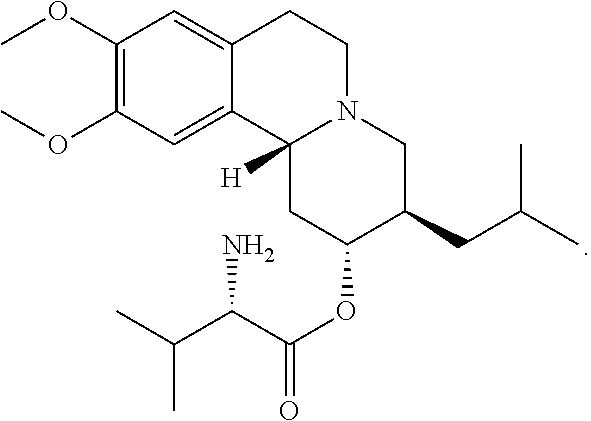
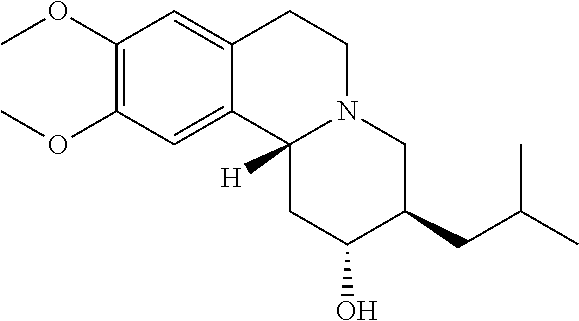
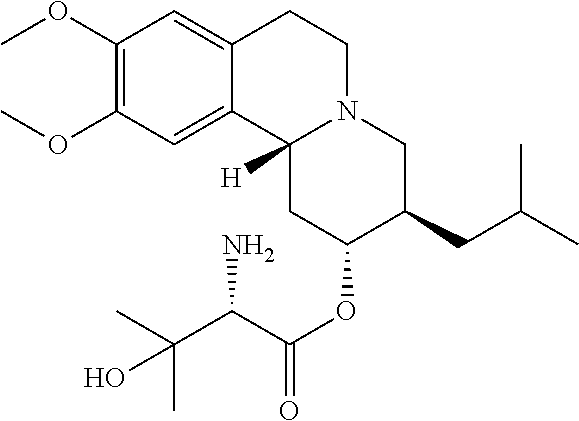
Methods for the Administration of Certain VMAT2 Inhibitors
a technology of vmat2 inhibitors and methods, applied in the direction of nervous disorders, drug compositions, organic chemistry, etc., can solve the problems of sub-therapeutic plasma levels of those drugs over tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Phase 1, Open-Label Study to Assess the Effect of Ketoconazole on the Pharmacokinetics of NBI-98854 in Healthy Subjects
[0234]This was a Phase 1, single-center, open-label, drug interaction study conducted in 24 healthy male and female subjects.
[0235]After providing informed consent, subjects were screened for eligibility to participate in the study. Screening started 21 days before Day 1 (the first day of study drug administration). Subjects who met the eligibility criteria were admitted to the research unit on Day −1 (the day prior to dosing) and remained at the study center until the end of the study (Day 10 or early termination). On Day −1, a blood sample was collected to determine cytochrome P450 2D6 (CYP2D6) status.
[0236]All subjects received the same treatment. Subjects received a single dose of 50 mg NBI-98854 on Days 1 and 6 at approximately 0800 hours. In addition, subjects received 200 mg ketoconazole twice daily (bid) on Days 5 through 9 at approximately 0800 and 2000 h...
example 2
Pharmacologic Characterization of Valbenazine, Tetrabenazine, and Metabolite Thereof
[0297]Upon oral administration, TBZ is reduced to form four discrete isomeric secondary alcohol metabolites, collectively referred to as dihydrotetrabenazine (DHTBZ), which contains three asymmetric carbon centers (C-2, C-3, and C-11β), which could hypothetically result in eight stereoisomers. However, because the C-3 and C-11β carbons have fixed relative configurations, only four stereoisomers are possible: (R,R,R-DHTBZ or (+)-α-DHTBZ (alternate nomenclature) or NBI-98782 (laboratory nomenclature); S,S,S-DHTBZ or (−)-α-DHTBZ or NBI-98771; S,R,R-DHTBZ or (+)-β-DHTBZ or NBI-98795; and R,S,S-DHTBZ or (−)-β-DHTBZ or NBI-98772.
[0298]The affinity of each compound was measured by inhibition of [3H]-DHTBZ binding to rat forebrain membranes. The affinities relative to R,R,R-DHTBZ were also calculated and are presented. Data are reported as both the negative logarithm of the Ki (pKi) for statistical calculati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| threshold concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com