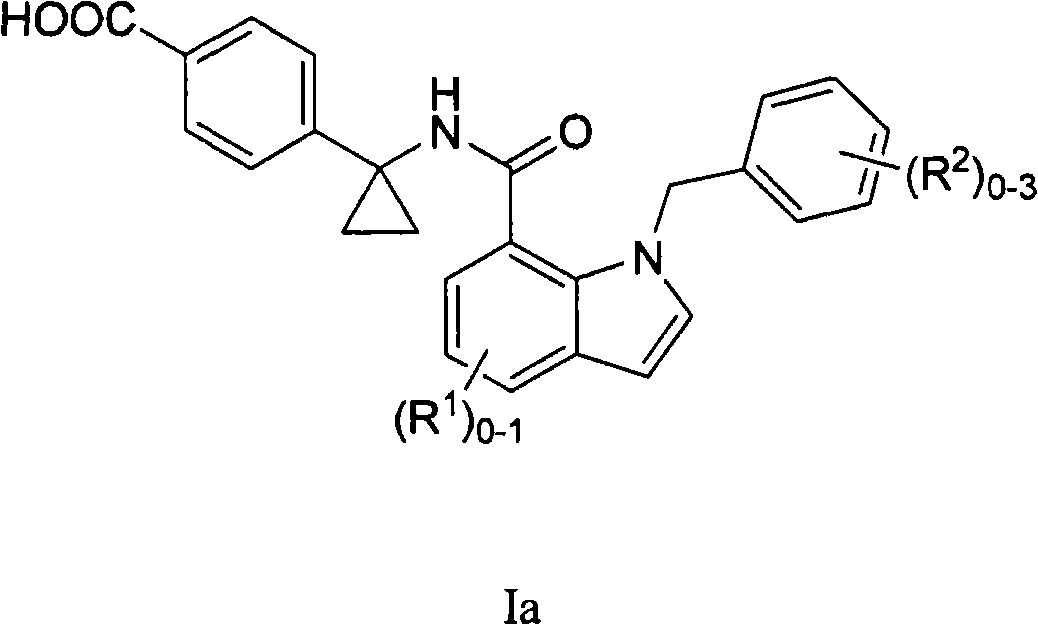
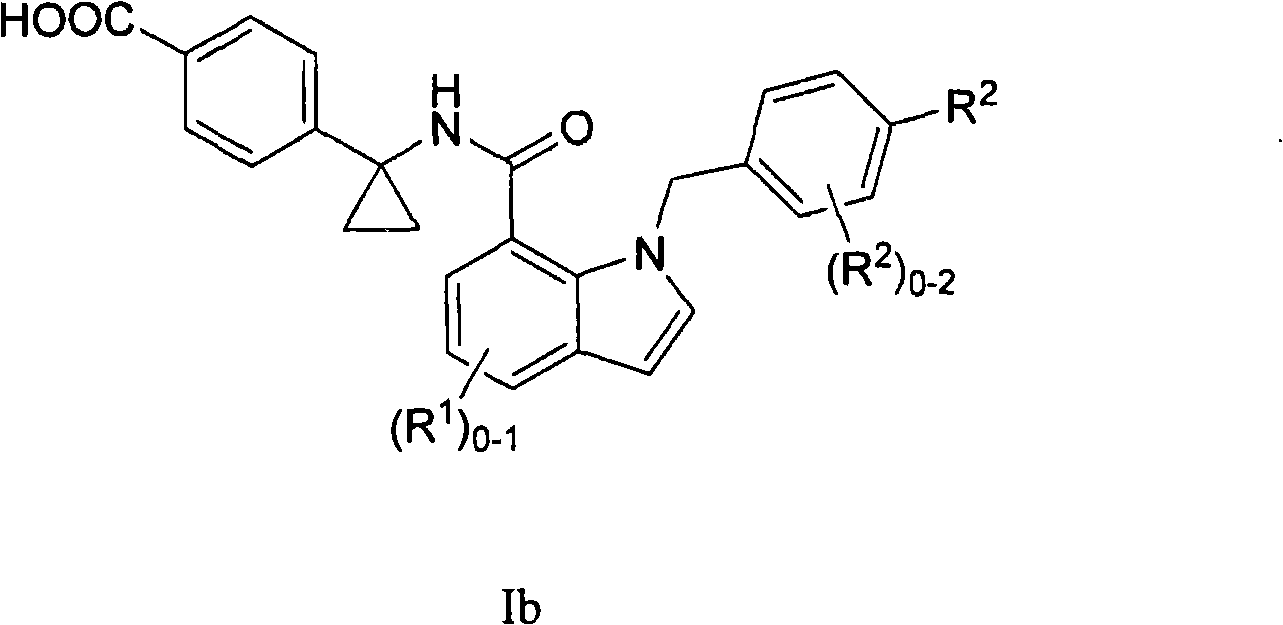
Indole and indoline cyclopropyl amide derivatives as ep4 receptor antagonists
An alkyl, compound technology, applied in pain and inflammation antagonists, GE2 receptors, E2 receptor fields, can solve problems such as deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Potassium 4-[1-({[1-(3,4-dichlorobenzyl)-2,3-dihydro-1H-indol-7-yl]carbonyl}amino)cyclopropyl]benzoate
[0132]
[0133] Step 1: 1-(tert-Butoxycarbonyl)indole-7-carboxylic acid
[0134]
[0135]Indoline-1-carboxylic acid tert-butyl ester (25 g, 114 mmol) and TMEDA (22.9 ml, 151 mmol) were added to 567 ml of diethyl ether. The resulting solution was cooled to -78°C, and s-BuLi in cyclohexane (1.2 equiv, 1.4M) was added dropwise. The mixture was stirred at the above temperature for 1 h. will CO 2 Gas was bubbled through the mixture for 5 min and the incubation was removed. After stirring for 10 min, the mixture was quenched with 1N HCl and warmed to RT, then extracted 3 times with EtOAc. The combined organic layers were washed with brine and washed over MgSO 4 Dry on top. The solvent was removed and the resulting solid was triturated with 1:1 ether / hexane.
[0136] 1 H NMR (500MHz, DMSO-d6): δ12.5(bs, 1H), 7.35(m, 2H), 7.05(t, 1H), 4.00(t, 2H), 3.00(t, 2H), ...
Embodiment 2
[0152] 1-(3,4-Dichlorobenzyl)-N-{1-[4-(1H-tetrazol-5-yl)phenyl]cyclopropyl}indoline-7-carboxamide
[0153]
[0154] Step 1: 4-(1-Aminocyclopropyl)benzonitrile
[0155]
[0156] Terephthalonitrile (500 mg, 390 mmol) was dissolved in 10 ml of DCM. Ti(OiPr) 4 (1.1 ml, 3.9 mmol) was added to the resulting solution, followed by a 3M solution of ethylmagnesium bromide in THF (2.3 ml, 7.0 mmol). The mixture was aged at RT for 45 min, and BF was added 3 Et 2 O (890ul, 7.0mmol). Then, the mixture was aged for another 2 h at RT. use NH 4 Cl and 3N HCl quenched the reaction. The layers were separated, and the aqueous layer was washed with ether. Then, the aqueous layer was basified with 10N NaOH (pH 9-10). EtOAc was added, and the biphasic mixture was filtered. The layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with MgSO 4 Dry, filter and concentrate. Yield = 15%.
[0157] Step 2: N-[1-(4-cyanophenyl)cycl...
Embodiment 3
[0169] 4-{1-[({1-[4-(trifluoromethyl)benzyl]-1H-indol-7-yl}carbonyl)amino]cyclopropyl}benzoic acid
[0170]
[0171] Step 1: Methyl 1-[4-(trifluoromethyl)benzyl]-1H-indole-7-carboxylate
[0172]
[0173] 1H-Indole-7-carboxylic acid methyl ester (47.8 g, 273 mmol) and 1-(bromomethyl)-4-(trifluoromethyl)benzene (81 g, 341 mmol) were dissolved in DMF ( 1.3L). 60% w / w NaH (12 g, 300 mmol) was added in portions. The ice bath was removed, and the mixture was stirred at 0 °C for 3 hours, then at RT overnight. The reaction mixture was washed with 3 L NH 4 It was quenched with Cl(sat), and the aqueous layer was extracted 3 times with 1 L ether. The organic layers were combined and washed with water and brine. The compound was purified by flash chromatography on silica gel.
[0174] 1 H NMR (500MHz, DMSO-d6): δ8.90(d, 1H), 7.70(s, 1H), 7.60(d, 2H), 7.40(d, 1H), 7.10(t, 1H), 7.00(d , 2H), 6.70(d, 1H), 5.70(s, 2H).
[0175] Step 2: 1-[4-(Trifluoromethyl)benzyl]-1H-indole-7-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com