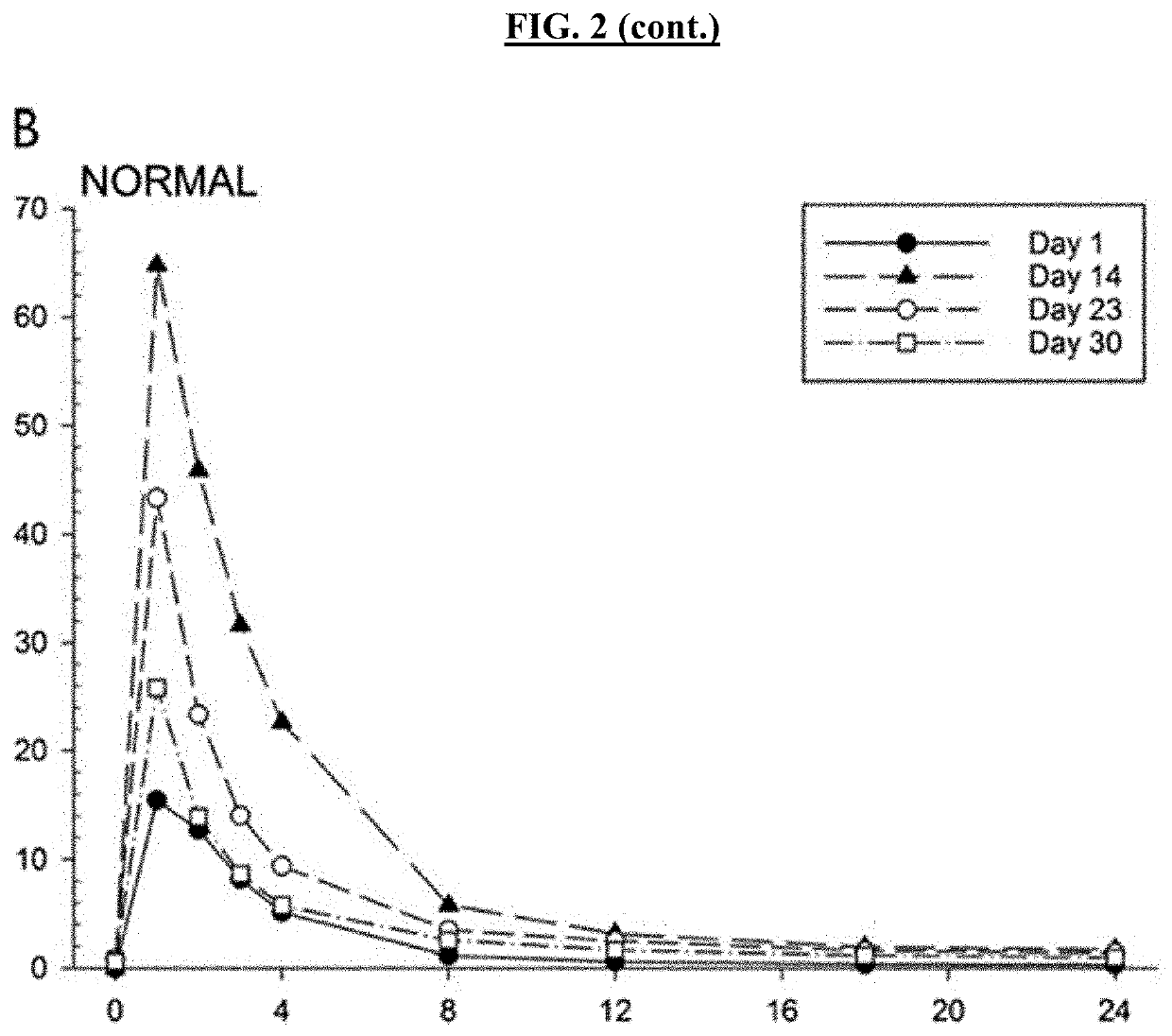
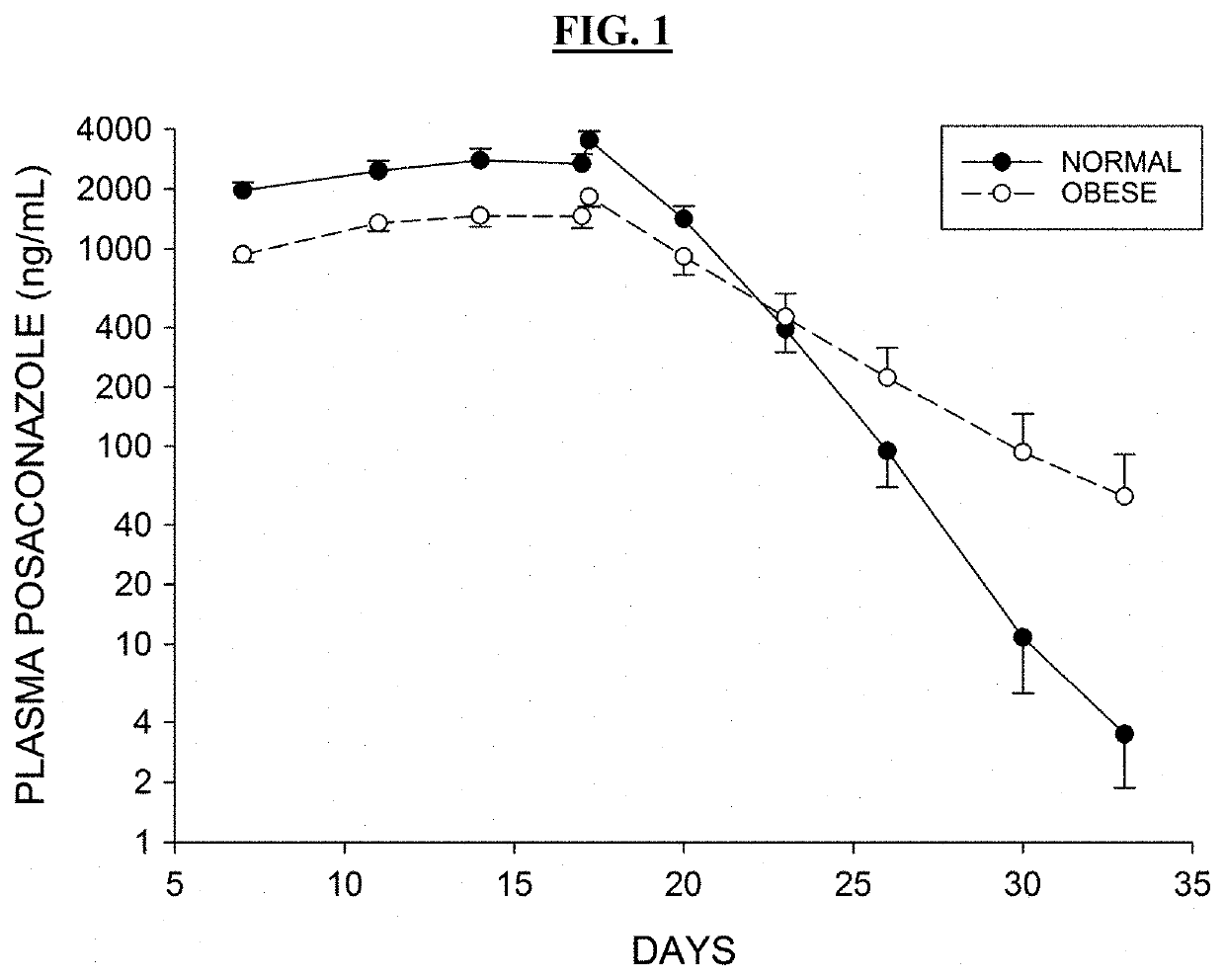
ActiveUS20190076425A1Avoid and reduce incidenceSafety managementOrganic active ingredientsPharmaceutical drugPosaconazole
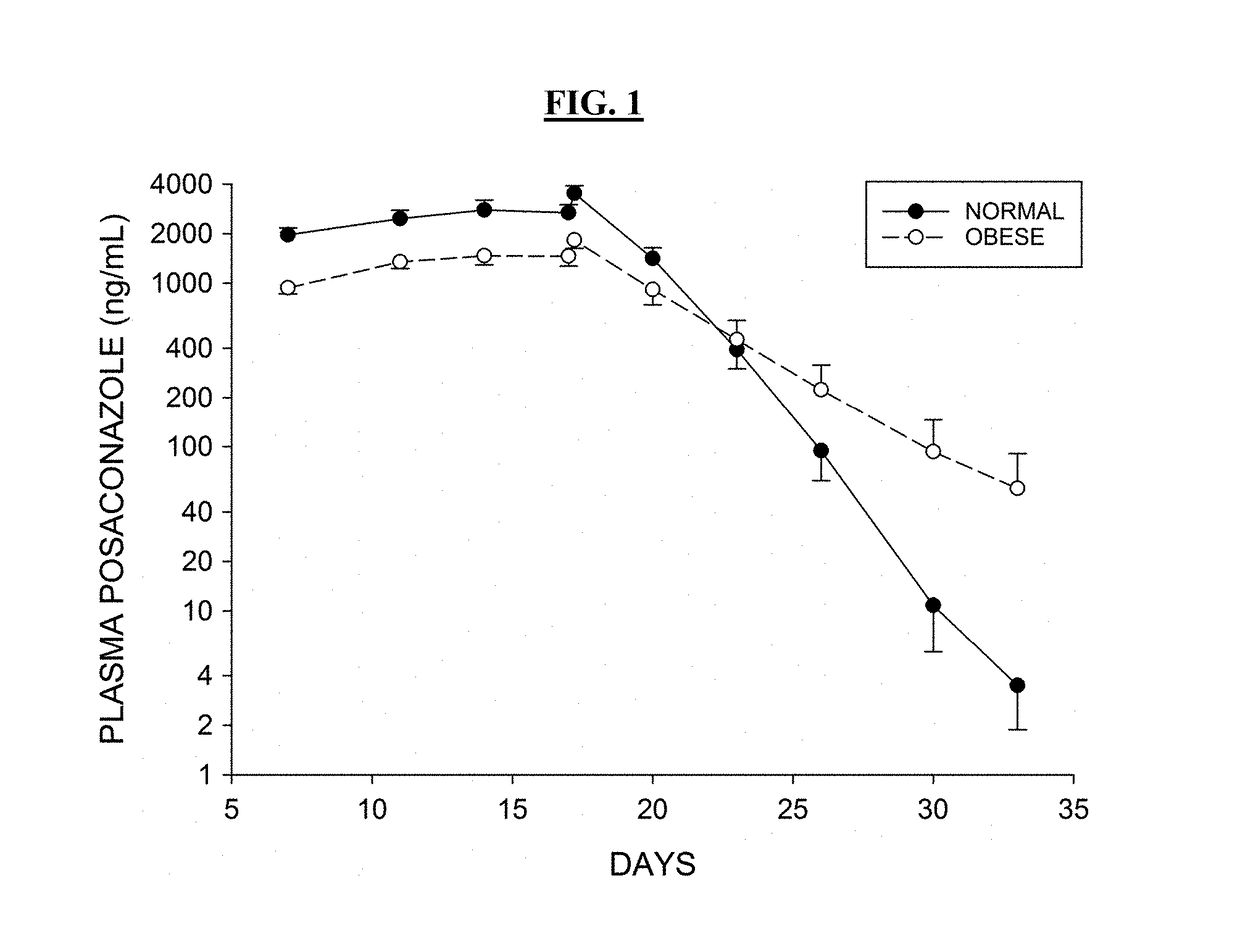
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug, wherein the patient is treated with posaconazole. In some embodiments, the patient stops posaconazole treatment, waits for at least 2 days, and then is treated with the CYP3A4 substrate drug as soon as it is safe to do so. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-42 days after stopping posaconazole. In some embodiments, the patient is treated with a reduced dose of the CYP3A4 substrate drug for about 2-42 days.
Owner:BOW RIVER LLC
Methods for the Administration of Certain VMAT2 Inhibitors
Provided are methods of administering a vesicular monoamine transport 2 (VMAT2) inhibitor chosen from valbenazine and (+)-α-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol, or a pharmaceutically acceptable salt and / or isotopic variant thereof, to a patient in need thereof wherein the patient is also being administered a strong cytochrome P450 3A4 (CYP3A4) inhibitor.
Owner:NEUROCRINE BIOSCI INC
Methods for the Administration of Certain VMAT2 Inhibitors
Provided are methods of administering a vesicular monoamine transport 2 (VMAT2) inhibitor chosen from valbenazine and (+)-a-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol, or a pharmaceutically acceptable salt and / or isotopic variant thereof, to a patient in need thereof wherein the patient is also being administered a strong cytochrome P450 3A4 (CYP3A4) inhibitor.
Owner:NEUROCRINE BIOSCIENCES INC
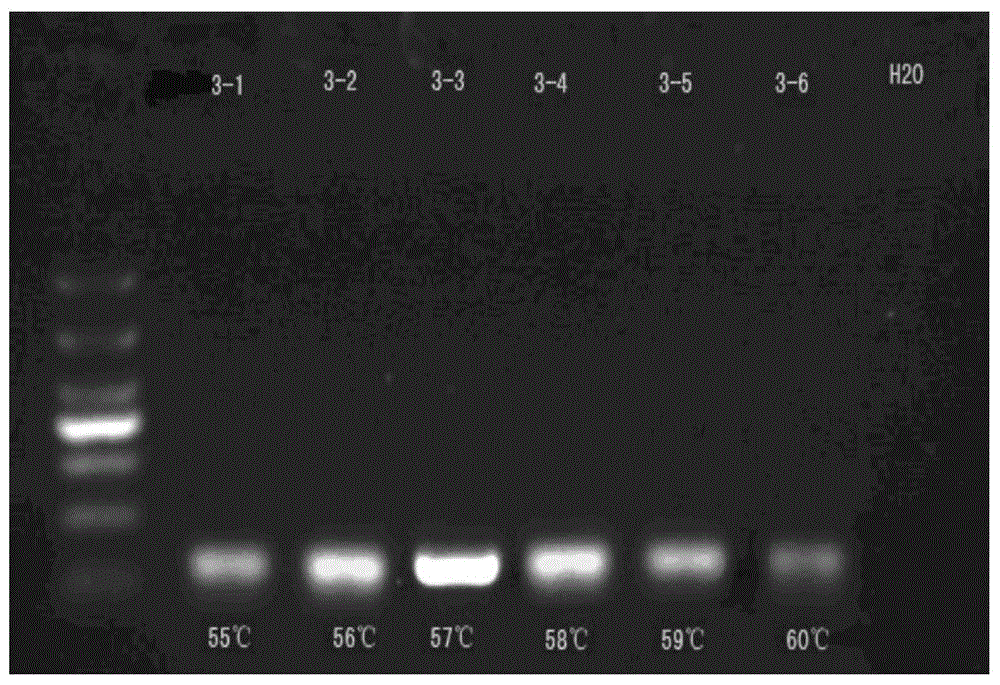
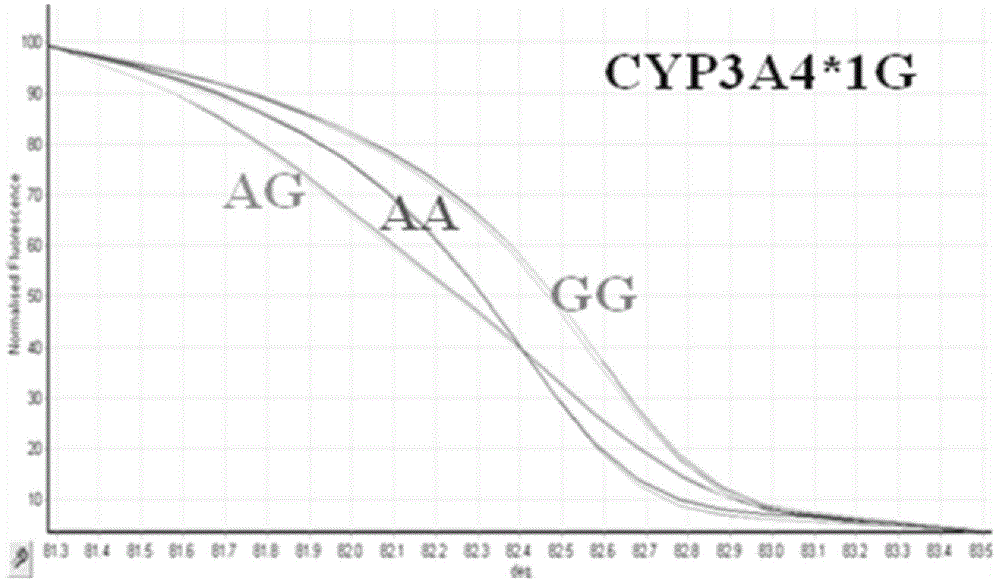
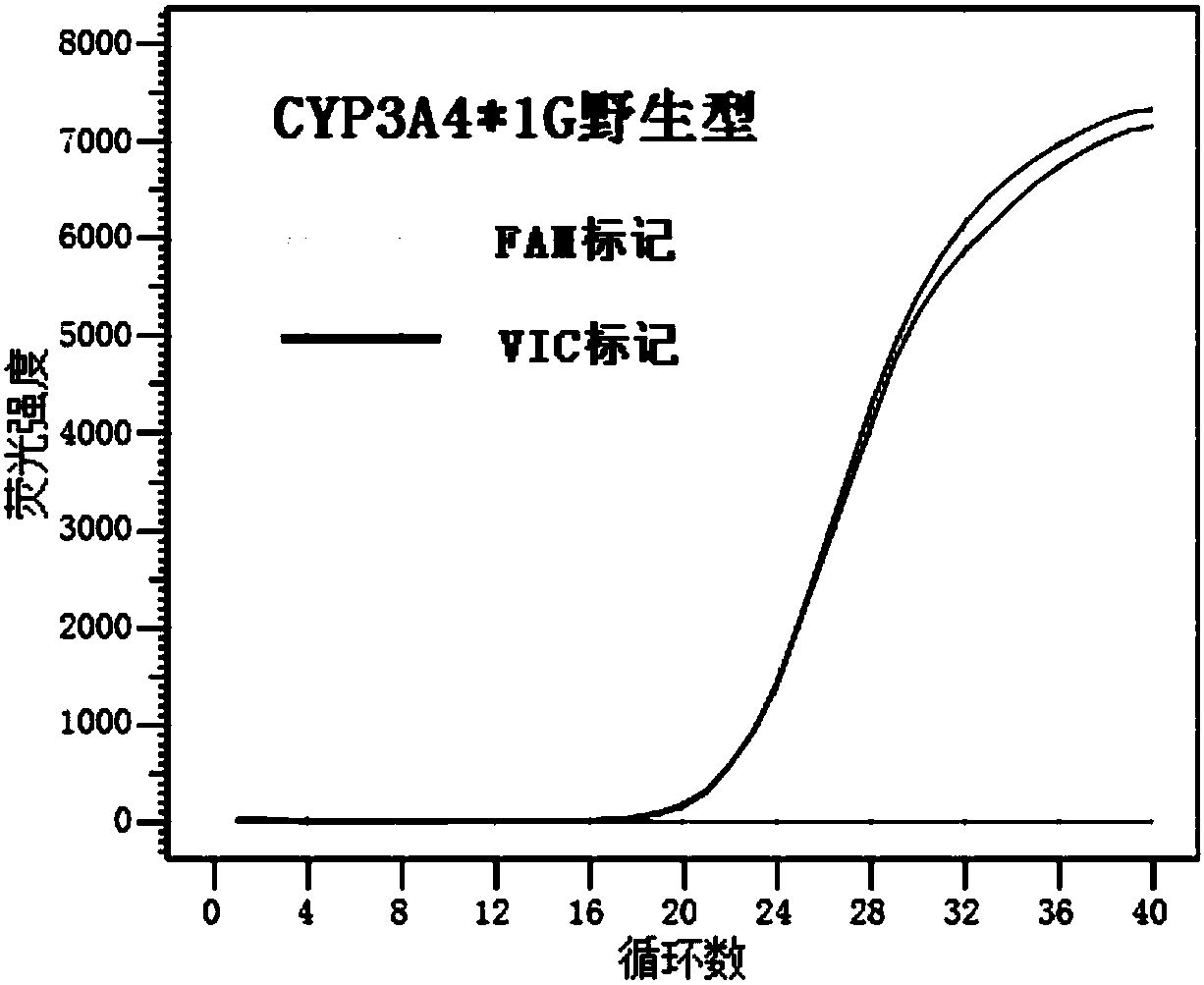
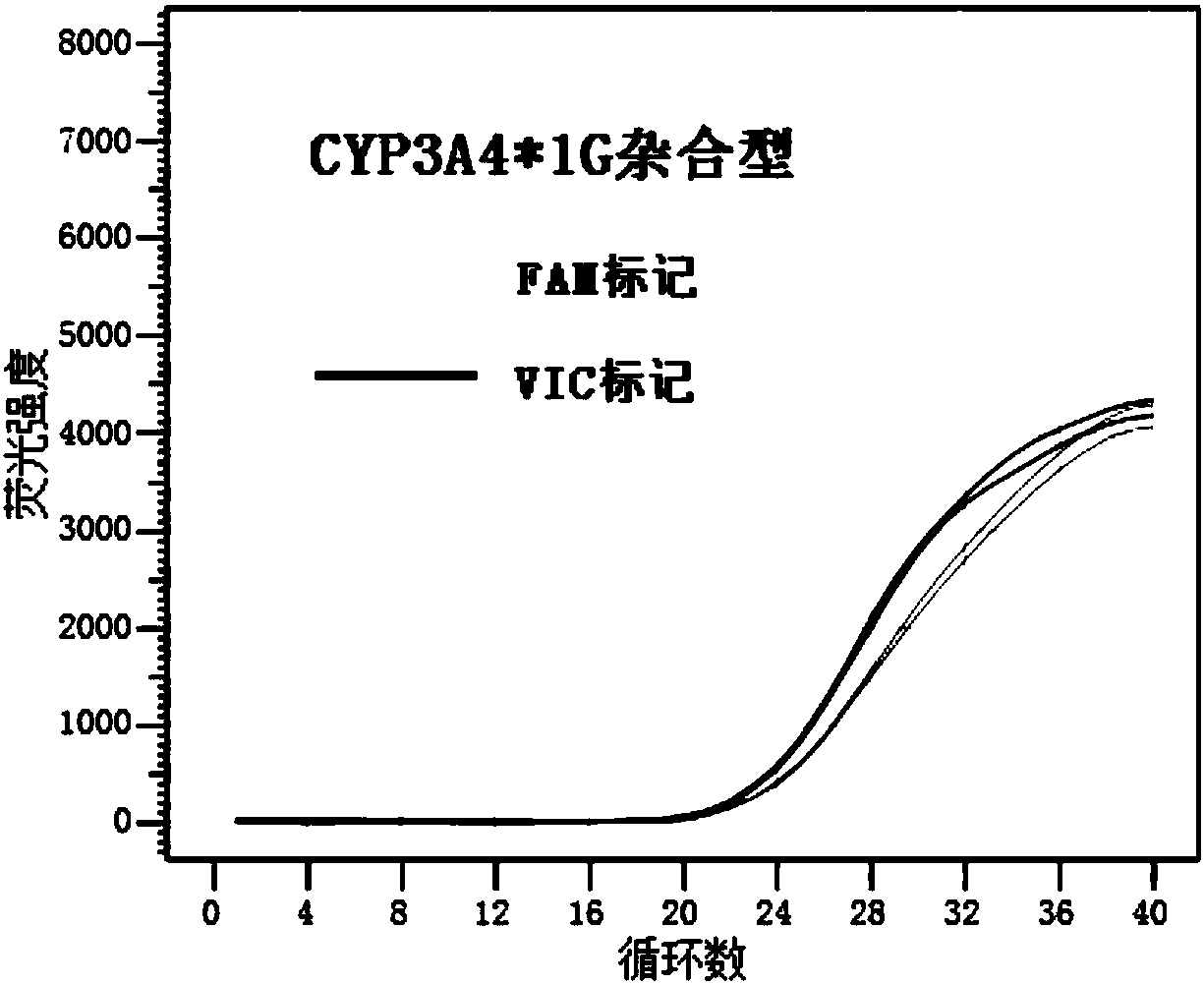
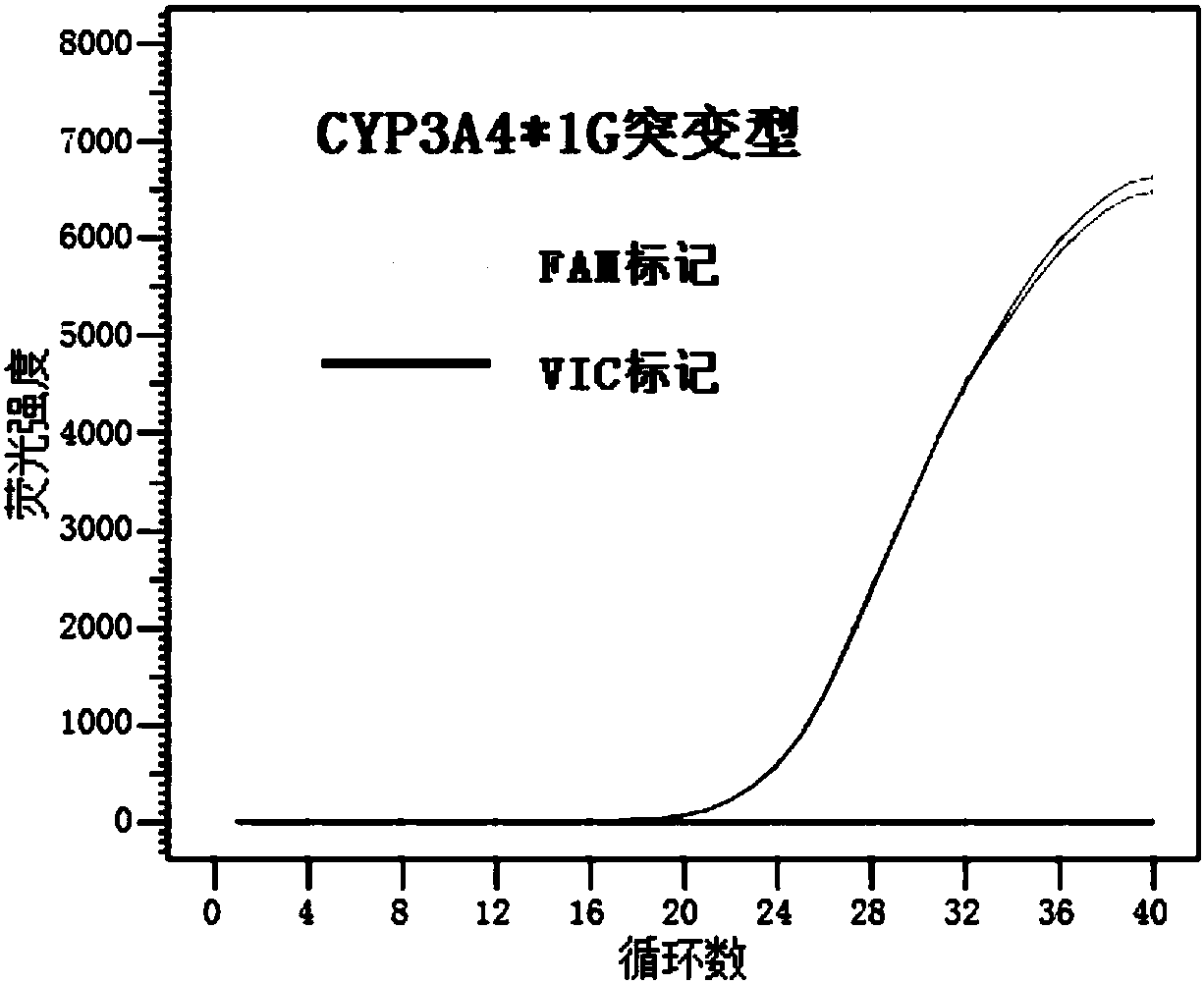
HRM method for detecting genetic polymorphism of CYP3A4*1G and MDR1C1236T
InactiveCN105274190AEasy and fast to provideMicrobiological testing/measurementUse medicationDissolution
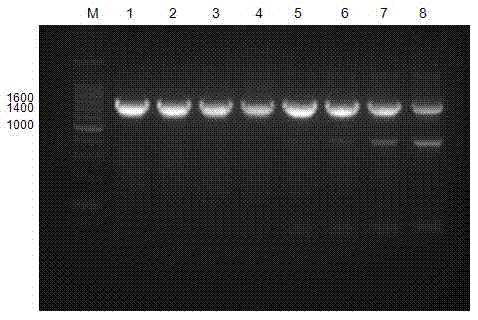
The invention belongs to the field of molecular biology and medicine, and discloses an HRM analysis technology for detecting genetic polymorphism of CYP3A4*1G and MDR1C1236T. The method includes the following steps: (1) searching DNA sequences of target genes; (2) designing and screening appropriate primer pairs aiming at mutation loci of the target genes; (3) extracting sample DNA, and carrying out gene amplification by using the primers screened in the step (2); (4) analyzing whether a PCR product is obtained through specific amplification by an agarose gel electrophoresis method; and (5) according to a high-resolution dissolution curve method, detecting the mutation loci of the amplified target genes, and selecting an SYTO-9 saturated fluorescent dye. A sequence specific probe is not needed during testing, sequencing is not needed, the test is not limited by mutation base loci and types, genetic information is simply and quickly provided for clinical individualized medication, and a help is provided for rational drug use and clinical drug monitoring.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Kit for detecting CYP3A4 and CYP3A5 polymorphic sites and method thereof
ActiveCN108410961AIncrease the Tm valueRaise the annealing temperatureMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceRepeatability
The invention discloses a kit for detecting CYP3A4 and CYP3A5 polymorphic sites and a method thereof. The kit comprises specificity upstream and downstream primer sequences of CYP3A4*1G shown in sequences 1-4 in a sequence table, specificity TaqMan-MGB bi-fluorescence probe sequences of CYP3A4*1G shown in sequences 5-8, specificity upstream and downstream primer sequences of CYP3A5*3 shown in sequences 9-12 and specificity TaqMan-MGB bi-fluorescence probe sequences of CYP3A5*3 shown in sequences 13-16. The kit has the advantages that the specificity is high, pollution is prevented, the sensitivity is high, the detection speed is high, result analysis is simple, and the accuracy is high; the kit can judge the metabolism capacity of to-be-detected objects to fentanyl medicine by detecting polymorphic sites of CYP3A4 and CYP3A5 genes, the using amount of medicine such as fentanyl can be more accurately and effectively guided accordingly, and the kit has the actual clinical application value. The kit is applied to clinical diagnosis, the fit degree between the kit and the prior art is higher, the cost is low, and repeatability is high.
Owner:浙江鼎创医疗科技有限公司
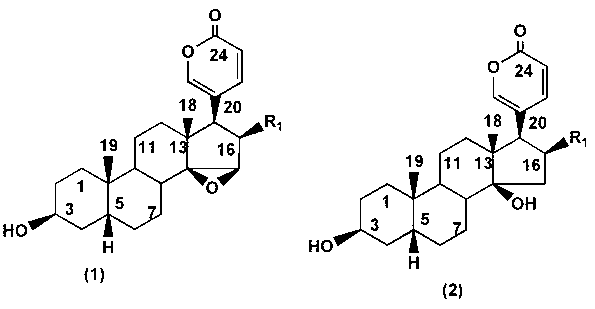
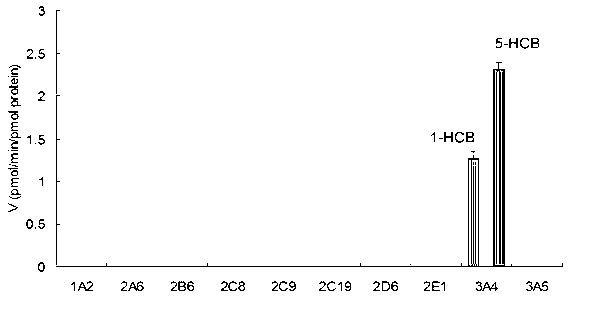
Specific probe substrate for cytochrome P450 3A4 enzyme and application of substrate
ActiveCN102993263AAdvantages of in vitro activityStrong specificityMicrobiological testing/measurementSteroidsQuantitative determinationHistiocyte
The invention provides a specific probe substrate for a cytochrome P450 3A4 enzyme and an application of the substrate in CYP3A4 enzyme activity measurement. The specific operation flow of the enzyme activity measurement comprises the following steps: carrying out the CYP catalytic reaction of the specific substrate by virtue of a CYP in-vitro incubation system by selecting any monomer in toad steroid series of compounds as a high-specificity probe substrate; and measuring the activity of the CYP3A4 enzyme in each biological sample and each cell by quantitatively detecting a product generated quantity or a substrate eliminated quantity in a unit time. The specific probe substrate provided by the invention can be used for quantitative evaluation of CYP3A4 enzyme activity in biological samples belonging to different species and deriving from different individual sources, and quantitative measurement of CYP3A4 enzyme activity in animal tissue cell culture fluids and cell products deriving from different sources.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI
Methods of treatment
ActiveUS10857144B2Avoid and reduce incidenceSafety managementOrganic active ingredientsNervous disorderPharmaceutical drugPosaconazole
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug, wherein the patient is treated with posaconazole. In some embodiments, the patient stops posaconazole treatment, waits for at least 2 days, and then is treated with the CYP3A4 substrate drug as soon as it is safe to do so. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-42 days after stopping posaconazole. In some embodiments, the patient is treated with a reduced dose of the CYP3A4 substrate drug for about 2-42 days.
Owner:BOW RIVER LLC
Methods of treatment with CYP3A4 substrate drugs
ActiveUS10835529B2Avoid and reduce incidenceSafety managementOrganic active ingredientsPharmaceutical drugPosaconazole
Owner:BOW RIVER LLC
Primer pair, kit and method for detection of related genes for guiding individualized administration of fentanyl drugs
InactiveCN107893114AEasy to useRapid determinationMicrobiological testing/measurementDNA/RNA fragmentationBiologyFentanyl
The invention relates to a primer pair for the detection of related genes for guiding the individualized administration of fentanyl drugs. The primer pair comprises specific primers and a probe aimingat a CYP3A4*1G gene site. The invention relates to a kit for the detection of related genes for guiding the individualized administration of fentanyl drugs. The kit comprises a PCR reaction solutioncontaining the primers and the probe mentioned above, PCR buffer, dNTP, nuclease-free water, and HS Tag enzymes. The invention also relates to a method for the detection of related genes for guiding the individualized administration of fentanyl drugs. The provided kit and detection method thereof have the advantages that the operation is simple, the detection is rapid, the accuracy is high, the specificity and sensitivity are good, the use is convenient, and the clinical requirements can be satisfied effectively.
Owner:韩林志
Method of providing aripiprazole to patients having impaired CYP2D6 or CYP3A4 enzyme function
The disclosed embodiments relate to methods of initiating aripiprazole treatment in a patient who is a CYP2D6 poor metabolizer or a CYP3A4 poor metabolizer, or both.
Owner:OTSUKA PHARM CO LTD
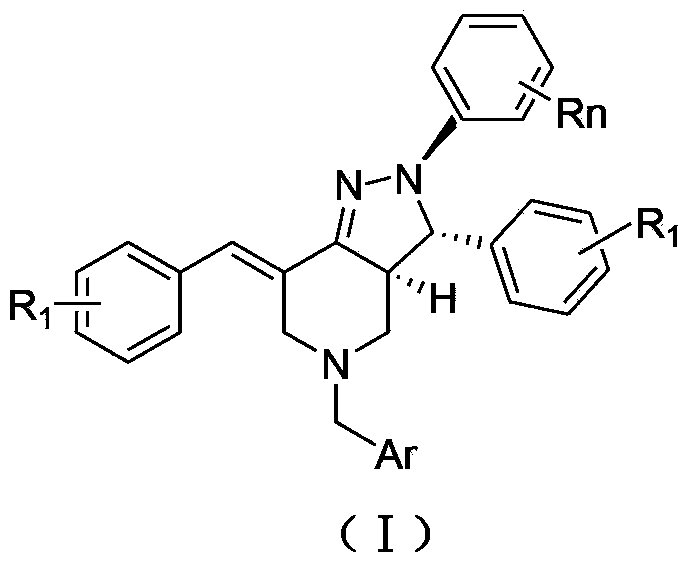
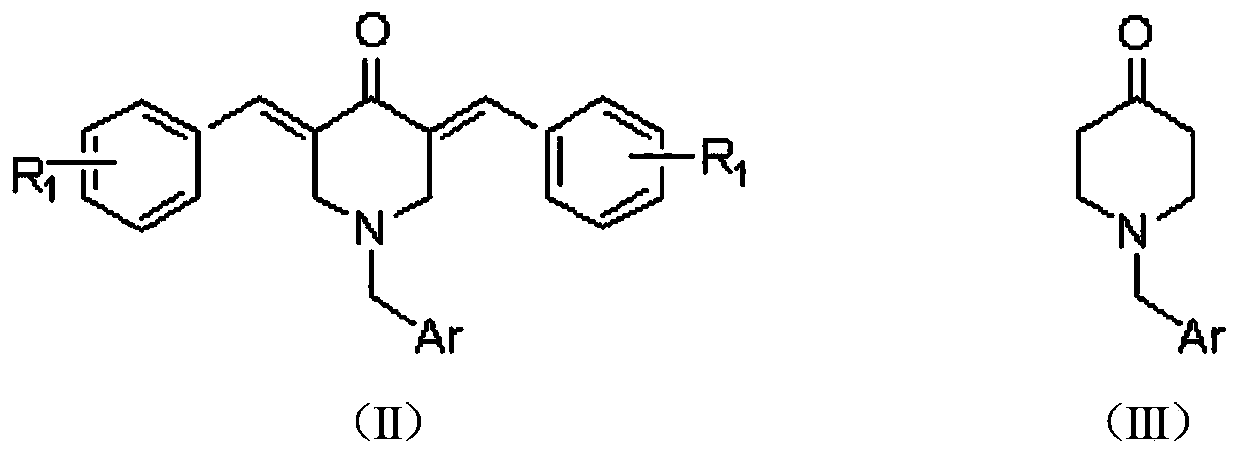
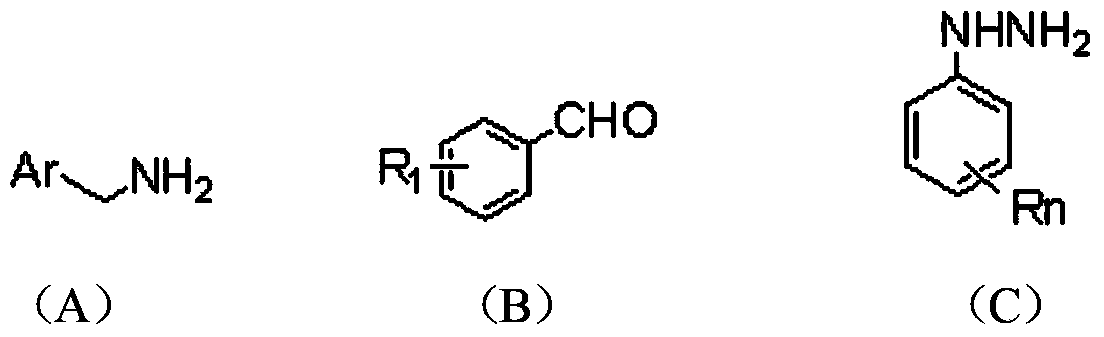
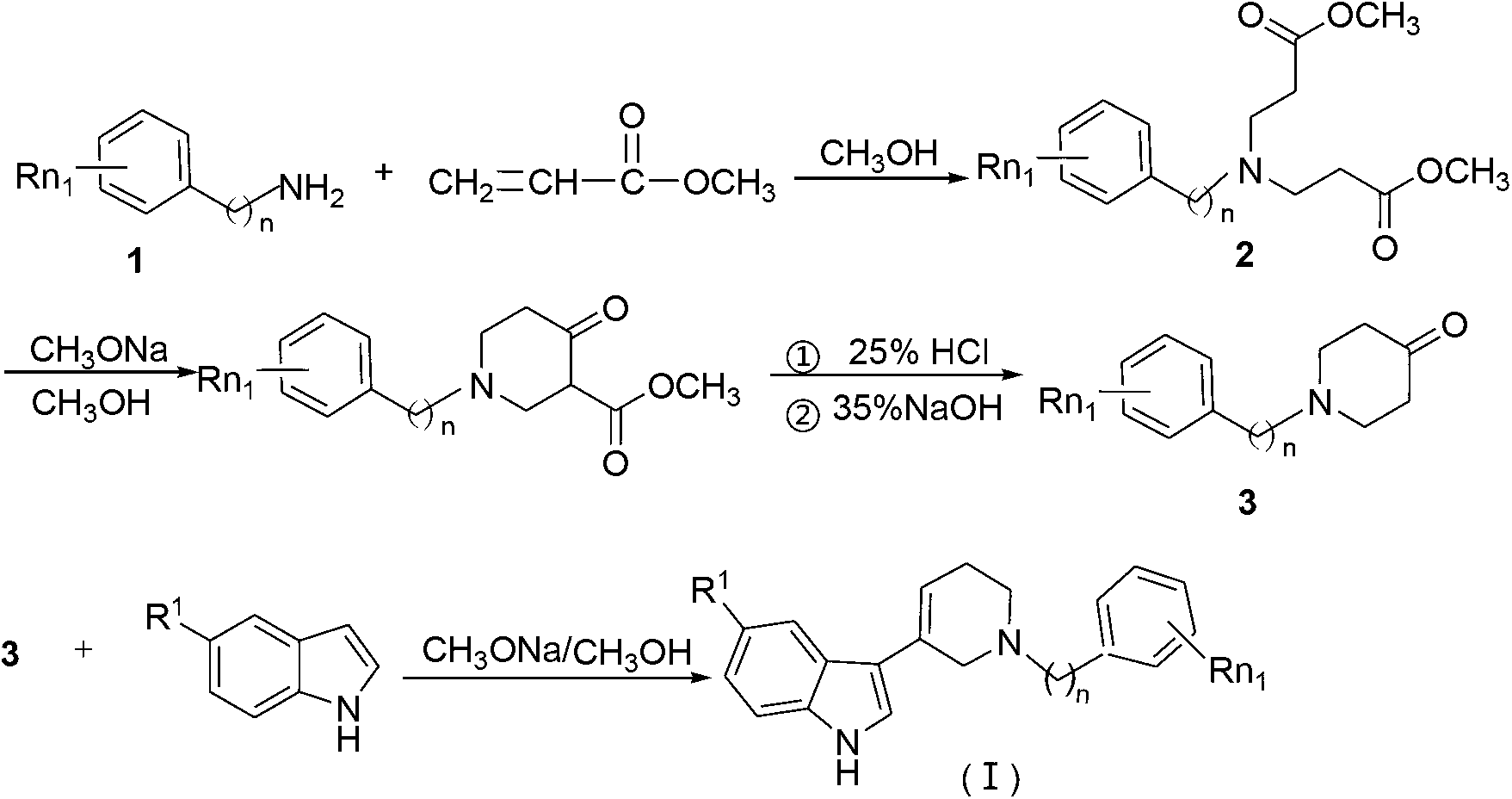
2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and preparation method and application thereof
The invention provides 2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and a preparation method and application thereof. The derivative is 2, 3-bis(substituted phenyl)-5-subsituted arylmethyl-7-substituted benzylidene dihydro-pyrazolo piperidine derivative, having the following formula (I). The preparation method includes using substituted arylmethyl amine and methyl acrylate as raw materials; subjecting the materials to Michael addition, Dieckmann condensation and hydrolysis-decarboxylation sequentially; allowing for Aldol reaction with substituted benzaldehyde to obtain intermediate N-substituted arylmethyl-3, 5-bis(substituted benzylidene)-4-piperidone; allowing for condensation with substituted phenylhydrazine to obtain a compound according to the formula (I). The derivative is efficient in inhibiting multiplication of various carcinoma cell lines such as leukemia, esophagus cancer, ovarian cancer and breast cancer in human, is well stably metabolic in liver microsomes of human and rat, is free of direct and competitive inhibition on five enzymes of liver microsomes, such as CYP3A4, CYP2D6, CYP2C9, CYP1A2 and CYP2C19, is highly bioavailable, is low in toxicity to normal cells, and is available for the preparation of drugs for the cancers.
Owner:SHANGHAI NORMAL UNIVERSITY
Derivatives of dillapiol and related monolignans and use thereof
InactiveUS20130012477A1Improve efficacyImprove bioavailabilityBiocideOrganic chemistryActive agentCytochrome p450 enzyme
Derivatives of dillapiol, sesamol and related monolignans having the following general formula:These compounds have synergistic properties, inhibit cytochrome P450 enzymes such as human CYP3A4, and can be used as pesticide synergists or pharmaco-enhancers. Accordingly, methods for increasing the efficacy and / or bioavailability of a pharmaceutically active agent and for increasing the potency of a pesticide are described, as are synergistic pesticidal and pharmaceutical compositions.
Owner:UNIVERSITY OF OTTAWA +1
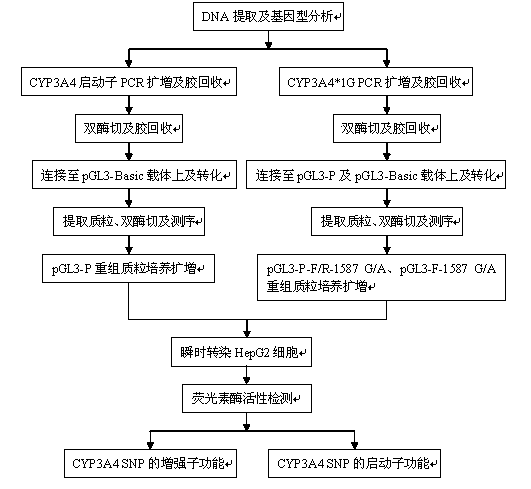
Reporter gene testing method for enhancer and promoter functions of intron SNP of CYP3A4
InactiveCN103525940AIncrease productionMicrobiological testing/measurementDNA/RNA fragmentationGene vectorPromoter
The invention relates to a reporter gene method for an intron sequence of human CYP3A4 and for testing whether the SNP of the sequence has enhancer and promoter expression enhancing functions at the same time. The method comprises the following steps: selecting the intron sequence from the genome of human CYP3A4; constructing a luciferase reporter gene plasmid of a promoter sequence of CYP3A4; constructing a luciferase reporter gene vector of an intron SNP enhancer of CYP3A4; constructing a luciferase reporter gene vector of an intron SNP promoter of CYP3A4; culturing and transfecting HepG 2 cells; and determining the activity of dual-luciferase. With the method, it is discovered that the intron SNP of human CYP3A4 has enhancer and promoter functions at the same time, SNP capable of improving output of CYP3A4 enzyme can be screened by using the method, and the method has a critical application value in further in-vitro research on or screening of drugs used for metabolism of the CYP3A4 enzyme.
Owner:ZHENGZHOU UNIV
Novel cytochrome CYP3A4 enzyme specific probe reaction and application thereof
InactiveCN104892563AStrong specificityLow toxicityOrganic chemistryMicrobiological testing/measurementGeneration rateIn vivo
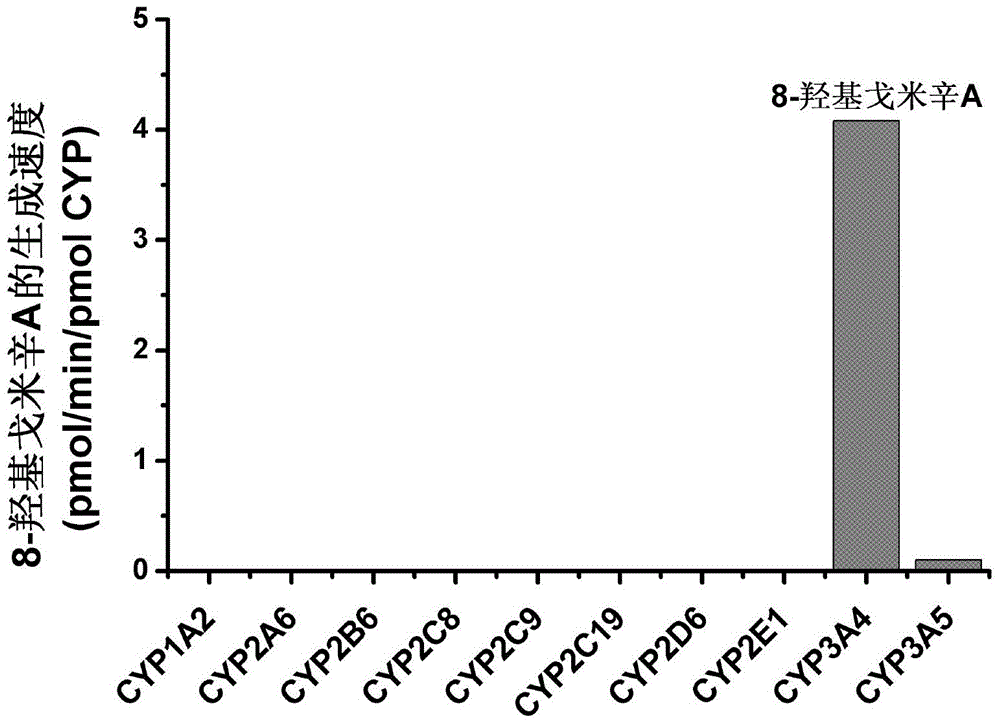
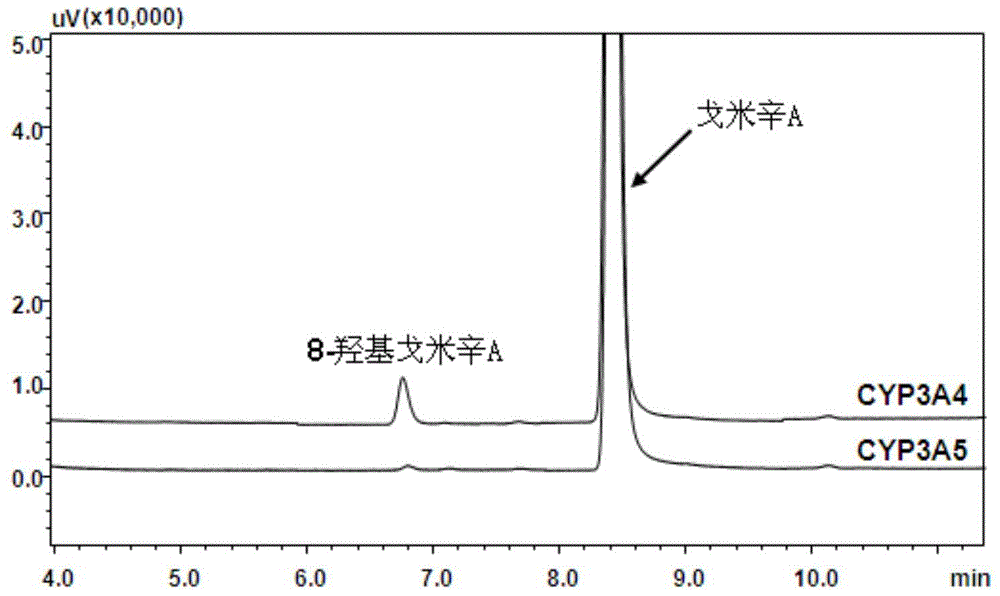
The invention provides a novel cytochrome CYP3A4 enzyme specific probe reaction and an application thereof; gomisin A can be used as a probe substrate of a CYP3A4 enzyme for detecting the activity of the enzyme; the gomisin A or a pharmaceutical preparation thereof is used as the specific probe substrate, a reduction rate of the substrate gomisin A or a generation rate of a product 8-hydroxy gomisin A is quantitatively determined in unit time and is used as an evaluation index of the cytochrome CYP3A4 enzyme activity. The probe substrate also has high security and can be used as an overall probe; a to-be-tested mammal is allowed to take 0.1-500 mg / kg body weight of the gomisin A or the pharmaceutical preparation thereof by intravenous injection; time points are selected in 0 to 24 hours, and plasma samples of the to-be-tested animal are collected; the reduction rate of the substrate gomisin A or the generation rate of the product 8-hydroxy gomisin A is determined and is used as the evaluation index of the overall cytochrome CYP3A4 enzyme activity. The quantitative evaluation of different-source biological samples and in-vivo CYP3A4 enzyme activity can be realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Lentiviral expression vector for specifically promoting high expression of CYP3A4 (cytochrome P450 3A4) gene in hepatocytes, and construction method and application thereof
InactiveCN102864171AIncrease useHigh transfection efficiencyGenetic material ingredientsFermentationCloning SitePromoter
The invention provides a lentiviral expression vector for specifically promoting high expression of CYP3A4 (cytochrome P450 3A4) gene in hepatocytes. The lentiviral expression vector for specifically promoting high expression of CYP3A4 (cytochrome P450 3A4) gene in hepatocytes comprises fundamental sequences, resistance gene sequences, multiple-cloning-site sequences, promoter subsequences, and CYP3A4 gene cDNA (complementary DNA) sequences in a pLVX-AcGFP-C1 expression vector. Multiple cloning sites include an XhoI restriction enzyme cutting site and an XmaI restriction enzyme cutting site. The CYP3A4 gene cDNA sequences include XhoI restriction enzyme cutting sites, CYP3A4 gene coded sequences and XmaI restriction enzyme cutting sites. The CYP3A4 gene cDNA sequences are forwardly inserted into the multiple-cloning-site sequences. The lentiviral expression vector is capable of expressing the CYP3A4 gene specifically, continuously, efficiently and stably with high transfection efficiency and low consumption, and can be used as a powerful tool applied to pharmaceutical research and development related to CYP3A4.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
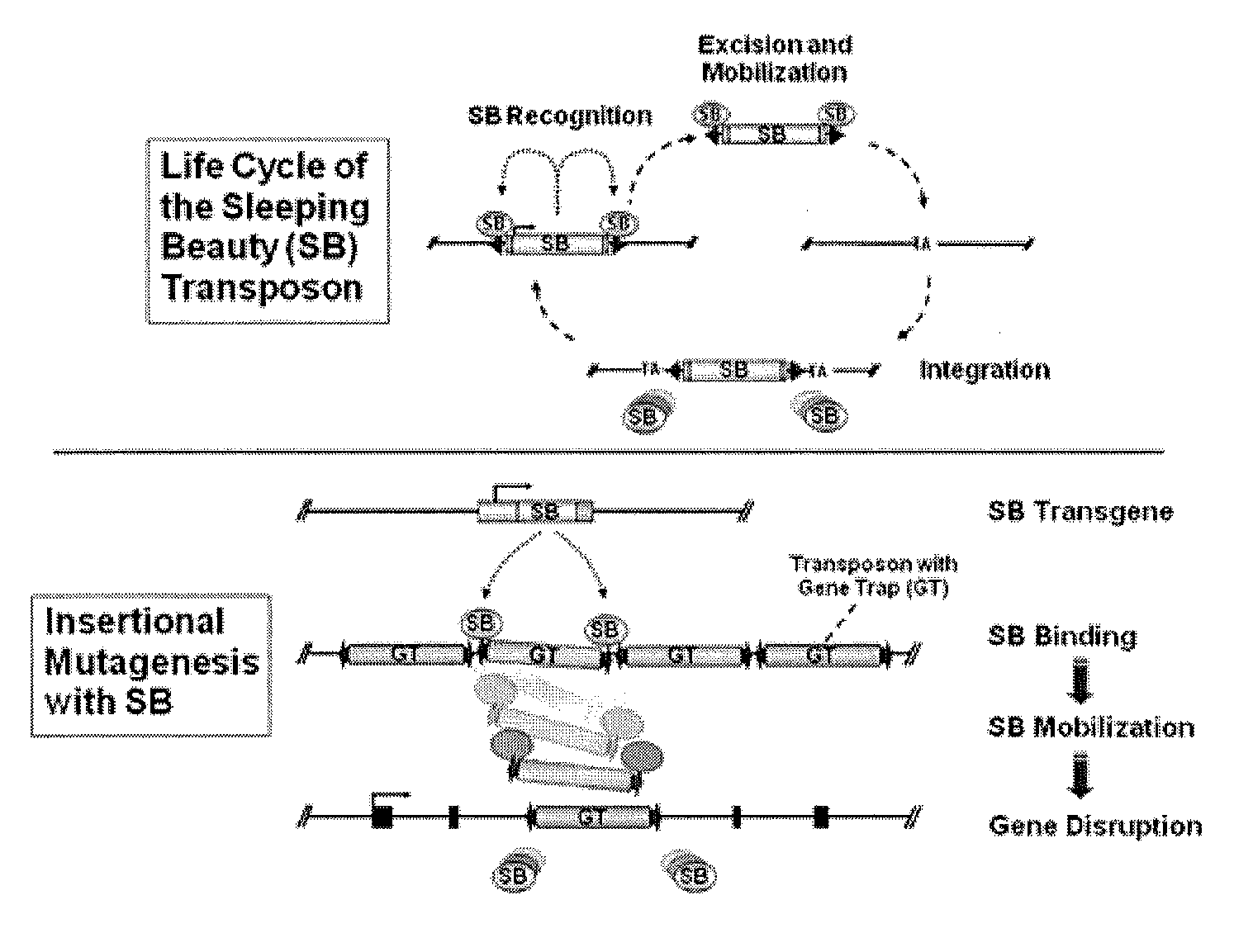
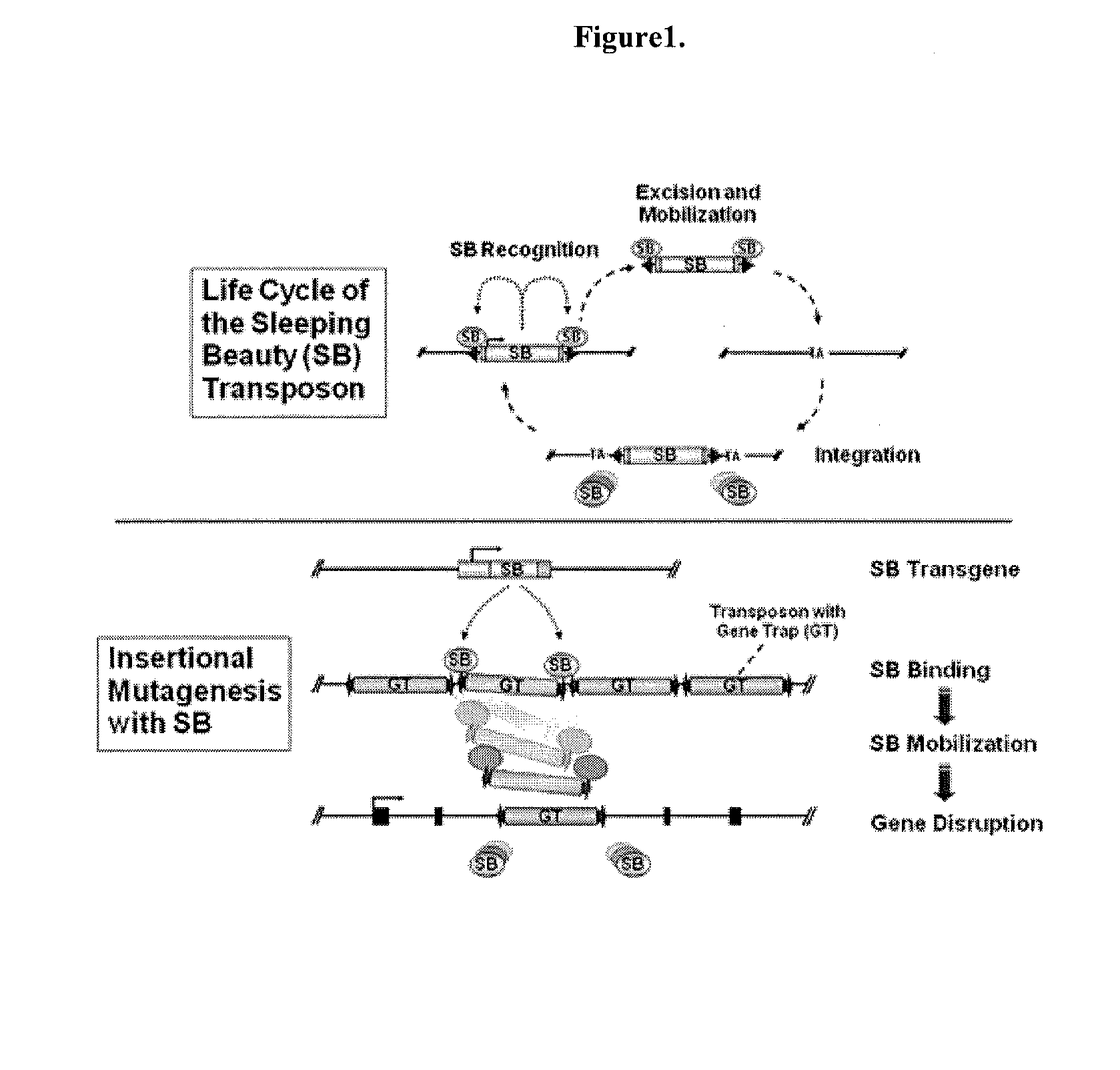
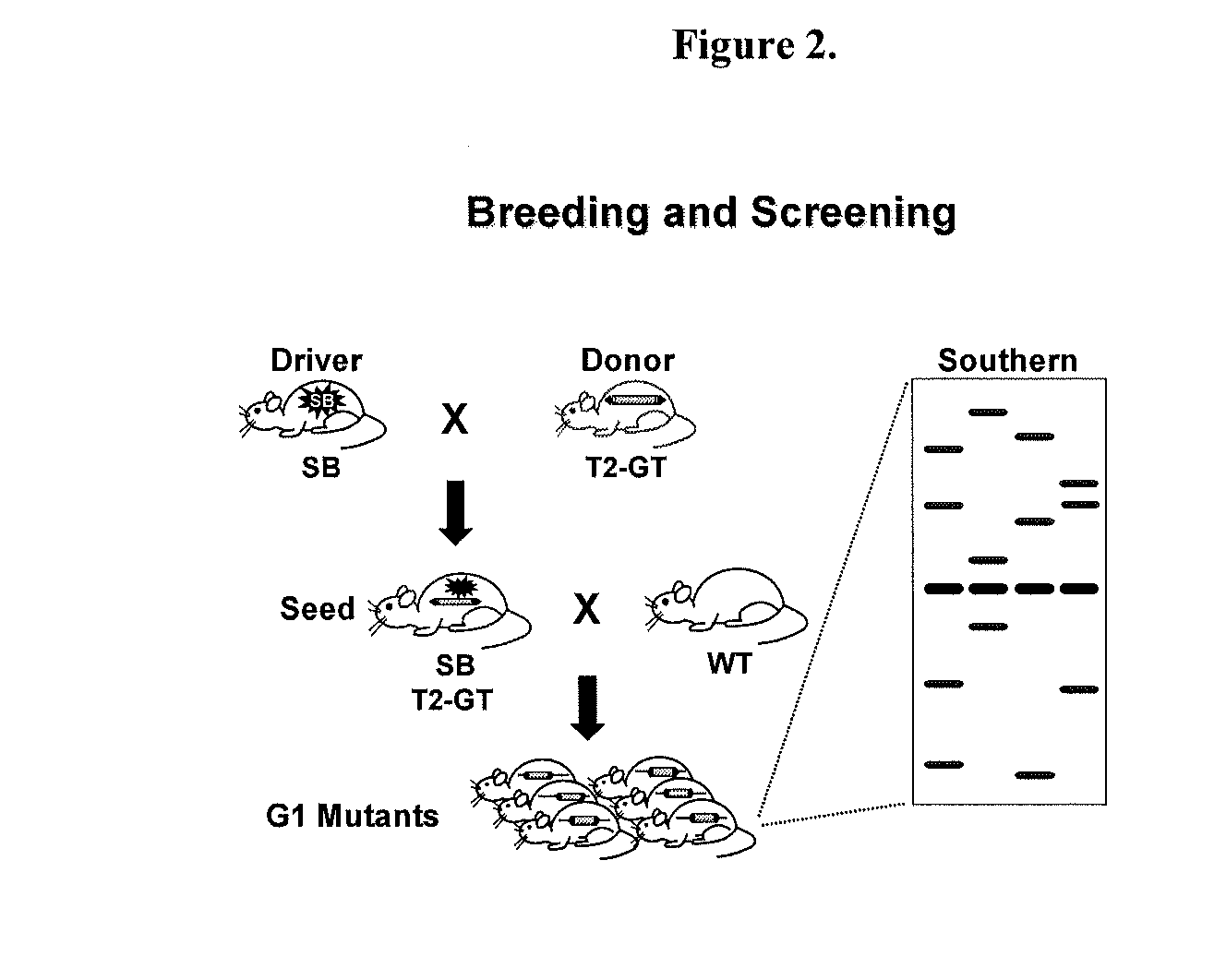
Genetically Modified Rat Models for Drug Metabolism
The present invention provides a desired rat or a rat cell which contains a predefined, specific and desired alteration rendering the rat or rat cell predisposed to alterations in drug and chemical metabolism by modification of its structure or mechanism. Specifically, the invention pertains to a genetically altered rat, or a rat cell in culture, that is defective in at least one of two alleles of a drug metabolism gene such as the Cyp7b1 gene, the Cyp3a4 gene, etc. In another embodiment, the rat cell is a somatic cell. The inactivation of at least one drug metabolism allele results in an animal with a higher susceptibility to altered drug and chemical metabolism. In one embodiment, the genetically altered animal is a rat of this type and is able to serve as a useful model for altered drug and chemical metabolism or toxicology and as a test animal for autoimmune and other studies. The invention additionally pertains to the use of such rats or rat cells, and their progeny in research and medicine. In one embodiment, the invention provides a genetically modified or chimeric rat cell whose genome comprises two chromosomal alleles of a drug metabolism gene wherein at least one of the two alleles contains a mutation, or the progeny of the cell.
Owner:TRANSPOSAGEN BIOPHARM
Method of providing aripiprazole to patients having impaired cyp2d6 or cyp3a4 enzyme function
The disclosed embodiments relate to methods of initiating aripiprazole treatment in a patient who is a CYP2D6 poor metabolizer or a CYP3A4 poor metabolizer, or both.
Owner:OTSUKA PHARM CO LTD
Plasticizer tracking biomarker, plasticizer tracking genetic chip, and plasticizer tracking biomarker confirmation method
The invention discloses a plasticizer tracking biomarker, a plasticizer tracking genetic chip, and a plasticizer tracking biomarker confirmation method. The biomarker found through the confirmation method is one or more of human gene / protein clusters composed of PPARG, CASP3, PPARA, NR1I2, ESR1, AR, CYP1B1, ABCB1, CYP1A1, VEGFA, ESR2, PPARD, LAMP3, BAX, BCL2, CDO1, CELSR2, CSNK1A1, CYP3A4, MAPK1, MAPK3, MYC, NCOA1, PAPSS1, PAPSS2, SUOX, SLC5A5, AKR1C1, IL4, INHA and PCNA, and is suitable for detecting that whether there is pollution of plasticizers, such as phthalate and the like, in a human body or not.
Owner:舍尔辛格
Application of product for detecting gene locus mutation in preparation of product for predicting or evaluating metabolic condition of patient taking tacrolimus
PendingCN111662975AImprove forecast accuracyThe test result is accurateMicrobiological testing/measurementStatistical analysisGenetics genomics
The invention discloses an application of a product for detecting CYP3A4 rs2242480 and CYP3A4 rs4646437 gene locus mutation in preparation of a product for predicting or evaluating the metabolic condition of a patient taking tacrolimus. According to the application, single nucleic acid polymorphisms (SNP) of CYP3A4 rs2242480 and rs4646437 loci of 221 patients with kidney transplantation are determined and clinical combined medication conditions are discussed; genomics and statistical analysis find that combined use of a Wuzhi-capsule (WZC) and a CYP3A4 rs22480-rs4646437 polyploidy is a main factor affecting in vivo metabolism of the tacrolimus; in the aspect of pharmacogenomics, individualized medication of TAC is considered and a dosage prediction scheme of the TAC is formulated; safe, effective, economical and appropriate individualized medication of the TAC is achieved; and a theoretical basis is provided for clinical individualized medication and medication scheme adjustment.
Owner:南昌大学第一附属医院
Compounds that inhibit trpv1 and uses thereof
Owner:ABBVIE INC
Specificity probe substrate of cytochrome P450-3A4 enzyme and application thereof
ActiveCN104975066AStrong specificityConvenient sourceMicrobiological testing/measurementActivity measurementsActivity measurement
The invention provides a specificity probe substrate Tenuifoliside A of cytochrome P450-3A4 enzyme and application thereof in CYP3A4 enzyme activity measurement. The enzyme activity measurement includes the following operation processes: (1) with the Tenuifoliside A as the high-specificity probe substrate, carrying out a CYP catalytic reaction of a specificity substrate by means of a CYP in-vitro incubation system; and (2) quantitatively detecting the product generation amount or substrate consumption amount in unit time for determining the activity of the CYP3A4 enzyme in various bio-samples and cells. The specificity probe substrate can be used for quantitative evaluation of the CYP3A4 enzyme activity in the bio-samples from different individual sources, and quantitative measurement of the CYP3A4 single-enzyme activity of cell culture fluids and cell prepared products from different animal tissue cell sources.
Owner:GENERAL HOSPITAL OF PLA
Method for detecting in-vivo CYP1A2 and CYP3A4 enzyme activity in earthworm through high-performance liquid chromatography-tandem mass spectrometry
The invention relates to a method for detecting in-vivo CYP1A2 and CYP3A4 enzyme activity in earthworm through high-performance liquid chromatography-tandem mass spectrometry, and belongs to the technical field of the enzyme activity detection. The method comprises the following steps: (1) preparing earthworm microsome protein suspension; (2) adding the earthworm microsome protein suspension prepared in the step (1) in an incubation system containing two specific probe primers, warming to start enzymatic reaction, after the enzymatic reaction is terminated, detecting two specific metabolites produced after the enzymatic reaction is terminated by utilizing high-performance liquid chromatography-tandem mass spectrometry, and respectively computing CYP1A2 and CYP3A4 enzyme activity. The detection method disclosed by the invention is high in accuracy and precision and sensitivity, strong in stability, capable of measuring multiple CYP subenzyme activities in the earthworm body, thereby providing a detection method for exploring response mode on the soil pollutant by different CYP subenzyme of the earthworm.
Owner:CHONGQING ACAD OF AGRI SCI
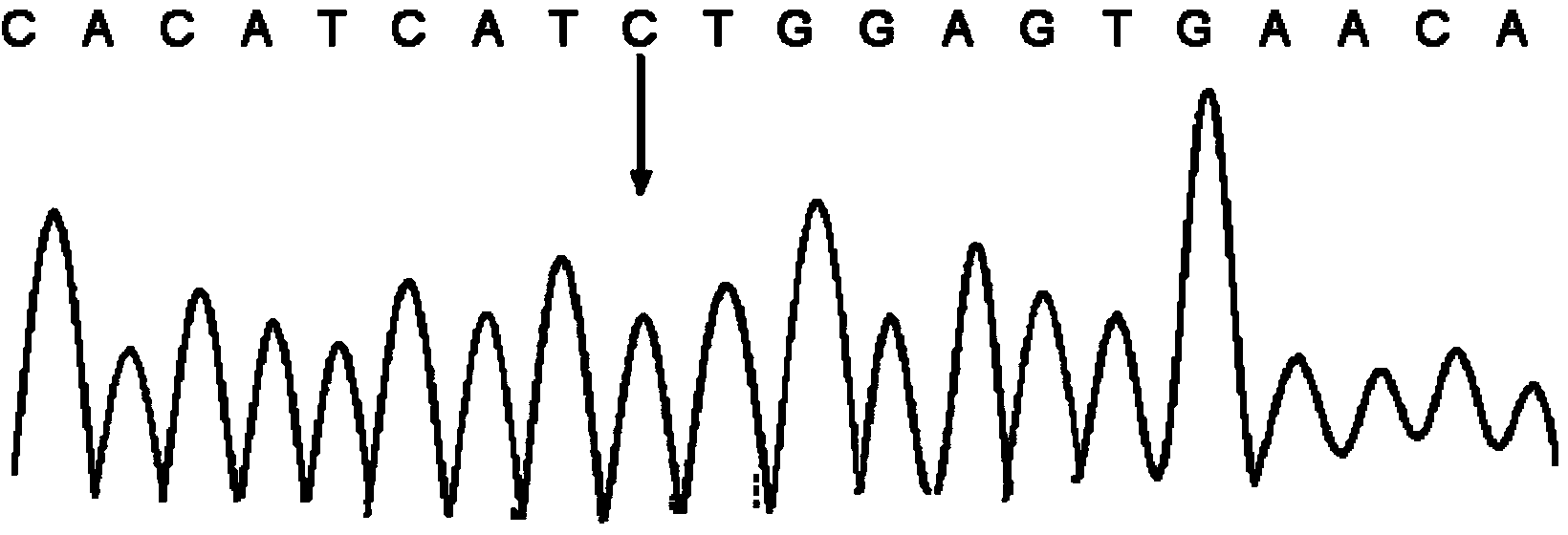
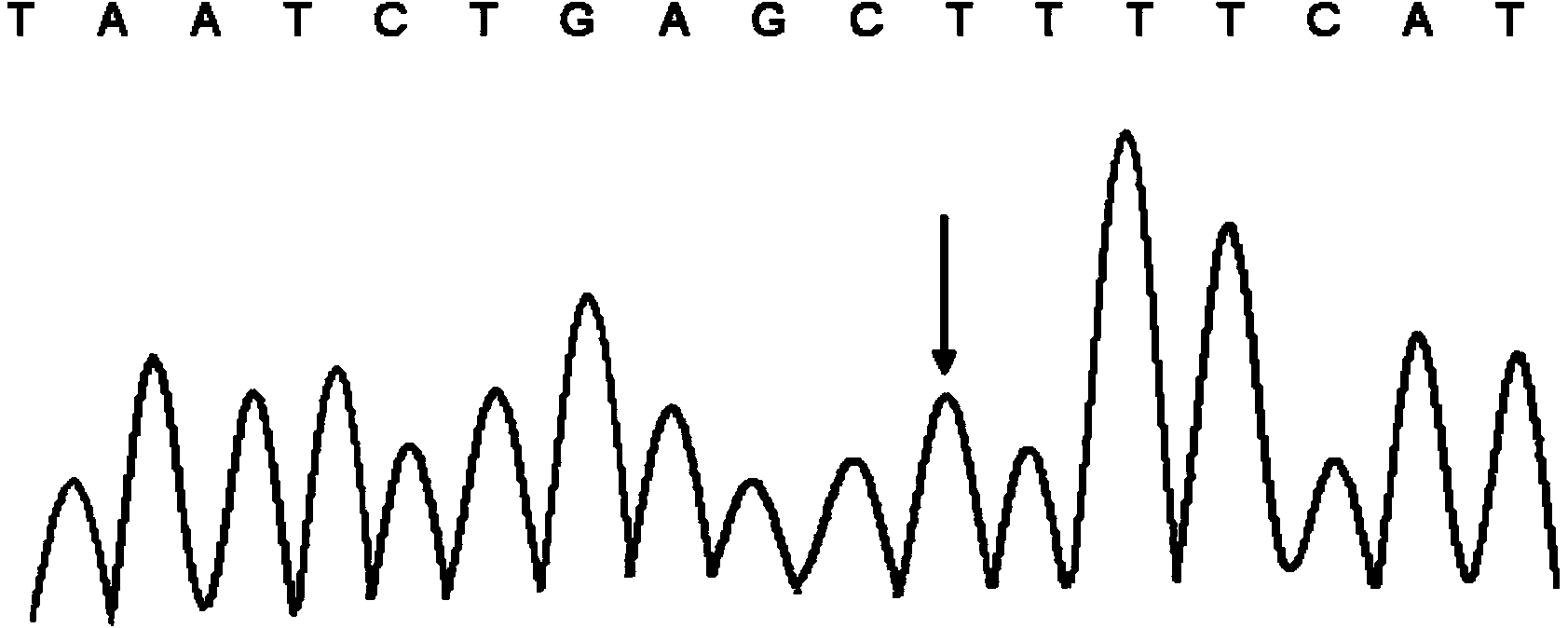
Kit and method for detecting polymorphism of CYP3A4 gene
ActiveCN103468814AIncreased sensitivityStrong specificityMicrobiological testing/measurement3-deoxyriboseExon
The invention relates to a kit and a method for detecting the polymorphism of a CYP3A4 gene and belongs to the technical field of gene sequencing. The kit comprises primers for detection, wherein the primers for the detection comprise at least one pair in long-fragment amplification specific forward and reverse primers for a first exon to a third exon of the CYP3A4 gene, long-fragment amplification specific forward and reverse primers for a fourth exon to a seventh exon of the CYP3A4 gene, long-fragment amplification specific forward and reverse primers for an eighth exon to an eleventh exon of the CYP3A4 gene and long-fragment amplification specific forward and reverse primers for a twelfth exon and a thirteenth exon of the CYP3A4 gene and at least one pair in forward and reverse sequencing primers for each exon of the CYP3A4 gene corresponding to long-fragment deoxyribose nucleic acid (DNA) of the first exon to the third exon, long-fragment DNA of the fourth exon to the seventh exon, long-fragment DNA of the eighth exon to the eleventh exon and long-fragment DNA of the twelfth exon and the thirteenth exon. According to the kit and the method, the detection time is short and the workload is low.
Owner:刘辉
Methods of treatment
ActiveUS20210401820A1Avoid and reduce incidenceSafety managementOrganic active ingredientsNervous disorderPharmaceutical drugPosaconazole
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug, wherein the patient is treated with posaconazole. In some embodiments, the patient stops posaconazole treatment, waits for at least 2 days, and then is treated with the CYP3A4 substrate drug as soon as it is safe to do so. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-42 days after stopping posaconazole. In some embodiments, the patient is treated with a reduced dose of the CYP3A4 substrate drug for about 2-42 days.
Owner:BOW RIVER LLC
N-substituted benzyl tetrahydropyridine with indole and preparation method and application thereof
InactiveCN103232433APrevent proliferationPromote apoptosisOrganic chemistryAntineoplastic agentsHuman leukemiaEsophagus Cancers
The invention discloses a N-substituted benzyl tetrahydropyridine with -5-substituted indole and preparation method and application thereof, and the structure is shown in the general formula (I): substituted benzene methylamine (ethylamine) and methyl acrylate are raw materials, and an intermediate N-substituted benzylpiperidine (phenylethylpiperidine)-4-ketone is obtained by three steps of reaction such as Michael addition, Dieckmann condensation and hydrolysis decarboxylation or the like in sequence, and the object (I) is obtained by condensation reaction of the intermediate and 5-substituted indole. The compound (I) can effectively inhibit proliferation of human leukemia K562, Jurkat, U937, THP-1 cell line, the human esophagus cancer ECA-109 cell line, human liver cancer SMMC-7721 cell line, human ovary cancer HO-8910 cell line, human breast cancer MCF-7 cell line, breast cancer MDA-MB-231 cell line; the compound has a good metabolism stability in the human and rat liver microsomes; The compound does not have mechanical inhibition effects for five enzymes of human liver microsomes such as CYP3A4, CYP 2D6, CYP2C9, CYP1A2 and CYP2C19 or the like; the compound can induce the cell cycle G2 / M retardance and promote cancer cell apoptosis and inhibit cancer cell propagation.
Owner:SHANGHAI NORMAL UNIVERSITY
Methods of treatment with cyp3a4 substrate drugs
PendingUS20220143011A1Avoid and reduce incidenceSafety managementOrganic active ingredientsNervous disorderPharmaceutical drugPosaconazole
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug, wherein the patient is treated with posaconazole. In some embodiments, the patient stops posaconazole treatment, waits for at least 2 days, and then is treated with the CYP3A4 substrate drug as soon as it is safe to do so. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-42 days after stopping posaconazole. In some embodiments, the patient is treated with a reduced dose of the CYP3A4 substrate drug for about 2-42 days.
Owner:BOW RIVER LLC
Inhibitors of cytochrome p450 (cyp3a4)
Owner:GILEAD SCI INC
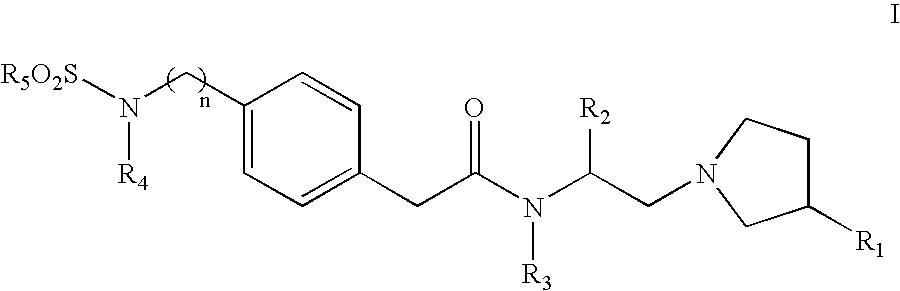
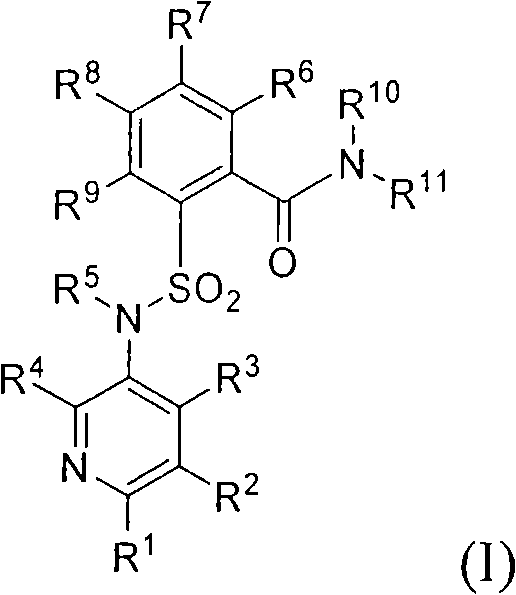
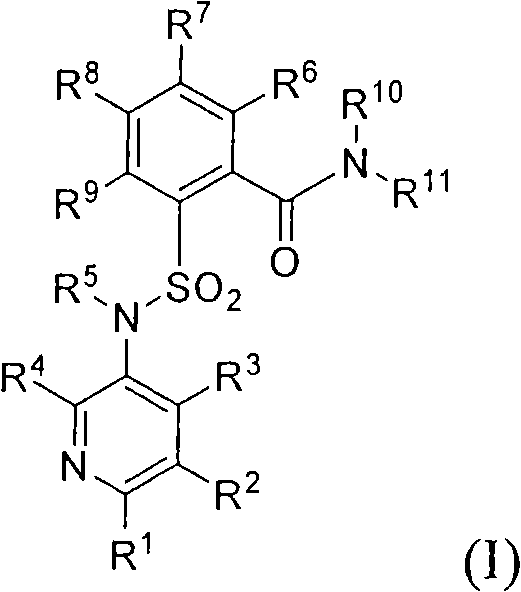
Sulfonylamino phenylacetamide derivatives and methods of their use
InactiveUS6992193B2Beneficial pharmaceuticalMinimizing undesirable side effectBiocideOrganic chemistryOpioid receptorHigh affinity binding
Sulfonylamino phenylacetamide derivatives of the general formula are disclosed. Pharmaceutical compositions containing the compounds and methods for their use are also disclosed. In certain embodiments, the compounds of the invention that, preferably:(1) bind with high affinity to κ opioid receptors;(2) display good opioid receptor selectivity of κ versus μ and κ versus δ; and(3) do not substantially inhibit cytochrome P450 enzymatic activity, in particular CYP2D6, CYP2C9 and CYP3A4.
Owner:APOLOR CORP
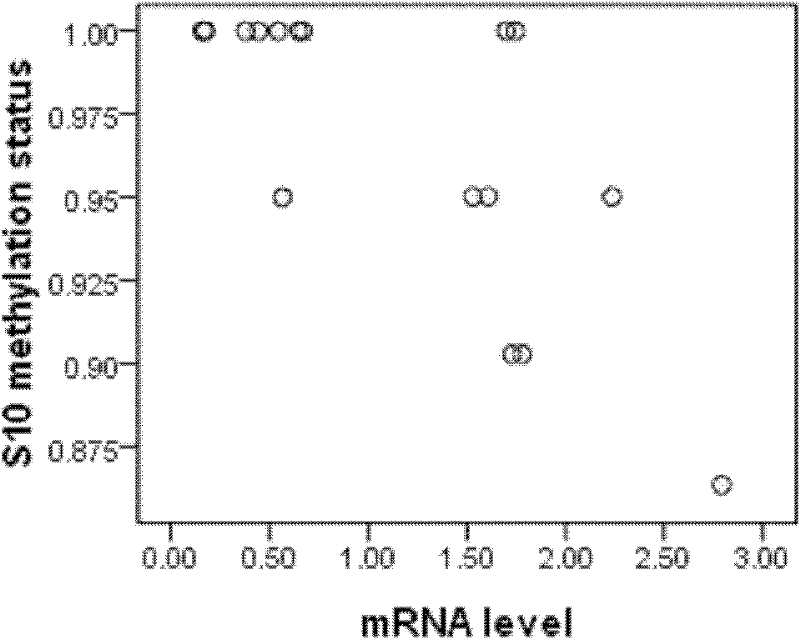
Method for deducting CYP3A4 gene expression quantity
The invention provides a method for deducting CYP3A4 gene expression quantity. The expression information of the CYP3A4 gene in human body can be deducted through detecting the methylation degree of a CpG site of the CYP3A4 gene in a human body blood DNA sample. The method is capable of deducting the activity of CYP3A4 enzyme in human body, explaining the individual difference of the drug effect and guiding the clinic and reasonable medication.
Owner:CHINESE NAT HUMAN GENOME CENT AT SHANGHAI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com