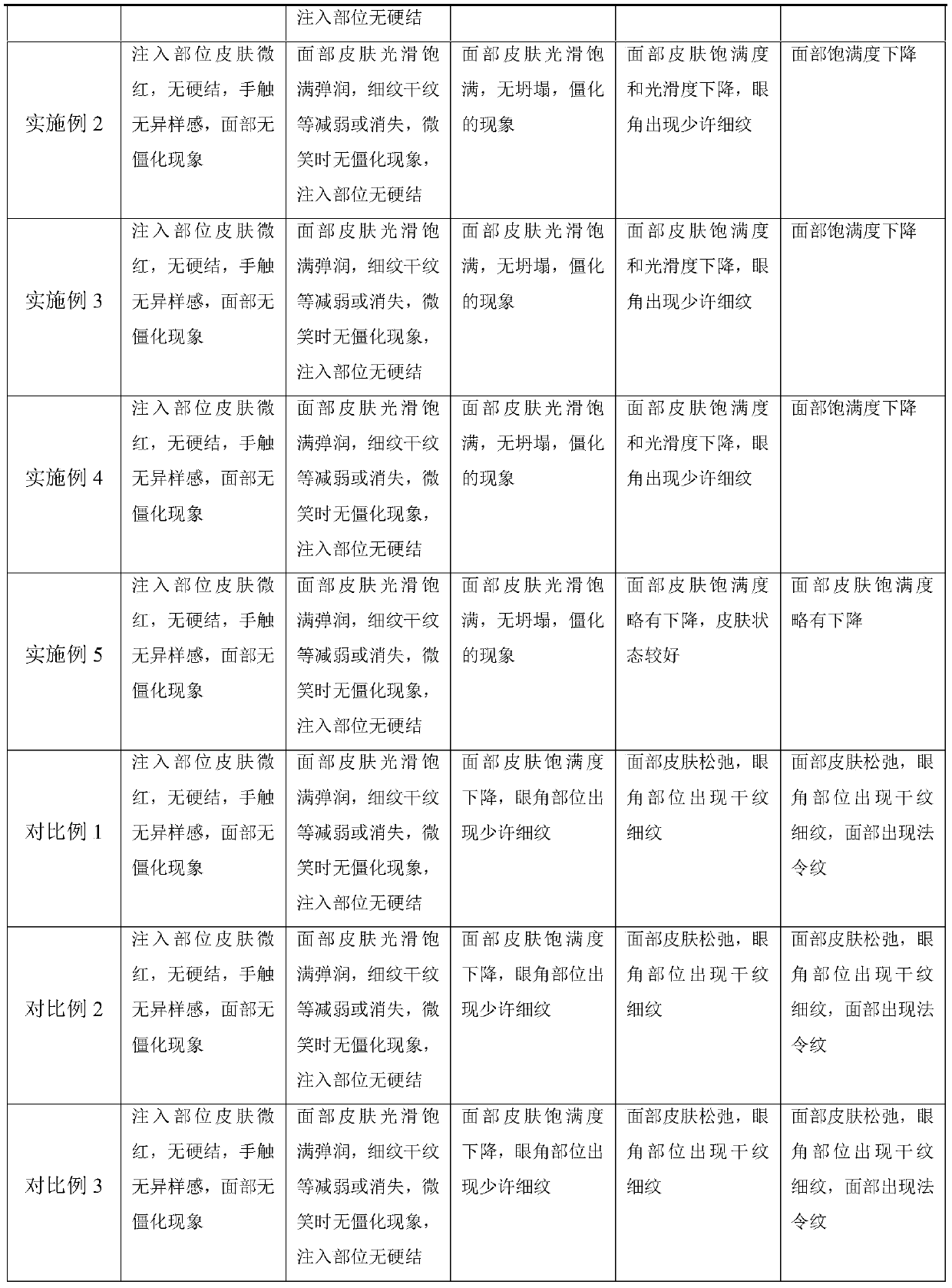
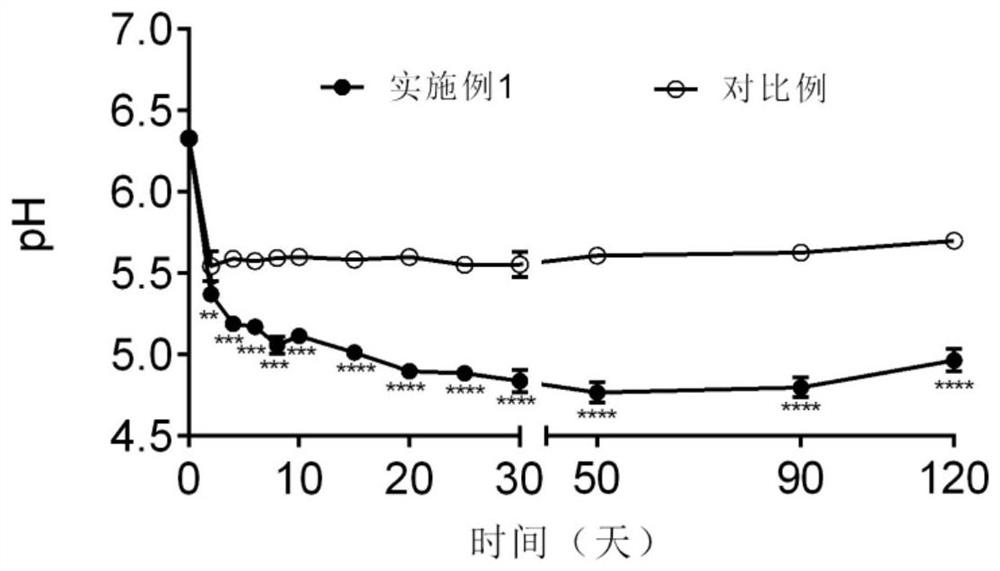
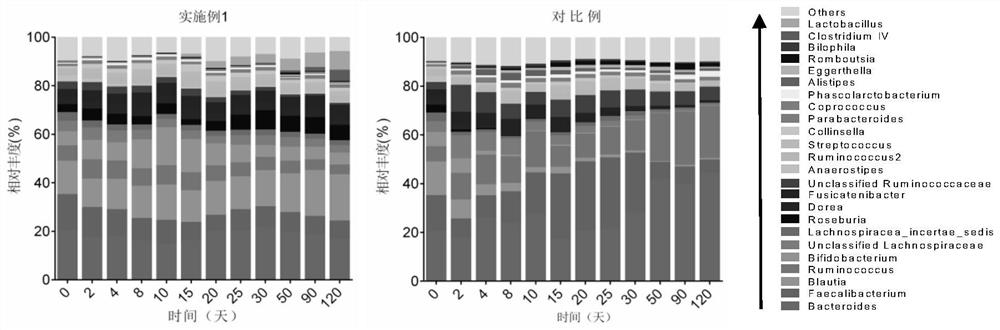
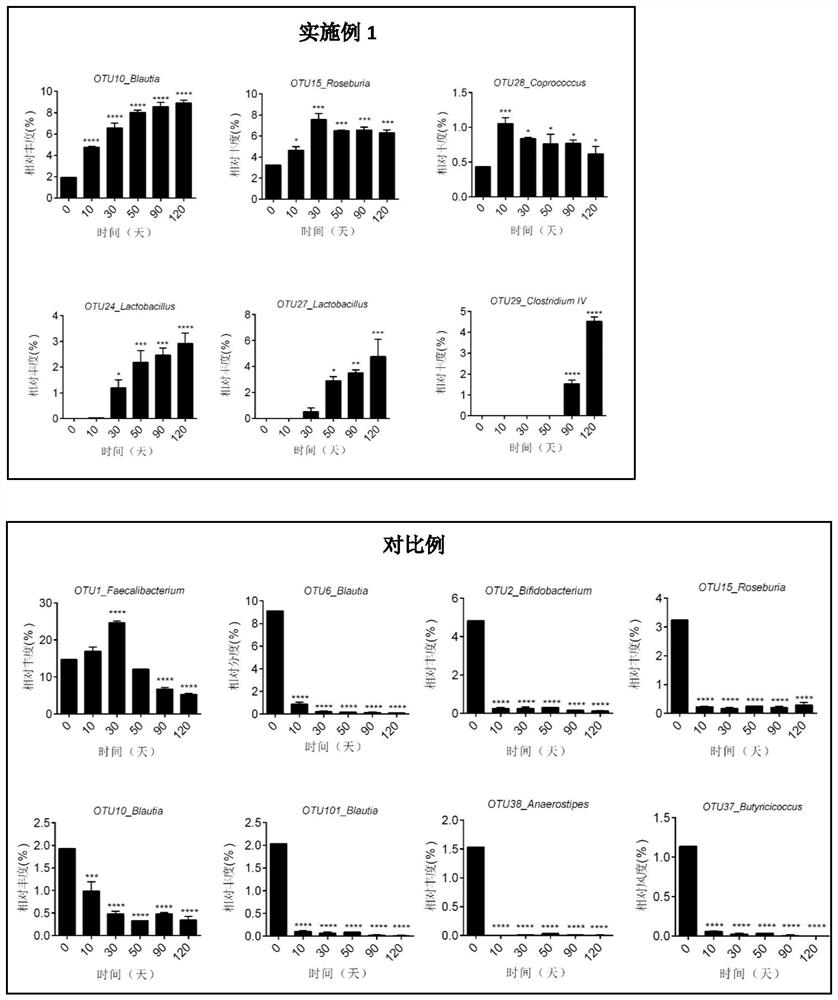
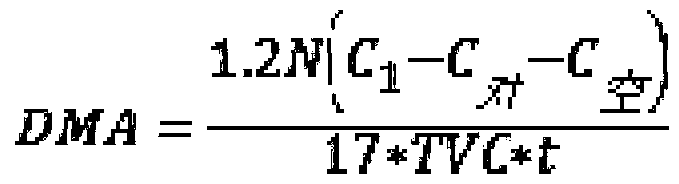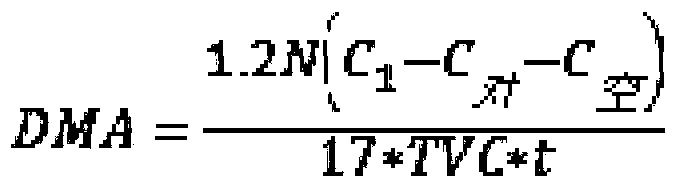Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
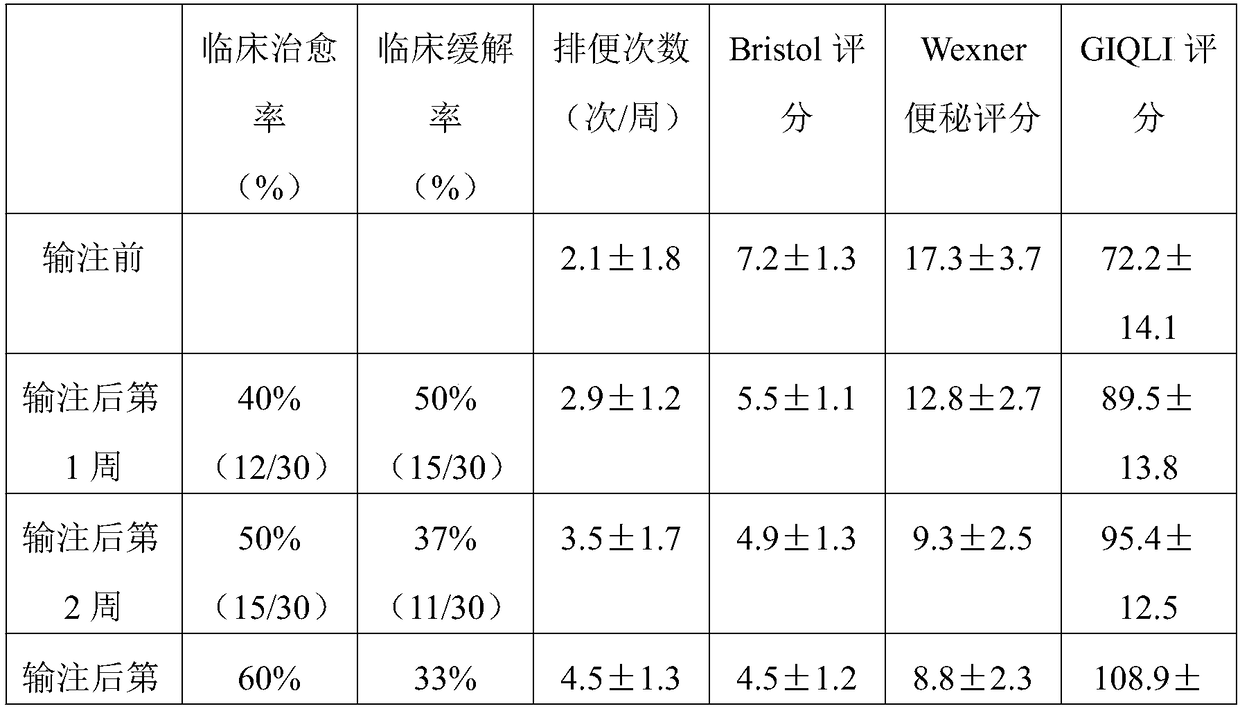
37 results about "STERILE SALINE SOLUTION" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and devices for sclerotherapy
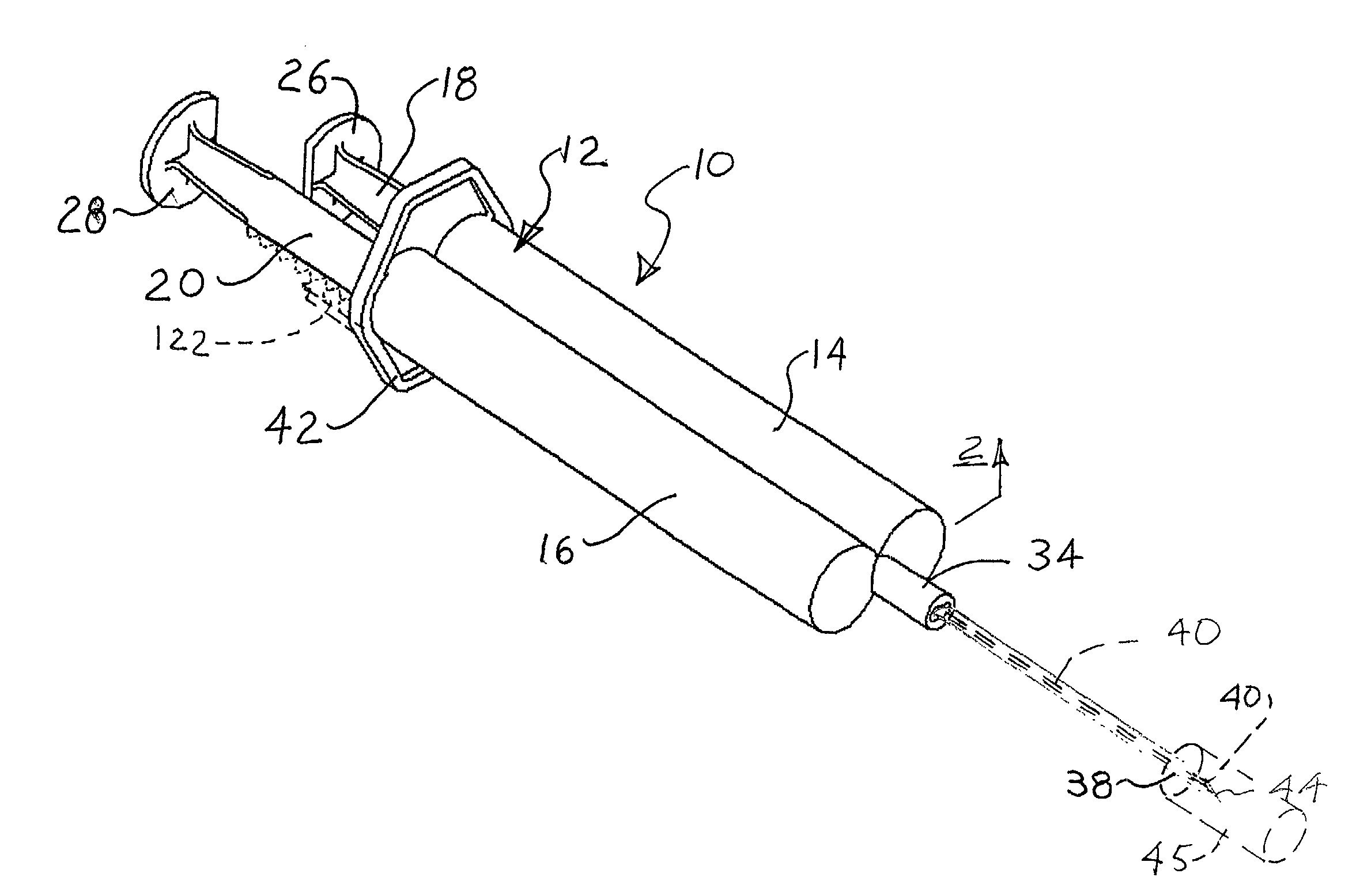
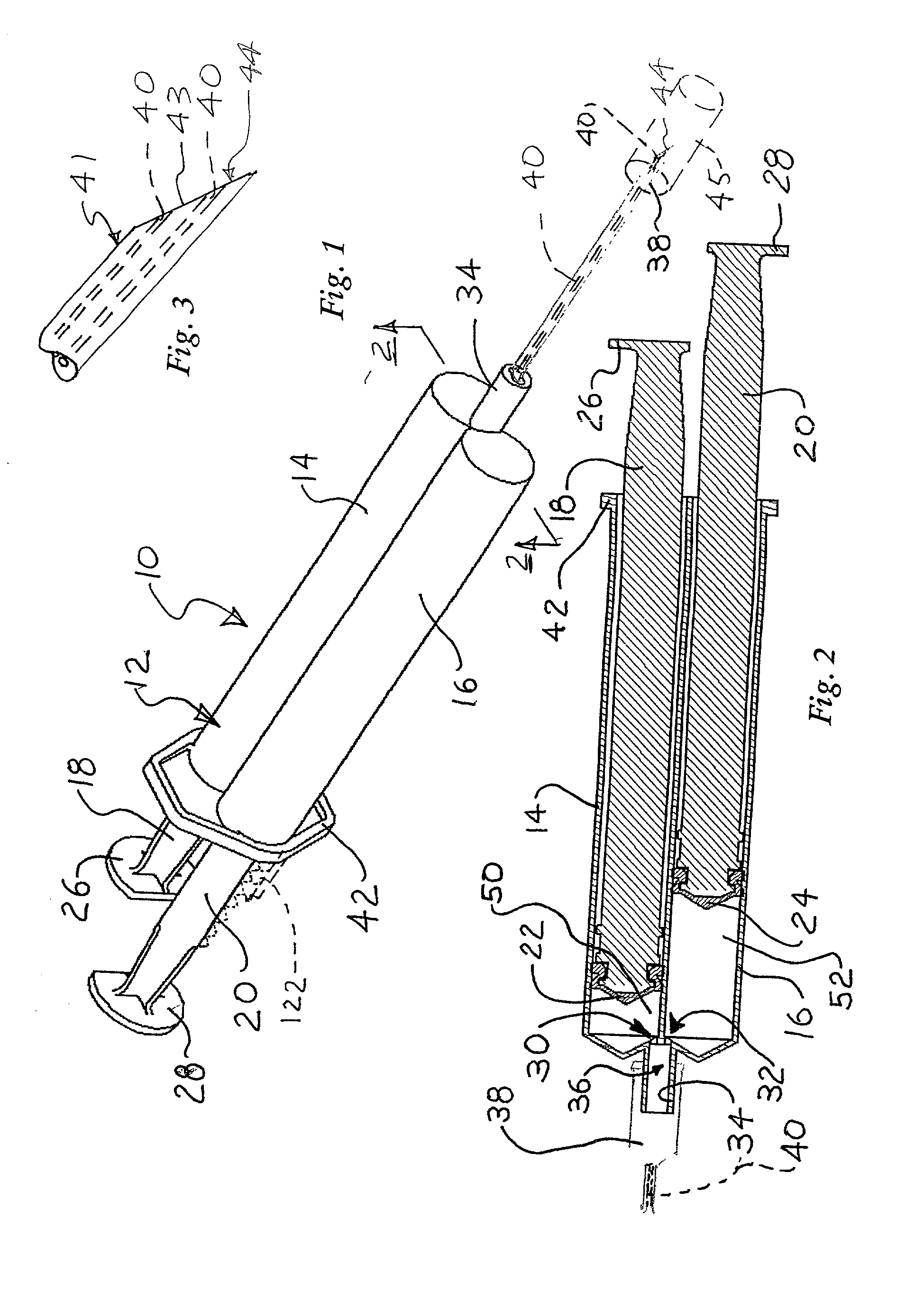
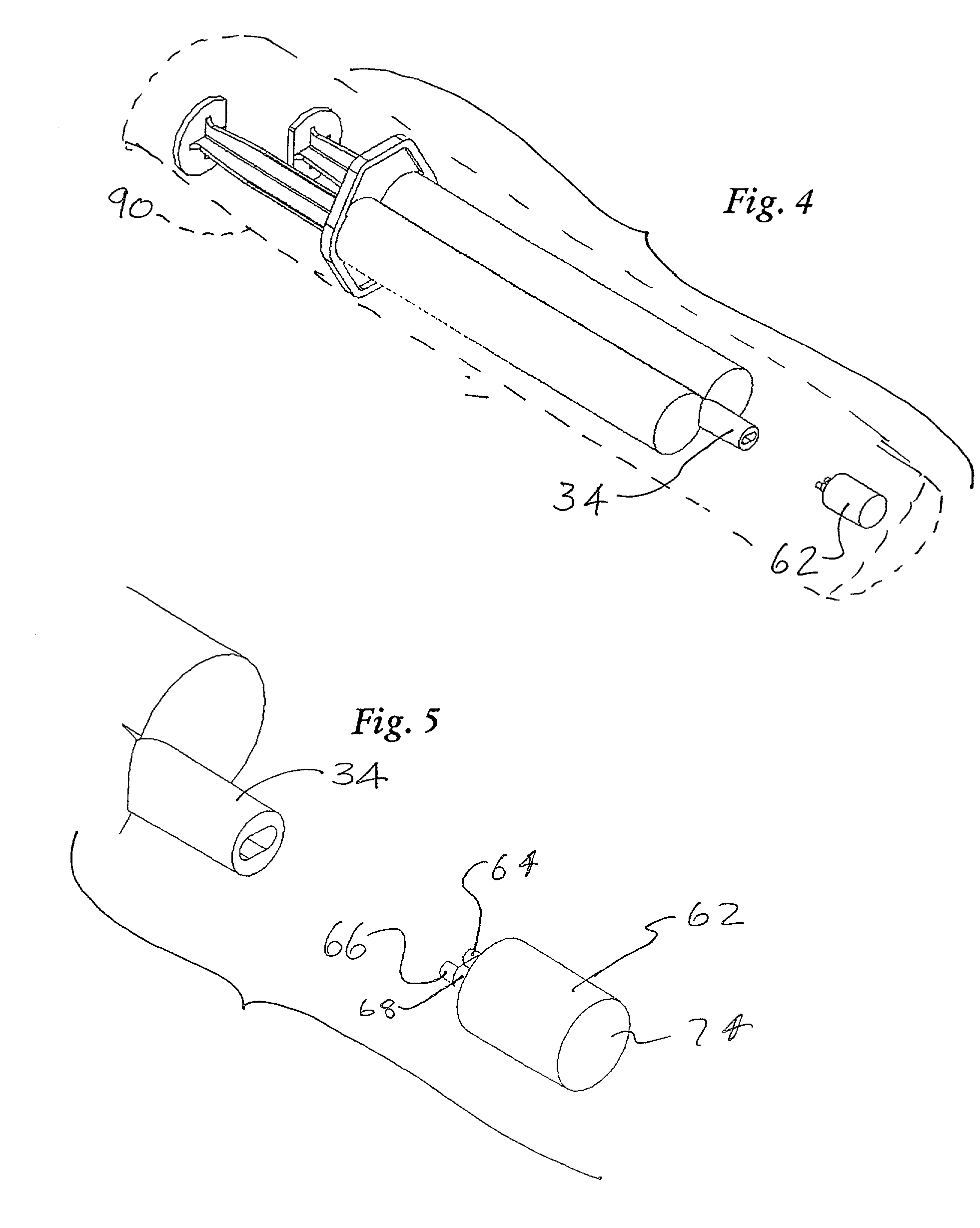
InactiveUS20030120201A1Cause more painProvide in timeEnemata/irrigatorsInfusion needlesVeinSCLEROSING AGENTS
In a method for sclerotherapy for treating varicose veins, a flushing solution, such as sterile saline solution, is initially injected into the vein or vessel being treated. The flushing solution displaces or flushes out blood from the treatment site of the vessel. A sclerosing agent is then injected into the treatment site. The displacement of blood before introduction of the sclerosing agent reduces complications. A syringe assembly useful for performing the method has first and second reservoirs sealed off from each by an end cap. The end cap is removed just before use. A needle is attached and is connected to both reservoirs. Flushing solution is delivered from the first reservoir followed by sclerosing solution delivered from the second reservoir, without removing the needle from the vessel.
Owner:ABERGEL R PATRICK
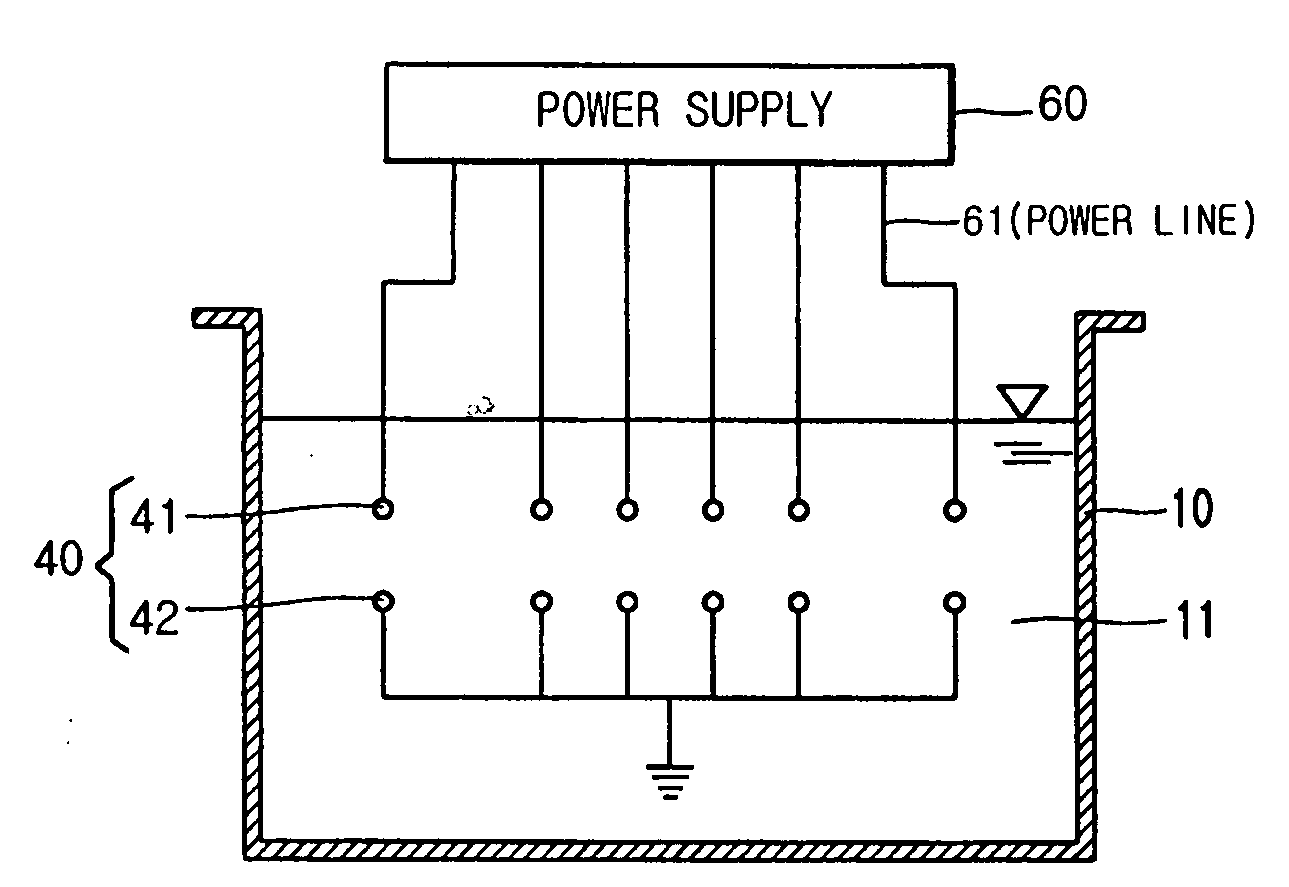
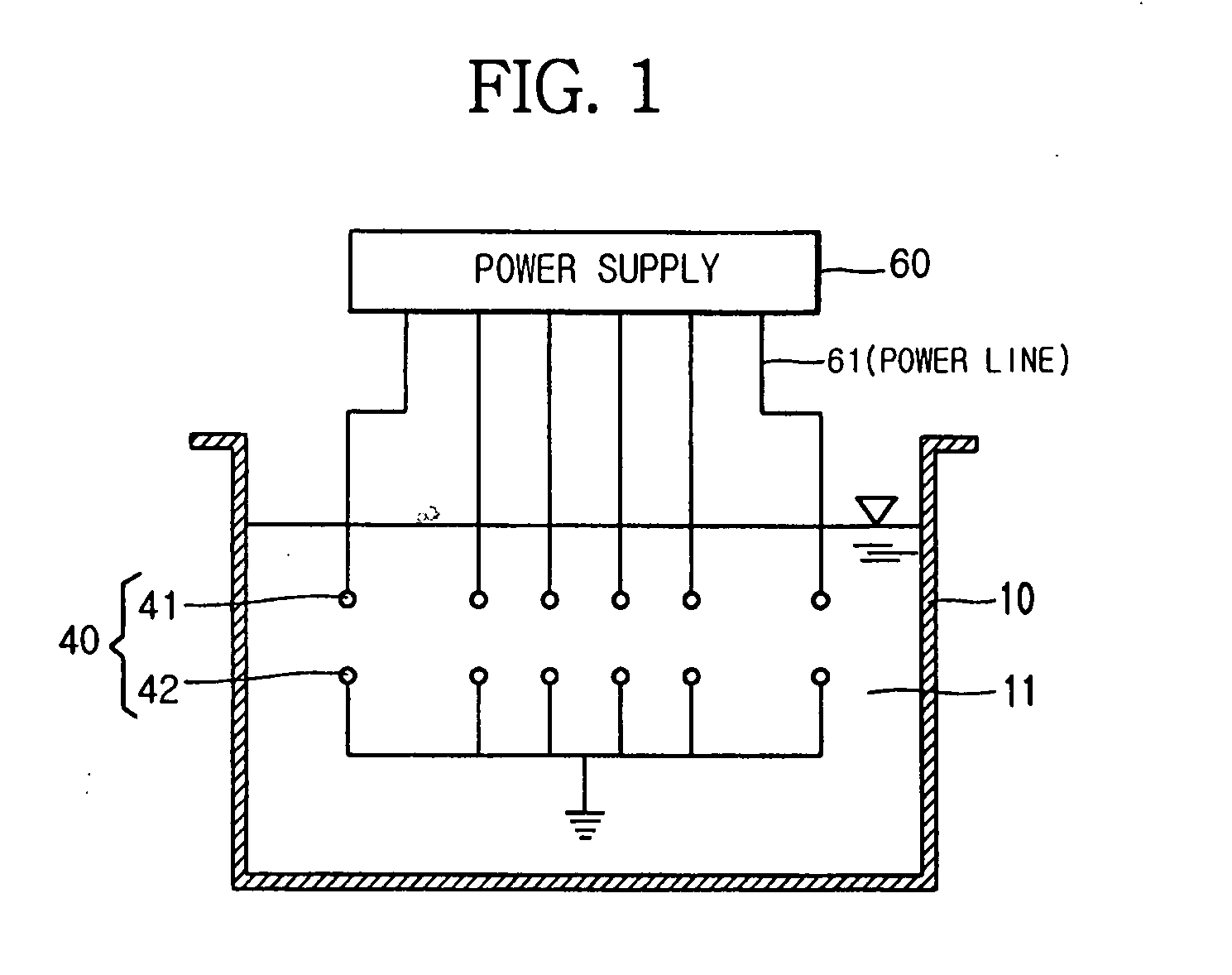
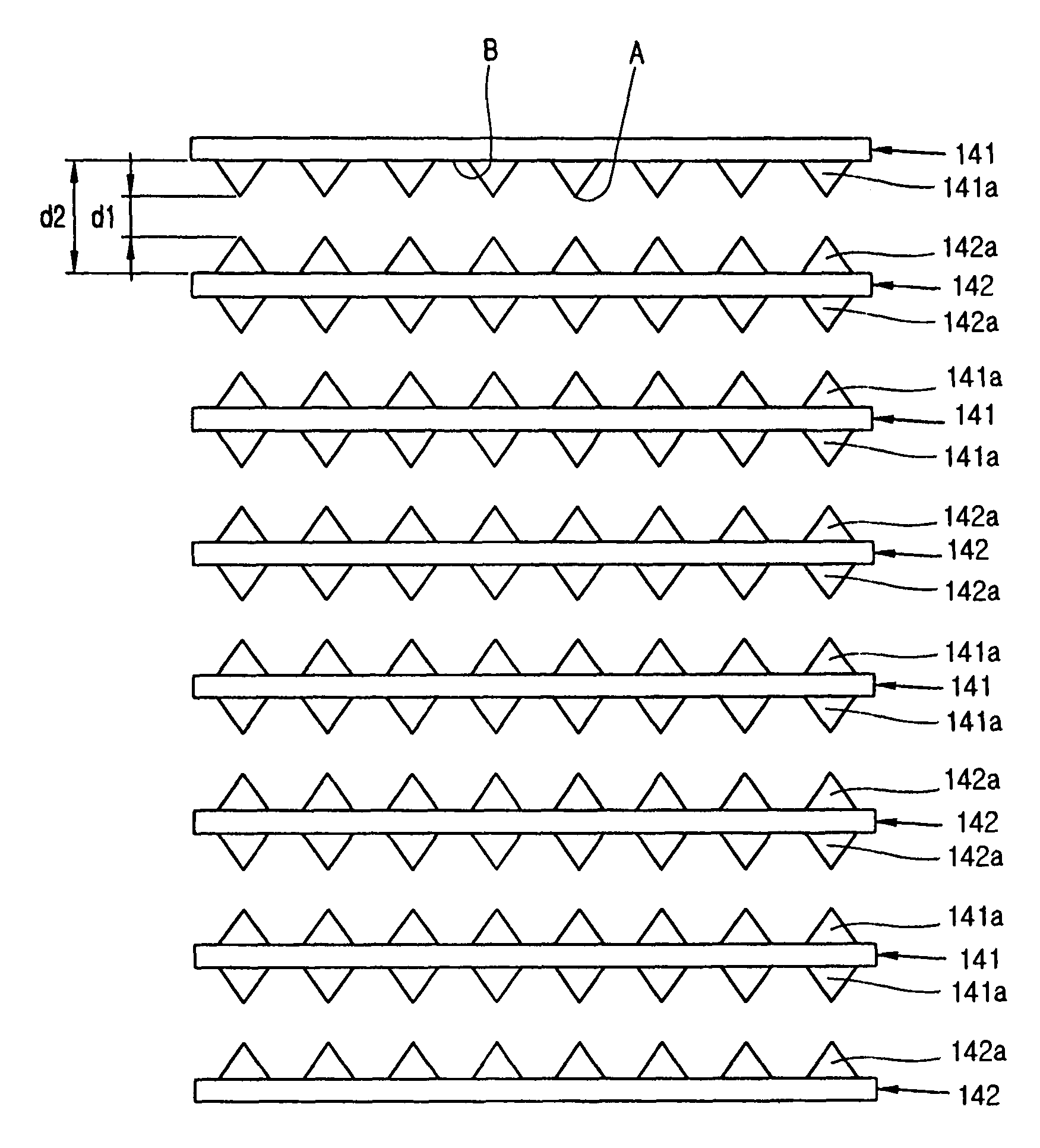
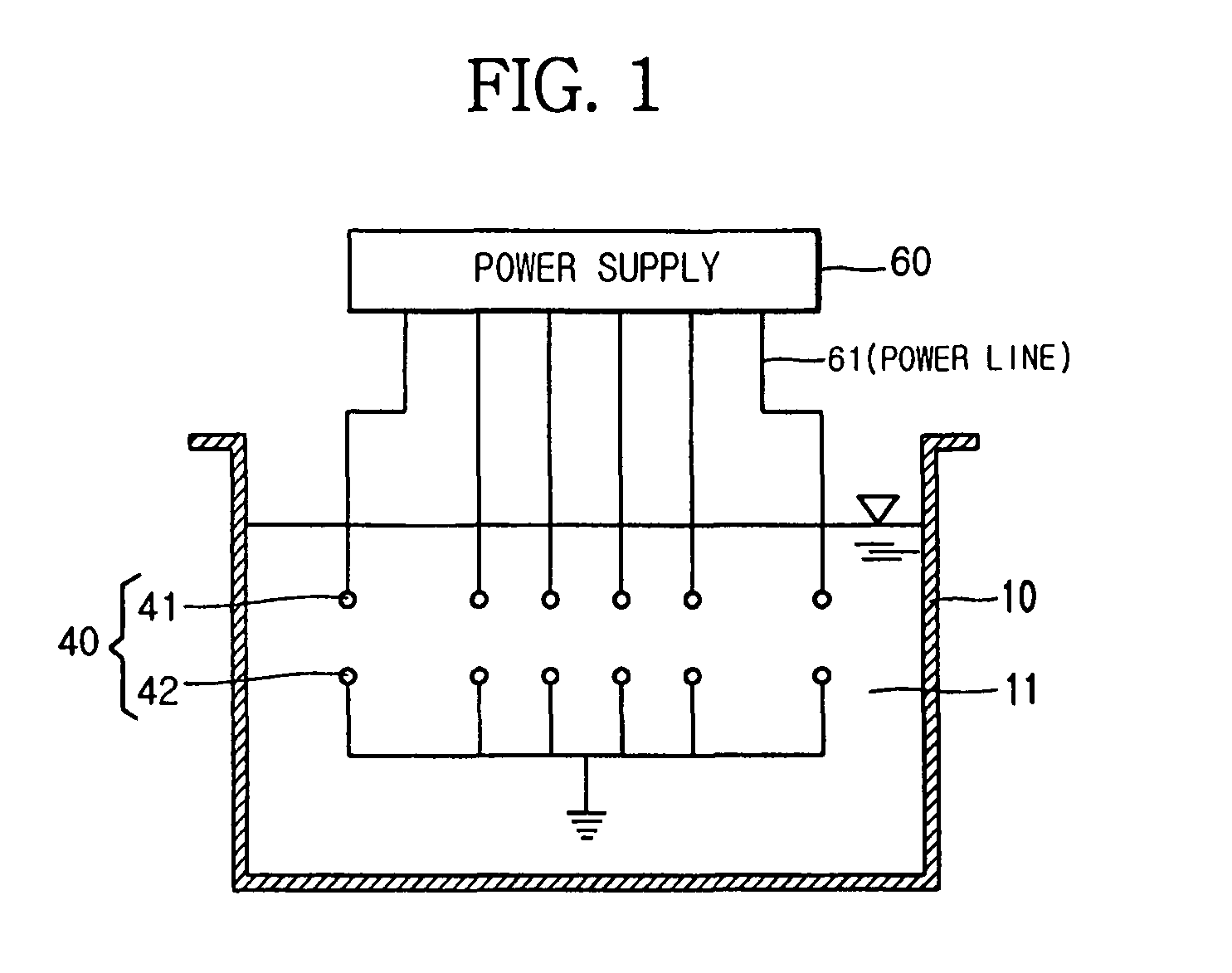
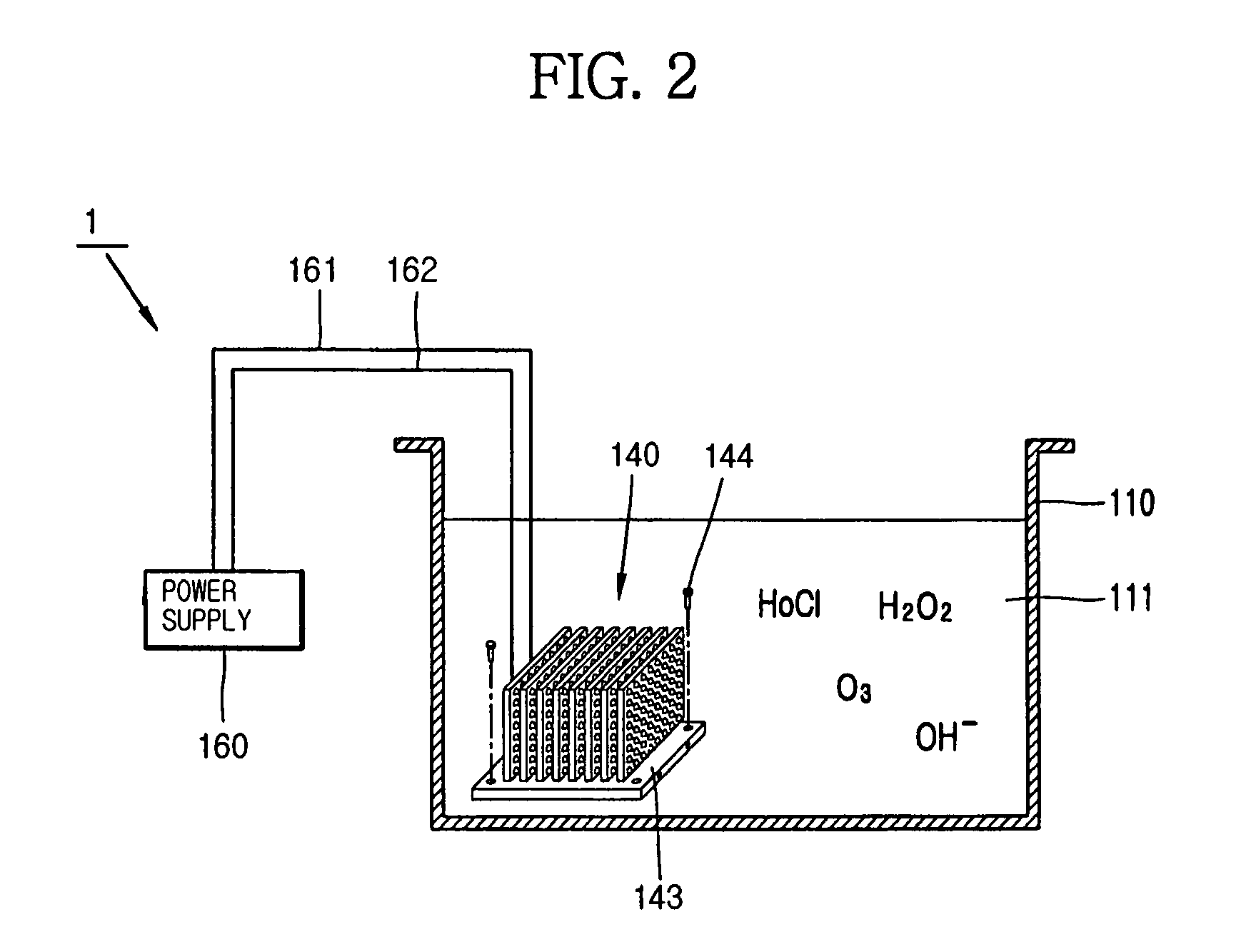
Apparatus for manufacturing sterilized water, and portable apparatus for manufacturing sterilized salt solution
ActiveUS20090314645A1Short timeReduce manufacturing costCellsSludge treatmentPower flowSTERILE SALINE SOLUTION
The present invention provides an apparatus for manufacturing sterilized water, spraying apparatus thereof and capsule containing salt using therein, more particularly, comprises a container having a water receiver for accommodating water; at least one negative electrode having at least one negative electrode projection formed thereon in the water receiver; at least one positive electrode having at least one positive electrode projection formed thereon arranged to face the negative electrode projection in the water receiver; and a power supply for supplying electric current to the negative electrode and the positive electrode, thereby promptly manufacturing a large amount of sterilized water within a short time, and thus, enabling users to use for disinfecting and sterilization the fresh sterilized water immediately after directly manufacturing the sterilized water without having aseptic to injured area or the inside of a nose for rhinitis' patients.
Owner:KIM CHIL YOUNG +1
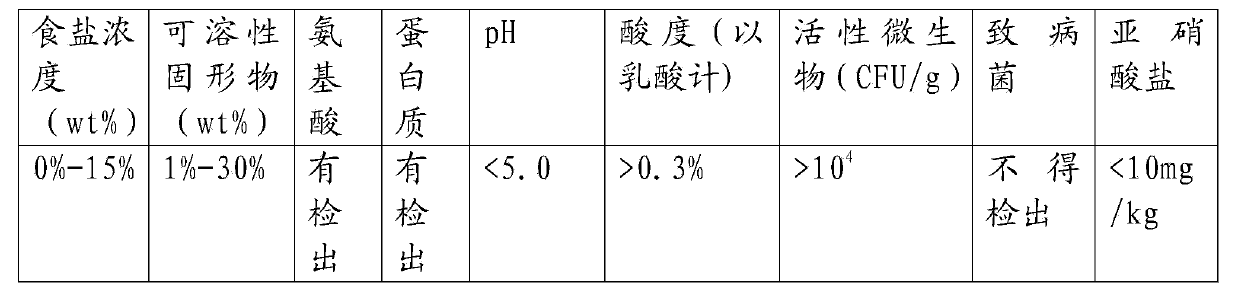
Pickle old brine dry powder
ActiveCN104187518AAdd flavorFermentation adaptationClimate change adaptationMulti-step food processesFlavorFreeze-drying
The invention belongs to the field of foods and particularly relates to pickle old brine dry powder. The pickle old brine dry powder comprises dry powder A and dry powder B in the mass ratio of 1:10 to 1:5000. The preparation method of the dry powder A and the dry powder B comprises the following steps: carrying out centrifugal separation on pickle old brine to respectively obtain supernate and precipitate; diluting the precipitate by using a sterile saline solution and carrying out freeze drying to obtain the dry powder A; and concentrating the separated supernate to obtain a concentrated solution and carrying out direct spray drying on the concentrated solution to obtain the dry powder B. The pickle old brine dry powder disclosed by the invention has the advantages that the production of instant pickles in different places is realized; compared with other pure lactobacillus fermented pickles, the pickles prepared by adopting the dry powder is better in flavor; moreover, natural (old brine) lactobacilli which are more suitable for the fermentation of the pickles are adopted; and the product is more convenient in transportation, long in storage life and rich in active lactobacilli and preservative is not needed to be added.
Owner:SICHUAN DONGPO CHINESE PAOCAI IND TECH RES INST
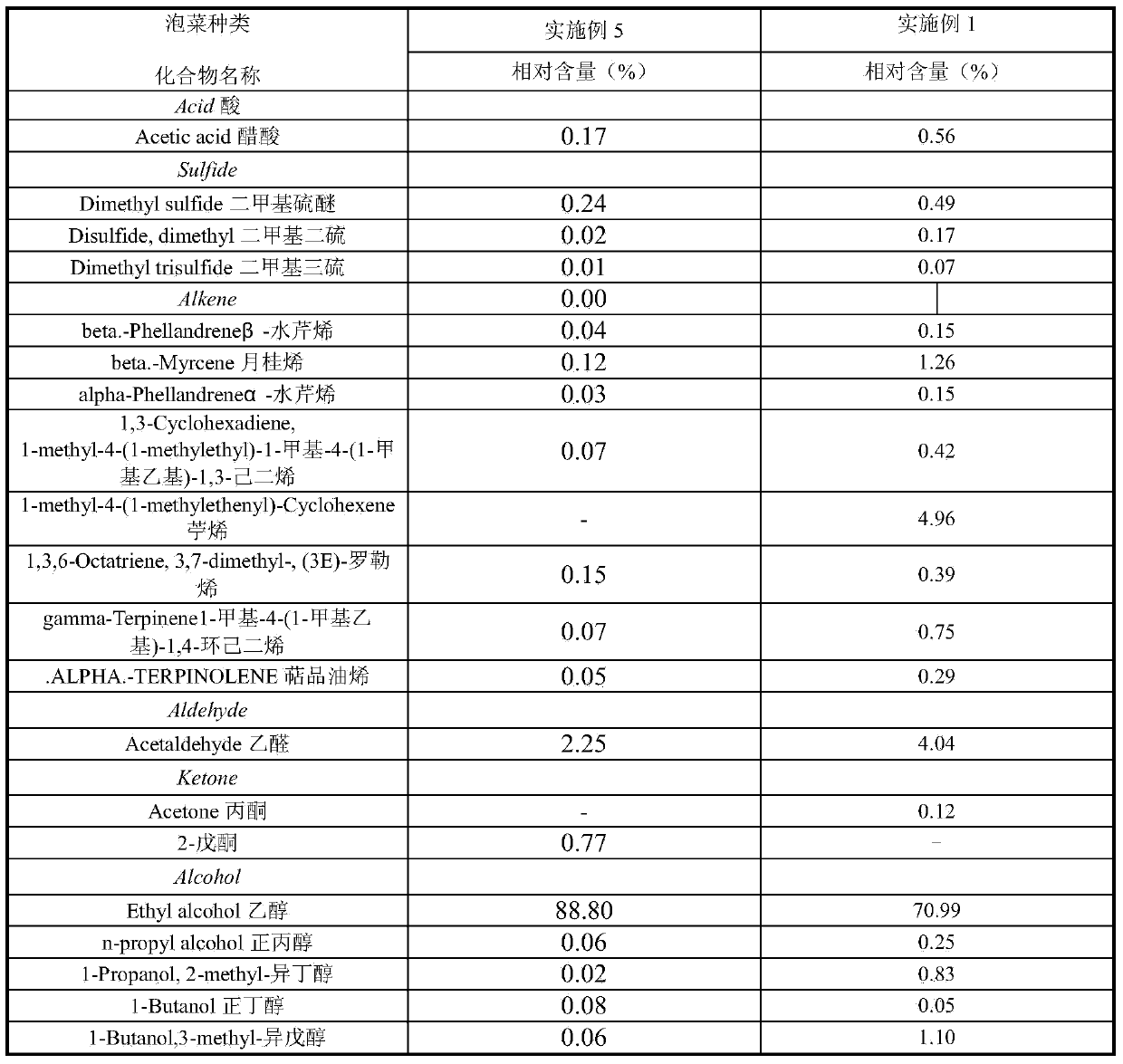
Vaginal speculum
InactiveUS20100016674A1SurgeryContainer/bottle contructionBlood pressure cuffsSTERILE SALINE SOLUTION
An inflatable vaginal speculum that includes diametrically and longitudinally expandable sidewalls with openings allowing observation of vaginal walls along the length of the speculum and a central opening allowing viewing down the length of the speculum to the cervix. The speculum may be positioned within an envelope together with a sterile saline solution. An array of the envelopes can be stored in a storage box together with bulb-type pump such as used with a blood pressure cuff. The envelope is opened by tearing down one side so that the speculum can be removed.
Owner:MILLS STEVEN C
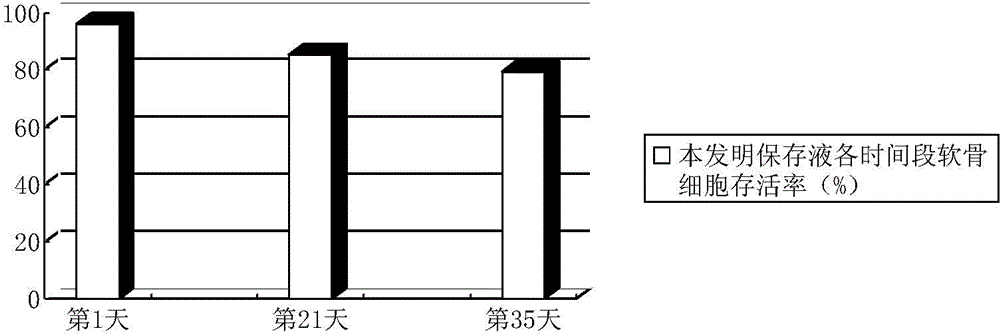
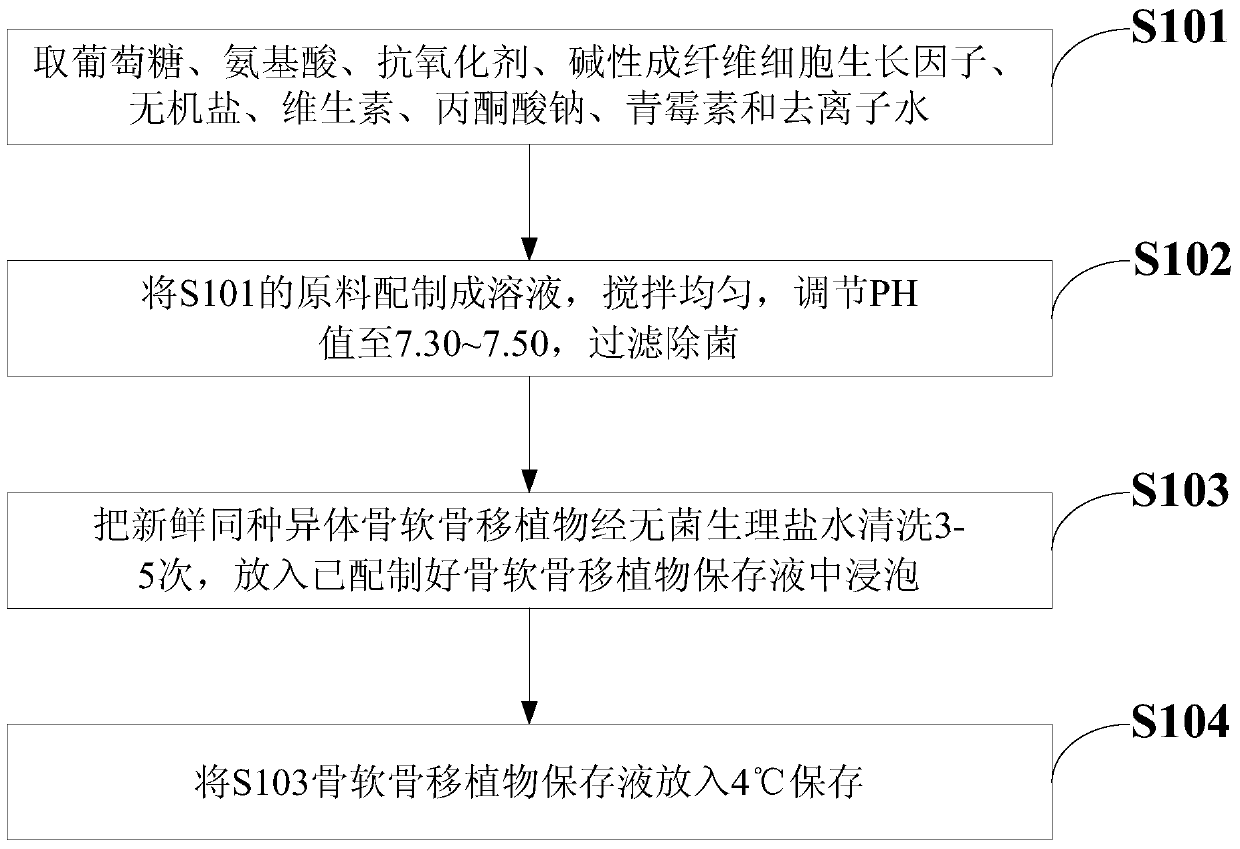
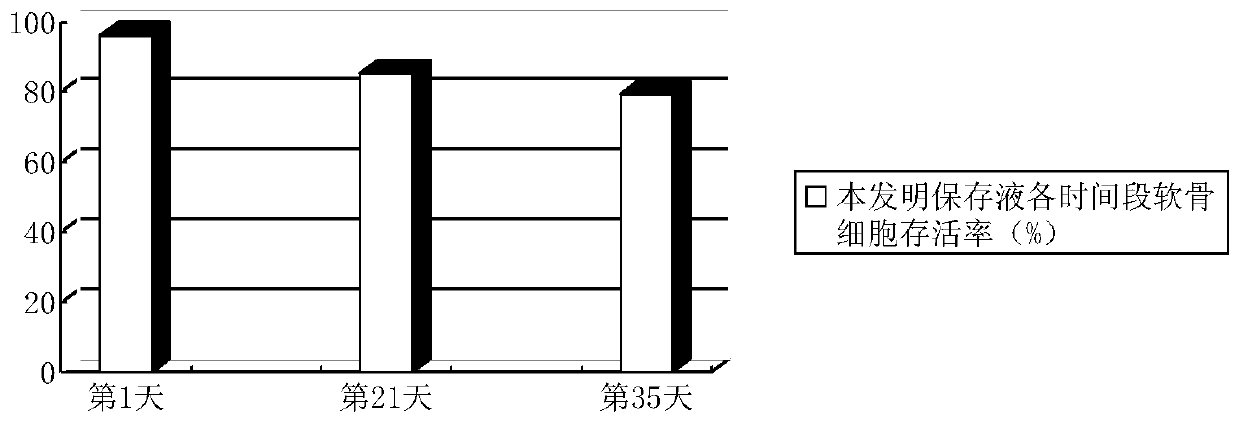
Osteochondral graft preservation liquid and preparation method thereof
ActiveCN104938476AImprove the preservation effectImprove survival rateDead animal preservationPenicillinAntioxidant
The invention discloses an osteochondral graft preservation liquid and a preparation method thereof. The osteochondral graft preservation liquid comprises the following raw materials: 80-125mmol of glucose, 0.1-2.0mmol of amino acid, 0.2-2.0mmol of an antioxidant, 5-60nmol of a basic fibroblast growth factor, 1-120mmol of inorganic salt, 1-9mmol of vitamin, 1-2mmol of sodium pyruvate, 50-70U of penicillin and the balance of deionized water. The method comprises the following steps: preparing a solution, adjusting the pH to be 7.30-7.50, filtering and degerming; cleaning fresh allogeneic osteochondral graft with a sterile saline solution for 3-5 times, and putting into the prepared osteochondral graft preservation liquid to soak; and storing at 4 DEG C. The osteochondral graft preservation liquid has the efficacies of resisting cartilage cells senescence in preservation, inhibiting cartilage cells apoptosis, keeping good biological activity of cartilage tissues, maintaining the survival rate of cartilage cells, prolonging the storage time and improving the storage effect of the osteochondral graft.
Owner:TAISHAN MEDICAL UNIV
Anti-gosling plague egg yolk antibody and preparation method thereof
The invention relates to an anti-gosling plague egg yolk antibody and a preparation method thereof, which adopt multiple inactivation technologies of physics, chemistry and the like and use the acidified water dilution method in combination with such modern biotechnics as the caprylic acid method, high speed centrifugal and superfiltration to effectively separate and purify immunoglobulin in egg yolk. The antibody contains no harmful substances and outer contaminations, has high safety and no residues at the injection position, has no irritation and toxic and side effects, and has no influence on carcass quality; the antibody is conveniently carried and needs no thawing when used, and is dissolved by using sterile saline solution to the volume prior to lyophilizing, and the injection of the antibody is convenient and fast; the antibody has high stress resistance and stable titer, and can be stored for long time; and the antibody is efficacious when stored for 24 months under the condition of room temperature, and the storage period of the antibody can be greatly prolonged under the condition of 2-8 DEG C. The antibody is a combined antibody, and has good prevention and treatment effect for multiple gosling infections such as gosling plague.
Owner:PU LIKE BIO ENG
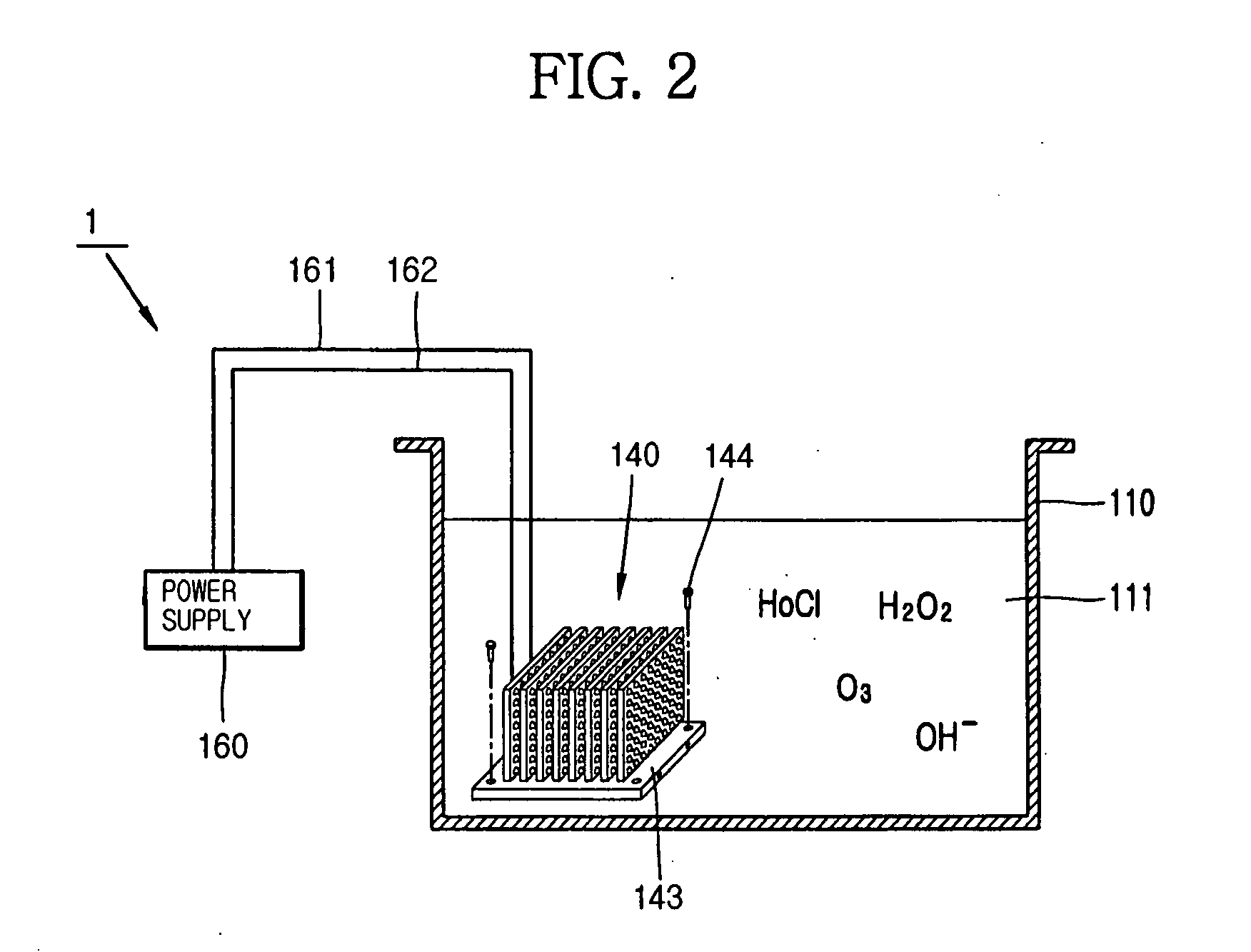
Apparatus for manufacturing sterilized water, and portable apparatus for manufacturing sterilized salt solution
The present invention provides an apparatus for manufacturing sterilized water, spraying apparatus thereof and capsule containing salt using therein, more particularly, comprises a container having a water receiver for accommodating water; at least one negative electrode having at least one negative electrode projection formed thereon in the water receiver; at least one positive electrode having at least one positive electrode projection formed thereon arranged to face the negative electrode projection in the water receiver; and a power supply for supplying electric current to the negative electrode and the positive electrode, thereby promptly manufacturing a large amount of sterilized water within a short time, and thus, enabling users to use for disinfecting and sterilization the fresh sterilized water immediately after directly manufacturing the sterilized water without having aseptic to injured area or the inside of a nose for rhinitis' patients.
Owner:KIM CHIL YOUNG +1
Decellularized fiber ring matrix preparation method
ActiveCN103007352AGood biocompatibilityPromote degradationProsthesisPhosphateSTERILE SALINE SOLUTION
The invention discloses a decellularized fiber ring matrix preparation method and belongs to the technical field of animal cells or tissues. The decellularized fiber ring matrix preparation method includes taking spinal fiber ring of a healthy adult pig, sufficiently rinsing the spinal fiber ring by sterile saline solution; placing the spinal fiber ring into Tris buffer solution of concentration 10mM and with 0.05-0.5% of EDTA (ethylene diamine tetraacetic acid) and 5-50KIU / ml of aprotinin and oscillating at the temperature of 4 DEG C; then placing the spinal fiber ring into the Tris buffer solution containing 1-5% of TritonX-100, oscillating at room temperature; placing the spinal fiber ring into Tris buffer solution containing 0.05-0.5mg / ml of deoxyribonuclease, 5-50microgram / ml of ribonuclease A, and oscillating; and finally, washing by sterile saline solution with oscillating, and storing in sterile PBS (phosphate buffer solution) at the temperature of 4 DEG C. The decellurlarized fiber ring matrix preparation method is simple in preparation process, thorough in decellurlarization and complete in reserved structure, similar in matrix content and mechanical performance as those of natural fibers, and is a good tissue engineering fiber ring support material.
Owner:TIANJIN HOSPITAL
Method for synergically removing sulfate and Cr (VI) in wastewater through sponge iron and microorganisms
ActiveCN106115932AMaintain reducing activityEffective recoveryWater contaminantsBiological water/sewage treatmentSulfate-reducing bacteriaSTERILE SALINE SOLUTION
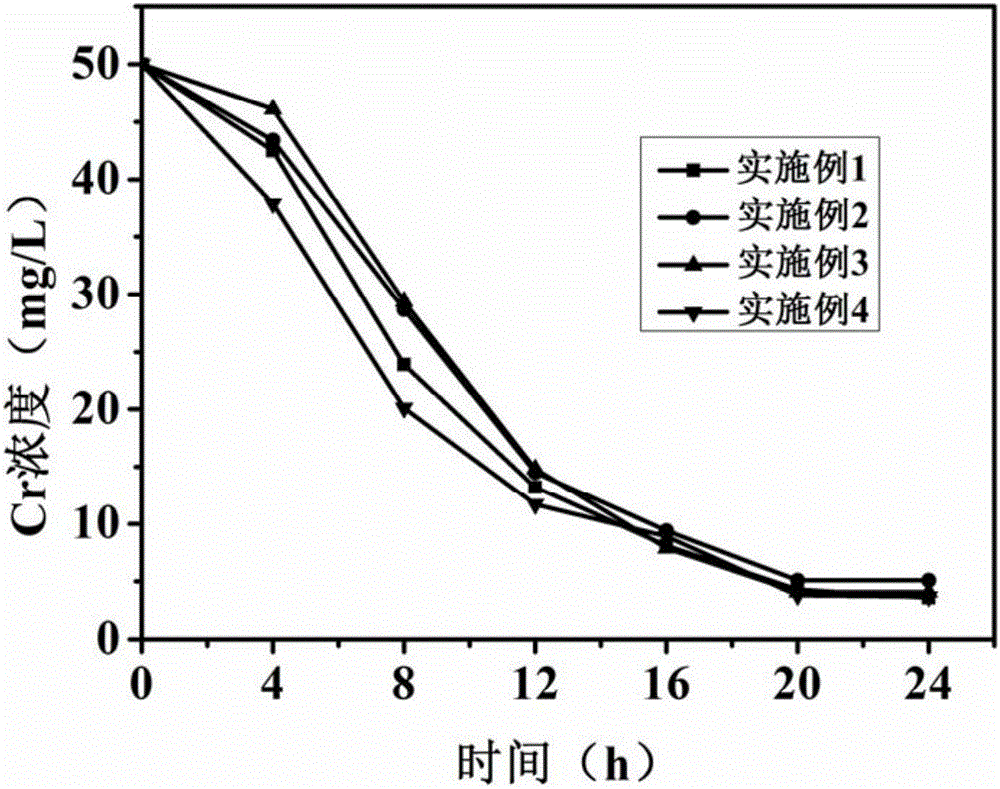
The invention discloses a method for synergically removing sulfate and Cr (VI) in wastewater through sponge iron and microorganisms. The method comprises the steps that under anaerobic conditions, a sponge iron solution A, a bacterial suspension B of sulfate reducing bacteria and a bacterial suspension C of sulfate reducing bacteria are mixed according to the volume ratio of 1 to 3 to 4, aging is performed for 30-60 minutes, sponge iron / microbial microspheres are repeatedly washed with deoxidized deionized water after the reaction finished and are soaked in a sterile saline solution to obtain a mixture of sponge iron and sulfate reducing bacteria / iron reducing bacteria; the mixture of sponge iron and sulfate reducing bacteria / iron reducing bacteria is mixed with sulfate and Cr (VI) in the wastewater, reaction is performed at room temperature for more than 24 hours, and meanwhile the sulphate and Cr (VI) in the wastewater are purified. The Cr (VI) metal removal rate of the method is above 89%, the required equipment is simple, reaction is completed at normal temperature and pressure, a product is a solid phase, a reaction system is a liquid phase, the product is easy to separate, and the method is used for large-scale industrial production.
Owner:SOUTH CHINA UNIV OF TECH
Method for detecting total bacteria count in milk
InactiveCN104928346AImprove relevanceThe test result is accurateMicrobiological testing/measurementSTERILE SALINE SOLUTIONHydrolysate
The invention discloses a method for detecting total bacteria count in milk. The method includes pouring milk into sterile saline solution, oscillating at 100-150 rpm for 2 min to obtain mixed solution, adding xylene into the mixed solution, stirring and centrifuging at 1000-1500 rpm for 5-15 min, and removing the upper layer; adding mixed enzyme solution containing trypsin, papain and chymotrypsin into the residual liquid, water-bathing 5 min at 40 degrees Centigrade to obtain enzymatic hydrolysate, concentrating by a nanofiltration membrane, and dyeing the concentrate by methylene blue dyeing liquid and detecting and counting by a microorganism detector. The method for detecting total bacteria count in milk has no remarkable difference as compared with an international method and is high in correlation, accurate in detecting results and short in detecting time and can be applied to actual detection, and detection limit is 20-106 cfu / mL.
Owner:DONGCHEN LEADER TESTING
Method for conducting fixation of denitrifying bacteria with Trichodermaviride as carrier
ActiveCN107058282AReduce nitrate nitrogen contentHigh removal rateWater contaminantsMicroorganism based processesSTERILE SALINE SOLUTIONWater quality
The invention discloses a method for conducting fixation of denitrifying bacteria with Trichodermaviride as a carrier. The method includes the following steps that Paracoccussp. is inoculated to an enrichment medium, shaking culture is conducted, the obtained bacteria are suspended in a sterile saline solution, and a bacterium suspension of the denitrifying bacteria is obtained; Trichodermaviride is inoculated to a solid medium for culture, washing is conducted with sterile water, the cultured Trichodermaviride is stored at low temperature, and a spore suspension is obtained; the bacterium suspension of the denitrifying bacteria and the spore suspension are inoculated to a mycelium pellet medium to conduct shaking culture, and mixed mycelium pellets of fixation of denitrifying bacteria with Trichodermaviride as the carrier are obtained. The method has the advantages of being simple and convenient in operation, short in culture time, reusable and good in sedimentation performance, the mixed mycelium pellets prepared can obviously reduce the nitrogen content of a water body, water quality conditions are improved, and sewage reaches the discharge standard; nitrite accumulation does not exist, environmental pollution is reduced, and the method has important significance for sewage treatment by means of Paracoccussp. on a large scale.
Owner:QUFU NORMAL UNIV
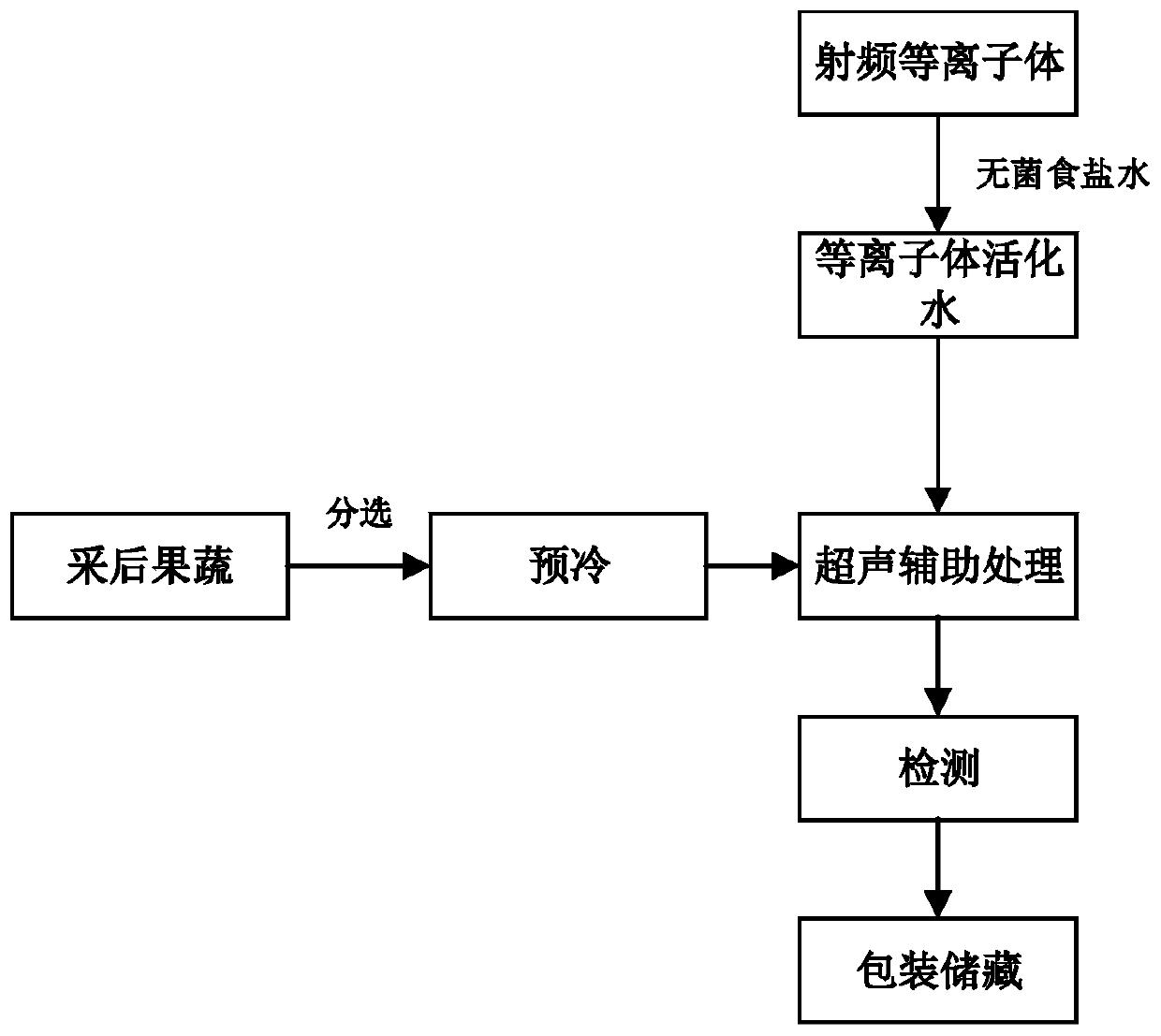
Method for cleaning and preserving fruits and vegetables with ultrasonic-assisted plasma activated water
InactiveCN110897076ADegradation of pesticide residues on the surfaceDegrade dirtFruits/vegetable preservation by irradiation/electric treatmentFood ultrasonic treatmentBiotechnologyPesticide residue
The invention belongs to the technical field of cleaning and preservation of fruits and vegetables, and relates to a method for cleaning and preserving the fruits and vegetables with ultrasonic-assisted plasma activated water. The method comprises the steps of S1, putting a sterile saline solution in a position below an atmospheric radio-frequency discharge plasma nozzle; S2, starting a high-pressure radio-frequency generator, introducing an air source into the atmospheric radio-frequency discharge plasma nozzle through a steel bottle, and performing treatment on the sterile saline solution byplasma generated by a central electrode to obtain the plasma activated water; S3, sorting, classifying and precooling freshly picked fruits and vegetables, and then, putting the sorted, classified and precooled fruits and vegetables in the plasma activated water, obtained in the step S2, according to a certain proportion for ultrasonic cleaning and sterilization; and S4, performing packaging andstorage after the cleaned and sterilized fruits and vegetables are air-dried. The method can overcome the defects of a preservation technology of single plasma activated water, effectively exerts theadvantages of the preservation technology, effectively degrades pesticide residue and dirt on the surfaces of the fruits and vegetables while increasing the sterilization preservation efficiency, andgreatly prolongs the preservation and storage period and enhances the edible safety of the fruits and vegetables.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of mink hemorrhagic pneumonia inactivated vaccine and application thereof
InactiveCN106390111AReduced immune responseReduce emergencyAntibacterial agentsAntibody medical ingredientsMinkAdjuvant
The invention relates to a preparation method of a mink hemorrhagic pneumonia inactivated vaccine and also relates to an inactivated vaccine prepared by the preparation method and an application of the inactivated vaccine. The preparation method of the inactivated vaccine comprises the following steps: (1) culturing the isolated and identified pseudomonas aeruginosa virulent strain ZHDL9; (2) inactivation treatment: slowly adding formaldehyde into the bacterial liquid for inactivation; and (3) seedling preparation: centrifuging the pseudomonas aeruginosa virulent strain ZHDL9 qualified according to inactivation inspection, fetching the supernate, adding a saturated ammonium sulfate solution into the supernate, centrifuging, dissolving the precipitated protein with a sterile saline solution, adjusting the concentration of the multi-component protein to 500mug / ml, and adding an adjuvant. The invention has the following advantages: the immunization range is wide, the cost is lowered, and the immunization frequency is reduced, so that the emergency is reduced, and the economic income is increased.
Owner:于彦强
Preparation method for gram staining solution quality control product
InactiveCN103471896ACheck for expirationOperational step supervision and inspectionPreparing sample for investigationAlcoholSTERILE SALINE SOLUTION
The invention relates to the technical field of biomedical treatment and in particular relates to a preparation method for a gram staining solution quality control product. The preparation method comprises the following steps: firstly, putting two standard strains of gram positive bacteria and gram negative bacteria into two test tubes, and freezing and drying the standard strains for storage in a refrigerator; secondly, taking out the two standard strains from the refrigerator, and adding normal saline respectively for dissolving the gram positive bacteria and the gram negative bacteria to prepare a bacterial suspension; thirdly, adding a sterile saline solution into the bacterial suspension; then adding the two standard strains into the bacterial suspension respectively, and uniformly mixing the bacterial suspension on a vibrator; fourthly, taking out the uniformly mixed bacterial suspension, diluting, and dripping on a slide; fifthly, naturally drying the slide at room temperature; sixthly, ensuring that the coating surface of the dried slide faces up, moving the slide back and forth on flame of an alcohol lamp, and fixing a bacterial film. The preparation method has the characteristics that the time is saved, the operation is simple, the work efficiency of workers is improved, the quality of a staining solution can be detected, and the workers can be supervised.
Owner:上海兰卫医学检验所股份有限公司
Intestinal flora capsule preparation method and intestine flora capsules
InactiveCN109394795ASignificantly regulates the balanceRelieve painBacteria material medical ingredientsDigestive systemEscherichia coliIntestinal structure
The invention relates to an intestinal flora capsule preparation method. The preparation method comprises the following steps: S1, collecting human excrement, and mixing the human excrement and sterile saline solution according to a certain proportion to obtain a mixed solution; S2, stirring the mixed solution to transform the mixed solution into a suspension; S3, performing micro-filtering treatment on the suspension to obtain filtrate; S4, centrifuging the filtrate to obtain a precipitate; S5, mixing the precipitate and a freeze-drying protective additive according to a certain proportion toobtain a pre-frozen solution, and performing freeze drying after the pre-frozen solution is pre-frozen to obtain freeze-dried powder; S6, adding the freeze-dried powder into capsules, sealing and packaging to obtain the intestinal flora capsules, and storing the intestinal flora capsules in an environment at a temperature of 20 DEG C below zero. The invention further relates to the intestinal bacterial capsules. The intestinal bacterial capsules have the advantages of containing a compound of probiotics, bifidobacterial, lactobacillus, aerobic bacteria, escherichia coli and anaerobic bacteria, being capable of remarkably regulating the intestinal flora balance and shortening the treatment period.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
Pork preservative and pork fresh-keeping method
InactiveCN110100875AAvoid negative effectsReduce processing timeMeat/fish preservation by freezing/coolingMeat/fish preservation using chemicalsPreservativePork meat
The invention relates to a pork fresh-keeping method, and belongs to the technical field of food processing and manufacturing. The pork fresh-keeping method comprises the following steps that a, freshpork chops are selected, cleaned and then drained off; b, flowing water immersion cleaning is carried out on the cleaned pork chops with a sterile saline solution for 10 minutes under the conditionsof the low temperature of 2-6 DEG C and the cyclical variation low pressure ranging from 0.01 Mpa to 0.05 Mpa; c, a specially prepared preservative is used for soaking the pork chops after immersion cleaning for 10 minutes under the conditions of the lower temperature of 2-6 DEG C and the lower pressure of 0.01-0.05 MPa; d, vacuum packaging and freezing are carried out. The pork fresh-keeping method has the advantages that by adopting a low-temperature low-pressure preservative impregnation technology, the treatment time of the preservative is shortened significantly, thereby reducing the occurrence of undesirable substances such as nitrosamines, and prolonging the quality ensuring time, so that the safety of the product is ensured.
Owner:HAINAN SIDAYUNTONG TECH LTD
Posterior scleral quantitative pressure block and using method thereof
PendingCN110302005AReduce distortionReduce astigmatismEye surgerySurgical treatmentSTERILE SALINE SOLUTION
The embodiments of the invention disclose a posterior scleral quantitative pressure block and a using method thereof, and belong to the technical field of ophthalmic surgical treatment appliances. According to the technical scheme key point, the posterior scleral quantitative pressure block comprises an implant body and an implant handle, one end of the implant handle is connected to the implant body, the implant body is of a flexible hollow structure, and a water flow pipeline is disposed in the implant handle, and the water flow pipeline is disposed in the length of the implant handle, one end of the water flow pipeline communicates with the implant body, the other end of the water flow pipeline extends to the end, away from the implant body, of the implant handle, and the posterior scleral quantitative pressure block and the using method thereof have the advantage of being able to perform sterile saline solution injection through the pipeline during the operation for quantitative pressure.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
A kind of osteochondral graft preservation solution and preparation method thereof
ActiveCN104938476BImprove the preservation effectImprove survival rateDead animal preservationCartilage cellsPenicillin
The invention discloses an osteochondral graft preservation solution and a preparation method thereof, comprising: 80-125 mmol of glucose, 0.1-2.0 mmol of amino acid, 0.2-2.0 mmol of antioxidant, 5-60 nmol of basic fibroblast growth factor, inorganic salt 1~120mmol, vitamin 1~9mmol, sodium pyruvate 1~2mmol, penicillin 50~70U, and the rest is deionized water; make a solution, adjust the pH value to 7.30~7.50, filter and sterilize; fresh allogeneic osteochondral The grafts were washed 3‑5 times with sterile saline, soaked in the prepared preservation solution for osteochondral grafts, and stored at 4°C. The invention has the functions of resisting chondrocyte aging during preservation, inhibiting chondrocyte apoptosis, maintaining good biological activity of cartilage tissue, maintaining chondrocyte survival rate, prolonging preservation time, and improving preservation effect of osteochondral grafts.
Owner:TAISHAN MEDICAL UNIV
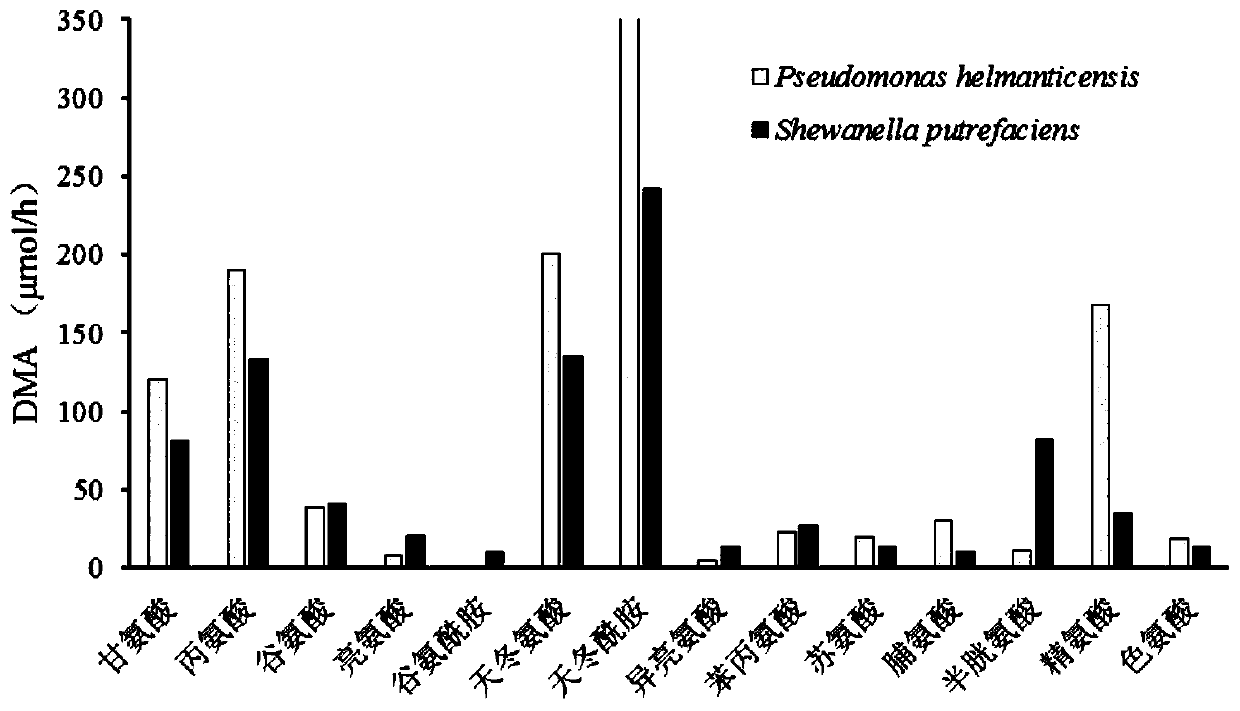
Method for detecting deamination capability of amino acid of spoilage bacteria in fish flesh
ActiveCN110724726AComponent separationMicrobiological testing/measurementSTERILE SALINE SOLUTIONSpoilage bacteria
The invention discloses a method for detecting deamination capability of an amino acid of spoilage bacteria in fish flesh. The method comprises the following steps: dissolving a to-be-detected amino acid substrate with a phosphate buffering solution to obtain a substrate solution of a to-be-detected amino acid; adding a bacteria suspension of to-be-detected spoilage bacteria into the substrate solution of the to-be-detected amino acid to obtain a mixture and using the mixture as an evaluation group; adding the bacteria suspension of the to-be-detected spoilage bacteria into the phosphate buffering solution to obtain a mixture and using the mixture as a control group, and adding a sterile saline solution into the substrate solution of the to-be-detected amino acid to obtain a mixture and using the mixture as a blank group; and separately culturing the evaluation group, the control group and the blank group under same conditions, and subsequently, performing detection to obtain evaluation C, control C and blank C of free ammonia concentration of the evaluation group, the control group and the blank group, and performing calculation to obtain the deamination capability of the amino acid of a variety of spoilage bacteria in various fish flesh. The method provided by the invention can be widely applied to detection of the deamination capability of the amino acid of the variety of spoilage bacteria in the various fish flesh, is applied to quantitative detection and evaluation of the deamination capability of a variety of specific amino acids through the spoilage bacteria, and isapplied to evaluation of the capability of free amino acids through utilization of metabolism of the spoilage bacteria in the fish flesh.
Owner:CHINA AGRI UNIV
In-vitro separation culture method for skeleton satellite cells of excellent ice and snow athletes
The invention relates to an in-vitro separation culture technique for the skeleton satellite cells of excellent ice and snow athletes, and belongs to the interdiscipline field of cytobiology, sports traumatology and tissue engineering. The technique comprises the following steps: cleaning muscular tissues by using 0.9% sterile saline solution; digesting and separating the skeleton satellite cells under the cooperative action of 1% of I-type collagenase and 0.1% of pronase under the condition of 37 degrees; culturing the skeleton satellite cells by using a high-glucose DMEM (Dulbeccos Modified Eagles Medium) culture medium containing 10% of fetal calf serum; and freezing and storing the skeleton satellite cells by using a freezing-stored liquid formed by the DMEM basal culture medium, dimethyl sulfoxide and the fetal calf serum which are in the ratio of 5:1:4. Thus, the simple, efficient and stably-inherited in-vitro separation cultivation method for the skeleton satellite cells of the excellent ice and snow athletes is provided.
Owner:HARBIN INST OF PHYSICAL EDUCATION
Method for rapid detection of drug resistance phenotypes of strains
PendingCN110699421AEasy to operateLess consumablesMicrobiological testing/measurementBiological material analysisNutritionSTERILE SALINE SOLUTION
The invention discloses a method for rapid detection of drug resistance phenotypes of strains. The method includes: firstly, preparing antibacterial drug diluent in gradient-changing concentration from nutrient broth, dropwise adding antibacterial drugs in different concentrates onto sample target plates of a mass spectrometer to form drug-sensitive target plates, and drying for standby application; secondly, preparing standard bacterial suspension of to-be-tested strain from sterile saline solution, and diluting with nutrient broth to keep for standby application; thirdly, sequentially dropwise adding the diluted standard bacterial suspension onto the prepared drug-sensitive target plates to form microdroplets, putting into a wet box, incubating for 2h-6h in an incubator, and discarding broth on the drug-sensitive target plates; fourthly, after drying, dropwise adding a mass spectrometry pretreatment agent, crystallizing, performing microbiological assay by adopting a mass spectrometer, and reading a result. The method is simple in operation, low in material consumption and independent from any drug resistance mechanisms, antibiotic coating and concentrations can be determined according to demands, quickness in detection is achieved, the result is direct and clear, excessive analysis is avoided, and the method is wide in application range.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Synergistic Removal of Sulphate and Cr(ⅵ) Wastewater by Sponge Iron and Microorganisms
ActiveCN106115932BLarge specific surface areaHigh specific surface energyWater contaminantsBiological water/sewage treatmentSulfate-reducing bacteriaSTERILE SALINE SOLUTION
The invention discloses a method for synergically removing sulfate and Cr (VI) in wastewater through sponge iron and microorganisms. The method comprises the steps that under anaerobic conditions, a sponge iron solution A, a bacterial suspension B of sulfate reducing bacteria and a bacterial suspension C of sulfate reducing bacteria are mixed according to the volume ratio of 1 to 3 to 4, aging is performed for 30-60 minutes, sponge iron / microbial microspheres are repeatedly washed with deoxidized deionized water after the reaction finished and are soaked in a sterile saline solution to obtain a mixture of sponge iron and sulfate reducing bacteria / iron reducing bacteria; the mixture of sponge iron and sulfate reducing bacteria / iron reducing bacteria is mixed with sulfate and Cr (VI) in the wastewater, reaction is performed at room temperature for more than 24 hours, and meanwhile the sulphate and Cr (VI) in the wastewater are purified. The Cr (VI) metal removal rate of the method is above 89%, the required equipment is simple, reaction is completed at normal temperature and pressure, a product is a solid phase, a reaction system is a liquid phase, the product is easy to separate, and the method is used for large-scale industrial production.
Owner:SOUTH CHINA UNIV OF TECH
Fermentation method of human intestinal flora and application
ActiveCN110343640AGrowth inhibitionIncreased content of producing bacteriaBacteriaAntipyreticFecesSTERILE SALINE SOLUTION
The invention provides a fermentation method of human intestinal flora and application. The method comprises the following steps of a, donor screening, wherein boys who are twelve to fourteen years old, are in good health, do not have the serious disease history or obvious gastrointestinal tract symptoms, do not take any antibiotics in at least six months and have a regular life are screened out;b, sample collection and treatment, wherein fresh excrement of the donors is collected, quickly transferred to an anaerobic working station and weighed, a pre-reduction sterile saline solution is added according to a proportion, and even mixing with sufficient vortex is conducted; then filtering is conducted, and an excrement suspension solution is collected; c, fermentation, wherein the excrementsuspension solution is transferred to a sterile serum bottle, sealed and placed in a culture box for standing fermentation. According to the fermentation method, the obtained pH-acidity fermentationproduct with multiple rich probiotics is a kind of optimized healthy human intestinal flora and can be applied to the excrement bacterium transplantation technology and development of medicines, health care products and the like with the human intestinal flora as a target point.
Owner:SHANGHAI JIAO TONG UNIV
Detection method for thermoduric bacteria in soybean protein and detection culture medium for thermoduric bacteria
InactiveCN107574223AThe detection method is accurateFast detection methodMicrobiological testing/measurementSTERILE SALINE SOLUTIONThermoduric Bacteria
The invention relates to a detection method for thermoduric bacteria in soybean protein and a detection culture medium for thermoduric bacteria. The method comprises the following steps: adding a soybean protein sample into sterile saline solution, thereby preparing a soybean protein diluent; boiling the soybean protein diluent and then keeping temperature and heating; quickly cooling to room temperature; uniformly mixing the cooled soybean protein diluent with the detection culture medium for thermoduric bacteria; and culturing for 48h+ / -2h at 36 DEG C+ / -1 and counting the colony count of theculture medium. The detection method provided by the invention is not restrained by soybean protein gelation and is capable of accurately detecting the residual thermoduric bacteria quantity in the soybean protein; the detection method is simple and accurate and the effect is obvious; the culture medium for thermoduric bacteria is simple, the colony characteristics are obvious; the counting is easy, especially for the addition and additive proportion of triphenyltetrazolium chloride; the culture medium is not influenced by the soybean protein gelation; the display for the thermoduric bacteriais obvious; the colony of the thermoduric bacteria can be obviously observed.
Owner:SHANDONG YUWANG ECOLOGY FOOD IND
Filling preparation and preparation method thereof
PendingCN110237305AImprove survival ratePromote proliferationProsthesisSTERILE SALINE SOLUTIONPlasma rich platelet
The invention discloses a filling preparation and a preparation method thereof. The preparation method comprises the following steps that peripheral blood is taken, centrifuging is conducted to obtain platelet-rich plasma, freezing is conducted, then the platelet-rich plasma is taken out and freeze-thawed, centrifuging continues to be conducted to remove membrane fragments at the bottom, and platelet-rich factor plasma is obtained for use; autologous subcutaneous fat is taken, fat granules are obtained and taken, DMSO refrigerating liquid is added in the granules for cryopreservation, then resuscitation is conducted, a sterile saline solution is added in the processed granules, a fat granule suspension is obtained, finally insulin is added in the fat granule suspension, the mixture is shaken evenly for use; the fat granules are centrifuged, upper oil is discarded, fat cells are obtained, and the fat cells are expanded and cultured to the P4 generation, and a fat stem cell resuspension solution is obtained for use; the platelet-rich factor plasma, the fat granule suspension and the fat stem cell resuspension solution are mixed evenly according to a certain volume ratio, and the filling preparation is obtained. According to the filling preparation, the problems that an existing filler material is low in survival rate, short in maintenance time after transplantation and poor in filling effect can effectively solved.
Owner:CHENGDU QINGKE BIOTECH
Medicine for preventing and treating acute radiation pneumonitis
InactiveCN103933028APromote infiltrationEasy to solveOrganic active ingredientsRespiratory disorderSTERILE SALINE SOLUTIONTherapeutic effect
The invention discloses a medicine for preventing and treating acute radiation pneumonitis; the effective component of the medicine is procyanidine. The medicine is a procyanidine solution with the concentration of 2.5-3.75mg / ml; the procyanidine solution comprises procyanidine with the purity of 95-99% and a sterile saline solution; the delivery manner of the medicine is aerosol inhalation. The medicine disclosed by the invention has a good prevention and treatment effect to acute radiation pneumonitis and is a plant raw material medicine with a prospect, namely, the procyanidine can be used for preventing and treating acute radiation pneumonitis. Aerosol inhalation of procyanidine can effectively implement targeted drug delivery of lung, and further can avoid the first pass effect, reduce the use level of the medicine and alleviate or avoid adverse drug reactions while directly reaching the site of action, so that the purpose of preventing or even treating acute radiation pneumonitis is achieved.
Owner:张维芬
A qualitative detection method for mold and yeast in Lactobacillus paracasei direct injection strain
ActiveCN104328162BAvoid distractionsThe test result is accurateMicrobiological testing/measurementMicroorganism based processesFiltrationSTERILE SALINE SOLUTION
The invention discloses a lactobacillus paracasei direct-fed type quantitative detection method for mold and yeast in bacteria, belonging to the technical field of microorganism verification. The detection method comprises the following verification steps in sequence: (1) preparing a sample, namely, preparing a uniform sample solution with the ratio of 1:20; (2) preparing a culture medium; (3) performing suction filtration for the first time, and pasting a filtered filter membrane on a prepared rose-bengal agar plate; (4) performing suction filtration for the second time, namely, diluting the filter membrane obtained from the first suction filtration in 20-40g of sterile saline solution, performing suction filtration for the second time, and pasting another filtered filter membrane on another prepared rose-bengal agar plate; (5) performing suction filtration for the third time; and (6) observing the result, namely, culturing the plate for 5 days at 28+ / -1 DEG C, and observing the growth situation of bacterial colonies on the filter membrane. The invention mainly aims at solving the problem that the detection result has great errors as an international method which is directly used can be interfered by factors such as the dilution ratio and the inhibition function.
Owner:YUNNAN HUANGSHI LESSON DAIRY IND
Fermentation method and application of human intestinal flora
ActiveCN110343640BGrowth inhibitionIncreased content of producing bacteriaBacteriaAntipyreticBiotechnologyDisease
The invention provides a fermentation method of human intestinal flora and application. The method comprises the following steps of a, donor screening, wherein boys who are twelve to fourteen years old, are in good health, do not have the serious disease history or obvious gastrointestinal tract symptoms, do not take any antibiotics in at least six months and have a regular life are screened out;b, sample collection and treatment, wherein fresh excrement of the donors is collected, quickly transferred to an anaerobic working station and weighed, a pre-reduction sterile saline solution is added according to a proportion, and even mixing with sufficient vortex is conducted; then filtering is conducted, and an excrement suspension solution is collected; c, fermentation, wherein the excrementsuspension solution is transferred to a sterile serum bottle, sealed and placed in a culture box for standing fermentation. According to the fermentation method, the obtained pH-acidity fermentationproduct with multiple rich probiotics is a kind of optimized healthy human intestinal flora and can be applied to the excrement bacterium transplantation technology and development of medicines, health care products and the like with the human intestinal flora as a target point.
Owner:SHANGHAI JIAOTONG UNIV
Lactobacillus paracasei direct-fed type quantitative detection method for mold and yeast in bacteria
ActiveCN104328162AAvoid distractionsThe test result is accurateMicrobiological testing/measurementMicroorganism based processesSTERILE SALINE SOLUTIONFiltration
The invention discloses a lactobacillus paracasei direct-fed type quantitative detection method for mold and yeast in bacteria, belonging to the technical field of microorganism verification. The detection method comprises the following verification steps in sequence: (1) preparing a sample, namely, preparing a uniform sample solution with the ratio of 1:20; (2) preparing a culture medium; (3) performing suction filtration for the first time, and pasting a filtered filter membrane on a prepared rose-bengal agar plate; (4) performing suction filtration for the second time, namely, diluting the filter membrane obtained from the first suction filtration in 20-40g of sterile saline solution, performing suction filtration for the second time, and pasting another filtered filter membrane on another prepared rose-bengal agar plate; (5) performing suction filtration for the third time; and (6) observing the result, namely, culturing the plate for 5 days at 28+ / -1 DEG C, and observing the growth situation of bacterial colonies on the filter membrane. The invention mainly aims at solving the problem that the detection result has great errors as an international method which is directly used can be interfered by factors such as the dilution ratio and the inhibition function.
Owner:YUNNAN HUANGSHI LESSON DAIRY IND
Fast detection method of salmonella
InactiveCN108103139AQuick checkEasy to operateMicrobiological testing/measurementBiological material analysisSTERILE SALINE SOLUTIONRoom temperature
The invention provides a fast detection method of salmonella. The method comprises the following steps of S1, treating samples: weighing 2 to 5g of solid samples; performing crushing; adding sterile saline solution into the crushed solid samples for dissolution; S2, preparing a culture medium; S3, after the culture medium is cooled to the room temperature, performing ultraviolet sterilization on the culture medium for 15 to 20min; S4, performing bacterium culture: putting the samples to be tested into the culture medium to be cultured for 4 to 6h; performing bacterium identification on the cultured product; taking the identified bacterial colony, adding the sterile saline solution for preparing a bacterial suspension. The method provided by the invention has the advantages that the operation steps are simple and convenient; the period is shorter; the salmonella can be fast detected; the detection time is saved; the practical detection use is convenient.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com