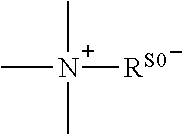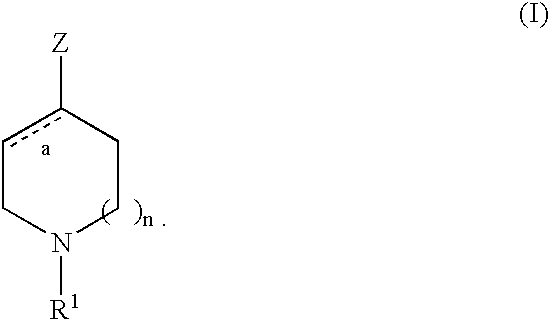Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
197 results about "Pain chronic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
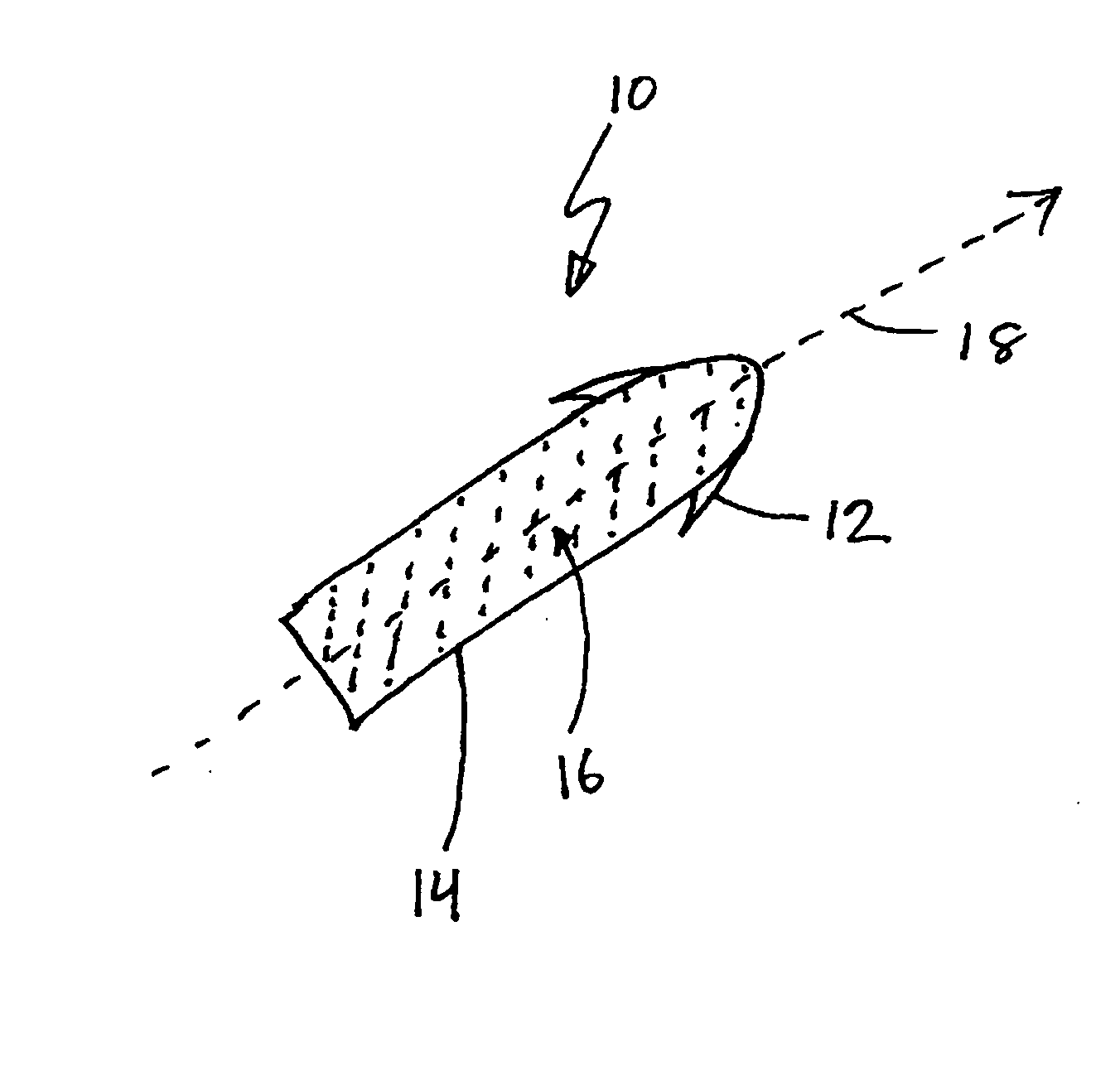
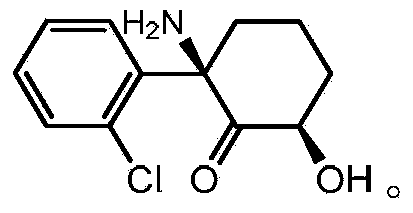
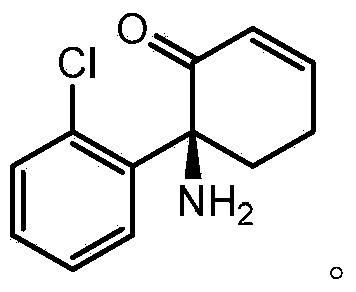
Chronic pain. Chronic pain was originally defined as pain that has lasted 6 months or longer. It is now defined as pain that persists longer than the normal course of time associated with a particular type of injury. Chronic pain is essentially caused by the bombardment of the central nervous system (CNS) with nociceptive impulses,...
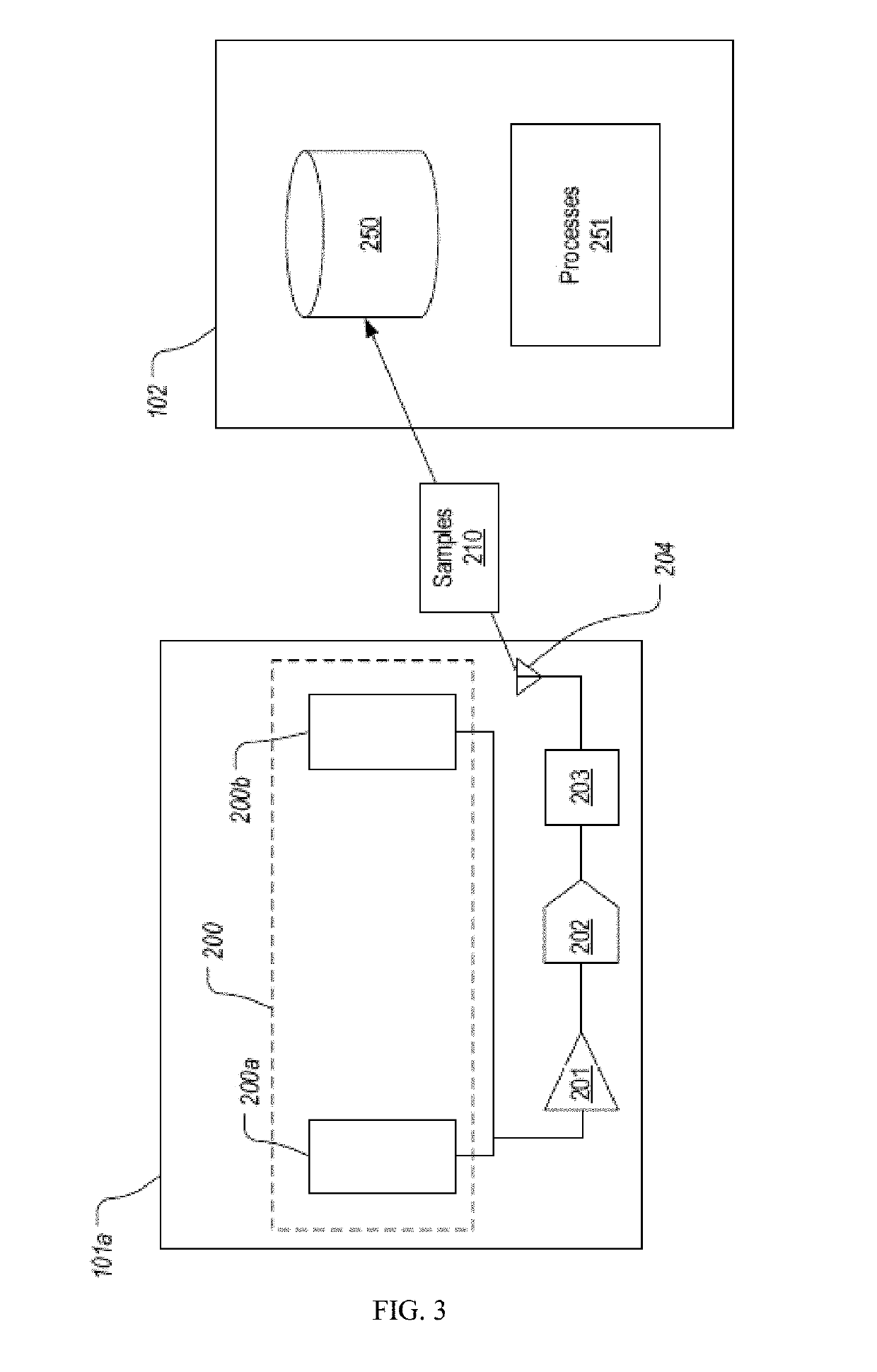
Drug depot implant designs and methods of implantation
ActiveUS20070243228A1Uniform drug distributionMinimal disruptionBiocidePeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
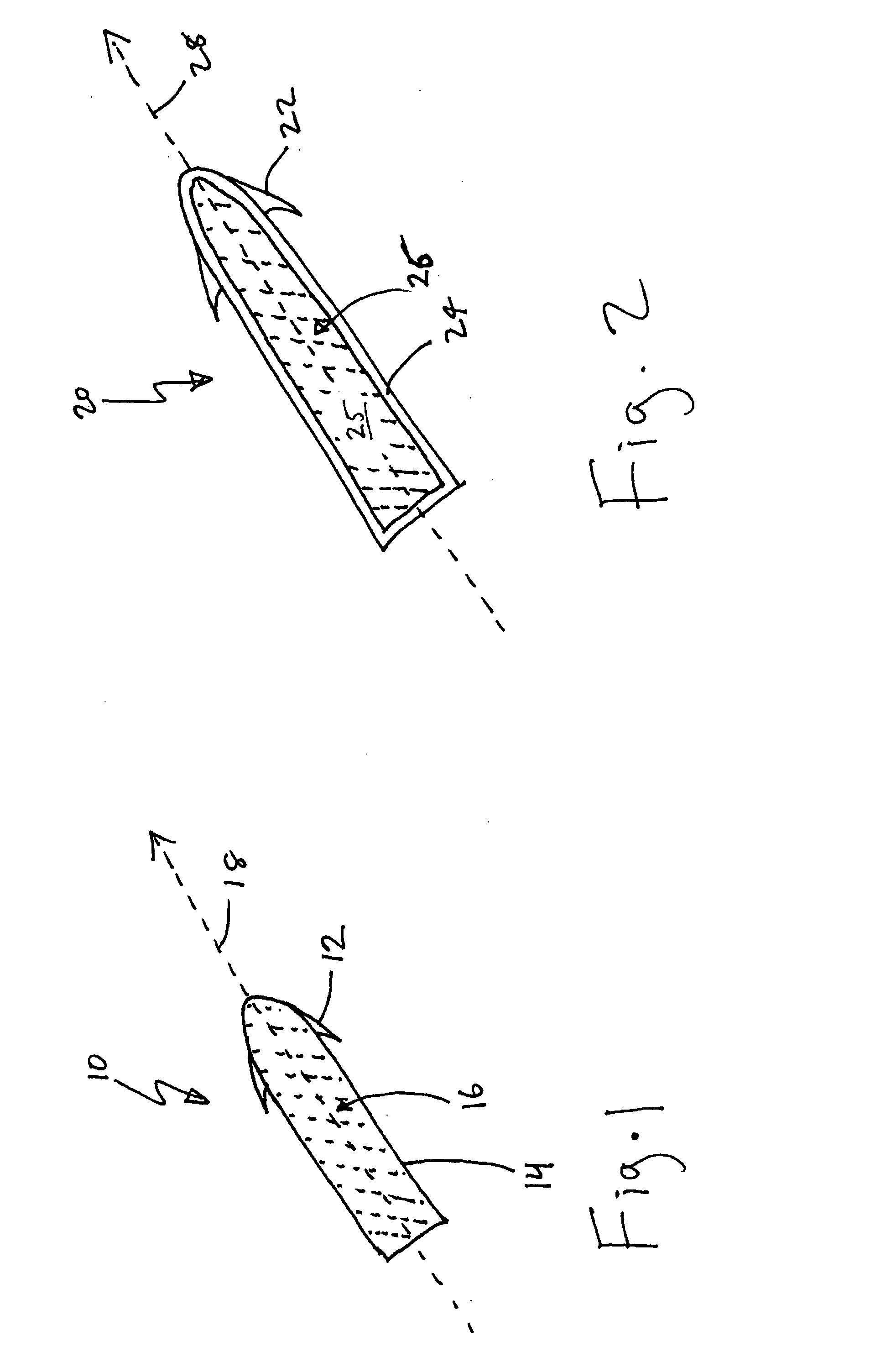
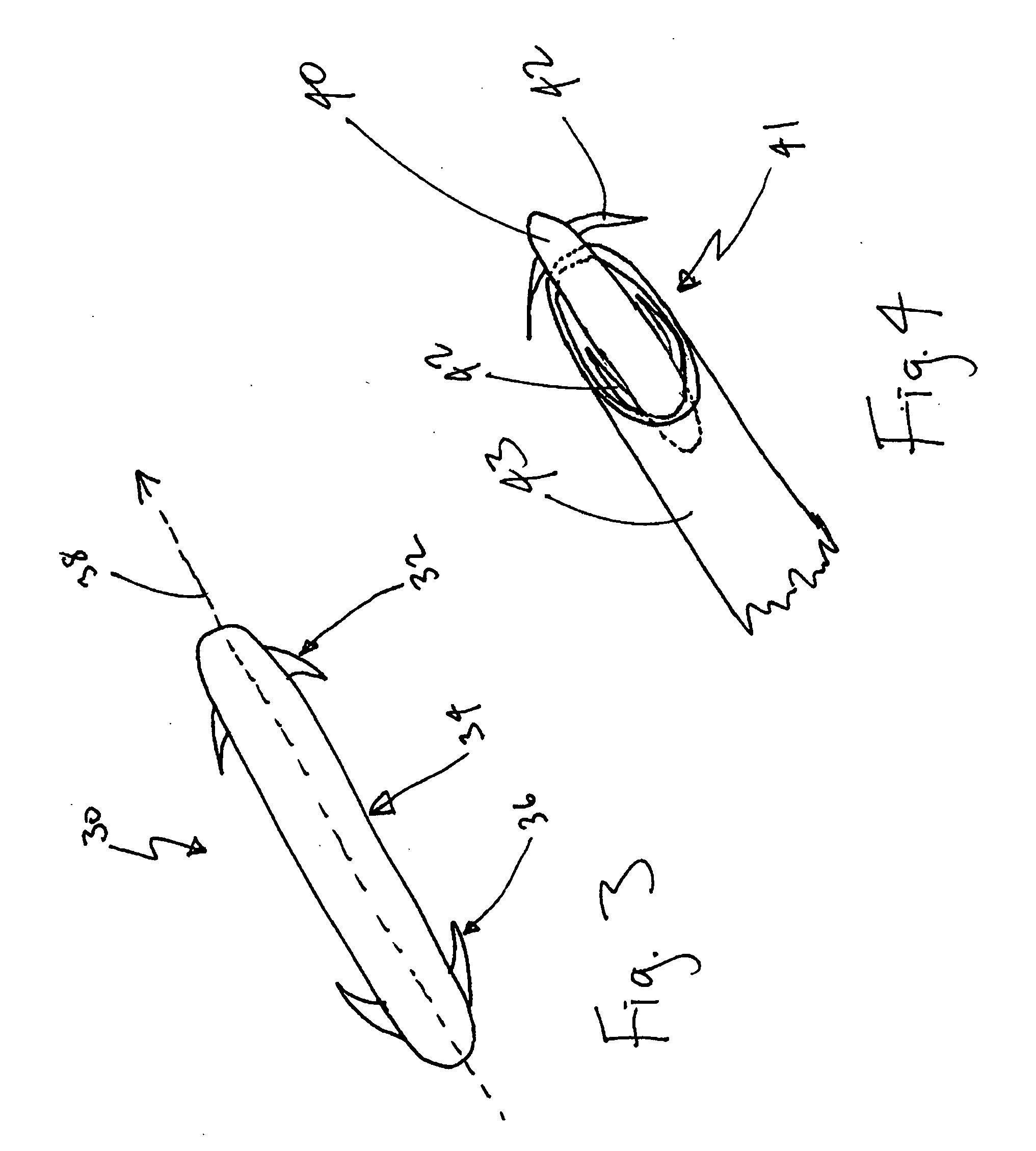
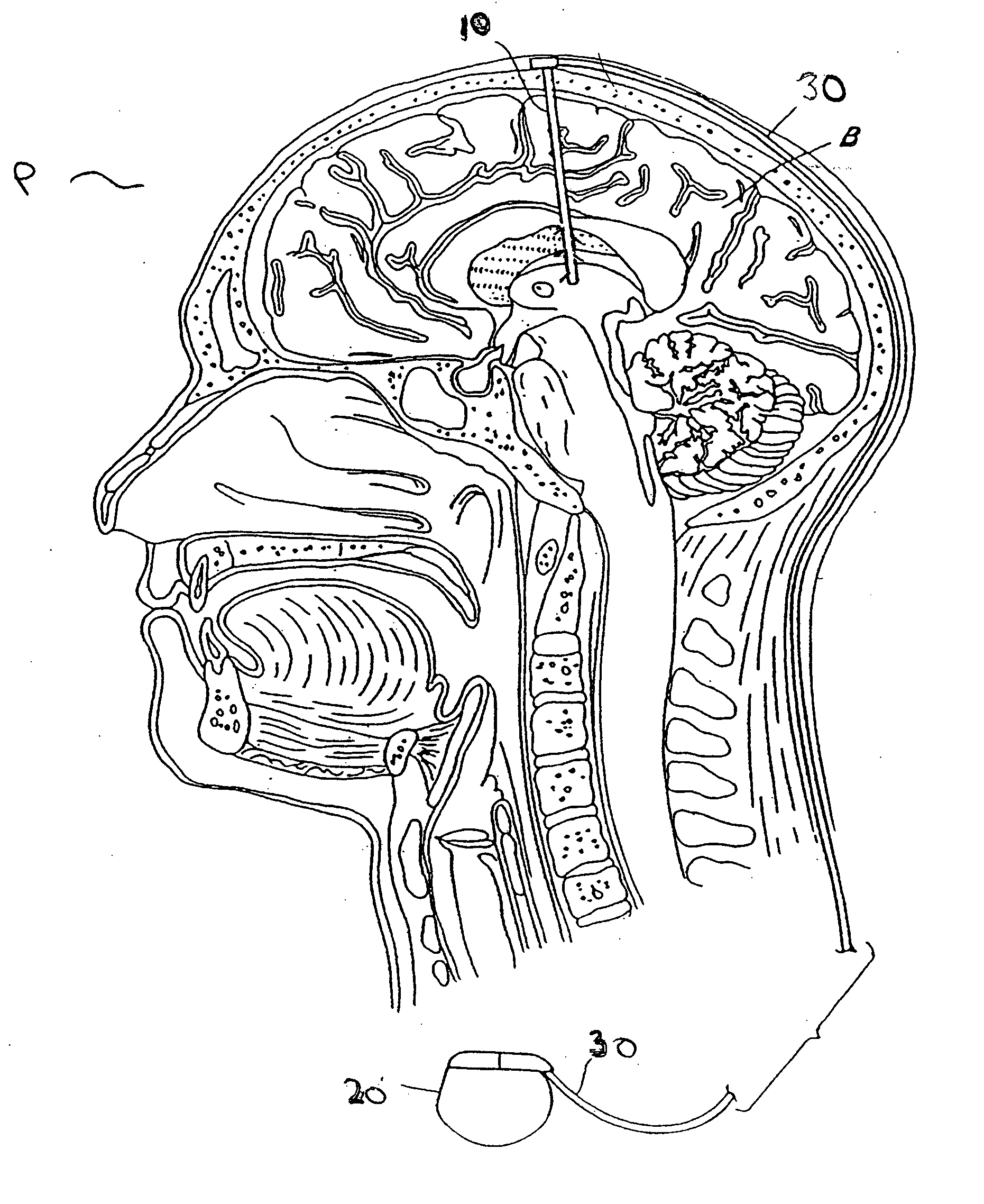
Modulation of the pain circuitry to affect chronic pain
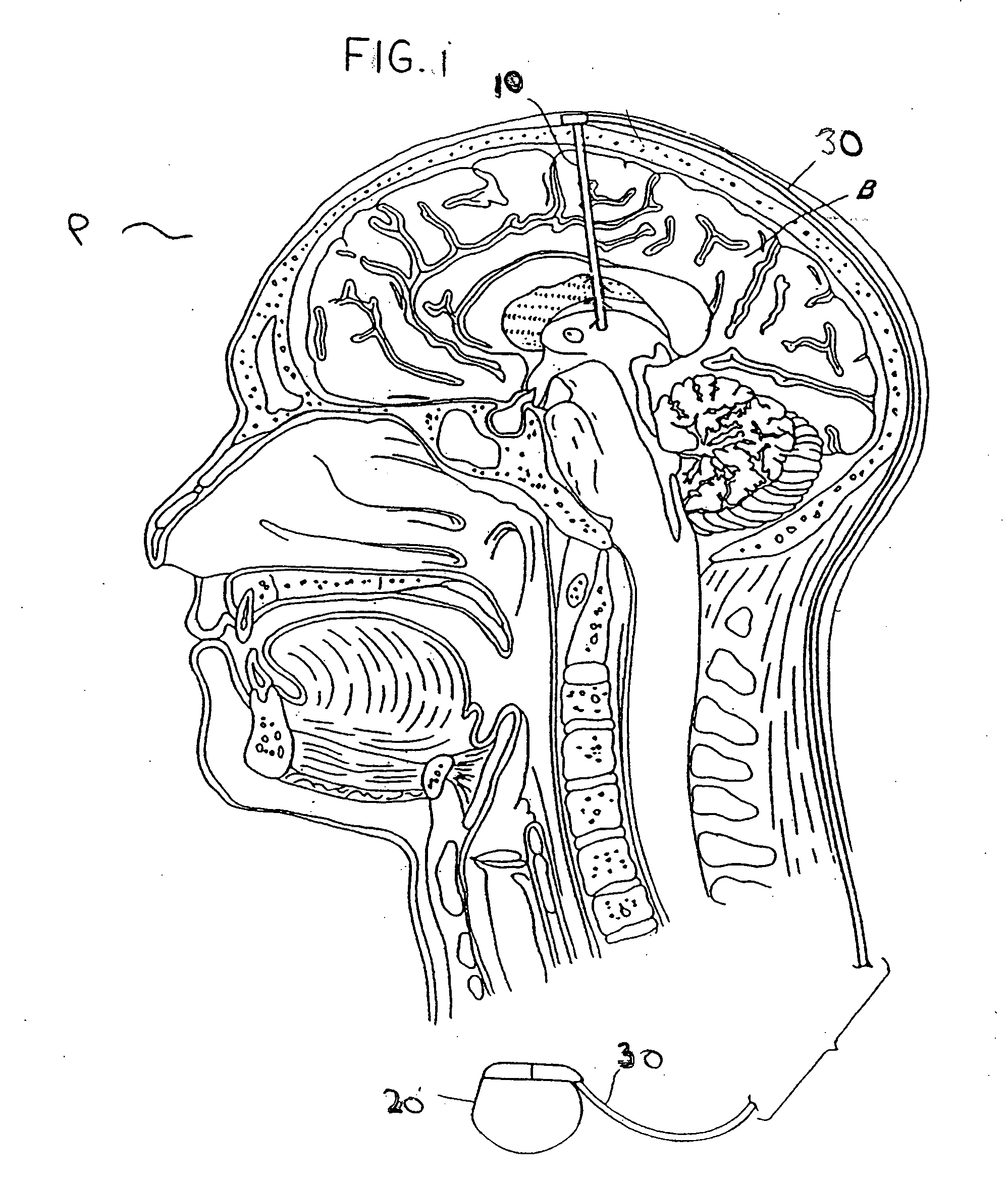
The present invention relates to methods of affecting chronic pain by electrically and / or chemically stimulating target sites of the pain circuitry associated with chronic pain. Such target sites include cerebral target sites, including limbic structures, associated with the emotional and suffering components of chronic pain, as well as deep brain target sites associated with the affective and sensory components of chronic pain. Also provided is a method of affecting chronic pain by stimulating a target site of the pain circuitry associated with chronic pain to stimulate the synthesis or release of endogenous opioids.
Owner:THE CLEVELAND CLINIC FOUND
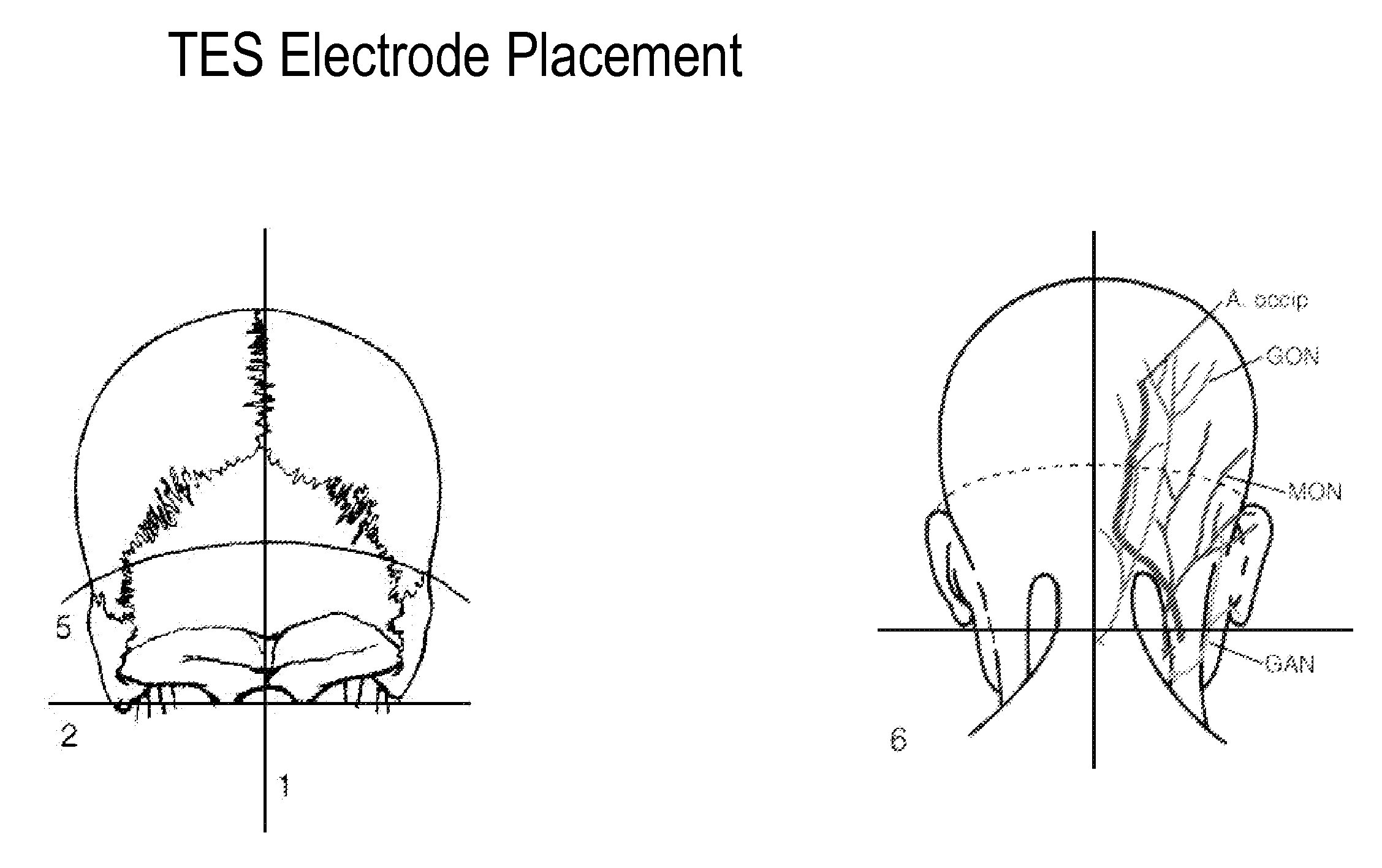
Eliciting analgesia by transcranial electrical stimulation
ActiveUS20110093033A1Promote healingFunction increaseInternal electrodesExternal electrodesTranscranial Electrical StimulationsAbsolute deviation
A method of eliciting analgesia in a human subject by Transcranial Electrical Stimulation (TCES, herein “TES”) is provided. Electrodes secured to the skin of the subject's head at particular sites provide an electrical current that includes a direct current combined with rectangular AC current pulses delivered at a particular frequency of between 10 and 100 Hz. In an embodiment the total current transmitted, a sum of the DC component and a Mean Absolute Deviation (MAD) of the current pulses, has a value between 0.2 and 20 mA. The method is used to produce analgesia during perioperative period, surgery and the post-operative procedure. It can also be used for treating acute chronic pain and a wide variety of other conditions.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
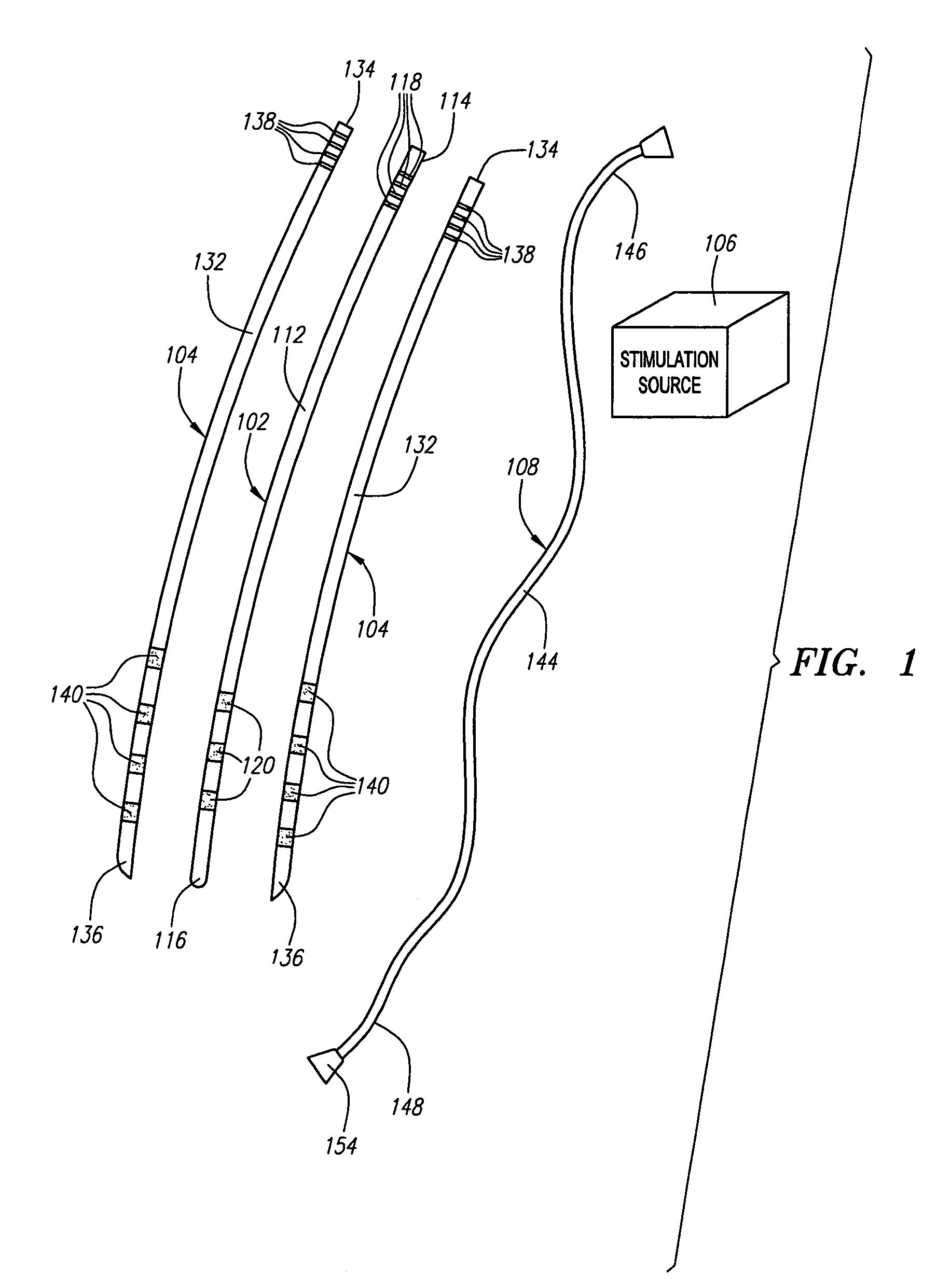
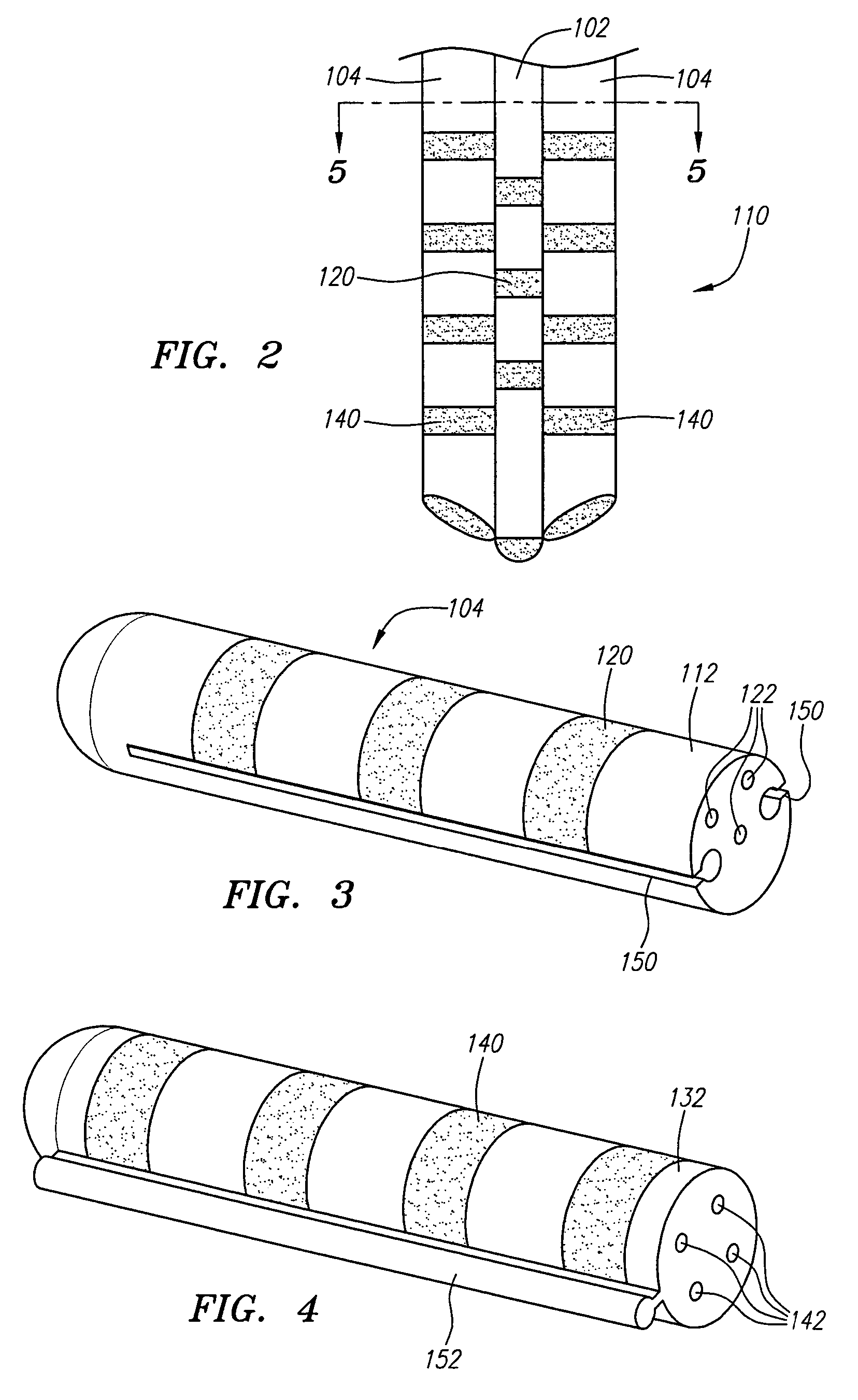
Modular stimulation lead network
InactiveUS7590454B2More stabilityMinimize damageSpinal electrodesExternal electrodesPhysical medicine and rehabilitationPhysical therapy
A medical kit and method for treating an ailment, such as chronic pain is provided. The kit comprises first and second medical leads, e.g., stimulation leads. Each lead comprises an elongated body and at least one operative element. The first medical lead comprises a coupling mechanism, such as a slot, and the second medical lead comprises a complementary mechanism, such as a rail, that slidably engages the coupling mechanism of the first medical lead. The method may comprise delivering the first medical lead into a patient's body, e.g., into the epidural space of the patient, and delivering the second medical lead into the patient's body by sliding the complementary coupling mechanism of the second medical lead along the coupling mechanism of the first medical lead.
Owner:BOSTON SCI SCIMED INC
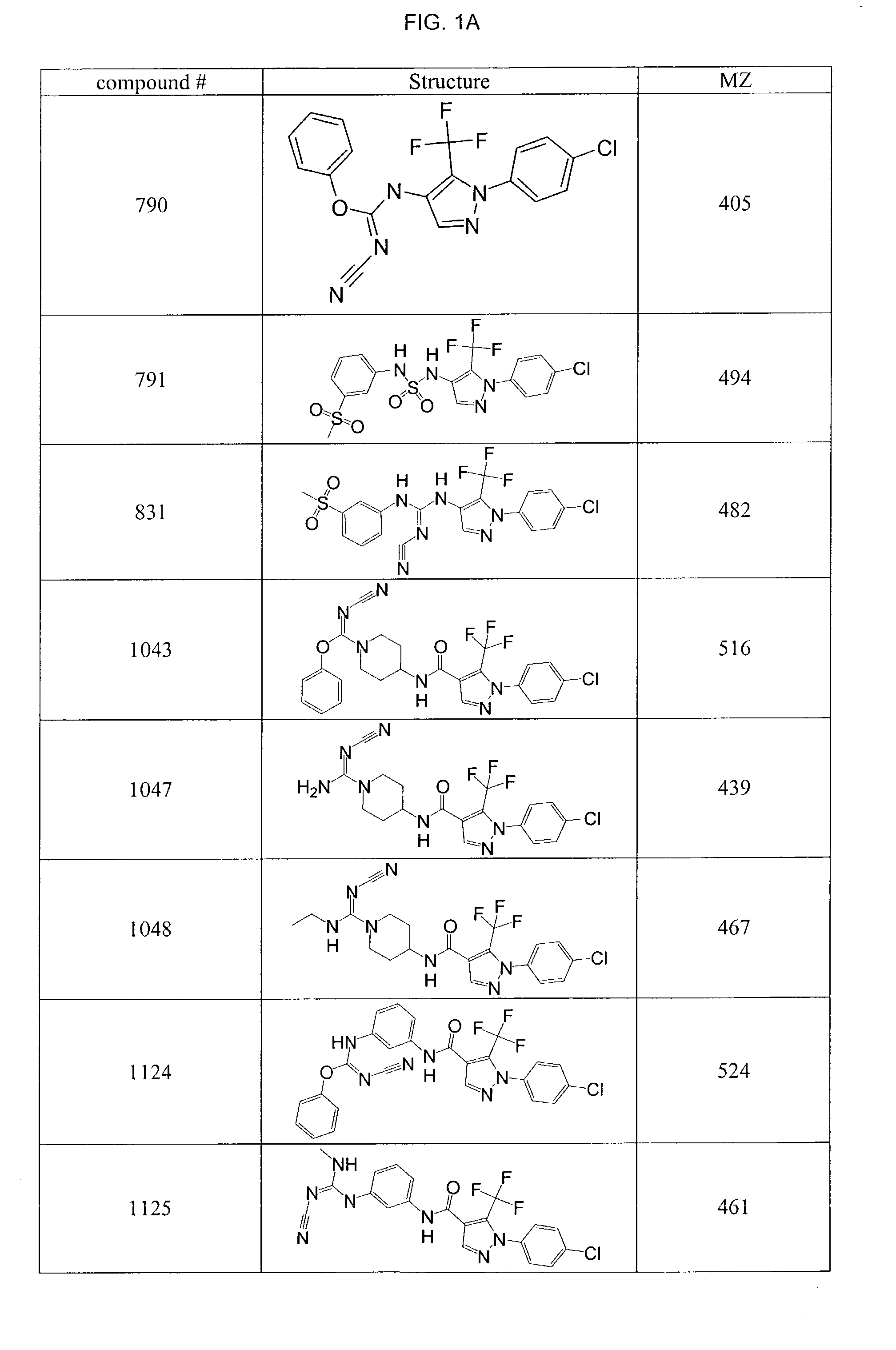
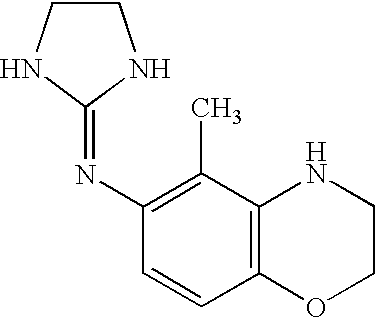
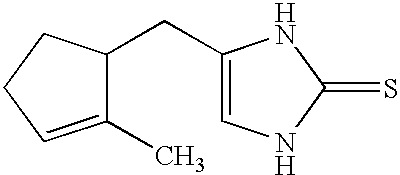
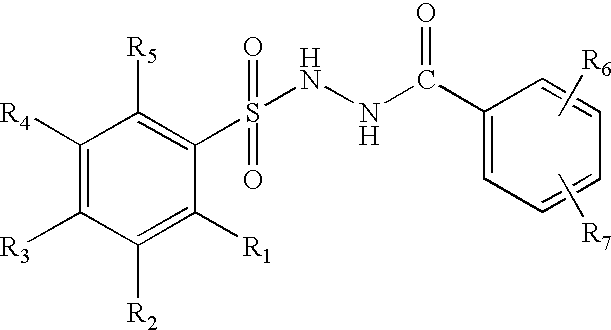
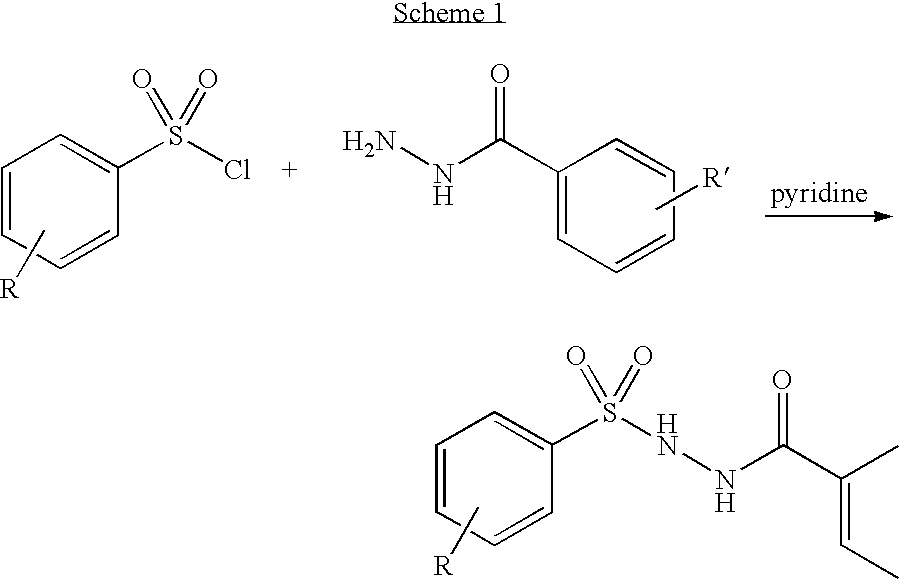
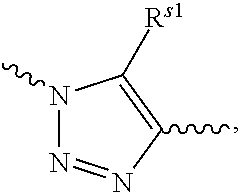
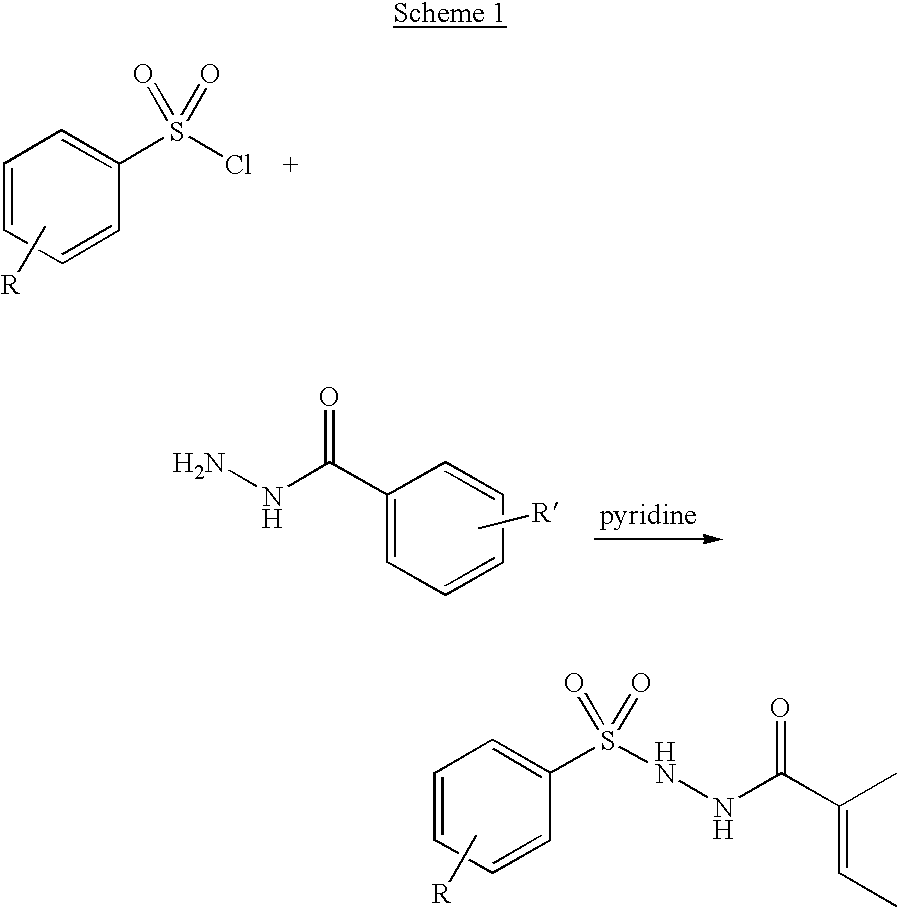
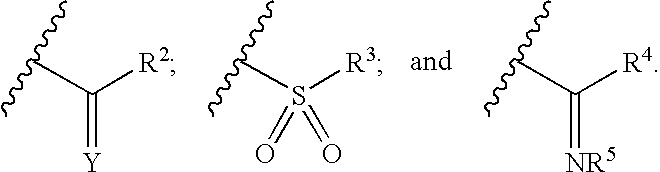
Pyrazole-amides and -sulfonamides
Owner:ICAGEN INC
The use of (2r, 6r)-hydroxynorketamine, (s)-dehydronorketamine and other stereoisomeric dehydro and hydroxylated metabolites of (r,s)- ketamine in the treatment of depression and neuropathic pain
InactiveCN104395283AInhibitory concentrationNervous disorderOrganic compound preparationMetaboliteRegional pain
Owner:UNITED STATES OF AMERICA +2
Device and system for monitoring and treating muscle tension-related medical conditions
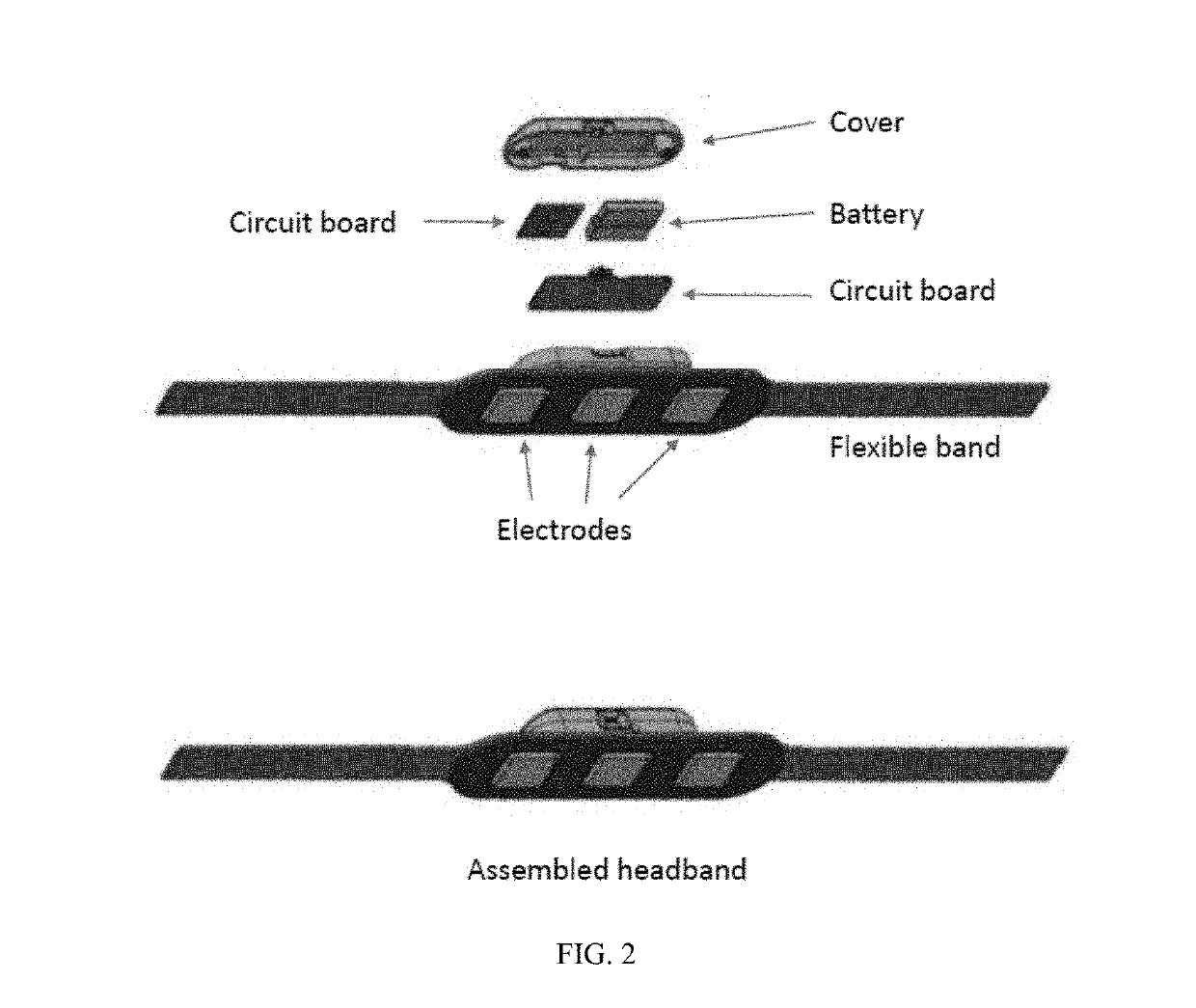
InactiveUS20190313934A1Relieve muscle tensionAvoid seizuresElectroencephalographyMedical communicationCognitive behavioral therapyChronic pain
Mobile systems and devices can employ surface electromyography (sEMG) technology and other sensing technologies for measuring muscle tension and managing chronic pain conditions. These systems and devices can sense and quantify excessive muscle tension and can facilitate management of excessive muscle tension and chronic pain through cognitive behavioral therapy, pharmacologic intervention, or a combination of both.
Owner:BIOTRAK HEALTH INC
Novel methods and compositions for alleviating pain
The present invention provides a method for the long-term relief of chronic pain in a subject by activating in the subject an analgesic alpha-adrenergic receptor in the absence of alpha-2A receptor activation over a period of at least three days, such that relief of chronic pain is maintained in the absence of continued activation of said receptor. The analgesic alpha-adrenergic receptor can be, for example, the alpha-2B receptor.
Owner:ALLERGAN INC
Injectable compositions and process of preparation thereof
Novel and highly stable injectable pharmaceutical compositions comprising at least one cyclooxygenase-II enzyme (COX-II) inhibitor or non-steroidal anti-inflammatory drug (NSAID) or COX / LOX inhibitor, or its tautomeric forms, analogues, isomers, polymorphs, solvates, prodrugs or salts thereof as active ingredient suitable for parenteral administration preferably by intramuscular (IM) or intravenous (IV) route; process of preparing such compositions and therapeutic methods of using such compositions are provided. The analgesic and anti-inflammatory injectable compositions of the present invention are very useful in mammals particularly in humans for the treatment of acute painful conditions like one or more of post-operative trauma, pain associated with cancer, sports injuries, migraine headache, neurological pain and pain associated with sciatica and spondylitis, and the like, and / or chronic painful conditions, and / or a variety of painful and inflammatory conditions like postoperative pain, primary dysmenorrhea and painful osteoarthritis, and / or other associated disorders such as inflammation, fever, allergy, or the like.
Owner:PANACEA BIOTEC
Branched chain amino acid-dependent aminotransferase inhibitors and their use in the treatment of neurodegenerative diseases
The invention relates to BCAT inhibitors and the use thereof for treating or preventing neuronal loss associated with stroke, ischemia, CNS trauma, hypoglycemia and surgery, as well as treating neurodegenerative diseases including Alzheimer's disease, amyotrophic lateral sclerosis, Huntington's disease and Down's syndrome, treating or preventing the adverse consequences of the overstimulation of the excitatory amino acids, treating anxiety, psychosis, convulsions, aminoglycoside antibiotics-induced hearing loss, migraine headache, chronic pain, neuropathic pain, Parkinson's disease, diabetic retinopathy, glaucoma, CMV retinitis, urinary incontinence, opioid tolerance or withdrawal, and inducing anesthesia, as well as for enhancing cognition.
Owner:WARNER LAMBERT CO LLC
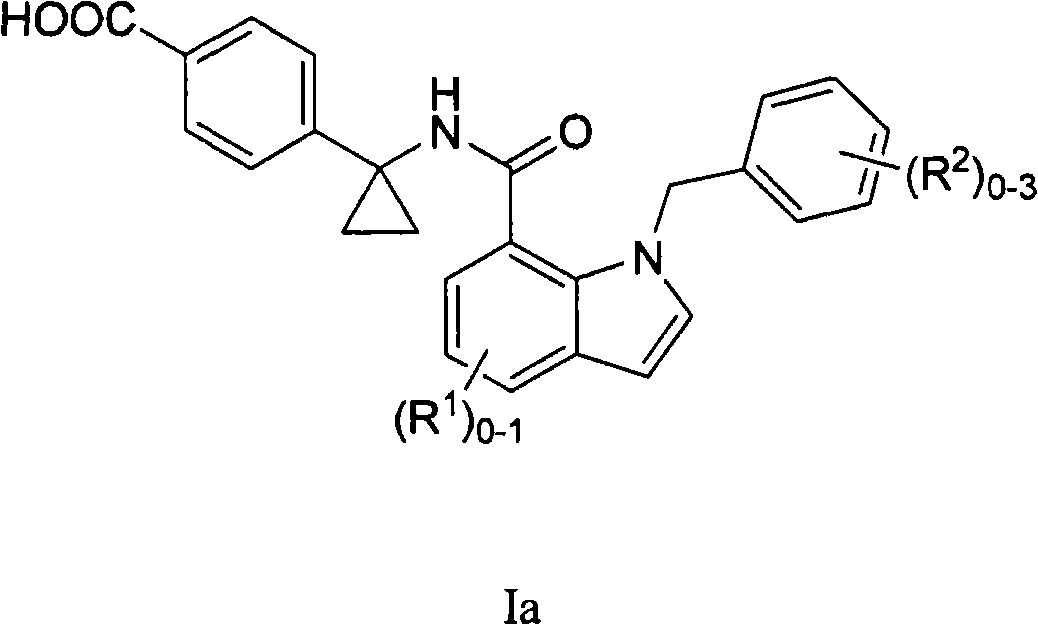
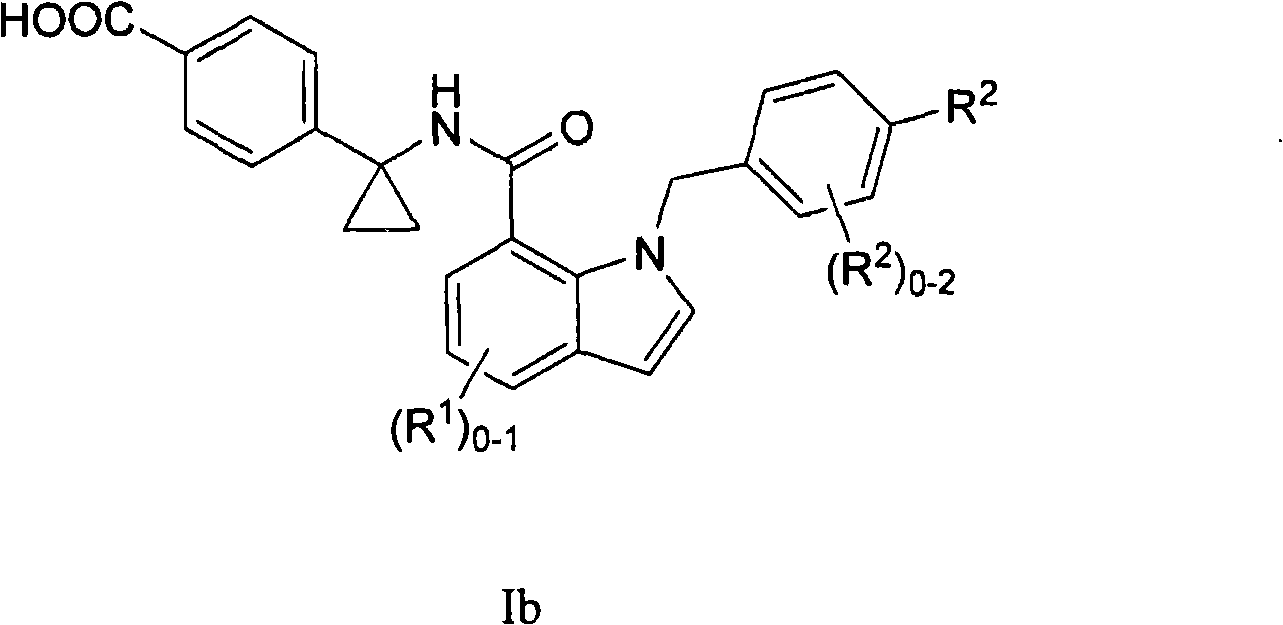
Indole and indoline cyclopropyl amide derivatives as ep4 receptor antagonists
The invention is directed to indole and indoline cyclopropyl amide derivatives as EP4 receptor antagonists useful for the treatment of EP4 mediated diseases or conditions, such as acute and chronic pain, osteoarthritis, rheumatoid arthritis and cancer. Pharmaceutical compositions and methods of use are also included.
Owner:MERCK CANADA
Conjugates of galactose-binding lectins and clostridial neurotoxins as analgesics
InactiveUS20060121056A1Pain reliefReduce and preferably prevent transmissionNervous disorderPeptide/protein ingredientsGalactose binding lectinAnalgesic agents
Owner:HEALTH PROTECTION AGENCY
Conjugate of polyethylene glycol and anesthetic, and preparation method thereof
ActiveUS10525143B2High drug loadingGood sustained releasePowder deliveryAntipyreticAnalgesia postoperativePolyethylene glycol
A conjugate represented by general formula (I), wherein R0 is a C1-6 alkyl, B is an anesthetic, and A is a linking group, and a quaternary ammonium salt is formed at the linking position between B and R0. The conjugate has a prolonged analgesic effect, and can be used in postoperative analgesia or treatment for chronic pain.
Owner:JENKEM TECH CO LTD TIANJIN
The use of (2r, 6r)-hydroxynorketamine, (s)-dehydronorketamine and other stereoisomeric dehydro and hydroxylated metabolites of (r,s)- ketamine in the treatment of depression and neuropathic pain
The disclosure provides pharmaceutical preparations containing (2R,6R)-hydroxynorketamine, or (R)- or (S)-dehydronorketamine, or other stereoisomeric dehydro or hydroxylated ketamine metabolite. (2R,6R)-hydroxynorketamine The disclosure also provides novel ketamine metabolite prodrugs. The disclosure provides methods of treating, bipolar depression, major depressive disorder, neuropathic and chronic pain, including complex regional pain disorder (CRPS) by administering a purified ketamine metabolite or a ketamine metabolite prodrug directly to patients in need of such treatment.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +2
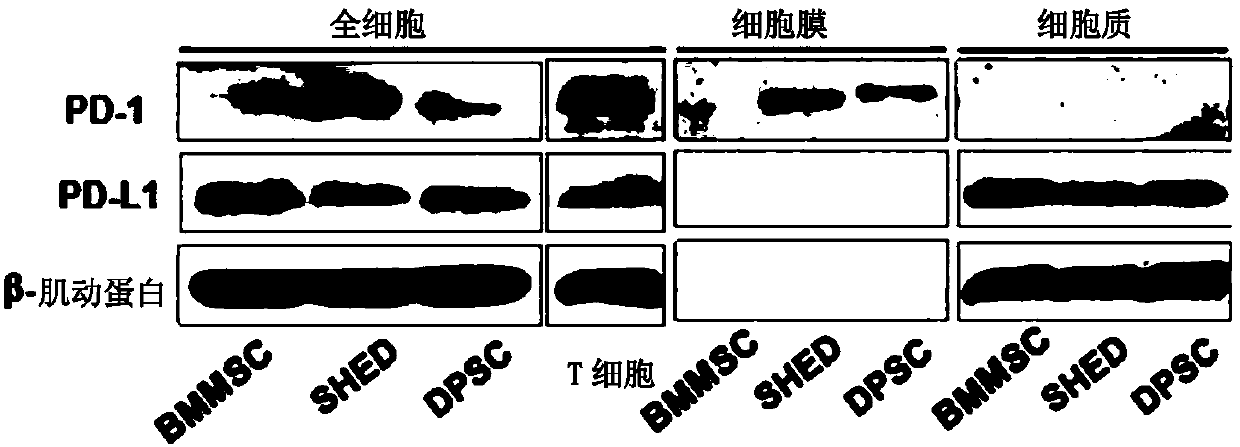
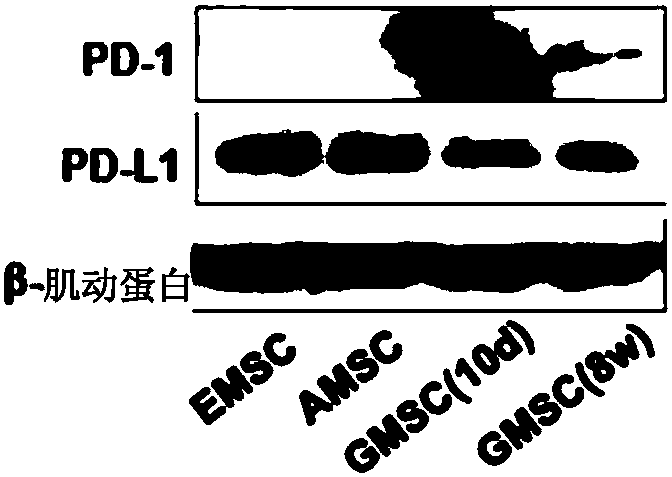
Stem cells for specifically expressing PD-1 as well as identifying and separating method and application of stem cells
The invention discloses stem cells for specifically expressing a programmed death receptor 1 (PD-1), and particularly relates to the stem cells from an organic cavity. The stem cells comprise but notlimited to dental pulp stem cells, gingival mesenchymal steam cells (GMSCs), periodontal ligament stem cells (PDLSCs), stem cells of apical papilla (SCAPs) and dental follicle stem cells (DFSCs) or any combination thereof, and preferably comprise stem cells from human exfoliated deciduous teeth (SHED) and / or dental pulp mesenchymal stem cells for permanent teeth (DPSC); the stem cells also includemesenchymal stem cells from other tissues, which do not express the PD-1 at first and express the PD-1 after being modified by CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), suchas PD-1 plus bone mesenchymal stem cells (BMMSC) modified by the CRISPR. The invention further discloses an identifying and separating method for the stem cells for specifically expressing the PD-1,a method for preparing the PD-1 plus mesenchymal stem cells modified by the CRISPR as well as application of the mesenchymal stem cells for expressing the PD-1, disclosed by the invention, in tissue regeneration, pain relief and treatment of chronic pain and a series of diseases.
Owner:北京泰盛生物科技有限公司
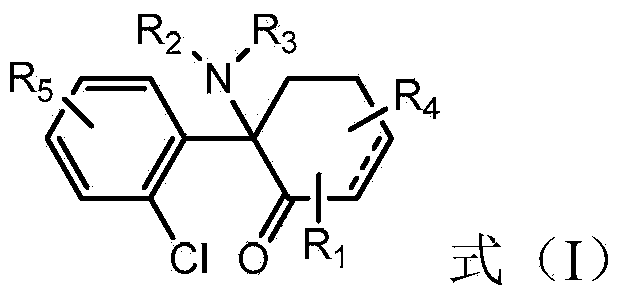
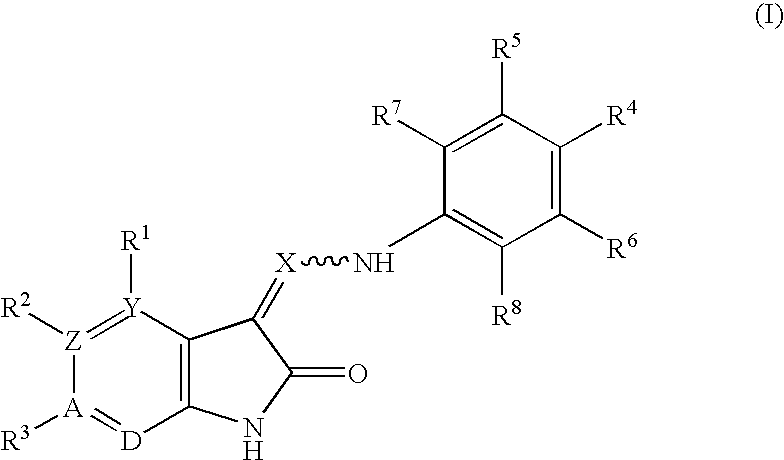
Oxindole derivatives
The present invention is related to oxindole derivatives of structure (I), compositions containing the same, and methods of use and manufacture of the same. Such compounds generally are useful pharmacologically as agents in those disease states alleviated by the alteration of mitogen activated signaling pathways in general, and in particular in the inhibition or antagonism of protein kinases, which pathologically involve aberrant cellular proliferation. Such disease states include tumor growth, restenosis, atherosclerosis, pain and thrombosis. In particular, the present invention relates to a series of substituted oxindole compounds, which exhibit Trk family protein tyrosine kinase inhibition, and which are useful in cancer therapy and chronic pain indications.
Owner:SMITHKLINE BECKMAN CORP
Analgesic tramadol hydrochloride oral disintegrating tablet and preparing method thereof
InactiveCN1528273AEasy to takeReduce adverse reactionsOrganic active ingredientsAntipyreticFreeze-dryingTramadol Hydrochloride
The present invention relates to an analgesic tramadol hydrochloride oral disintegrant tablet. It is made up by using main medicine tramadol hydrochloride and auxiliary material gelatin and / or mannitol and / or aspartame and / or glucose and / or microcrystal cellulose and / or crosslinked povidone and essence, etc. and adopting freeze-drying or coating, granulating and pressing processes. When it is taken in, it has no need of drinking water, it can be quickly disintegrated in oral cavity, and can be used for curing acute and chronic pain, moderate and light pain due to cancer, pain due to fracture and pain after various operations and toothache, and can obtain good therapeutic effect.
Owner:周宇
Branched chain amino acid-dependent aminotransferase inhibitors and their use in the treatment of neurodegenerative diseases
The invention relates to BCAT inhibitors and the use thereof for treating or preventing neuronal loss associated with stroke, ischemia, CNS trauma, hypoglycemia and surgery, as well as treating neurodegenerative diseases including Alzheimer's disease, amyotrophic lateral sclerosis, Huntington's disease and Down's syndrome, treating or preventing the adverse consequences of the overstimulation of the excitatory amino acids, treating anxiety, psychosis, convulsions, aminoglycoside antibiotics-induced hearing loss, migraine headache, chronic pain, neuropathic pain, Parkinson's disease, diabetic retinopathy, glaucoma, CMV retinitis, urinary incontinence, opioid tolerance or withdrawal, and inducing anesthesia, as well as for enhancing cognition.
Owner:WARNER-LAMBERT CO
Substituted oxindole derivatives as tyrosine kinase inhibitors
The present invention is related to oxindole derivatives of structure (I), compositions containing the same, and methods of use and manufacture of the same. Such compounds generally are useful pharmacologically as agents in those disease states alleviated by the alteration of mitogen activated signaling pathways in general, and in particular in the inhibition or antagonism of protein kinases, which pathologically involve aberrant cellular proliferation. Such disease states include tumor growth, restenosis, atherosclerosis, pain and thrombosis, In particular, the present invention relates to a series of substituted oxindole compounds, which exhibit Trk family protein tyrosine kinase inhibition, and which are useful in cancer therapy and chronic pain indications.
Owner:SMITHKLINE BECKMAN CORP
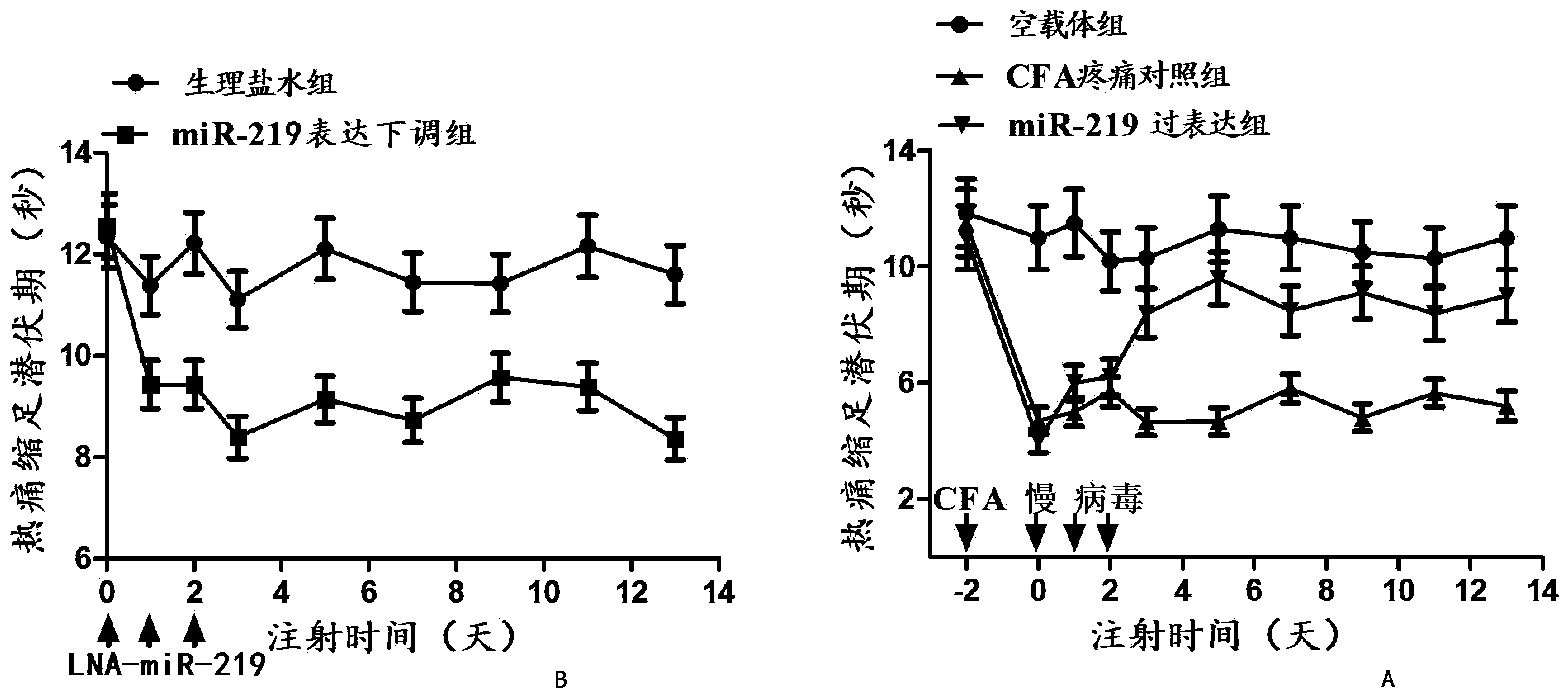
Application of miRNA-219 (micro-ribonucleic acid) compound to preparation of marker for diagnosing chronic pain and medicament for treating chronic pain
The invention discloses an application of a miRNA-219 (micro-ribonucleic acid) compound to preparation of a marker for clinically diagnosing chronic pain disease and a medicament for treating chronic pain. After screening a large number of experiments, an experimental result shows that expression of miRNA-219 in blood of a chronic pain patient is regulated down, generation and formation of the chronic pain can be obviously restrained by regulating the miRNA-219 up, and quantitative analysis is further carried out on a clinical blood specimen of the chronic pain patient to show that the miRNA-219 can be used as the marker of generation of the chronic pain.
Owner:XUZHOU MEDICAL COLLEGE
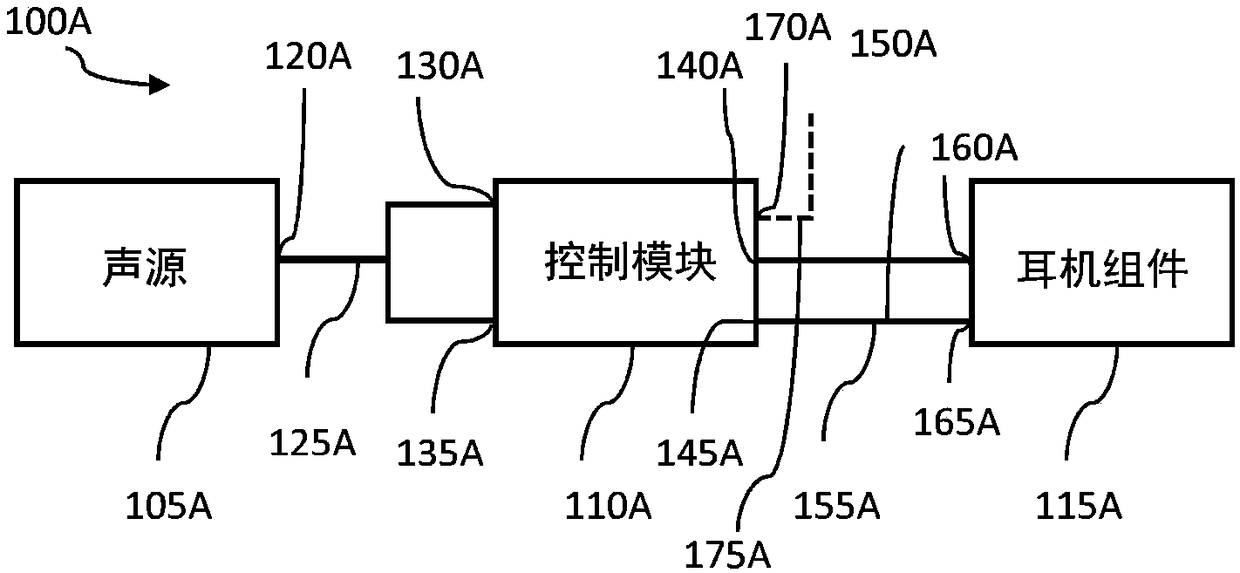
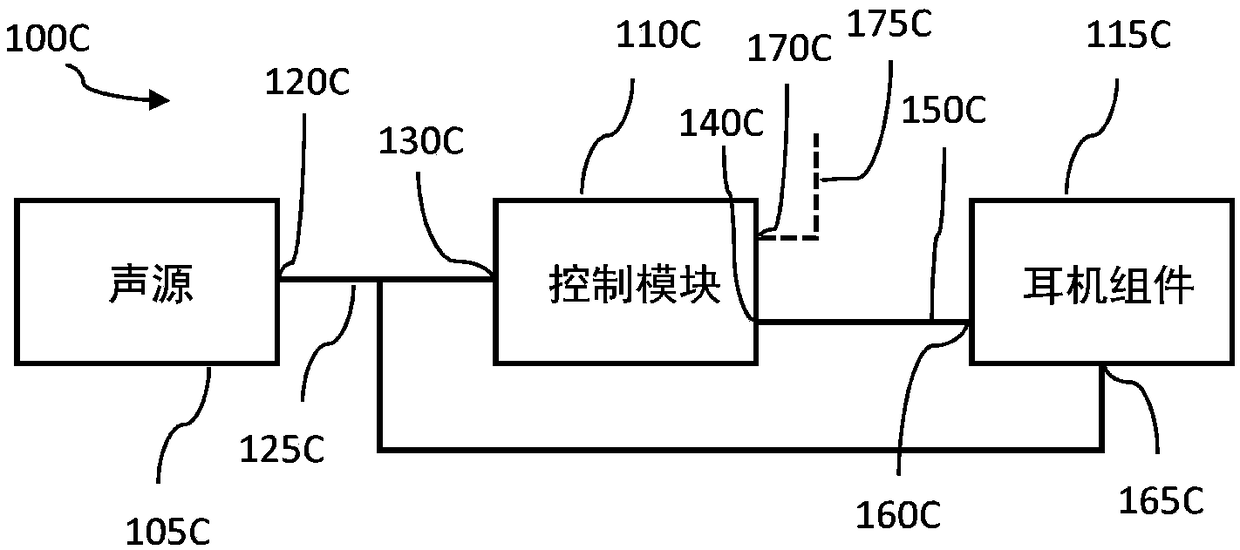
System and method for ear-arranged transcutaneous vagus nerve stimulation
InactiveCN109069297AConvenient treatmentImprove convenienceElectrotherapyMedical devicesSound sourcesEngineering
A transcutaneous nerve stimulation system includes a multifunctional earphone assembly that receives a sound signal from a sound source or other sources (such as a signal generator). An amplifier receiving the sound signal from the sound source or independent signal generator amplifies the sound signal to generate an amplified electrical signal. A stimulator, which includes multiple conductive electrode contacts, is coupled to the amplifier to receive the amplified electrical signal. The electrode contacts may protrude from an earbud / headphone or extend from an adjustable probe arm. The amplified electrical signal is used to apply electrical stimulation while the earphone assembly emits audible sounds according to the sound signal. The combined functionality enhances compliance with treatment regimens involving electrical stimulation of vagus nerves and other regions of a subject. Potential indications include, without limitation, major depressive disorder, epilepsy, chronic pain, pre-diabetes, insomnia, cardiovascular disorders, tinnitus, autism, daily stress, and anxiety.
Owner:THE GENERAL HOSPITAL CORP
Mu opioid receptor agonist analogs of the endomorphins
ActiveUS20120322740A1Improve solubilityBetter therapeutic ratioCompound screeningNervous disorderSide effectMorphine
The invention relates to cyclic peptide agonists that bind to the mu (morphine) opioid receptor and their use in the treatment of acute and / or chronic pain. Embodiments of the invention are directed to cyclic pentapeptide and hexapeptide analogs of endomorphin that have (i) a carboxy-terminal extension with an amidated hydrophilic amino acid and (ii) a substitution in amino acid position 2. These peptide analogs exhibit decreased tolerance relative to morphine, increased solubility compared to similar tetrapeptide analogs, while maintaining favorable or improved therapeutic ratios of analgesia to side effects.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND +1
Benzimidazole derivatives as selective blockers of persistent sodium current
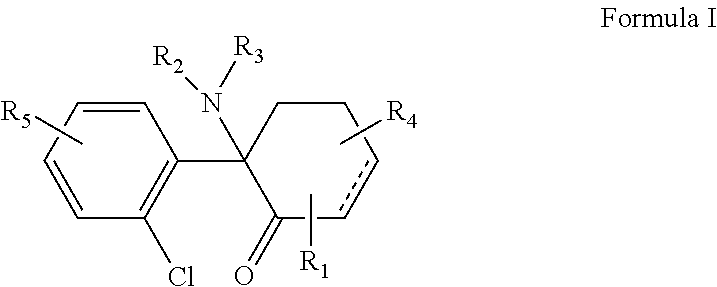
ActiveUS20130172389A1Reduce compoundingReduces capsaicin-induced thermal hyperalgesiaBiocideSenses disorderBenzimidazole derivativeDisease
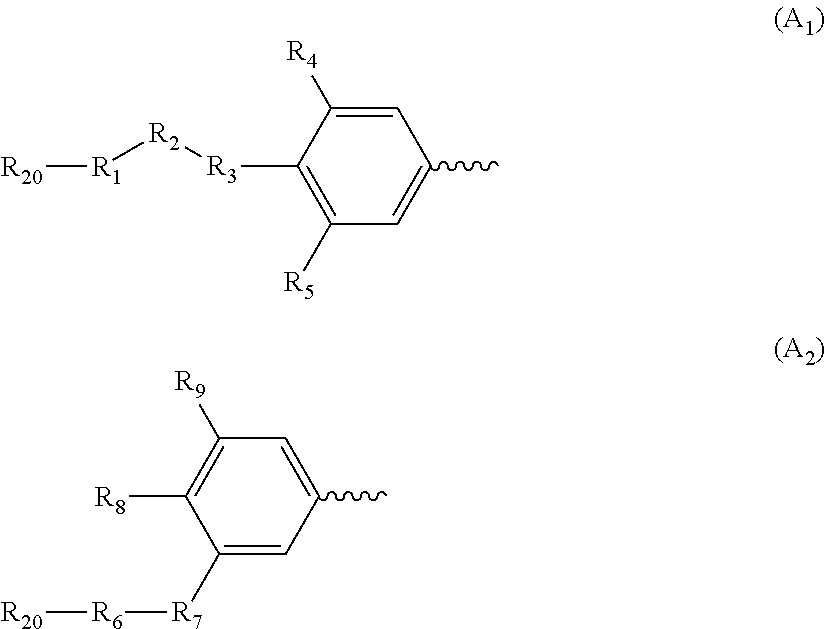
The present invention is directed to a compound of Formula Ior a pharmaceutically acceptable salt thereof; wherein R, R1, R2, R3, R4, m, and n are as defined herein, to pharmaceutical compositions comprising said compound, and to methods of treating diseases or conditions mediated by elevated persistent sodium current, such as an ocular disorder, multiple sclerosis, seizure disorder, and chronic pain.
Owner:ALLERGAN INC
Pharmaceutically acceptable salts of psilocin and uses thereof
ActiveUS11312684B1Sufficient amountImprove diseaseOrganic active ingredientsOrganic chemistryPsilocinDisease
The present invention composition features pharmaceutically acceptable salts of psilocin and compositions thereof. The pharmaceutically acceptable salts of psilocin may be used to treat a disease or condition, such as a neurological injury, an inflammatory condition, chronic pain, or a psychological condition, in a subject in need thereof.
Owner:ELEUSIS THERAPEUTICS US INC
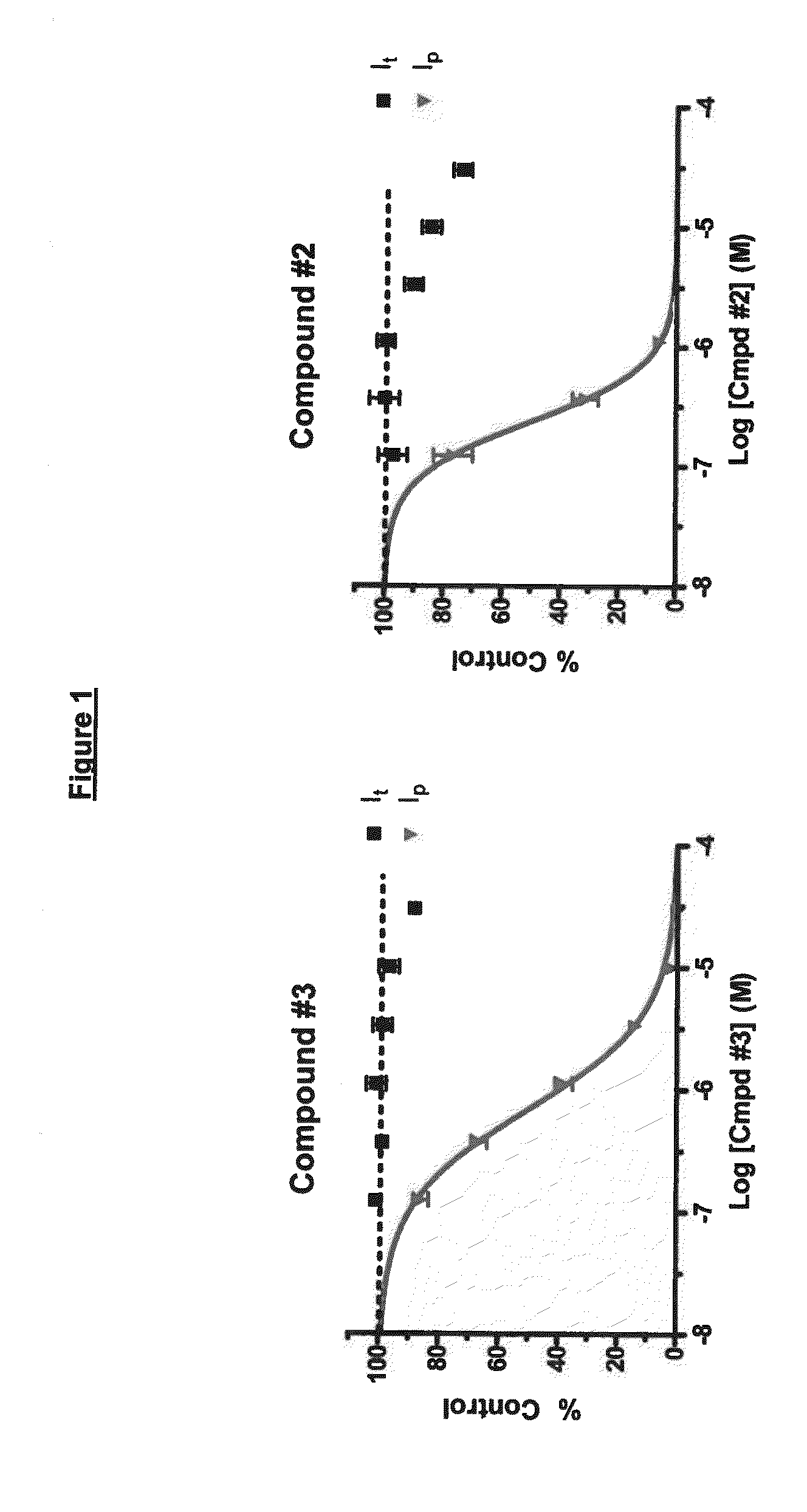
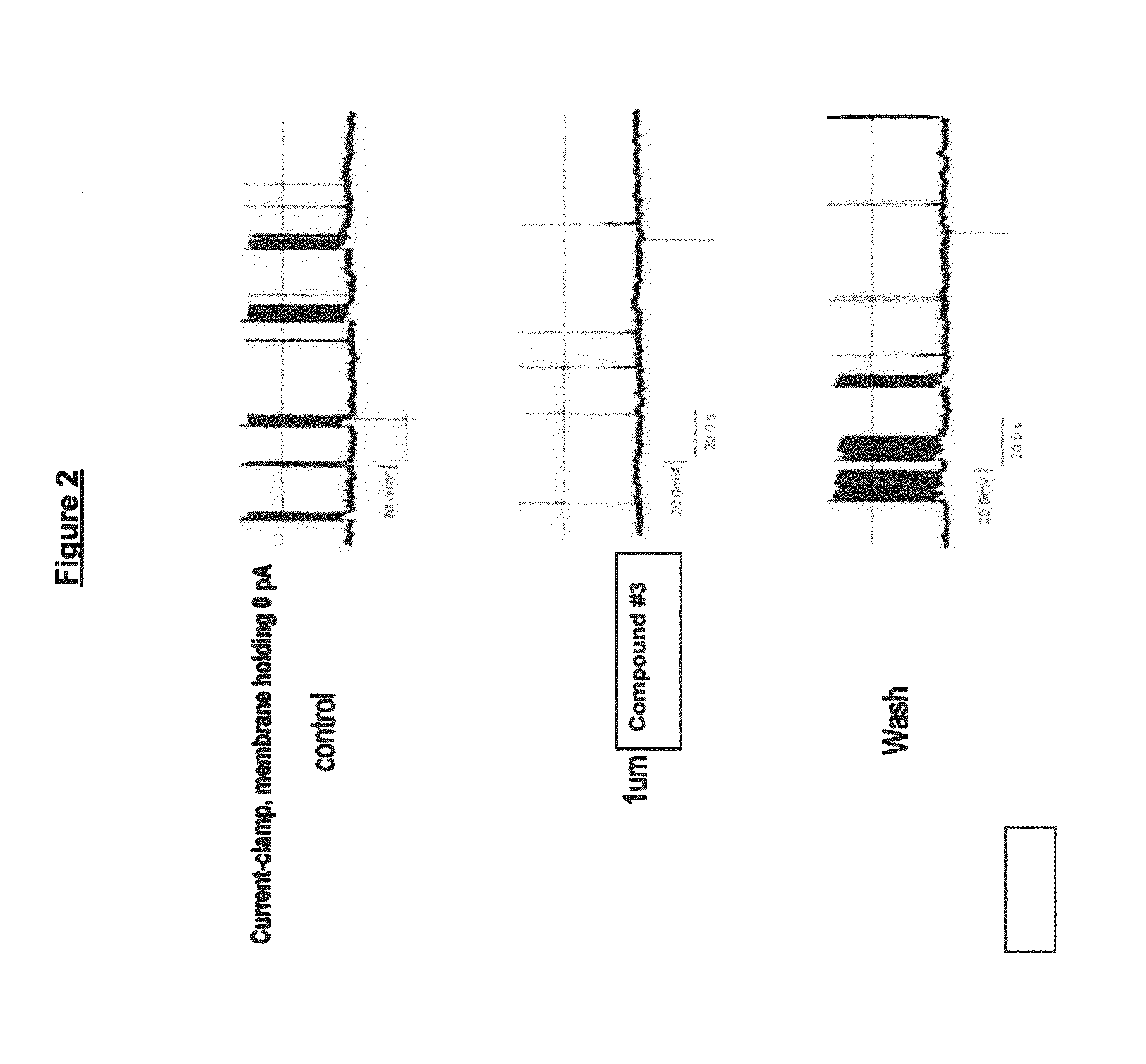
Methods for the treatment of chronic pain and compositions therefor
InactiveUS20070129366A1Inhibit cathepsin S gene expressionInhibit enzyme activityBiocideCompound screeningCathepsin S activityProteinase activity
The invention discloses cathepsin S as a suitable target for the development of new therapeutics to treat or ameliorate chronic pain. The invention relates to methods to treat and / or ameliorate chronic pain and pharmaceutical compositions therefor comprising modulators with inhibitory effect on cathepsin S enzyme activity and / or cathepsin S gene expression. The invention also relates to a method to identify compounds with therapeutic usefulness to treat chronic pain, comprising identifying compounds that can inhibit cathepsin S activity and / or gene expression which can also reverse the pathological effects of chronic pain in vivo.
Owner:BUXTON FRANCIS PAUL +3
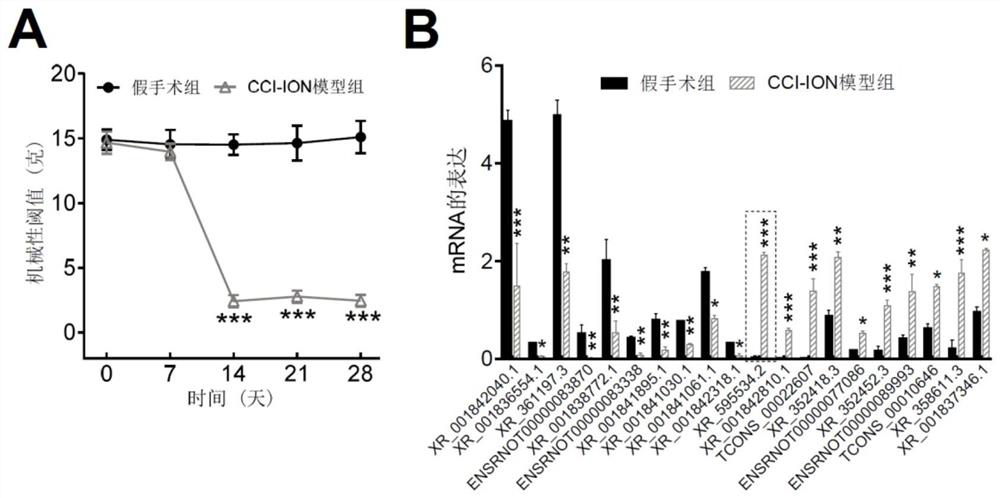
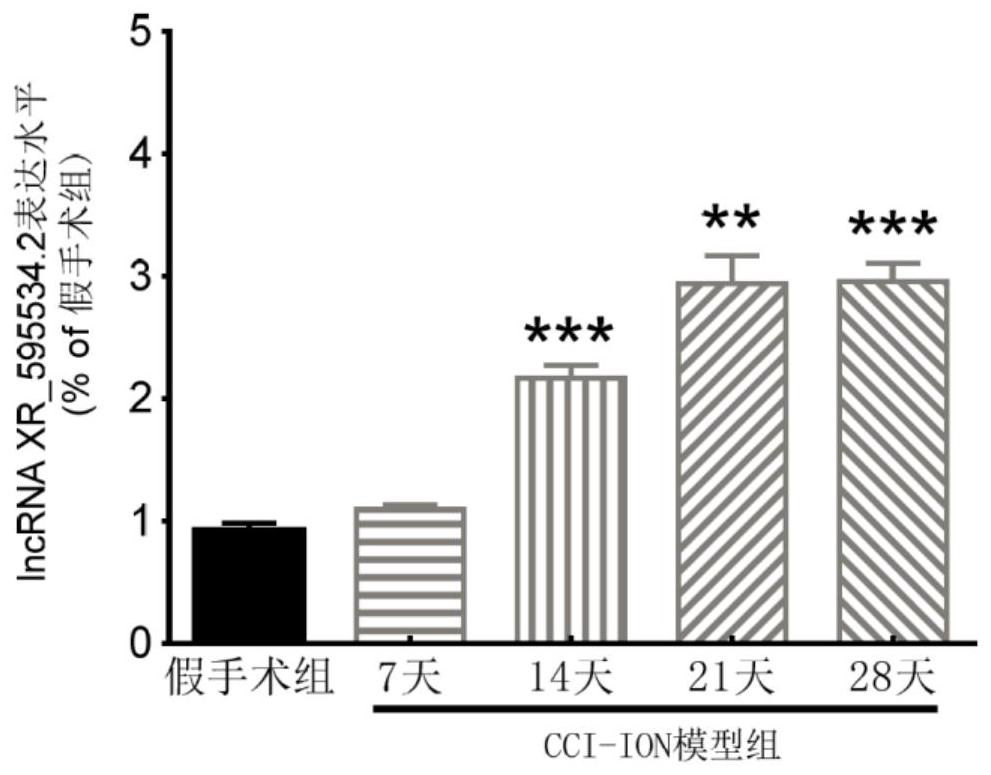
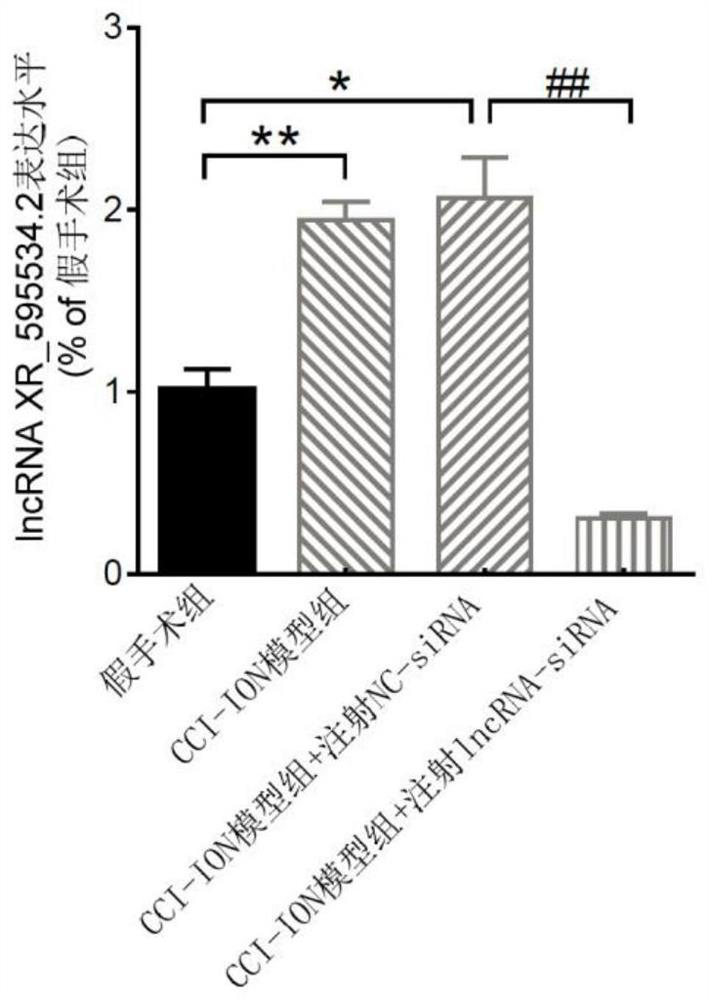
application of lncRNA XR595534.2 in preparation of medicine for treating or preventing chronic pain
ActiveCN112716971ABehavioral response to pain reliefOrganic active ingredientsNervous disorderDiseasePain behavior
The invention discloses an application of lncRNA (long non-coding ribonucleic acid) XR595534.2 in preparation of a medicine for treating or preventing chronic pain. According to the invention, trigeminal neuralgia induced by rat suborbital nerve chronic compression injury is taken as a pain model, a long-chain non-coding RNA gene lncRNA XR595534.2 with high specificity and difference expression in the model is obtained by screening, and interfering RNA for treating diseases taking lncRNA XR59534.2 as a target point is provided. The lncRNA XR595534.2 highly expressed in specificity and difference in a pain model is discovered for the first time, expression of the lncRNA XR59534.2 can be remarkably reduced by positioning injection of interfering RNA, pain behavior reaction is relieved, and the lncRNA XR595534.2 can be used for preparing medicines for preventing or treating prosopalgia, neuropathic pain, migraine and cancer pain.
Owner:SUZHOU UNIV
Piperidines
Compounds, compositions and methods are provided which are useful in the treatment of diseases through the inhibition of sodium ion flux through voltage-dependent sodium channels. More particularly, the invention provides substituted piperidines, and compositions containing these compounds. Also provided are methods using the compounds of the invention for the treatment of central or peripheral nervous system disorders, particularly pain and chronic pain by blocking sodium channels associated with the onset or recurrance of the indicated conditions. The compounds, compositions and methods of the present invention are of particular use for treating neuropathic or inflammatory pain by the inhibition of ion flux through a channel that includes a PN3 subunit.
Owner:ICAGEN INC
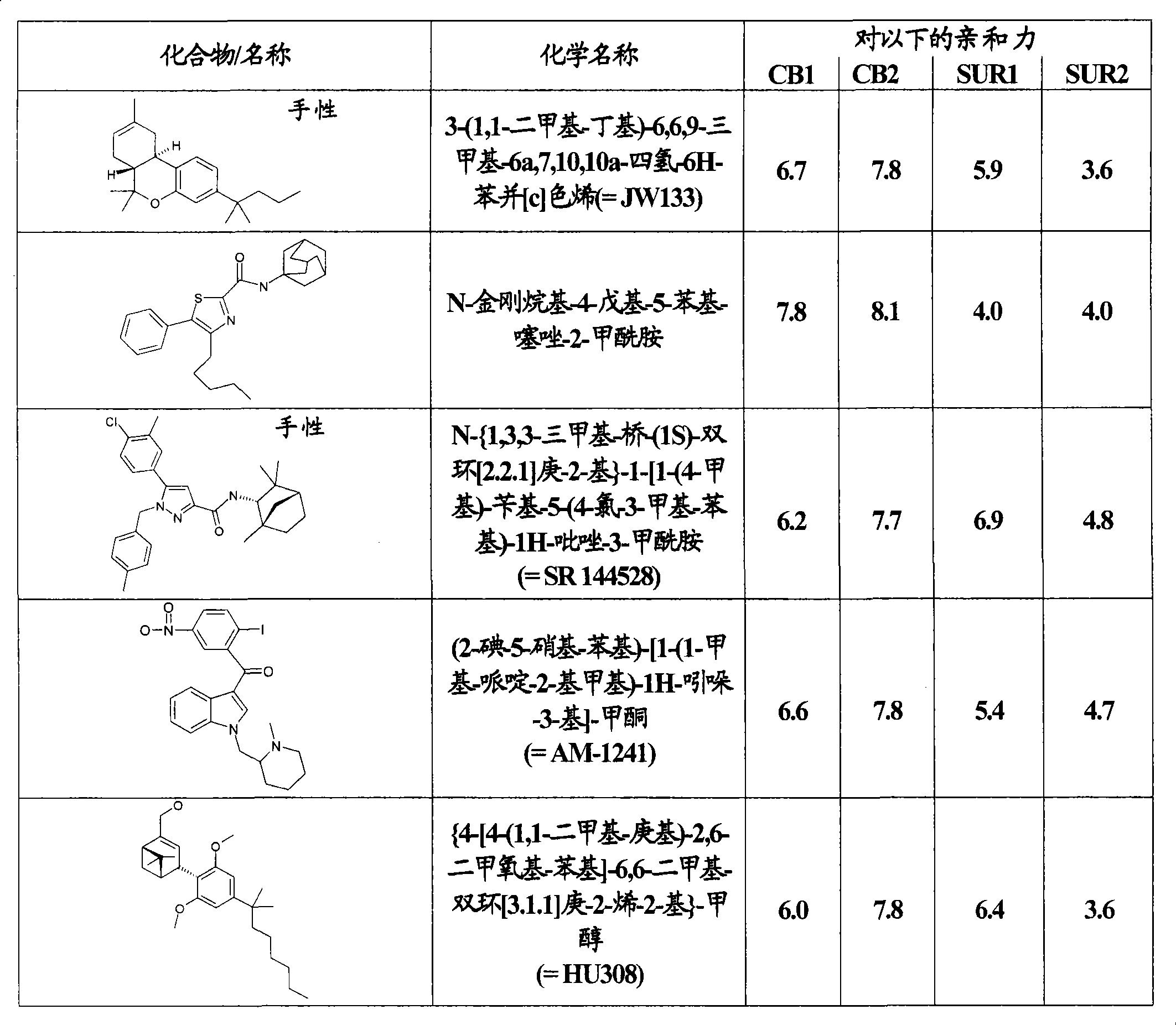
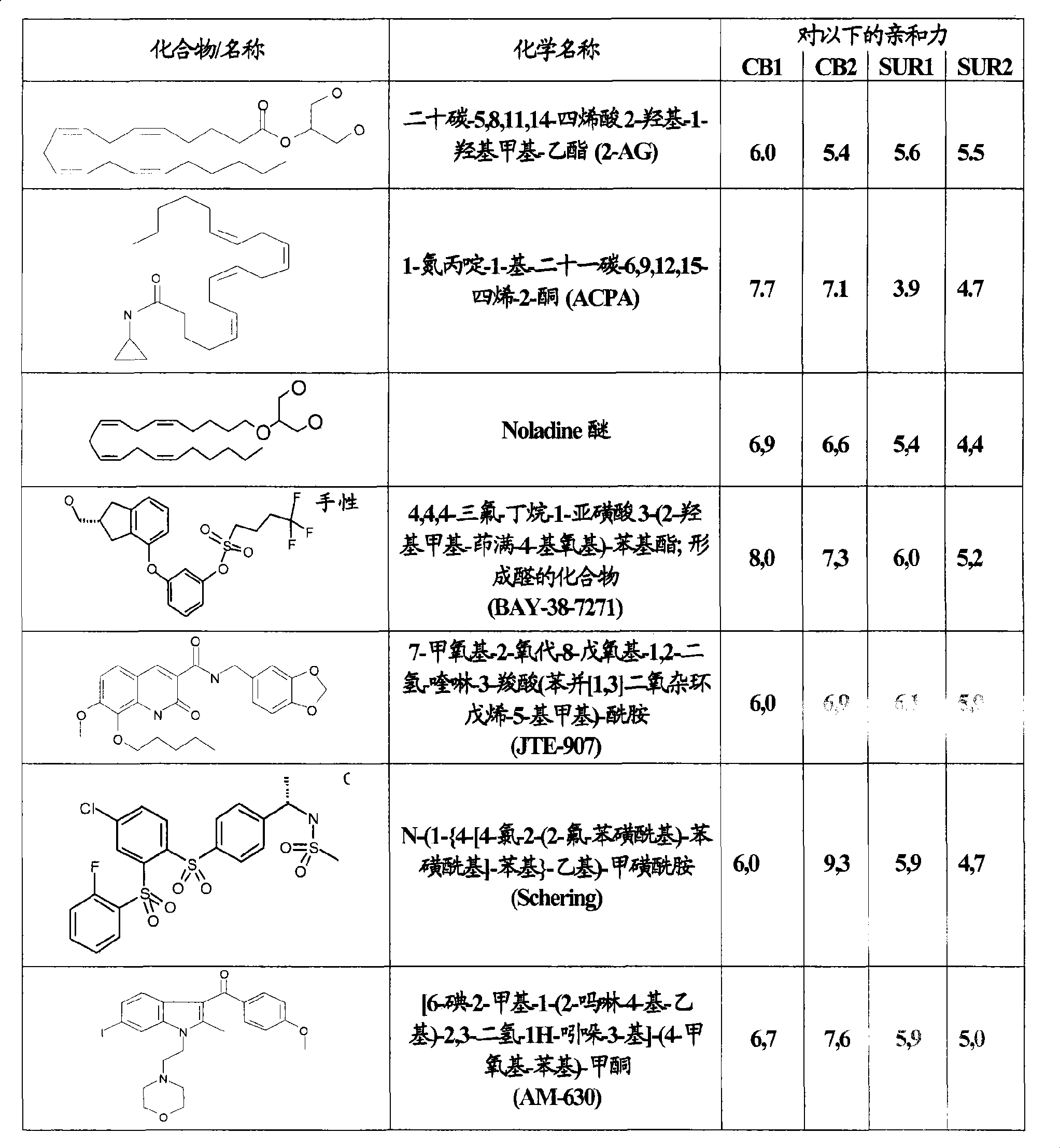
Use of CBx cannabinoid receptor modulators as potassium channel modulators
InactiveCN101431994AReduce processReduce seizuresNervous disorderMetabolism disorderAppetite regulationEpilepsy
The invention is directed to the use of at least one CBx modulator wherein the CBx modulator is selected from the group consisting of CB1 agonists; CB2 agonists; CB2 partial agonists; CB2 antagonists; CB2 inverse agonists; and dually acting compounds which are both a CB1 agonist and a CB2 agonist; and mixtures thereof, as KATP channel modulator for the prophylaxis, treatment, delayed progression, delayed onset and / or inhibition of a variety of disease conditions including obesity, diabetes mellitus, metabolic syndrome, syndrome X, insulinoma, familial hyperinsulemic hypoglycemia, male pattern baldness, detrusor hyperreactivity, asthma, neuroprotection, epilepsy, analgesia, cardioprotection, angina, cardioplegia, arrhythmia, coronary spasm, peripheral vascular disease, cerebral vasospasm, appetite regulation, neurodegeneration, pain - including neuropathic pain and chronic pain - and impotence in mammals and humans. The invention further relates to methods of treating, preventing, delaying progression of, delaying onset of and / or inhibiting a variety of disease conditions including obesity, diabetes mellitus, metabolic syndrome, syndrome X, insulinoma, familial hyperinsulemic hypoglycemia, male pattern baldness, detrusor hyperreactivity, asthma, neuroprotection, epilepsy, analgesia, cardioprotection, angina, cardioplegia, arrhythmia, coronary spasm, peripheral vascular disease, cerebral vasospasm, appetite regulation, neurodegeneration, pain - including neuropathic pain and chronic pain - and impotence in mammals and humans comprising administering to a subject in need thereof an effective amount of at least one CBx modulator having KATP channel modulating properties.
Owner:SOLVAY PHARMA GMBH
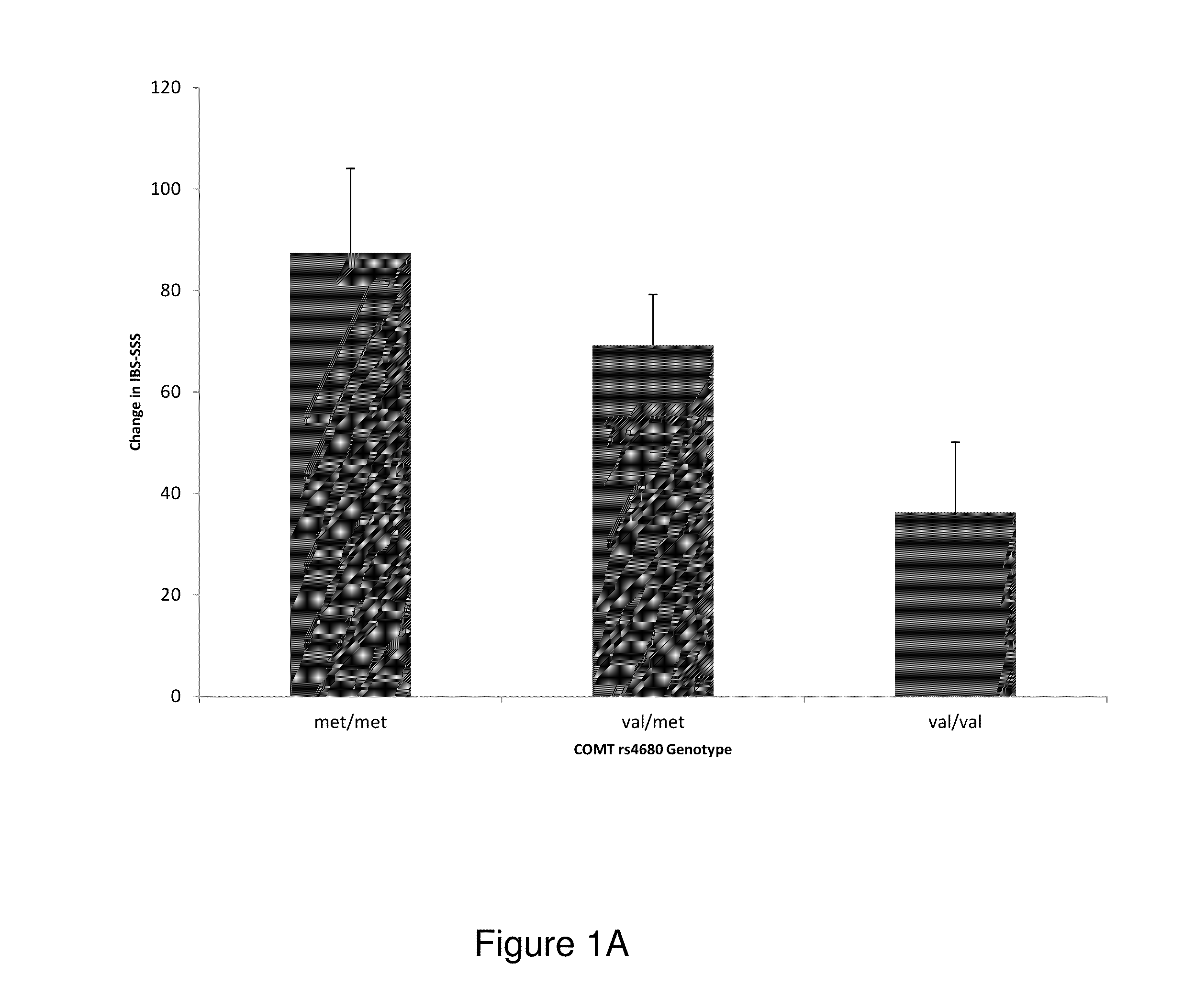
Methods and kits for determining a placebo profile in subjects for clinical trials and for treatment of patients
The present invention is directed to methods and assays for identifying subjects participating in clinical trials that may exhibit a placebo response and identifying treatments for subjects with varying degrees of placebo responses. In one aspect, a method of selecting subjects to participate in a clinical trial is disclosed. In another aspect, methods for treating a subject and determining a treatment dosage are disclosed. In an exemplary embodiment, a method for determining a response to a treatment of a subject having, suspected of having, or at risk for developing a disorder, such as cardiovascular disorder, irritable bowel syndrome, diabetes, autoimmune disorders, inflammation, neurological disorders, chronic pain, cancer, cancer treatments, allergies, depression, migraines, addiction, obesity, and other disorders, syndromes, or diseases, is disclosed.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Novel Pharmaceutical Compositions for Treating Acquired Chronic Pain and Associated Dysphoria
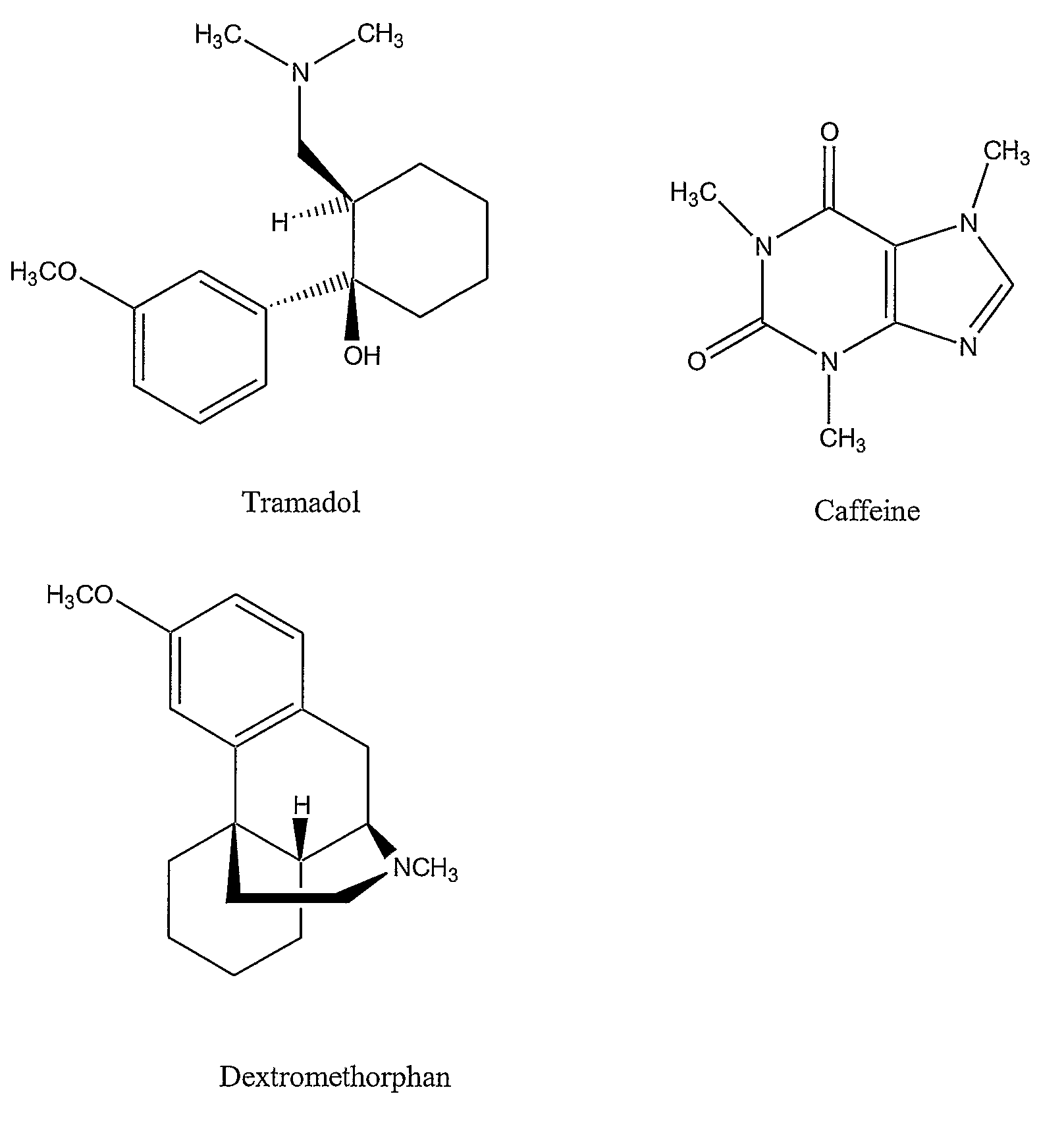
InactiveUS20080176873A1Good analgesic effectEffective pain managementBiocideNervous disorderDextrorphanSustained release drug
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a methylxanthine such as caffeine, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com