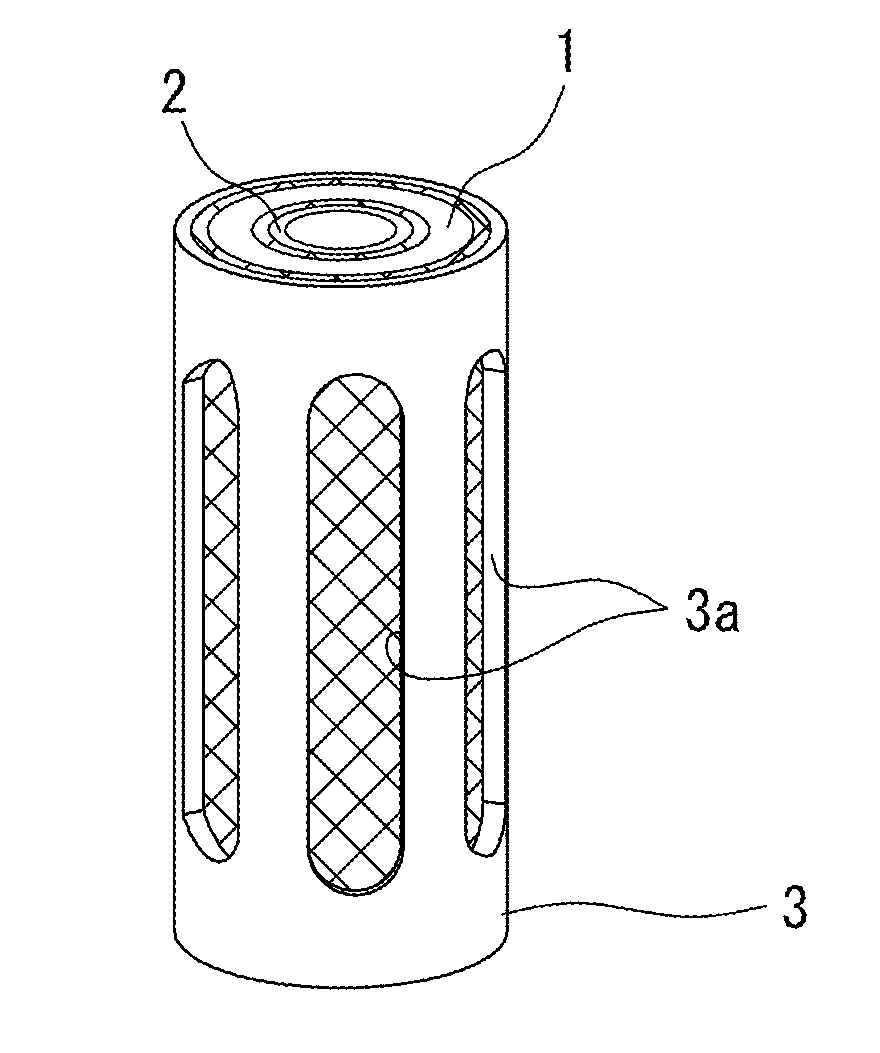
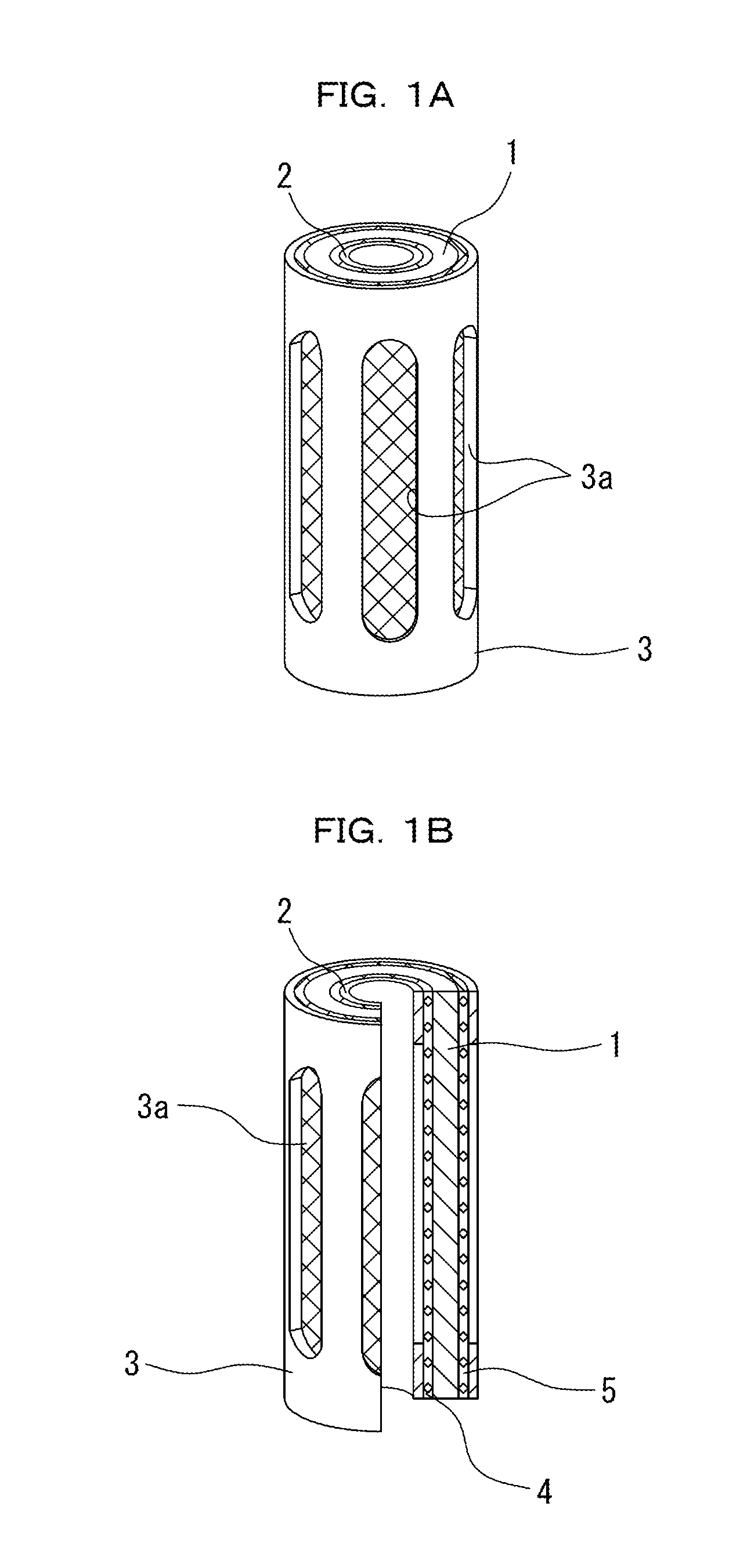
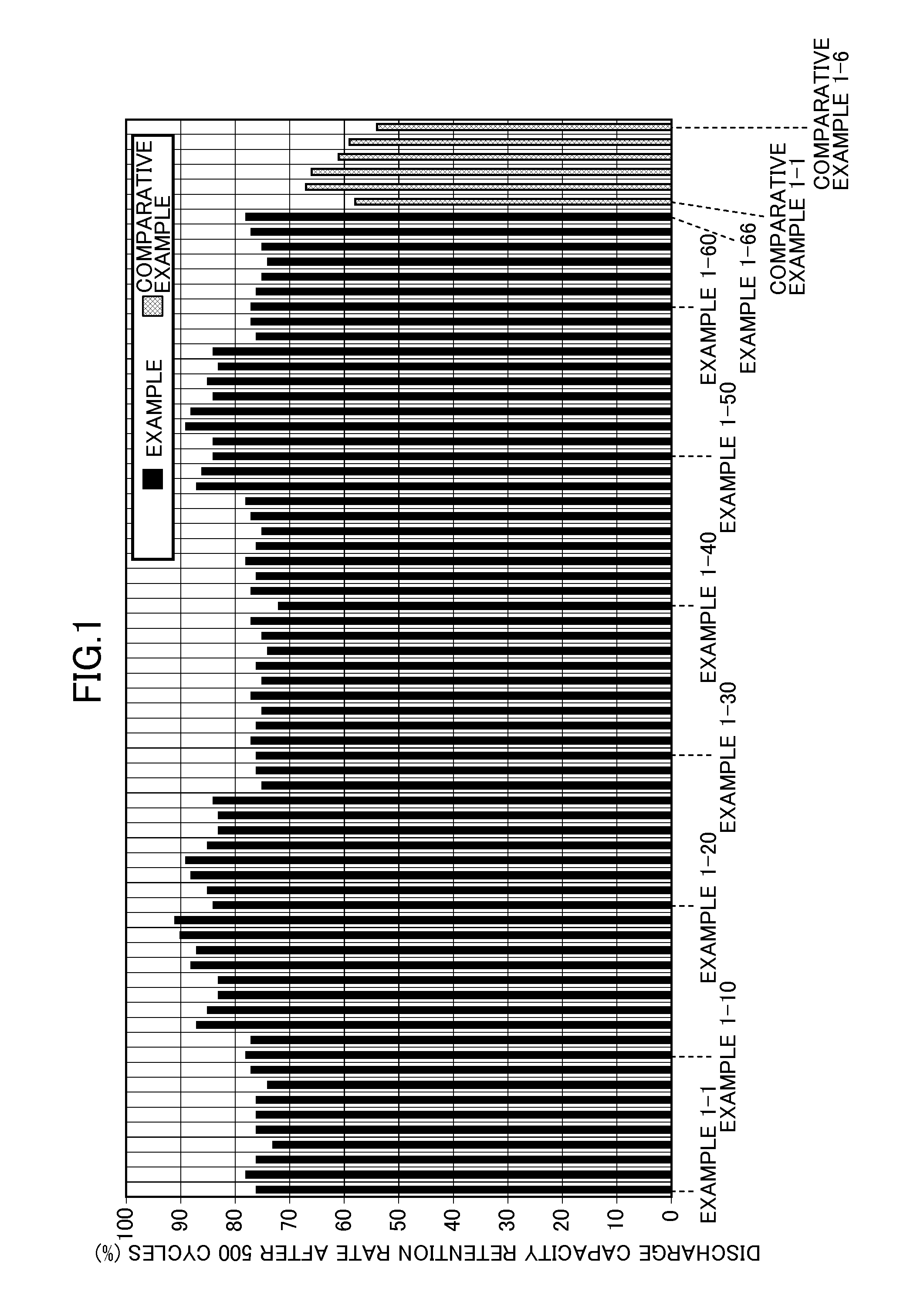
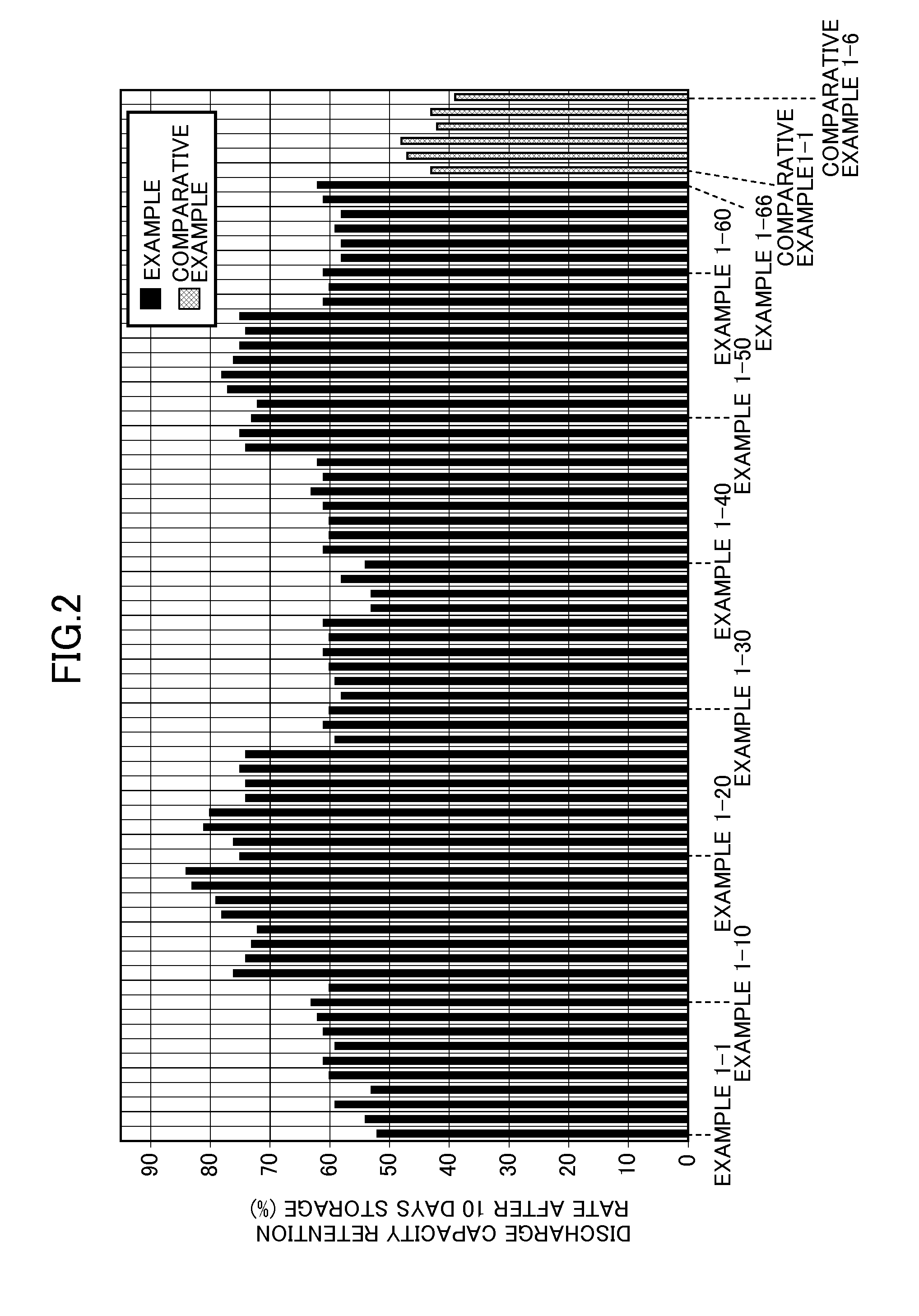
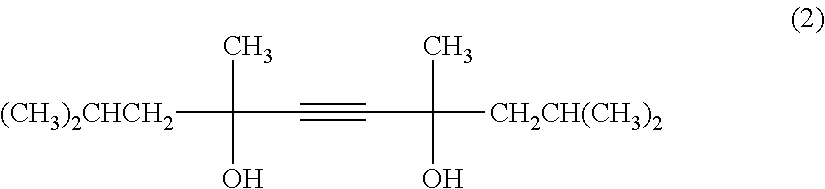
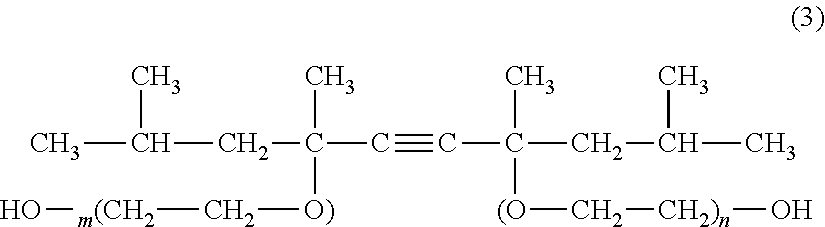
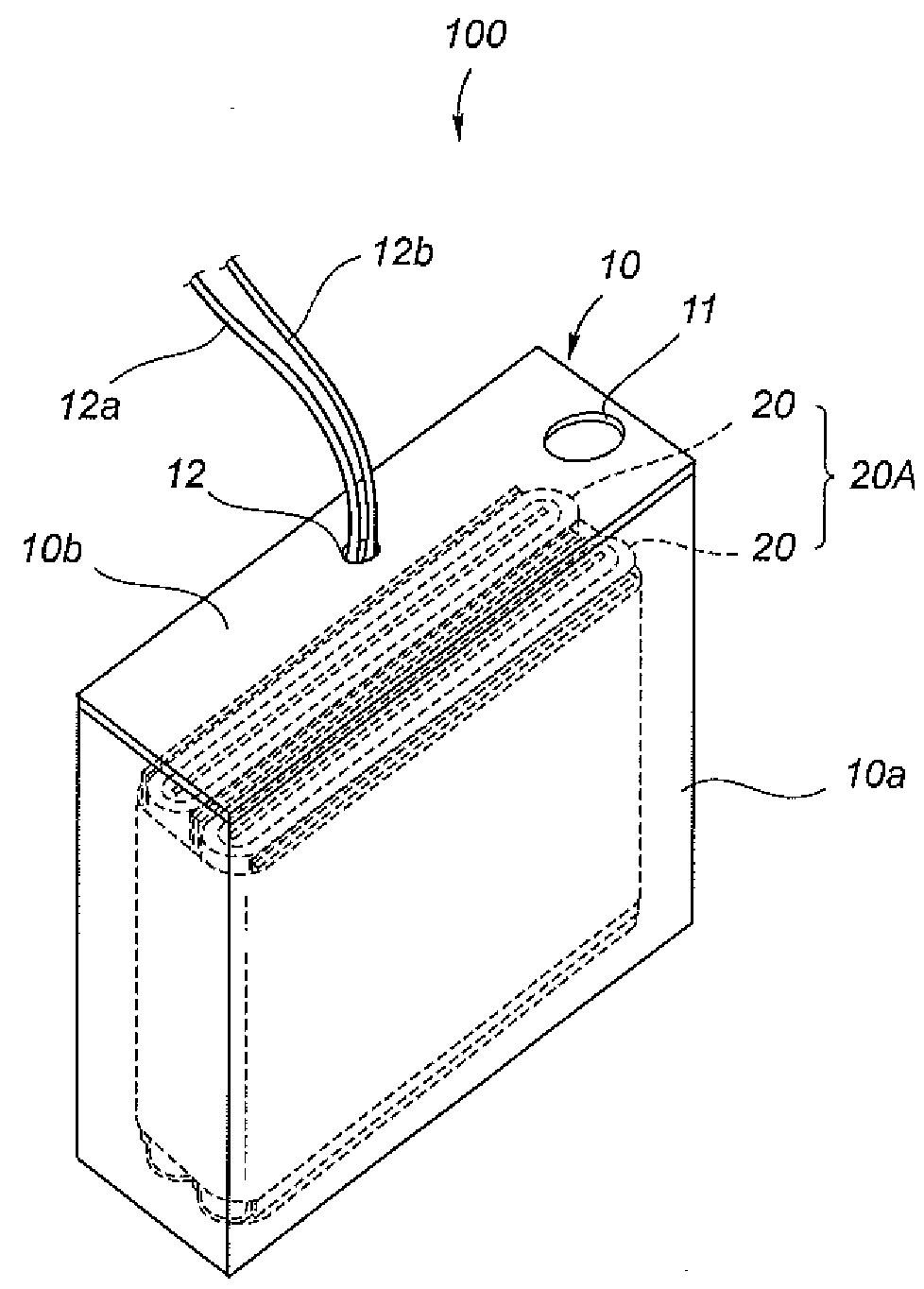
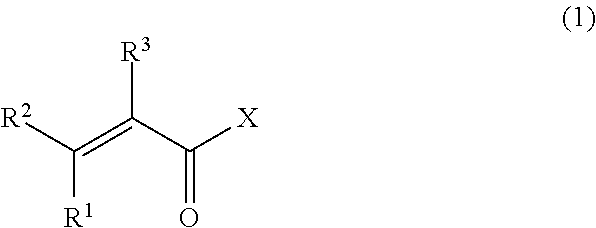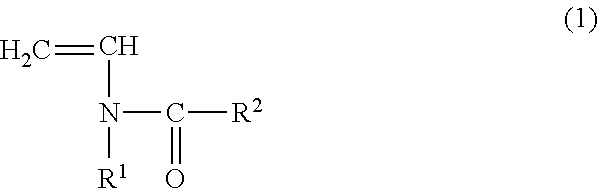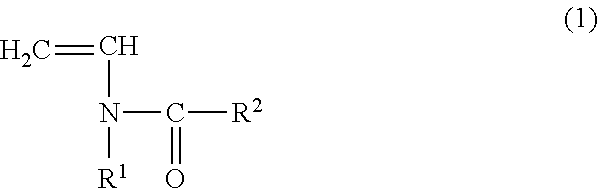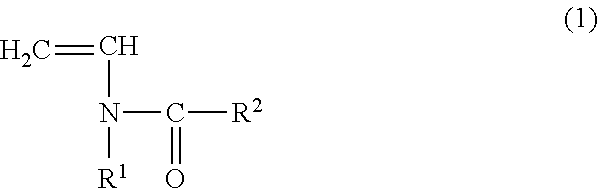Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
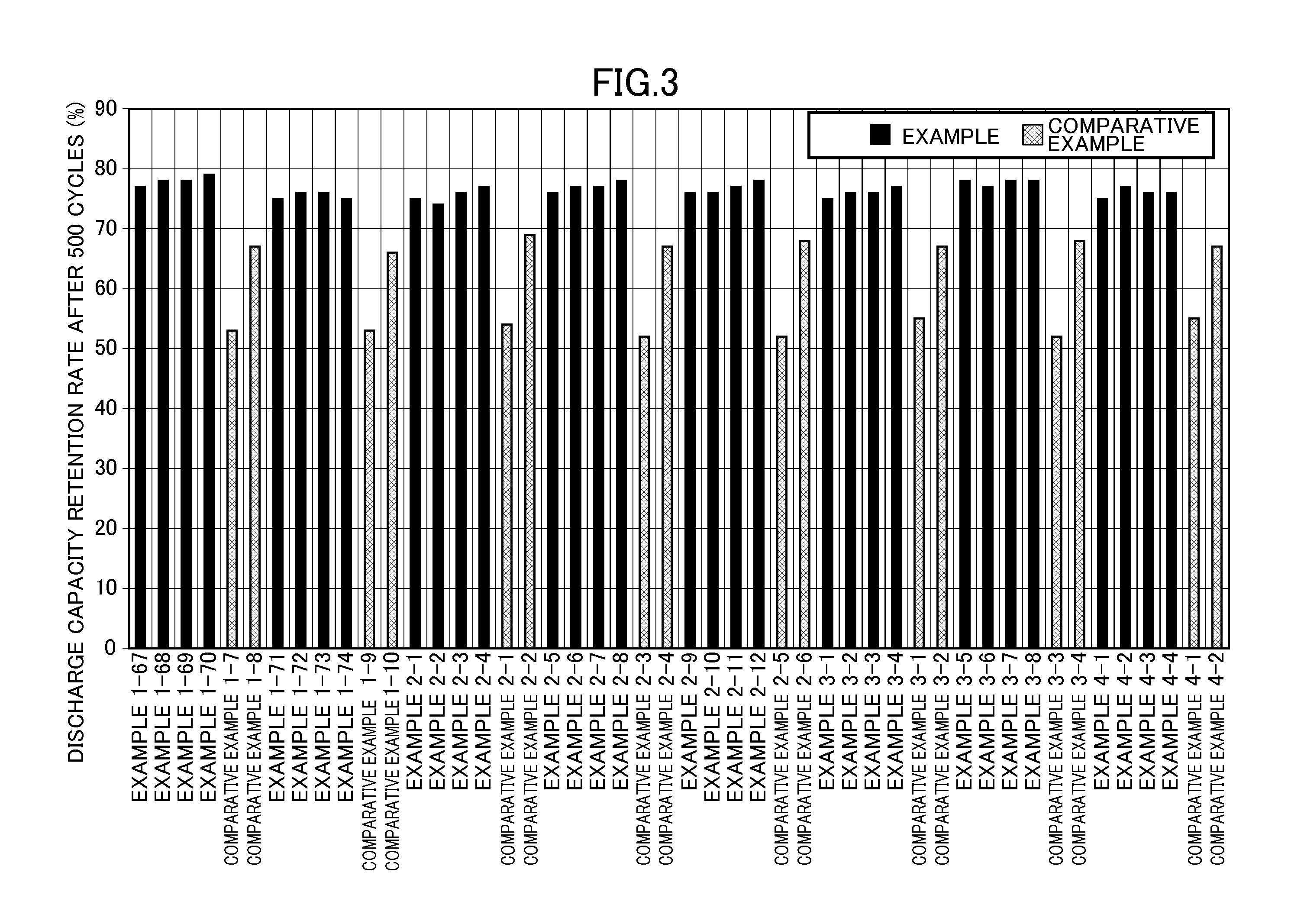
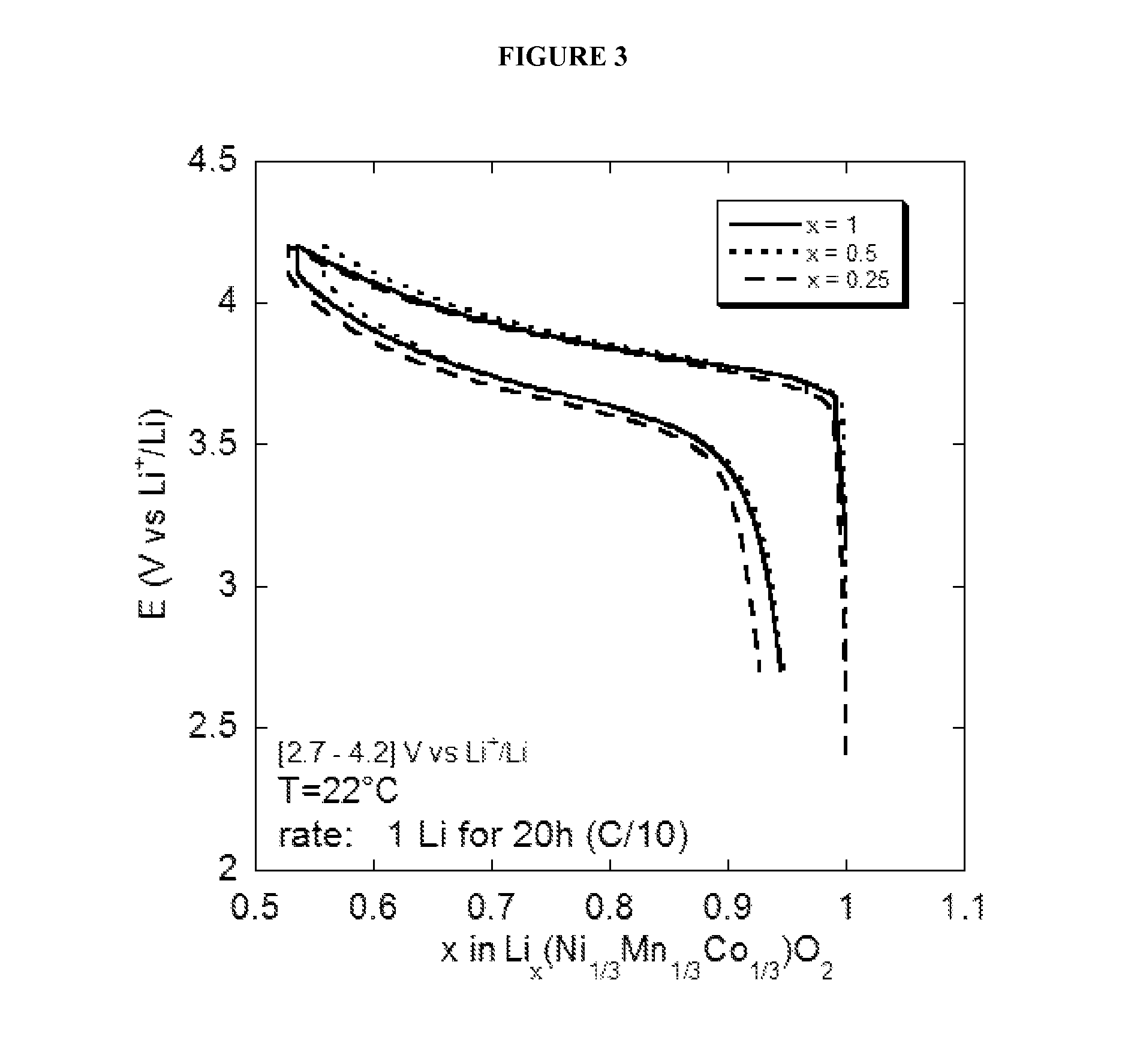
192 results about "Water battery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A water battery is a water based battery developed in 2014 by researchers at University of Southern California. It is capable of large energy density, tough cycle durability and costs 10 times less than comparable lithium ion batteries.
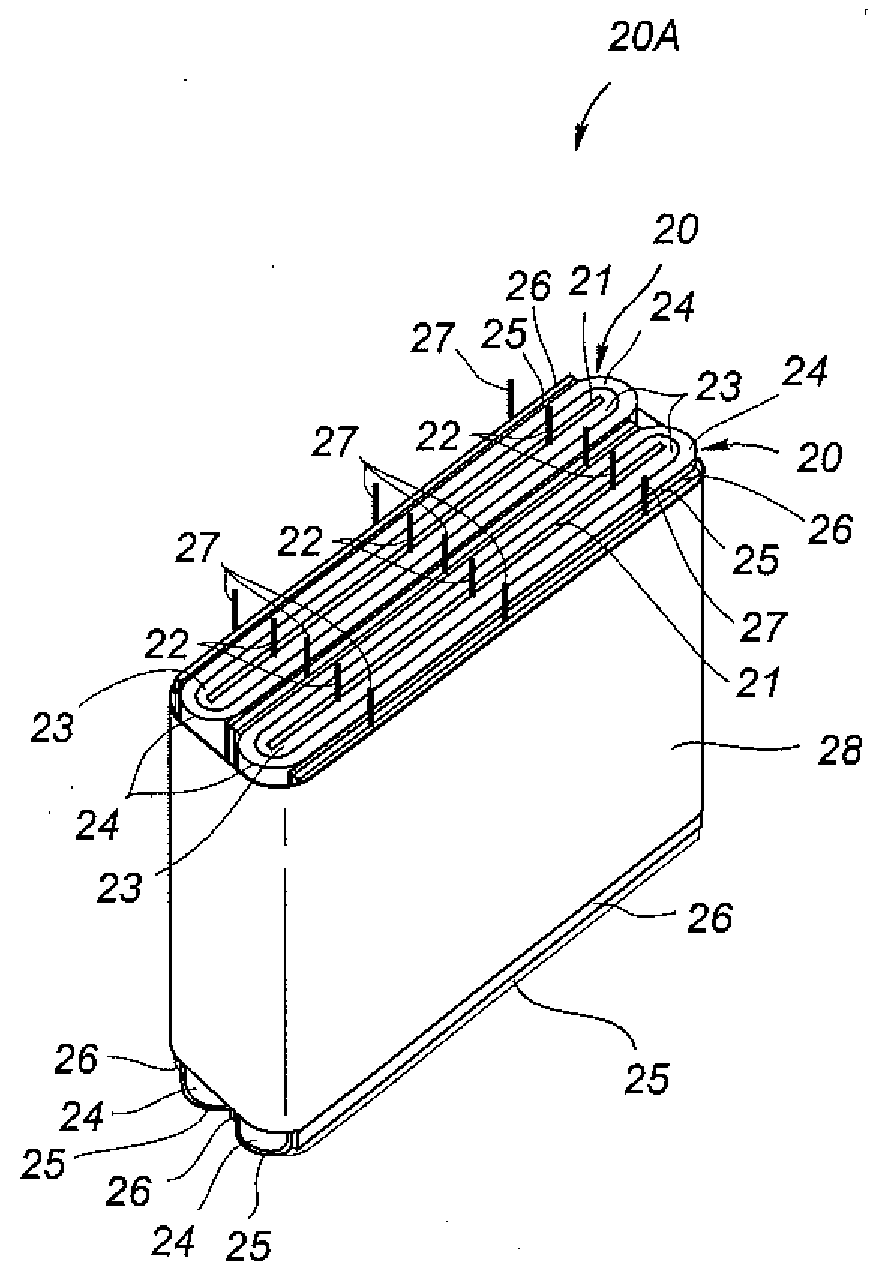
Non-aqueous battery of a thin configuration
InactiveUS6461757B1Small-sized cells cases/jacketsActive material electrodesMetallic foilElectrical battery
A non-aqueous battery is provided in a pouchy casing comprising opposing sheets of at least three-layer laminates, each laminate comprising (1) an inner thermoplastic resin layer, (2) a middle metal foil layer, and (3) an outer electrically insulating material layer, wherein the pouchy casing has an elongated, hermetic adhesion area along a periphery of the pouchy casing, and the middle metal foil layer has a peripheral elongated region in the elongated, hermetic adhesion area of the pouchy casing, and at least a pair of terminals electrically connected to the cathode and anode of the battery extends through and protrudes from the terminal-withdrawal sites in the elongated, hermetic adhesion area toward the outside of the pouchy casing, and the battery has at least one of the following features: (i) the peripheral elongated region of the middle metal foil layer has cut-out portions around the terminal-withdrawal sites and (ii) the surface of the peripheral edge of the pouchy casing is provided with electric insulation at least at portions around the terminal-withdrawal sites.
Owner:ASAHI KASEI ELECTRONICS CO LTD
Solid polymer battery electrolyte and reactive metal-water battery
In one implementation, a reactive metal-water battery includes an anode comprising a metal in atomic or alloy form selected from the group consisting of periodic table Group 1A metals, periodic table Group 2A metals and mixtures thereof. The battery includes a cathode comprising water. Such also includes a solid polymer electrolyte comprising a polyphosphazene comprising ligands bonded with a phosphazene polymer backbone. The ligands comprise an aromatic ring containing hydrophobic portion and a metal ion carrier portion. The metal ion carrier portion is bonded at one location with the polymer backbone and at another location with the aromatic ring containing hydrophobic portion. The invention also contemplates such solid polymer electrolytes use in reactive metal / water batteries, and in any other battery.
Owner:BATTELLE ENERGY ALLIANCE LLC
Polyolefin and Ceramic Battery Separator for Non-Aqueous Battery Applications
InactiveUS20110171523A1Improve heat resistanceImprove carrying capacityCell component detailsVehicular energy storagePolyolefinPolymer science
A ceramic microporous polyolefin battery separator membrane, high in air permeability, low in shrinkage and improved temperature resistance addresses the safety requirements of lithium ion batteries. The separators made by the current invention consists of one or more polyolefin polymers and kaolin fillers comprised of aluminum oxide and silicon oxide. The membranes of current invention have a thickness of 5-200 microns, air permeability of 1-200 sec / 10 cc (Gurley seconds), and average pore diameter of less than 1 micron.
Owner:ADVANCED MEMBRANE SYST INC
Magnesium sea water battery
This invention relates to a Mg sea-water cell based on the fuel cell technology theory which converts the dissolved oxygen in the sea to a cathode active substance by inert cathode, takes the sea water as the cell electrolyte and applies high potential Mg alloy as the anode to make it an independent chemical cell after immerging it in the sea. The cell only consumes Mg alloy anode when working and changes a new one after the Mg alloy anode is finished. The cell can work for a long time at a reliable and stable situation since the cell has a large capacity not necessary to have any pressure container, one Mg alloy anode can work continuously for 1-2 years, especially suitable for the work in the deep sea or the bottom.
Owner:李华伦 +3
Method for preparing battery-level lithium carbonate by using salt lake brine
ActiveCN102976367ALow impurity indexImprove product qualityLithium carbonates/bicarbonatesLithium carbonatePhysical chemistry
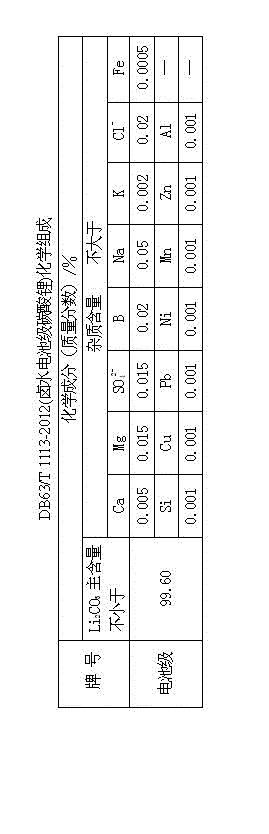
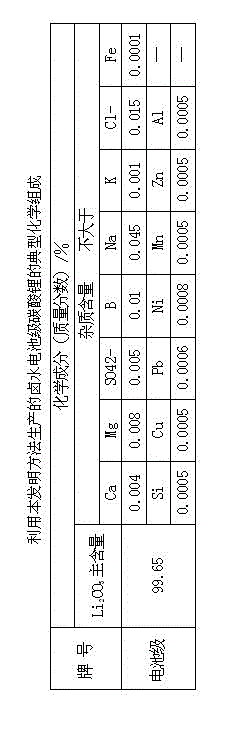
The invention provides a method for preparing battery-level lithium carbonate by using salt lake brine. The method uses salt lake brine in Qinghai as a raw material and uses an ion selectivity separation device to transfer magnesium and lithium ions in the raw material of the brine under the effect of an electric field; when the raw material of the brine passes through a separation membrane with selectivity, monovalent ions, such as the lithium ions and sodium ions can pass through, but divalent ions, such as magnesium ions and calcium ions can be separated, so that lithium-enrichment brine with a low ratio of magnesium to lithium is obtained after the separation; impurities, such as Ca<2+>, K<+>, SO4<2-> and Mg<2+>, in the lithium-enrichment brine with the low ratio of the magnesium to the lithium can be deeply removed; an auxiliary material of a pure alkali solution can be purified; the lithium-enrichment brine after deep removal of the impurities is subjected to three-effect evaporation and concentration after neutralizing with acid; alkali is added into the concentrated lithium-enrichment brine at a certain temperature for lithium sedimentation; and finally, the finished product of the battery-level lithium carbonate can be obtained by drying and cooling after filter press, washing and centrifugal separation washing are carried out. The battery-level lithium carbonate meets the requirements of the local standard DB63 / T1113-2012 (brine battery lithium carbonate) in the Qinghai Province.
Owner:QINGHAI LITHIUM IND
Non-aqueous cell having a mixture of fluorinated carbon cathode materials
The present disclosure relates generally to a cathode material suitable for use in a non-aqueous electrochemical cell that comprises a mixture of fluorinated carbon materials, and more particularly such a cell that comprises a mixture of three fluorinated carbon materials that each have distinct (from each other) discharge profiles (e.g., distinct voltages and capacities). The present disclosure additionally relates to a non-aqueous electrochemical cell comprising such cathode material and, in particular, to such a non-aqueous electrochemical cell that is lithium-based (i.e., a lithium, or lithium ion, non-aqueous electrochemical cell).
Owner:EAGLE PICHER TECH LLC
Hydrogen evolution electrode, preparation method and applications thereof
ActiveCN108172850ALarge specific surface areaIncrease active siteCell electrodesImmersion cellsAlloyCobalt
The invention relates to a hydrogen evolution electrode, a preparation method and applications thereof. The preparation method specifically comprises: washing a foam metal foam nickel as a substrate,and electrochemically depositing a layer of a granular alloy containing one or more than two selected from nickel, cobalt and molybdenum on the surface; carrying out room temperature aging in a solution containing chlorine ions to from a layer of a nano-sheet-like hydroxide with a nano-scale thickness on the surface of the electroplating layer, wherein the hydroxide is corresponding to the electrodeposition metal; and electrochemically depositing trace platinum, and continuously carrying out room temperature aging to increase the thickness of the hydroxide so as to obtain the hydrogen evolution electrode with the multi-level pore channel structure. According to the present invention, the prepared hydrogen evolution cathode has the low platinum loading, has the excellent hydrogen evolutionmass specific activity at the platinum loading of lower than 10 [mu]g / cm<2>, and has good stability in the application of magnesium-water batteries.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Anode plate material for magnesium cell
The anode plate material of magnesium cell is a continuously cast or continuously cast-rolled magnesium alloy, and its metallographic structure is a column crystal imward grown from surface layer andits structure is compact. Said anode plate material is an alloy using magnesium as base body and adding several elements, and said plate material is applicable to magnesium manganese laminated battery, cylindrical magnesium manganese dry battery, magnesium air cell and magnesium sea-water battery.
Owner:FUJIAN HUAMEI NEW TECH DEV
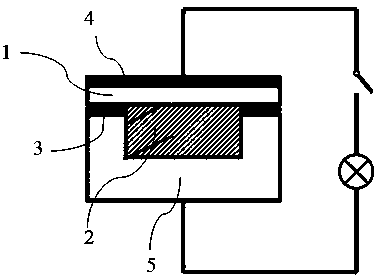
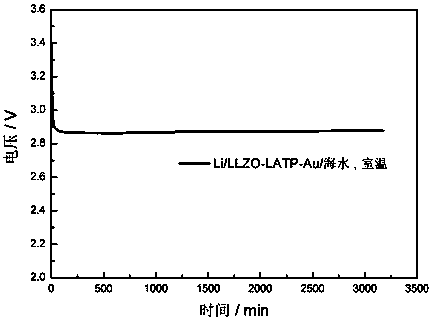
Preparation method and application of double-layer oxide solid electrolyte
InactiveCN110165236ACorrosion protectionExcellent duty cycle stabilityFuel and primary cellsSolid electrolyte cellsSolid state electrolyteElectron
The invention discloses a preparation method and application of a double-layer oxide solid electrolyte. A double-layer LLZO / LATP multifunctional oxide solid electrolyte comprises a dense LLZO ceramicelectrolyte layer and a porous LATP solid electrolyte layer. The dense LLZO electrolyte layer is stable to a metal lithium negative electrode and prevents corrosion of the metal lithium negative electrode by seawater. The porous LATP solid electrolyte layer is stable to seawater and air and provides an ionic conductive framework for the air positive electrode reaction. The double-layer oxide solidelectrolyte can be used for preparing a lithium seawater battery pack. The lithium seawater battery comprises the LLZO / LATP multifunctional oxide solid electrolyte, a lithium negative electrode packaged in a dense garnet electrolyte layer and an air positive electrode material having an ion / electron conductive network. Compared with the present lithium seawater battery, the structure design of the lithium seawater battery is more suitable for complicated seawater condition and has better working stability.
Owner:QINGDAO UNIV
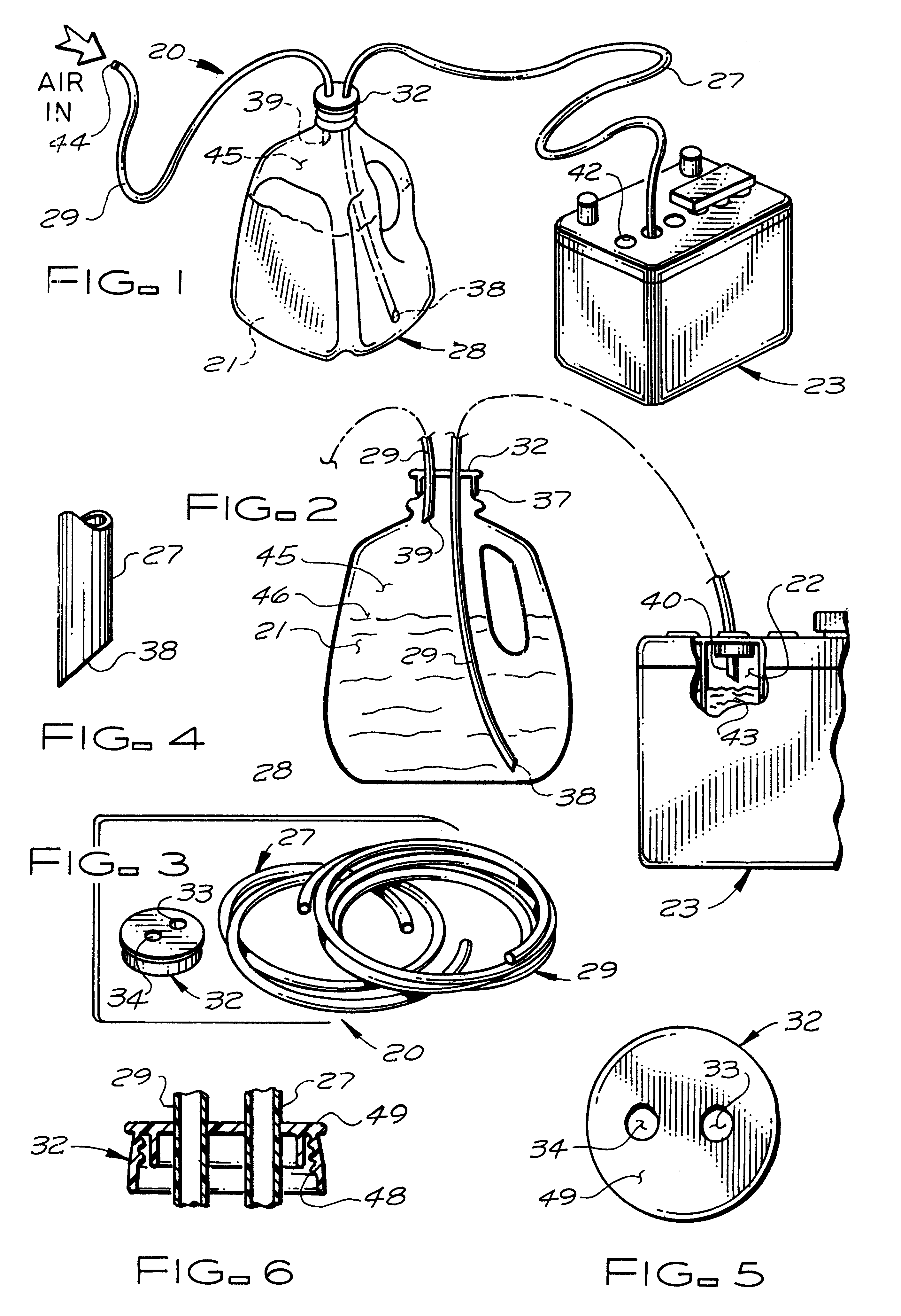
Battery filling system
InactiveUS6341628B1Easy to useEasy to manufactureLiquid fillingPressure pumpsBattery chargeMarine engineering
A battery filling system for use in efficiently adding water to, for example, those lead-acid batteries which are located in inconvenient or hard-to-reach places. The battery filling system includes a first flexible tube for use in transferring distilled water from the interior of a water-filled vessel to the hard-to-reach cell of a lead-acid battery. A second flexible tube transfers pressurized air from an external pressurized air source (typically human lungs) to the interior of the vessel in a quantity sufficient to cause the water to advance into and fill the first tube. A cap is used to snugly attach both tubes to the vessel. A means is also provided to minimize loss of air pressure from the vessel.
Owner:BURSON WILLIAM C
Cell catalyzed by titanium dioxide, preparation method of cell and application
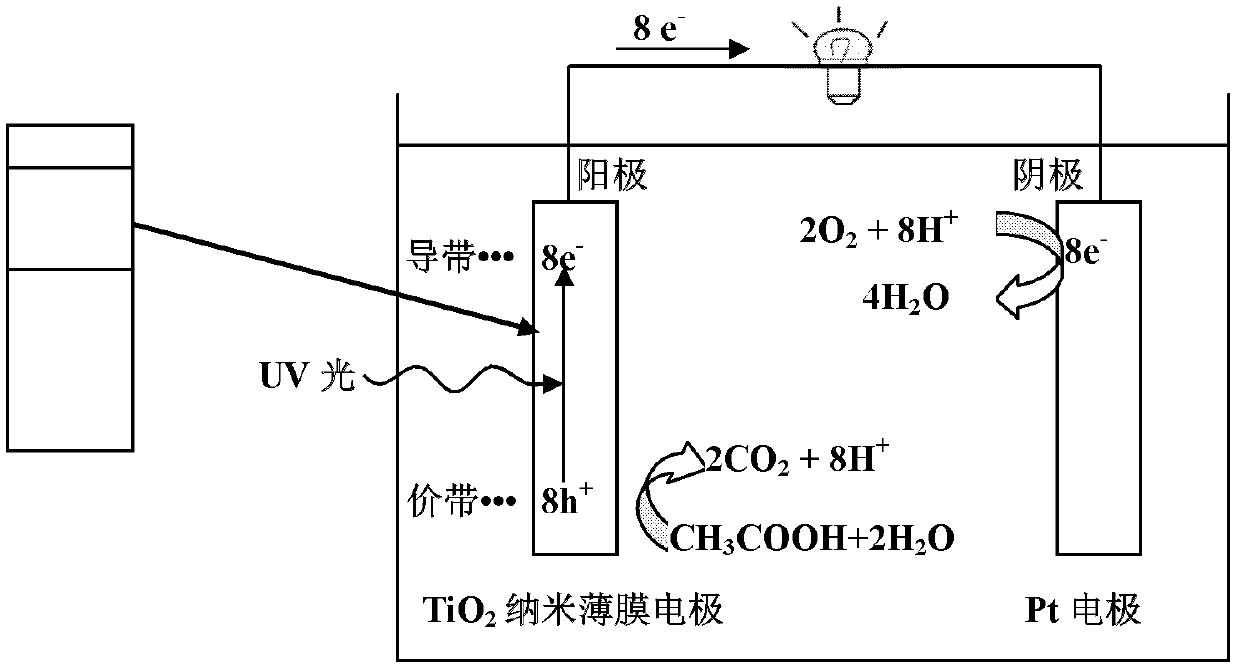
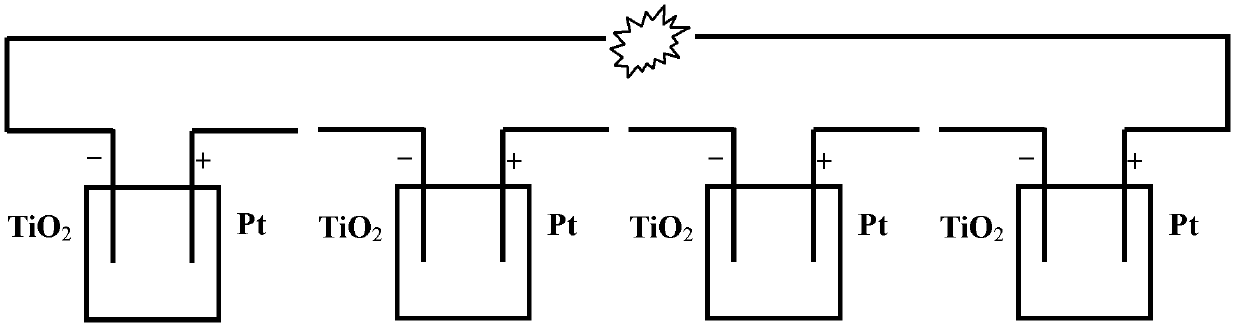
InactiveCN102820497APromotes rapid oxidationRealize processingCell electrodesWater/sewage treatmentWastewaterEnergy conversion efficiency
The invention discloses a power generation unit and particularly relates to a wastewater organic cell catalyzed by titanium dioxide films. A layer of nano-level titanium dioxide films is coated on conductive glass to serve as an anode, the anode and a cathode form the cell, and the cell can process organic substances in wastewater, recycle biological energy sources and convert biological energy sources to power generation. The cell has good energy conversion efficiency, the performance of the cell is better than that of an existing microorganism wastewater cell, and the current performance is increased by about 102-103 times. Besides, the cell has the advantages of being simple in structure, convenient to operate, easy to control and wide in application.
Owner:SHANGHAI QIBAO HIGH SCHOOL
Nonaqueous electrolyte cell and its manufacturing method
InactiveUS20030175583A1Improve securityEasy transferElectrode manufacturing processesFinal product manufactureCarbon dioxide contentElectrolyte
A non-aqueous cell according to the present invention has an assembly element comprising a positive electrode, a negative electrode, and a separator in a sealed case with the features: an amount of electrolyte is greater than or equal to 30% and less than or equal to 100% of the total pore volume of said assembly element; and a carbon dioxide content is greater than or equal to 1 volume % of the total gas contained in said sealed case.
Owner:GS YUASA CORP
Adhesive tape for non-aqueous battery
InactiveUS20120202051A1Prevent deformationGood anchor propertyFilm/foil adhesivesFinal product manufacturePolyolefinAdhesive
The present invention provides an adhesive tape for non-aqueous battery, comprising a substrate and an adhesive layer laminated on at least one surface of the substrate, wherein the adhesive layer is made of an adhesive comprising a polyolefin (a), a hydroxyl group-containing polyolefin (b) and a crosslinking agent (c) having a functional group capable of reacting with the hydroxyl group.
Owner:NITTO DENKO CORP
Magnesium water battery
The invention relates to a magnesium water battery which comprises a magnesium or magnesium alloy anode, a diaphragm, a hydrogen-evolution-catalyst-carried cathode and a cathode reactant water, wherein the anode and cathode are arranged in opposite; the diaphragm is arranged between the anode and cathode; and the anode, cathode and diaphragm are arranged in water. Compared with the prior art, the magnesium water battery is simple in structure and convenient to use, and is not restricted by the dissolved oxygen concentration in seawater when being used in seawater. The underwater magnesium water battery solves the problem of dependence of the traditional magnesium dissolved oxygen seawater battery on the oxygen concentration in seawater, and can operate normally even in an oxygen-free environment as long as the anode and cathode of the battery are isolated by the diaphragm and a hydrogen evacuation channel is left. Compared with the traditional magnesium dissolved oxygen seawater battery which needs a larger open space to ensure the continuous renewal of fresh seawater, the magnesium water battery has the advantages of compact structure, small size and obviously higher volumetric specific energy. The battery can operate normally as long as seawater enters.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
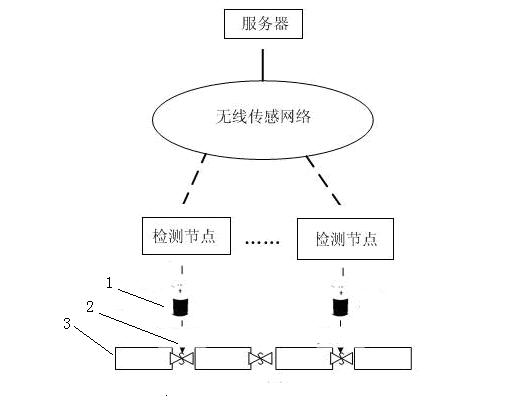
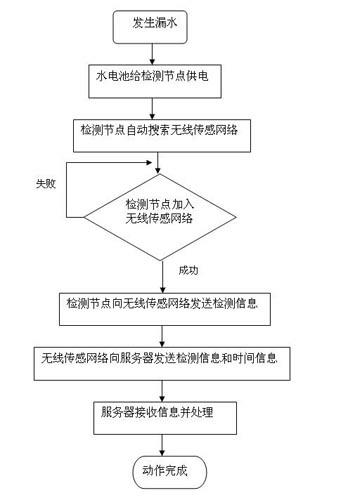
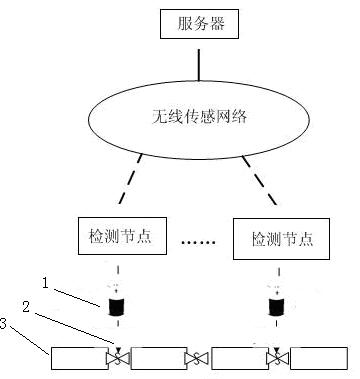
Automatic detection device and detection method for water leakage of water pipeline
InactiveCN102635787AReduce use costReduce maintenance costsPipeline systemsWater leakageNetwork monitoring
The invention provides an automatic detection device and a detection method for water leakage of a water pipeline, well solving the problem of pipe network monitoring. The detection device is simple in structure and energy-saving, instantly reports leakage and does not need to maintain in fixed time. The automatic detection device for the water leakage of the water pipeline comprises a detection unit, a transporting unit and a processing unit, wherein the detection unit detects water leakage information and converts the water leakage information into an electronic or digital signal, and after being initialized, the detection unit automatically searches the transporting unit; the transporting unit receives detection information and transports the detection information to the processing unit; the processing unit receives and processes the detection information transferred by the transporting unit; and power is supplied for the detection unit by a water battery; and the water in the water battery is supplied by water leaked from a water pipeline. The automatic detection device has the characteristics that the water battery is applied to leakage detection of a water pipeline, and application scenes comprise families, office buildings and municipal engineering.
Owner:SUN YAT SEN UNIV
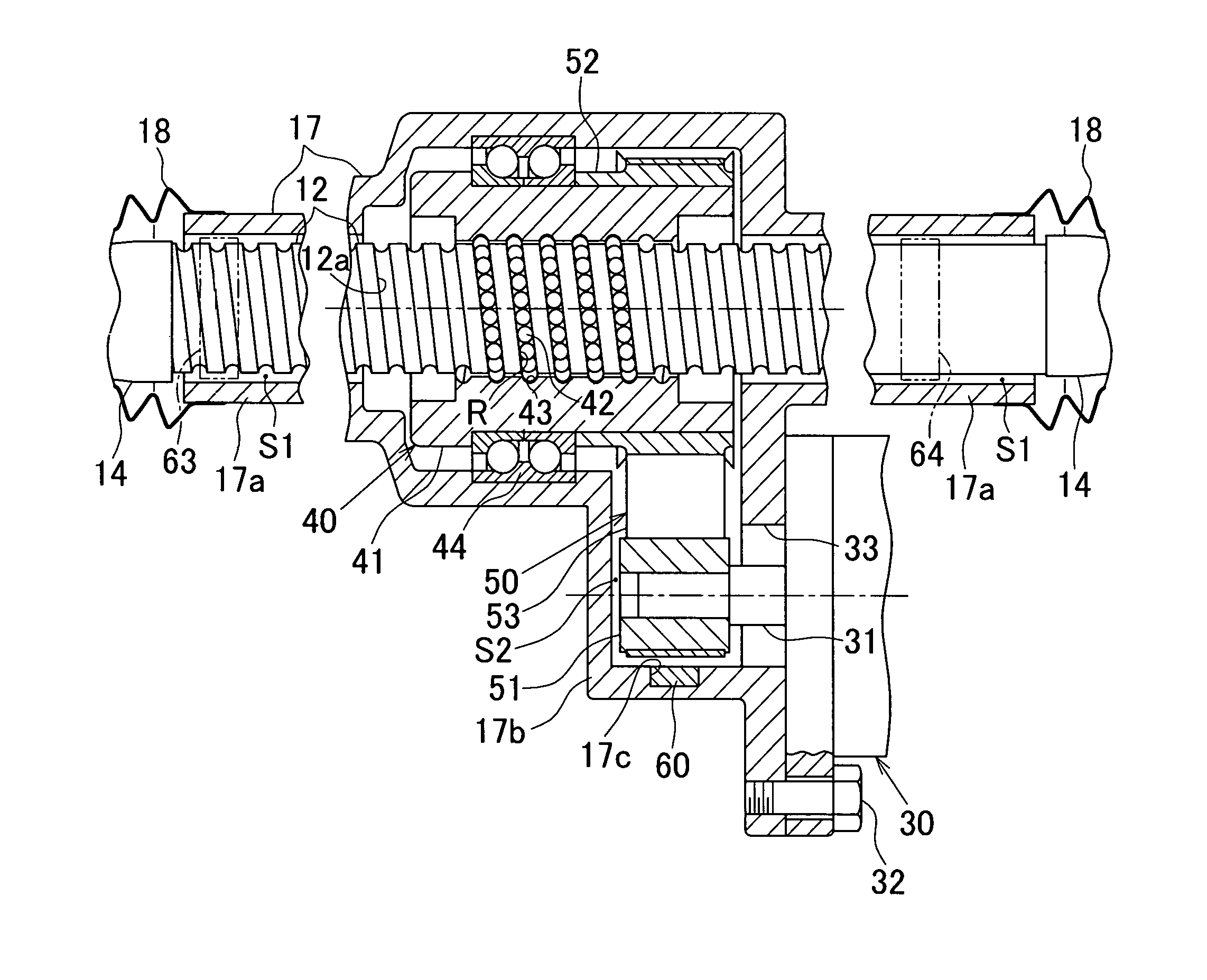
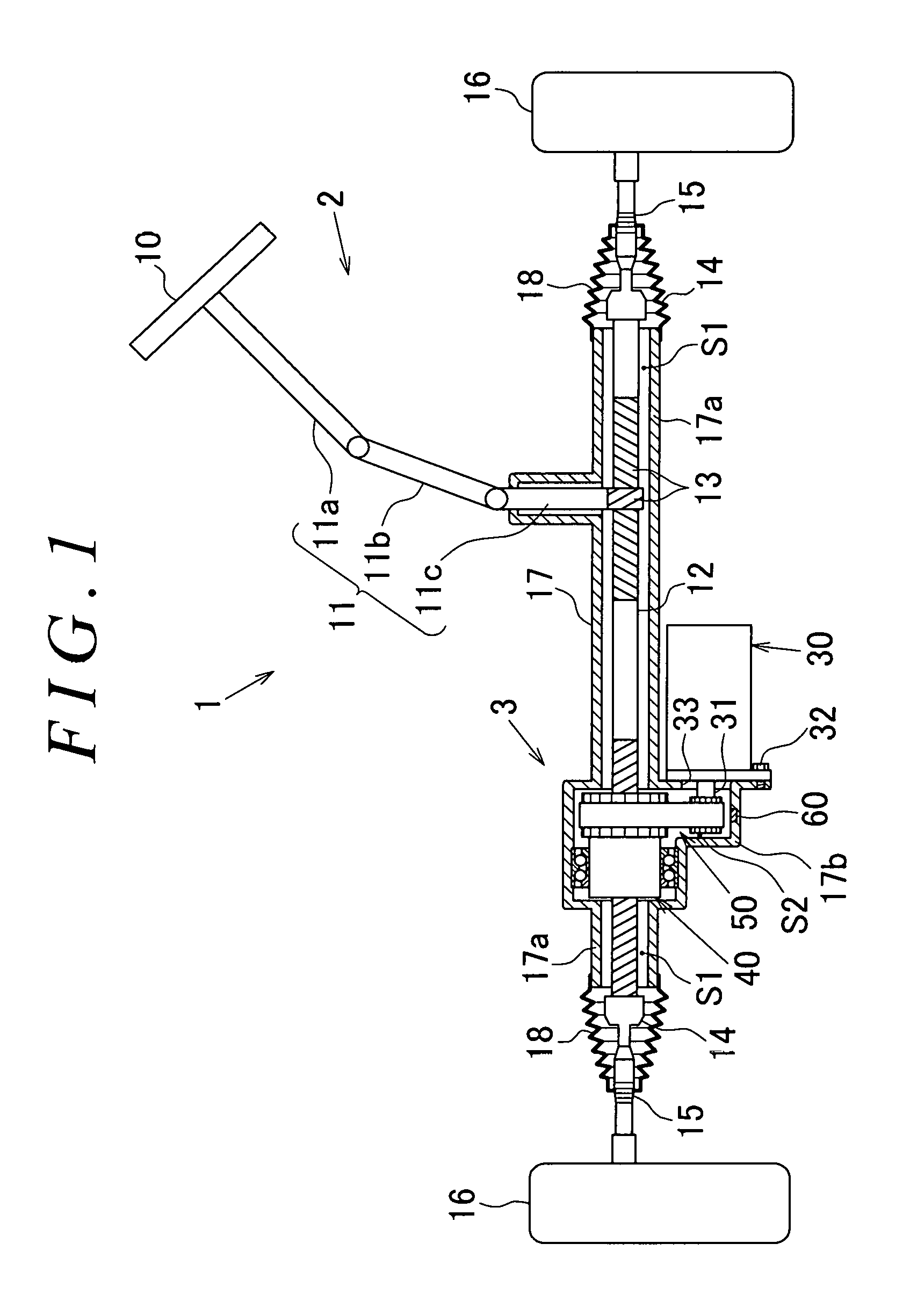
Moisture sensor and steering system
ActiveUS20170050669A1Reduce power consumptionAverage power consumptionCells structural combinationElectrical steeringWater flowEngineering
A recessed portion is formed in an inner wall surface of a lower portion of a reduction gear housing (a lower wall in FIG. 2) in the direction of gravity and between a bearing 44 and an outer wall of the reduction gear housing (a right side wall in FIG. 2). The recessed portion is formed to have such a depth that the recessed portion does not penetrate the reduction gear housing. The recessed portion is formed in the reduction gear housing within a given range in a circumferential direction with respect to the axis of a rack shaft serving as the center. A moisture sensor is mounted in the recessed portion to detect water infiltrating into a housing. The moisture sensor has a transmission circuit that operates using a water battery as a power supply source. The water battery generates power when water flows into the water battery.
Owner:JTEKT CORP
Vinylidene fluoride copolymer, method for producing the same, gel electrolyte, and non-aqueous battery
ActiveUS20170015772A1Improve balanceSecondary cellsElectrolye immobilisationAtomic groupHexafluoropropylene
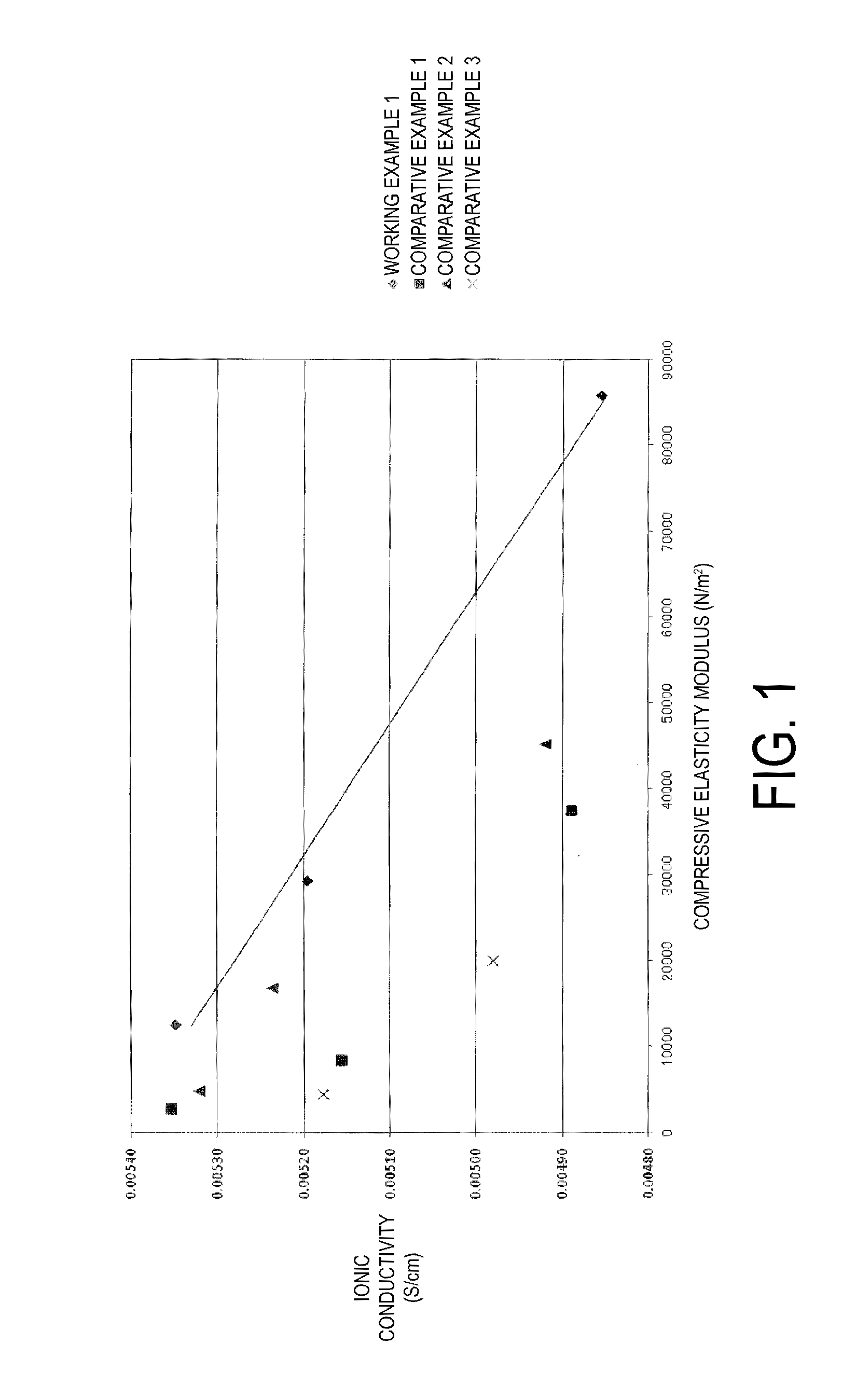
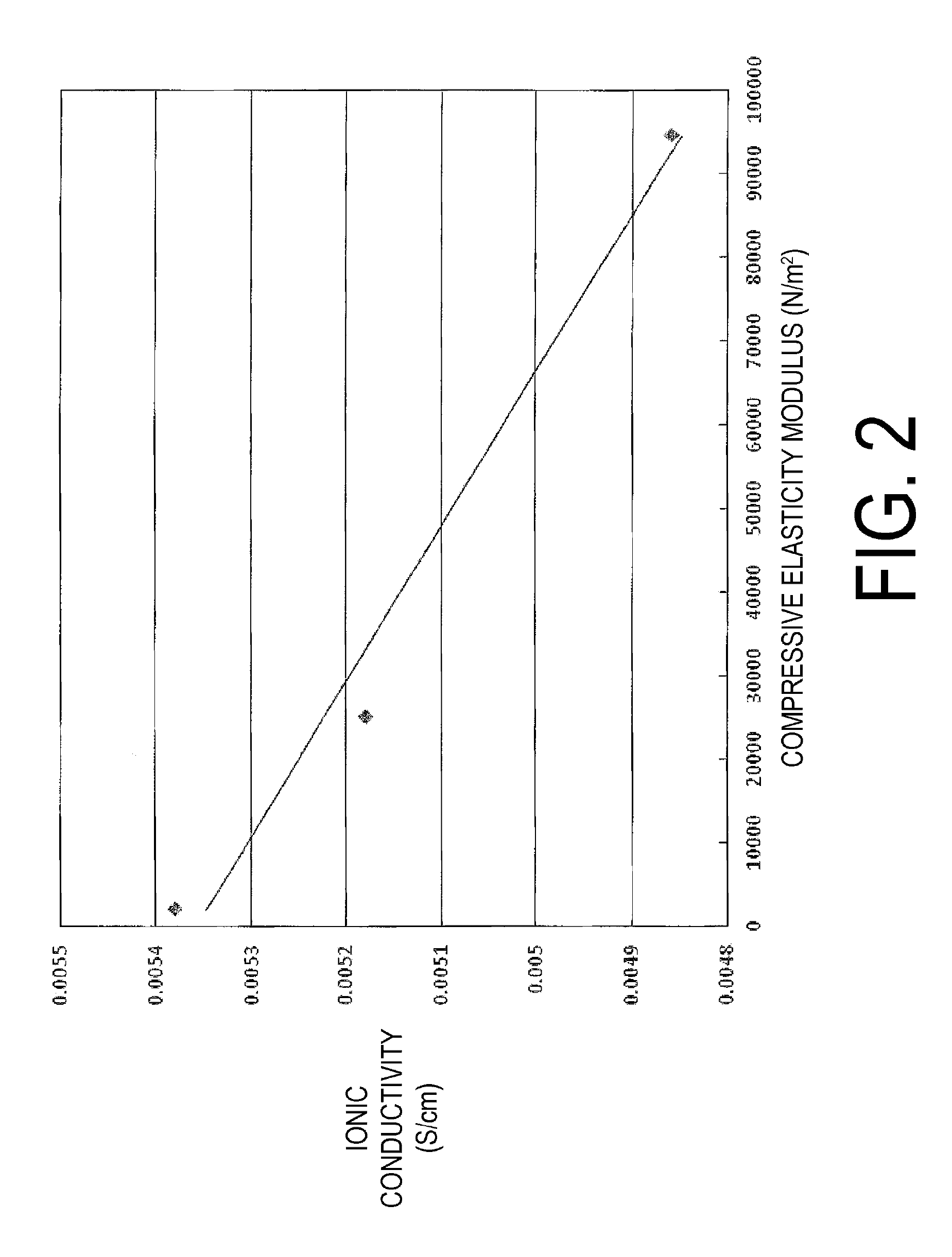
The vinylidene fluoride copolymer of the present invention is a polymer obtained by copolymerizing at least one type of fluorine-based monomer selected from hexafluoropropylene and chlorotrifluoroethylene, vinylidene fluoride, and a compound represented by formula (1) (wherein X is an atomic group having a hydroxyl group or a carboxyl group and having a molecular weight of not greater than 517 with a main chain having from 1 to 19 atoms) and is obtained by adding the compound represented by formula (1) to the fluorine-based monomer and vinylidene fluoride in divided portions or continuously during copolymerization. The gel electrolyte of the present invention contains the vinylidene fluoride copolymer and a non-aqueous electrolyte solution, and the gel electrolyte has an excellent balance of ionic conductivity and gel strength.
Owner:KUREHA KAGAKU KOGYO KK
Water battery device
ActiveUS20110180397A1Inhibits biofilm formationInhibition formationCellsSpecific water treatment objectivesElectrical batteryEngineering
The water battery device has a sterilizing unit composed of a non-noble metal body and a noble metal body that are overlapped coaxially via an interval keeping member. The sterilizing unit is disposed in a passing water or flowing water or immersed in a stored water. Then, a battery action is generated via the water at a uniform clearance space between the non-noble metal body and the noble metal body. Thereby, metal ions are dissolved in a complete ionization state from the non-noble metal body, thereby giving sterilization effect to the water. A container has a container structure that generates an electric current in the water between the non-noble metal body and the noble metal body and that maintains an oxygen concentration in the water required to continually produce the battery action water. Moreover, with the container structure, the concentration of the battery action water produced between the non-noble metal body and the noble metal body becomes not less than a predetermined concentration at which an oxide film or biofilm is formed on a facing surface of the non-noble metal body. Thus, the container structure surrounds the non-noble metal body and the noble metal body to keep the concentration of the battery action water at the concentration not less than the predetermined concentration.
Owner:YOUJI HAYAKAWA
Electrolytic Solution for Nonaqueous Electrolyte Batteries and Nonaqueous Electrolyte Battery Using the Same
InactiveUS20150118579A1Excellent cycle characteristicsExcellent characteristic high-temperature storageElectrolytic capacitorsOrganic electrolyte cellsHigh temperature storageLithium
The present invention provides an electrolytic solution for a nonaqueous electrolyte battery and a nonaqueous electrolyte battery having excellent cycle characteristics and high-temperature storage characteristics without causing hydrolysis of a fluorine-containing lithium salt, such as LiPF6, contained as a solute and containing a less amount of free fluorine ions, as well as a method of producing the electrolytic solution for a nonaqueous electrolyte battery. The electrolytic solution for a nonaqueous battery of the present invention includes a nonaqueous solvent and at least one fluorine-containing lithium salt as a solute and further includes an oxalato salt represented by Formula (1), wherein the content of a hexafluoro salt represented by Formula (2) is 150 mass ppm or less,LixMF(6-2y)(C2O4)y (1)LixMF6 (2)wherein M represents Fe, Sn, Si, Ge or Ti; x is 3 when M is Fe, and 2 when M is Sn, Si, Ge or Ti; and y is an integer of 1 to 3, wherein the content of the oxalato salt is 6500 mass ppm or less and the content of free fluorine ions is 50 mass ppm or less.
Owner:CENT GLASS CO LTD
Copolymer for binders for nonaqueous battery electrodes, slurry for nonaqueous battery electrodes, nonaqueous battery electrode, and nonaqueous battery
ActiveUS20190058195A1Decreasing resistance of batterySufficient binding propertyNegative electrodesElectrode collector coatingMeth-Organic chemistry
The invention relates to a copolymer for binders, which is capable of reducing the internal resistance of a battery, while ensuring sufficient binding properties between active materials and between an active material and a collector in a nonaqueous battery electrode; a composition for binders; a slurry for nonaqueous battery electrodes; a nonaqueous battery electrode; and a nonaqueous battery. This copolymer for binders is a copolymer for binders (P) of a monomer mixture (M) that contains at least a monomer (A) represented by general formula (1) and a (meth)acrylate salt monomer (B); and an amount of structure derived from the monomer (A) based on the copolymer for binders (P) is set to 0.5 to 20.0% by mass. (In the formula, each of R1 and R2 independently represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms.)
Owner:RESONAC CORP
Binder composition for nonaqueous battery electrodes, slurry for nonaqueous battery electrodes, nonaqueous battery electrode, and nonaqueous battery
ActiveUS20160248095A1Improve bindingReduce the amount requiredNon-aqueous electrolyte accumulator electrodesMaterials scienceCarboxylate
A binder composition for nonaqueous battery electrodes is prepared by adding a small amount of an acetylene glycol compound to an aqueous polymer emulsion obtained by emulsion polymerization of a monomer mixture comprising from 15 to 70% by mass of styrene (a), from 20 to 80% by mass of an ethylenically unsaturated carboxylate (b), from 1 to 10% by mass of an ethylenically unsaturated carboxylic acid (c), from 0.1 to 5% by mass of a crosslinkable ethylenically unsaturated monomer (d), and from 0 to 20% by mass of another monoethylenically unsaturated monomer (e). When the above binder composition is used, an active material is not peeled off in the step of cutting a collector even when a small amount of the binder is used, and a nonaqueous battery excellent in a charge-discharge cycle property can be produced.
Owner:RESONAC CORP
Polymer electrolyte containing a vinylidene fluoride copolymer and a nonaqueous electrolytic solution, and nonaqueous battery containing the polymer electrolyte
InactiveUS6824927B1High strengthRetention stabilityElectrolytic capacitorsConductive materialPolymer electrolytesPolymer science
A nonaqueous battery, such as a lithium ion battery, is formed from a polymer electrolyte comprising: a vinylidene fluoride copolymer comprises 80 to 97 wt. % of vinylidene fluoride monomer units and 3 to 20 wt. % of units of at least one monomer copolymerizable with the vinylidene fluoride monomer and has an inherent viscosity of 1.5 to 10 dl / g. The polymer electrolyte stably retains the nonaqueous electrolytic solution in a large amount and has excellent strength in this state.
Owner:KUREHA KAGAKU KOGYO KK
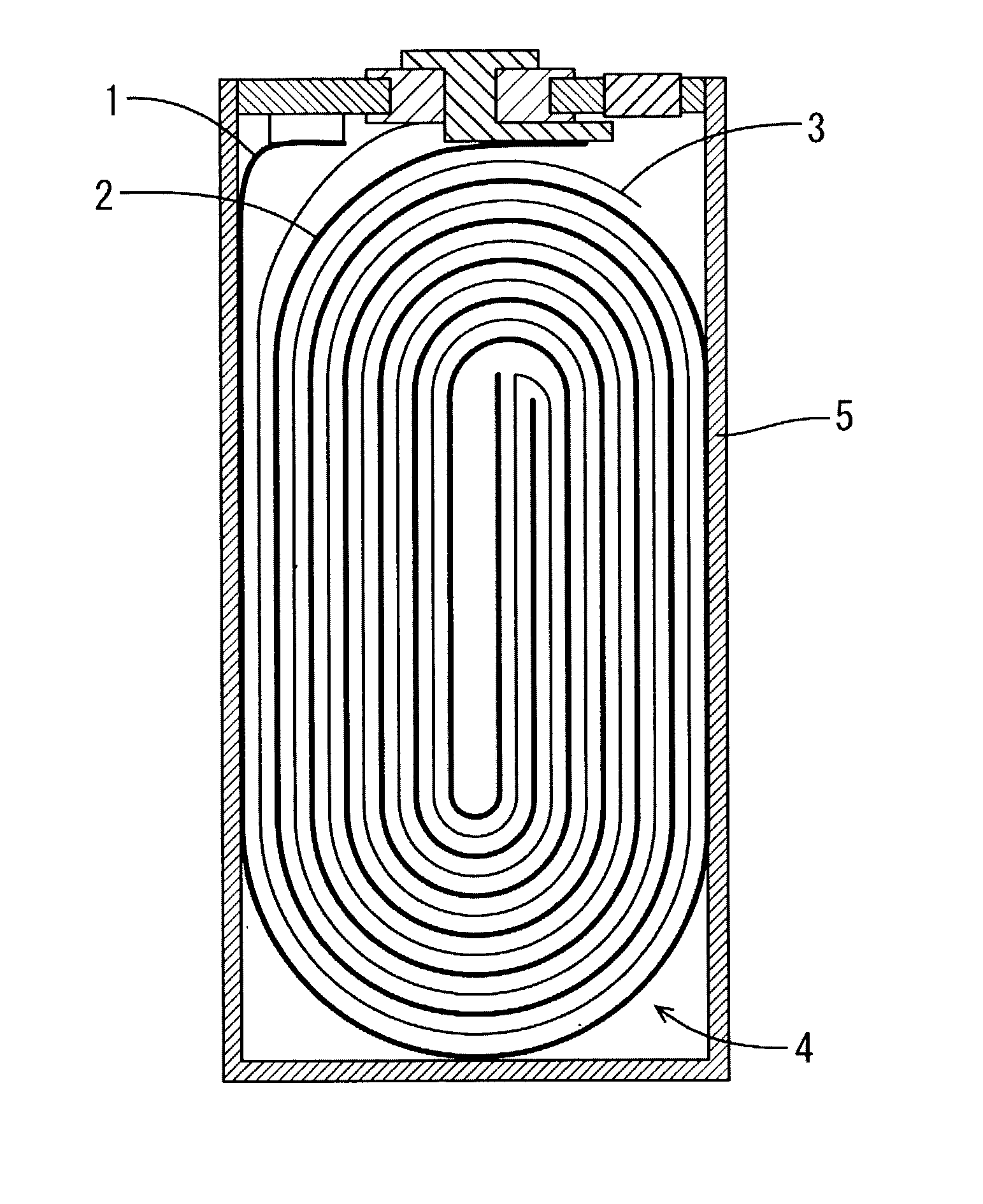
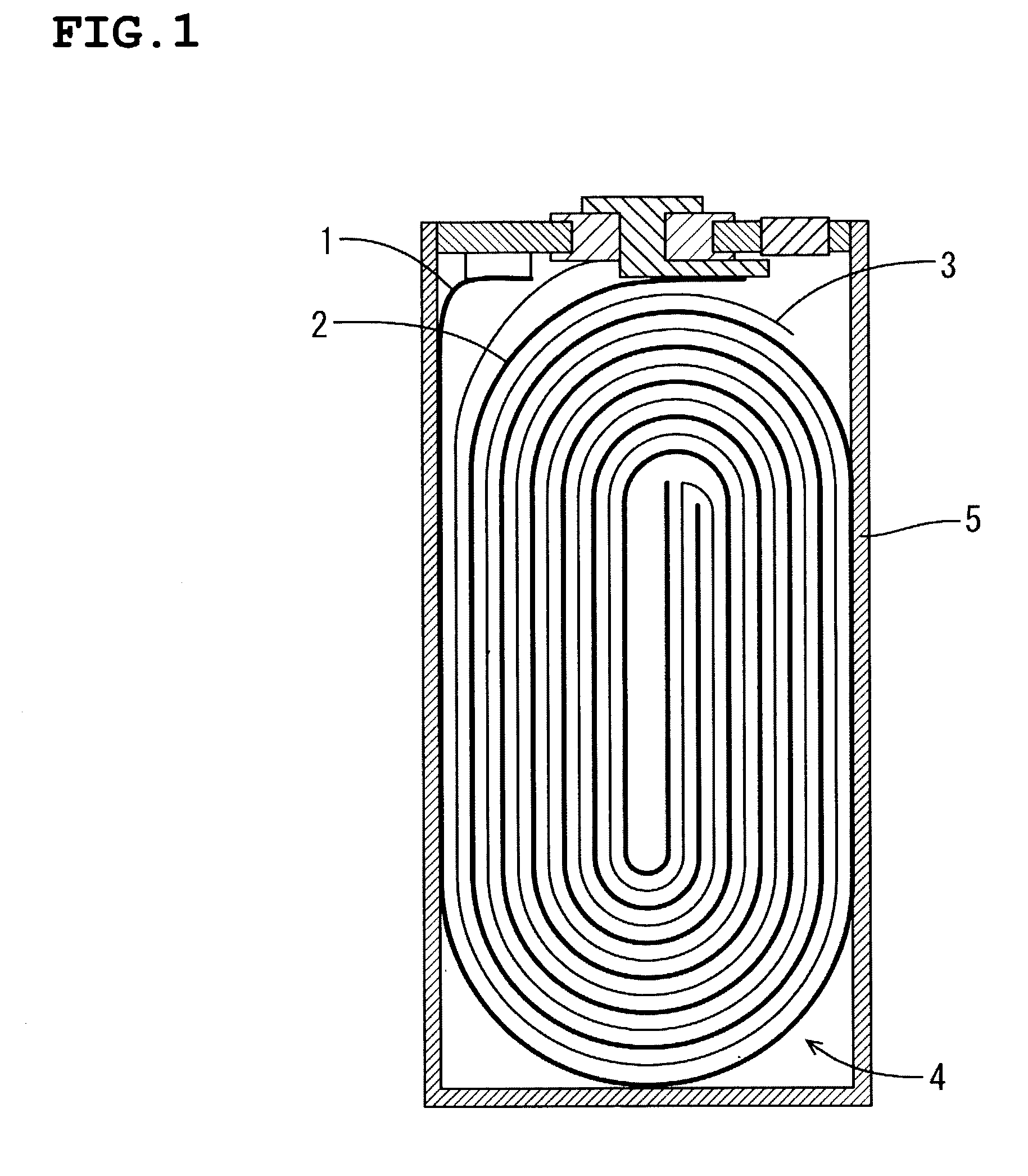
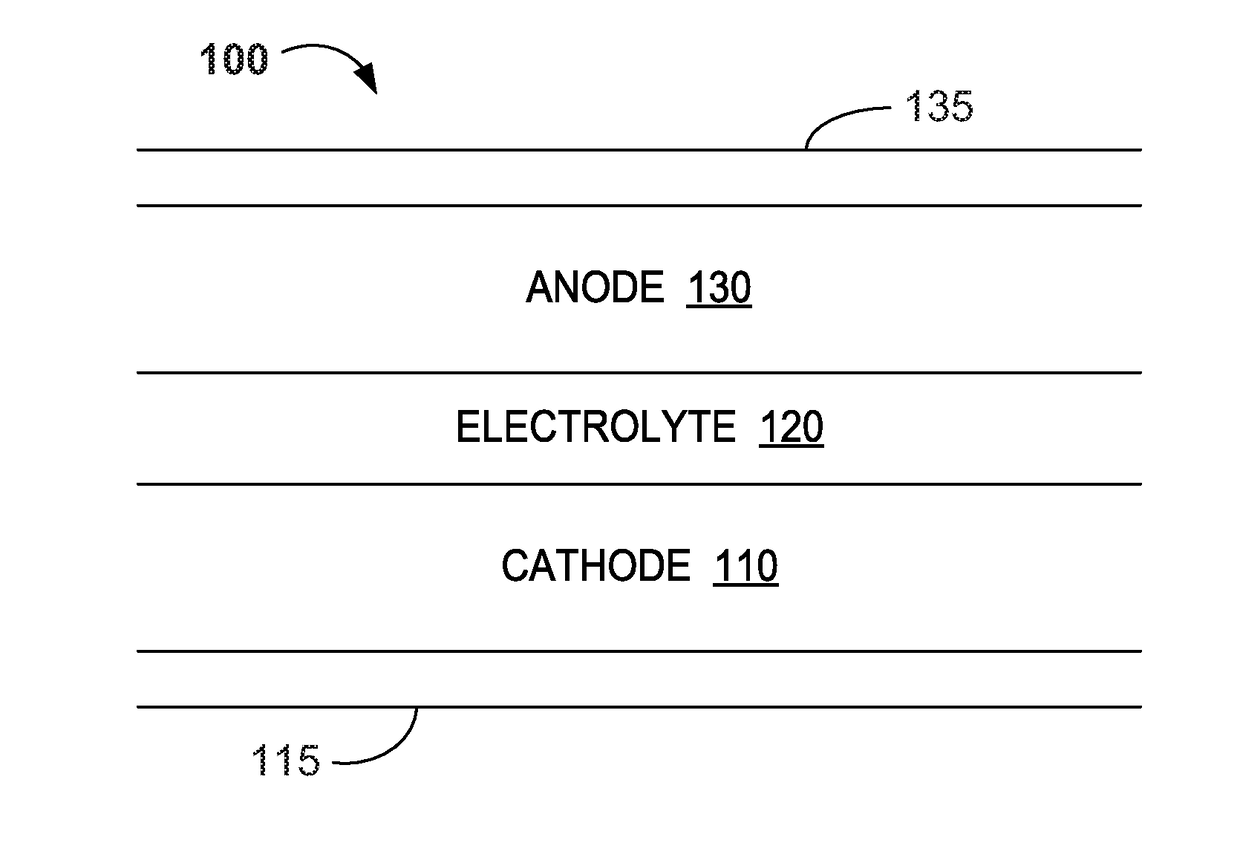
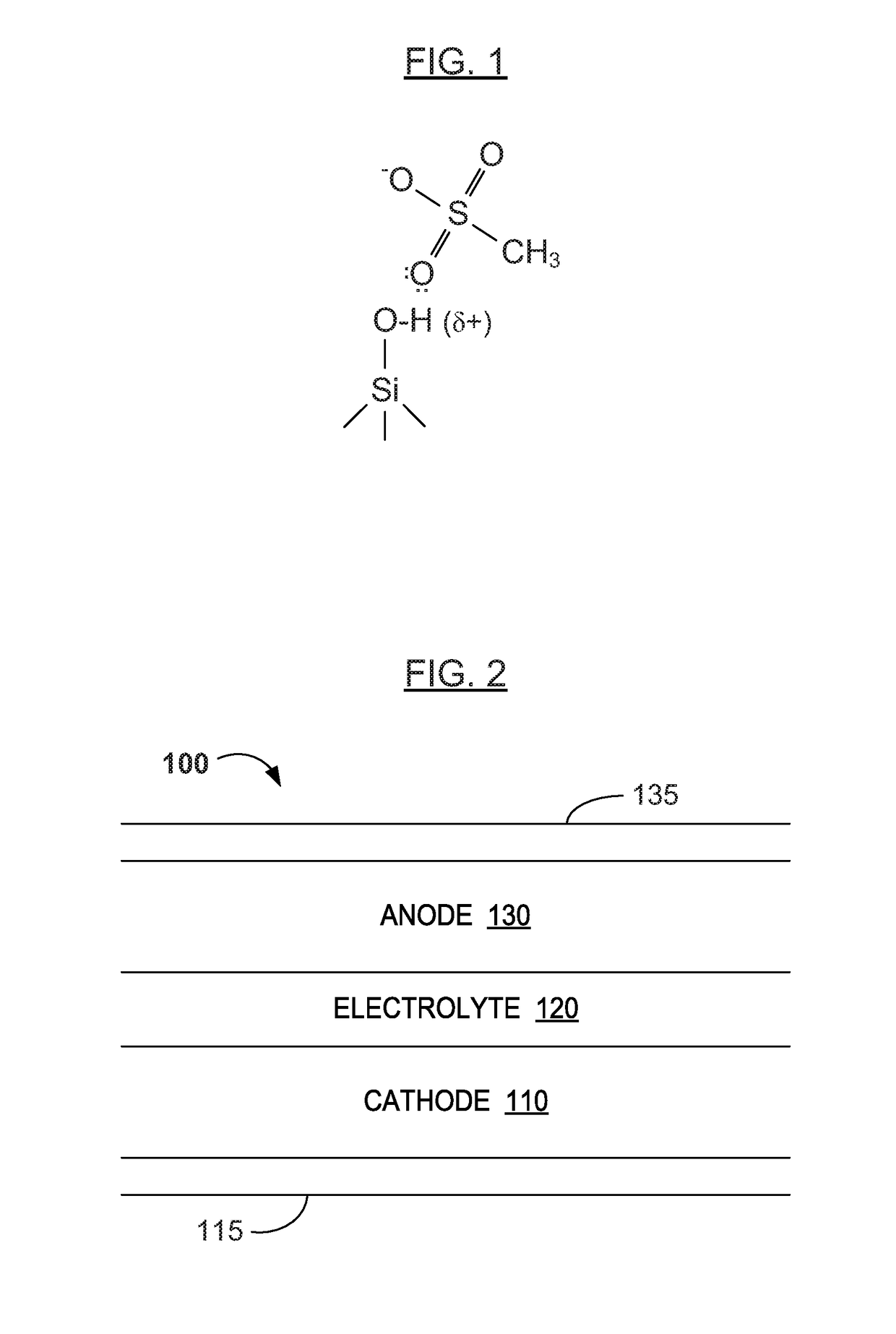
Water Battery
ActiveUS20160056477A1Improved electromotive force characteristicSmooth dischargeDeferred-action cellsPositive electrodesPolyvinyl alcoholMetallic materials
A water battery includes a housing and at least one battery cell mounted in the housing, capable of operating by injecting water into the housing at the time of use. The at least one battery cell includes an anode made of a metal material with a lower ionization tendency than that of a magnesium, an anode drawer electrode electrically connected to the anode, a cathode made of a magnesium material, a cathode drawer electrode electrically connected to the cathode, a collector electrode mounted between the anode and the cathode, a sheet member with water absorptivity and water retention, closely attached to the collector electrode, and a fixing member for pressing to each other and fixing together the anode, the collector electrode, the sheet member and the cathode. The sheet member includes an electrolyte containing nitrophenol, sodium, citric acid and polyvinyl alcohol.
Owner:MISHIMADENSHI
Electrolyte for a nonaqueous battery
ActiveUS20040131933A1Low heat generationCarrying out of the reaction simple and easyNon-aqueous electrolyte accumulatorsElectrolytic capacitorsOrganic solventMolten salt
An electrolyte containing calcium bistrifluoromethanesulfonimide [Ca((CF3SO2)2N)2] for a nonaqueous battery. The calcium bistrifluoromethanesulfonimide is soluble in an organic solvent and a molten salt having a melting point of not greater than 60° C.
Owner:SANYO ELECTRIC CO LTD
Ionic Liquid Gel for Electrolyte, Method of and Ink for Making the Same, and Printed Batteries Including Such Ionic Liquid Gels and/or Electrolytes
The disclosure concerns an electrolyte, an electrolyte ink, a battery or other electrochemical cell including the same, and methods of making the electrolyte and electrochemical cell. The electrolyte includes an ionic liquid comprising a hydrophilic or hydrophobic anion, a multi-valent metal cation suitable for use in a battery cell, a polymer binder, and optional additives (e.g., a solid filler). The electrolyte ink includes components of the electrolyte and a solvent. The solvent and the polymer binder (or, when present, the solid filler) have a hydrophilicity, hydrophobicity or polarity similar to or matching that of the ionic liquid's anion, or form hydrogen bonds with the ionic liquid's anion. The electrolyte includes a solid inorganic filler that provides mechanical support form hydrogen bonds with the anion and / or a counterpart anion of the multi-valent metal cation, and links with a material in an adjacent layer of the electrochemical cell.
Owner:IMPRINT ENERGY
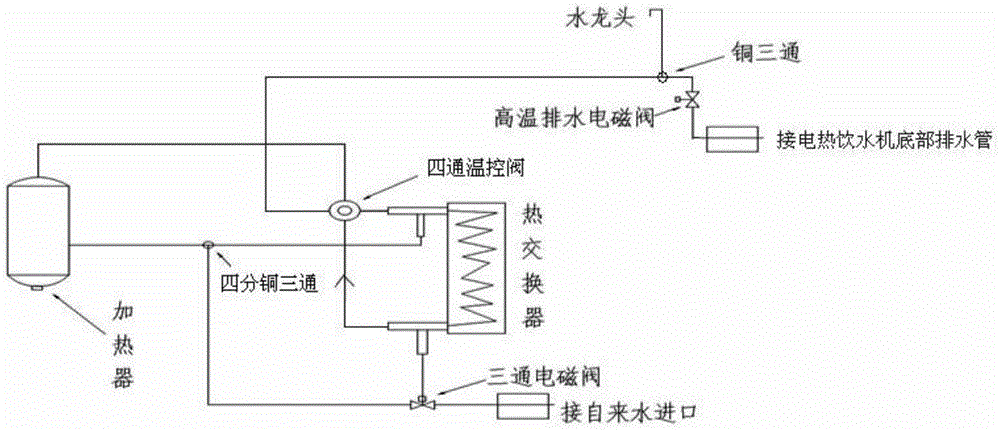
Sterilization system and method of electric heating water dispenser
InactiveCN104856569AReduce the temperatureAchieve the purpose of sterilizationBeverage vesselsHeatTemperature controlMagnetic valve
The invention discloses a sterilization system of an electric heating water dispenser. The sterilization system comprises a heat exchanger, a heater and a plurality of water pipes, wherein the heat exchanger consists of an inner tube and an outer tube which are reverse in inlet and outlet, a three-way magnetic valve is installed at the position of a water inlet of the outer tube of the heat exchanger, one water outlet of the three-way magnetic valve is connected with the water inlet of the outer tube of the heat exchanger, a water outlet of the outer tube of the heat exchanger is connected with the heater, a 15 millimeter copper three-way joint is arranged between the water outlet of the outer tube of the heat exchanger and the heater, the other water outlet of the three-way magnetic valve is connected with the15 millimeter copper three-way joint, the heater is connected with a water inlet of the inner tube of the heat exchanger, a four-way temperature control valve is arranged between the heater and the water inlet of the inner tube of the heat exchanger, a water outlet of the inner tube of the heat exchanger is connected with a faucet of the electric heating water dispenser, a copper three-way joint is arranged on a faucet interface water pipe and is sequentially connected with a high-temperature drainage battery valve and a draining pipe at the bottom of the electric heating water dispenser. The invention further provides a sterilization method of the sterilization system of the electric heating water dispenser. The problem that the bacterial colony number of the electric heating water dispenser exceeds the standard is solved by means of the sterilization system, and the sterilization method of the sterilization system consumes short time and is low in cost.
Owner:NANJING SHUIBEIZI DIFFERENT QUANTITY PIPELINE WATER SUPPLY ENG
A self-powering smart water meter system and method
PendingCN108431550ATariff metering apparatusPower network operation systems integrationFrequency spectrumTransceiver
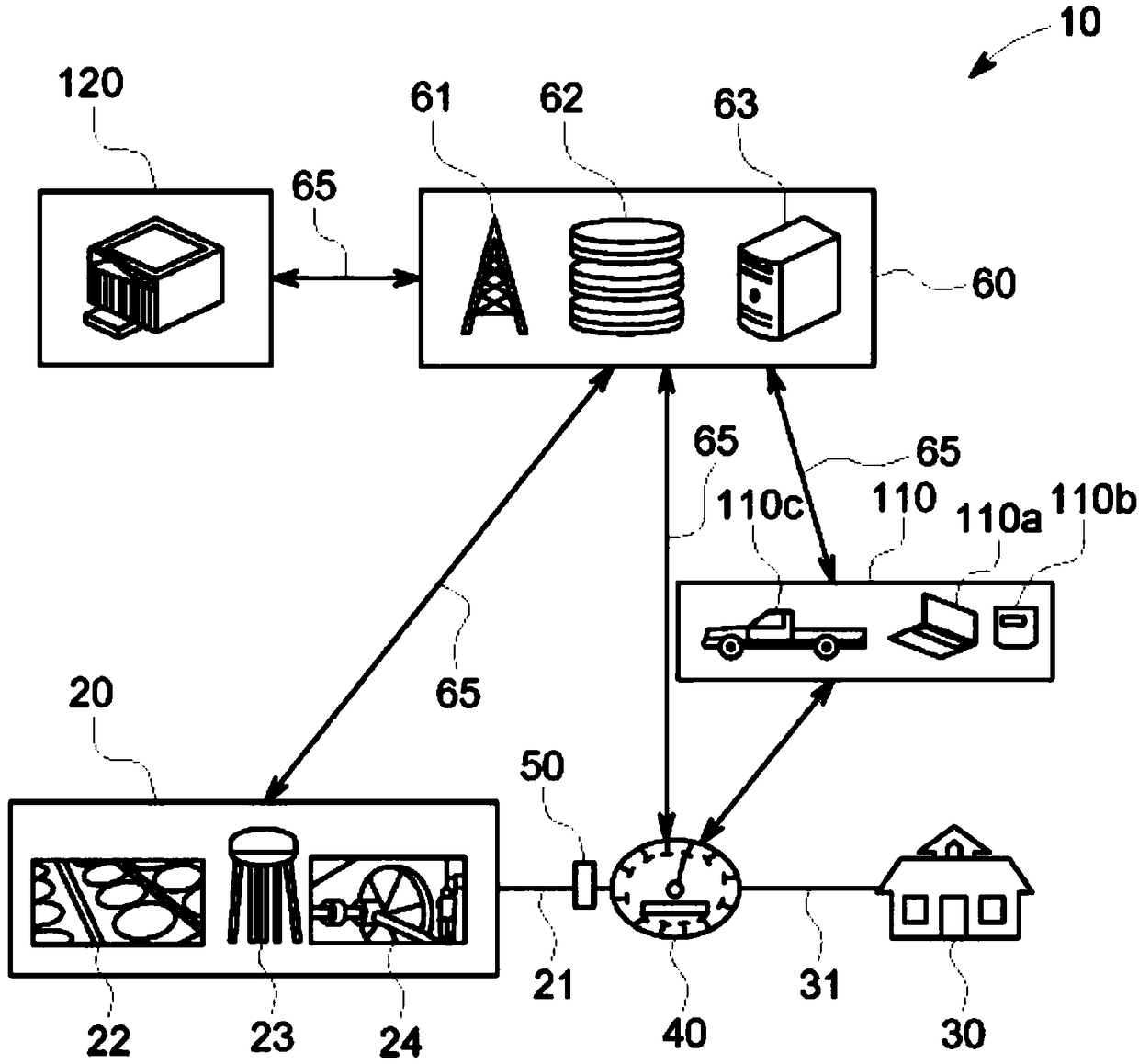
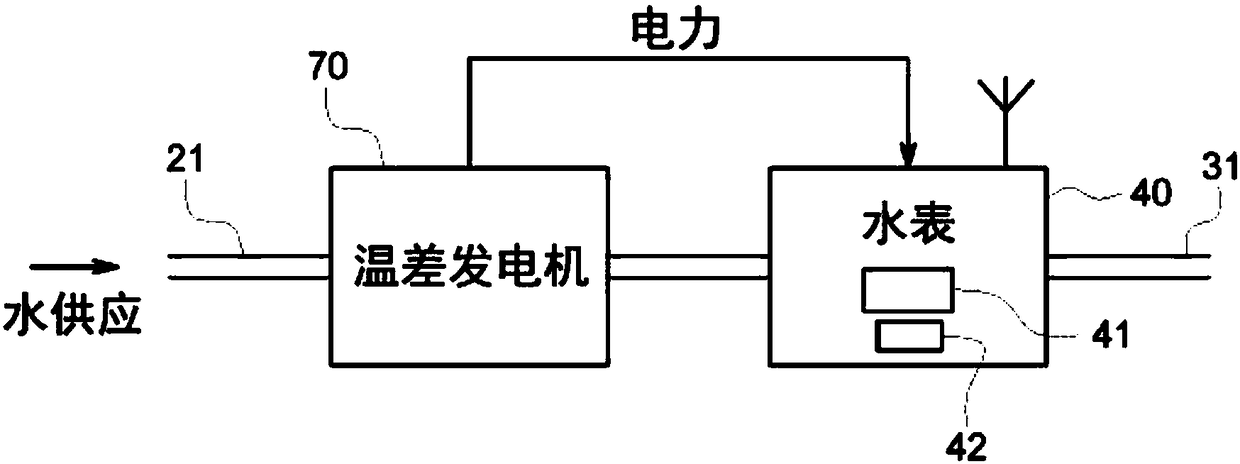
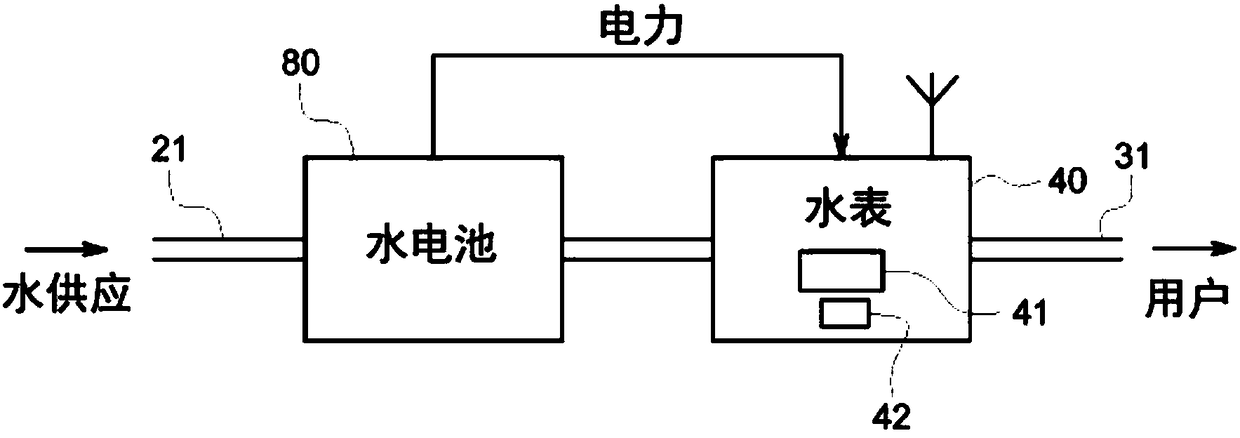
A smart fluid or water meter system 10 includes fluid supplying and receiving centers 20, 30 connected via a supply line 21. Further, a fluid metering member 40 is coupled to the supply line 21, and includes a fluid regulating member 41 to regulate the fluid therethrough, and a data transceiver 42, coupled to the fluid regulating member 41 to regulate the supply of fluid. A self-power generating source 50 is also provided to continuously charge the fluid metering member 40 for continuous operation. Various self- power generating source, such as, a thermoelectric generator, a water battery, a turbine power, and radio spectrum may be used for said purpose. The system may be provided with rechargeable battery to for recharging the battery and storing the power. Moreover, a network operation center 60 is coupled to receive and process the data to exchange relevant information or command to centers 20, 30 and the fluid metering member 40. Further in an optional embodiment, the smart systemoptionally comprises an Artificial Neural Network (ANN) module 2030 for self-training. The self-trained smart system identifies individual events (toilet flush, shower, washing machine cycle etc.) andautomatically initiate the related action such as but not limited to reporting the event, restricting the flow rate or restricting the total volume per day etc.
Owner:AQUA COMMAND LTD
Emergency metal seawater battery applied to sea surface
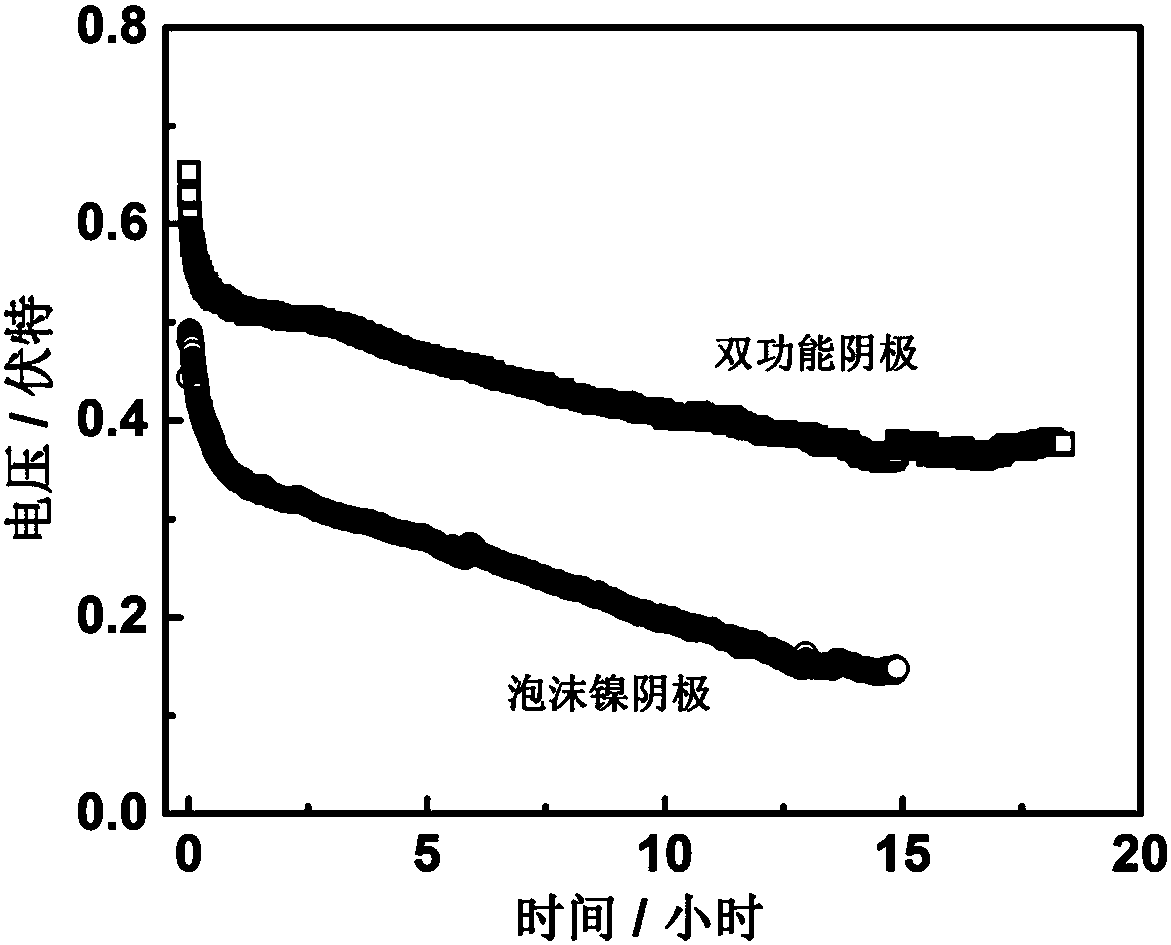
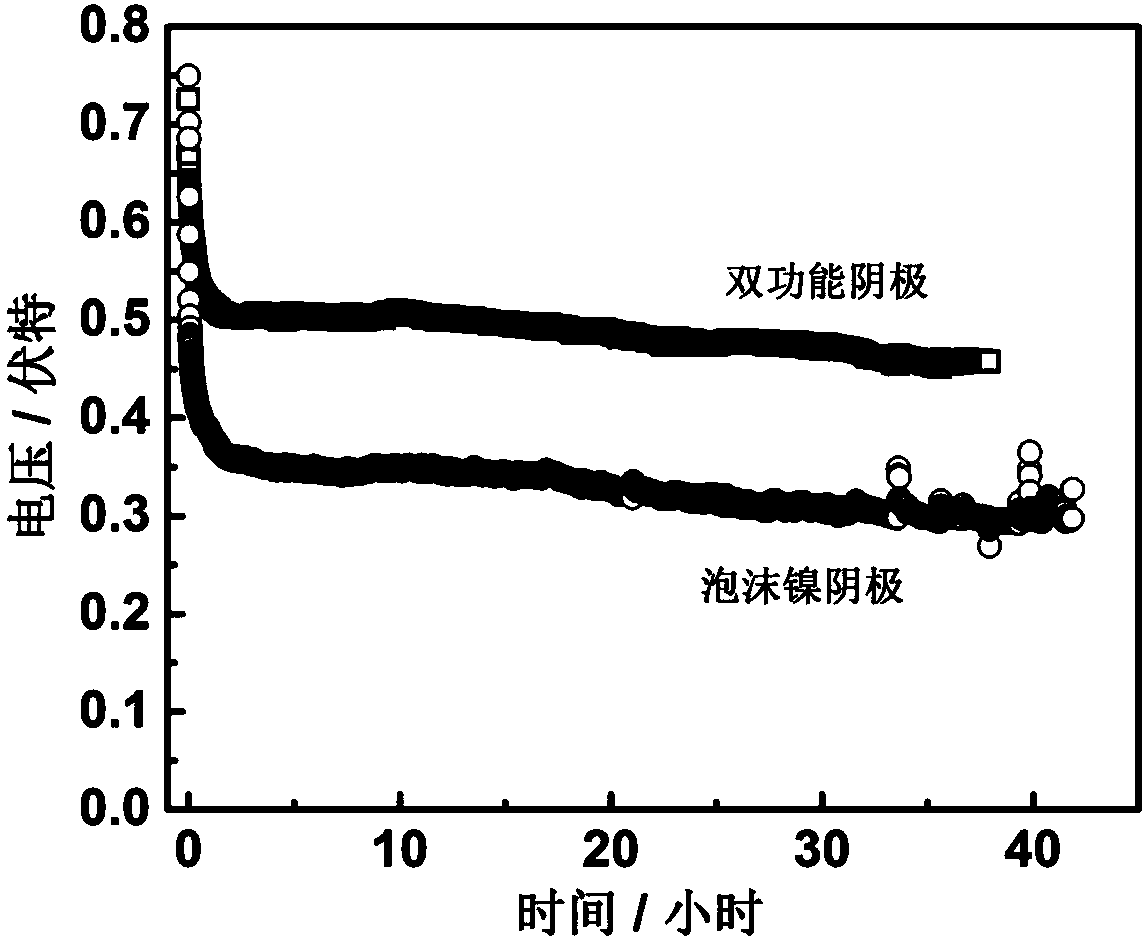
The invention relates to an emergency power supply, in particular to a metal / seawater battery. The battery has the characteristics of a metal / water battery and a metal / air battery at the same time; and the battery mainly comprises double-function cathodes, an alloy anode, diaphragms and a battery shell. The battery structure is of a sandwich configuration, wherein one anode is clamped by two cathodes, electrodes are separated by the diaphragms, and the battery shell is of a hollow structure. According to the battery, the advantages of the metal / water battery and the metal / air battery are combined effectively; when the battery floats on the sea surface, the dual-mode discharging of the metal / air battery and the metal / water battery is adopted; when the battery is fully immersed with seawater, the metal / water battery mode is adopted for discharging; by virtue of the dual-mode battery, a complex working environment of the sea surface can be handled. The battery is long in storage life, high in stability, high in mass specific energy and simple in structure, and is an ideal sea surface emergency power supply.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
High-energy non-aqueous batteries containing ion-conducting gels, and method for preparing and using same
ActiveUS8915975B2Improve performanceShorten the timeSolid electrolytesPrimary cellsLithiumHigh energy
Method for the preparation of a composite electrode and accumulator or battery including at least one composite electrode, the method includes a step of pouring a medium including at least one ionic liquid, a lithium, sodium or magnesium salt with at least one inorganic molecular precursor or a polymerizable monomer, the medium being in excess, and an in situ polycondensation or polymerization step.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Water battery device
The water battery device has a sterilizing unit composed of a non-noble metal body and a noble metal body that are overlapped coaxially via an interval keeping member. The sterilizing unit is disposed in a passing water or flowing water or immersed in a stored water. Then, a battery action is generated via the water at a uniform clearance space between the non-noble metal body and the noble metal body. Thereby, metal ions are dissolved in a complete ionization state from the non-noble metal body, thereby giving sterilization effect to the water. A container has a container structure that generates an electric current in the water between the non-noble metal body and the noble metal body and that maintains an oxygen concentration in the water required to continually produce the battery action water. Moreover, with the container structure, the concentration of the battery action water produced between the non-noble metal body and the noble metal body becomes not less than a predetermined concentration at which an oxide film or biofilm is formed on a facing surface of the non-noble metal body. Thus, the container structure surrounds the non-noble metal body and the noble metal body to keep the concentration of the battery action water at the concentration not less than the predetermined concentration.
Owner:YOUJI HAYAKAWA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com