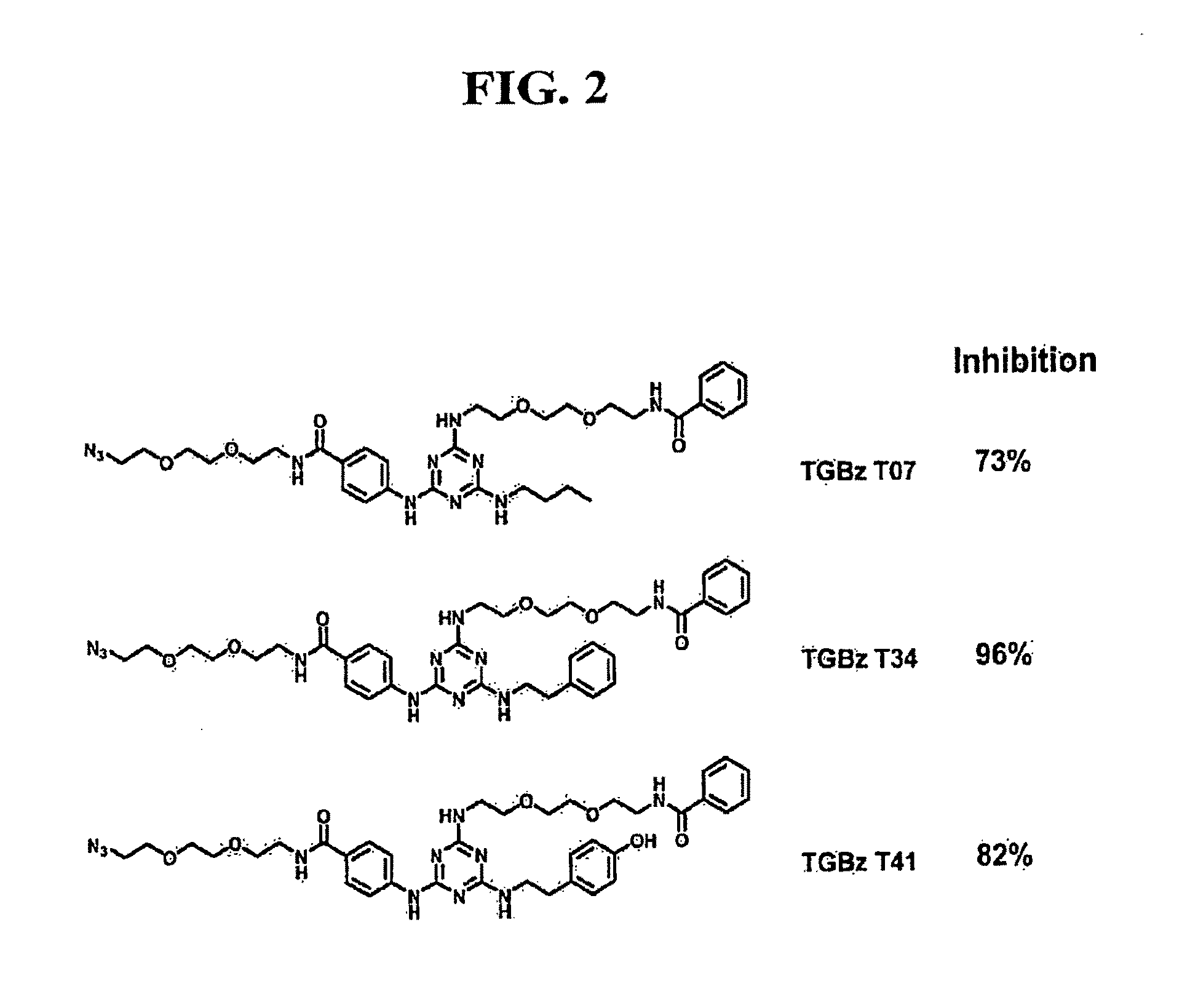
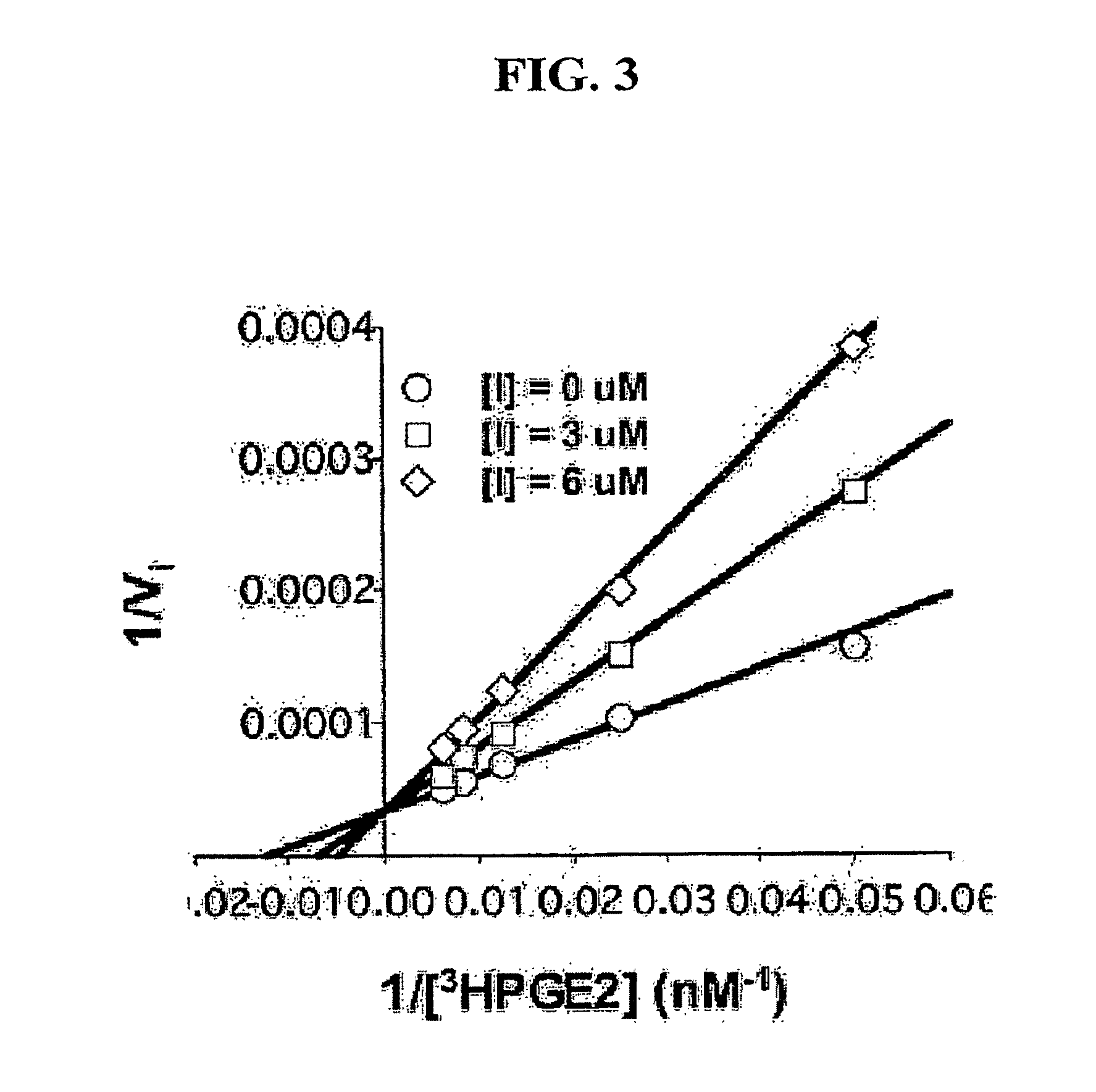
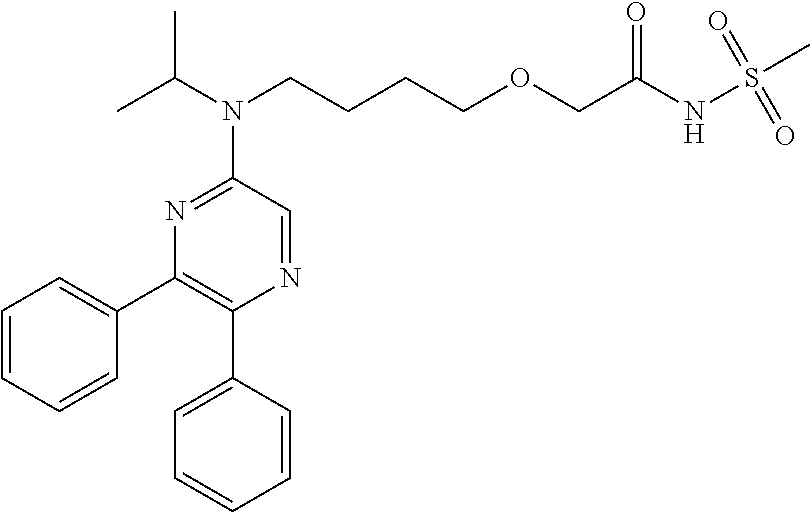
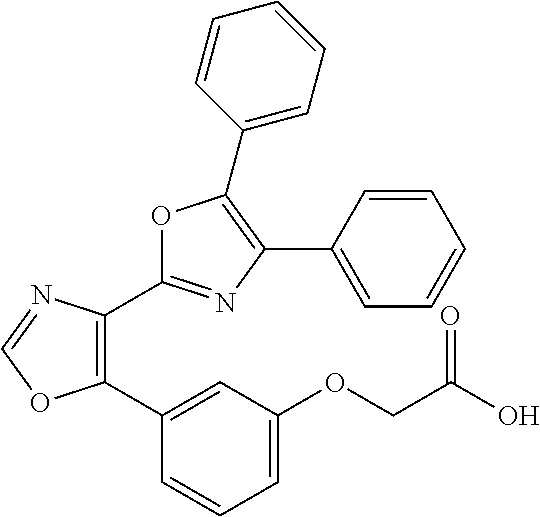
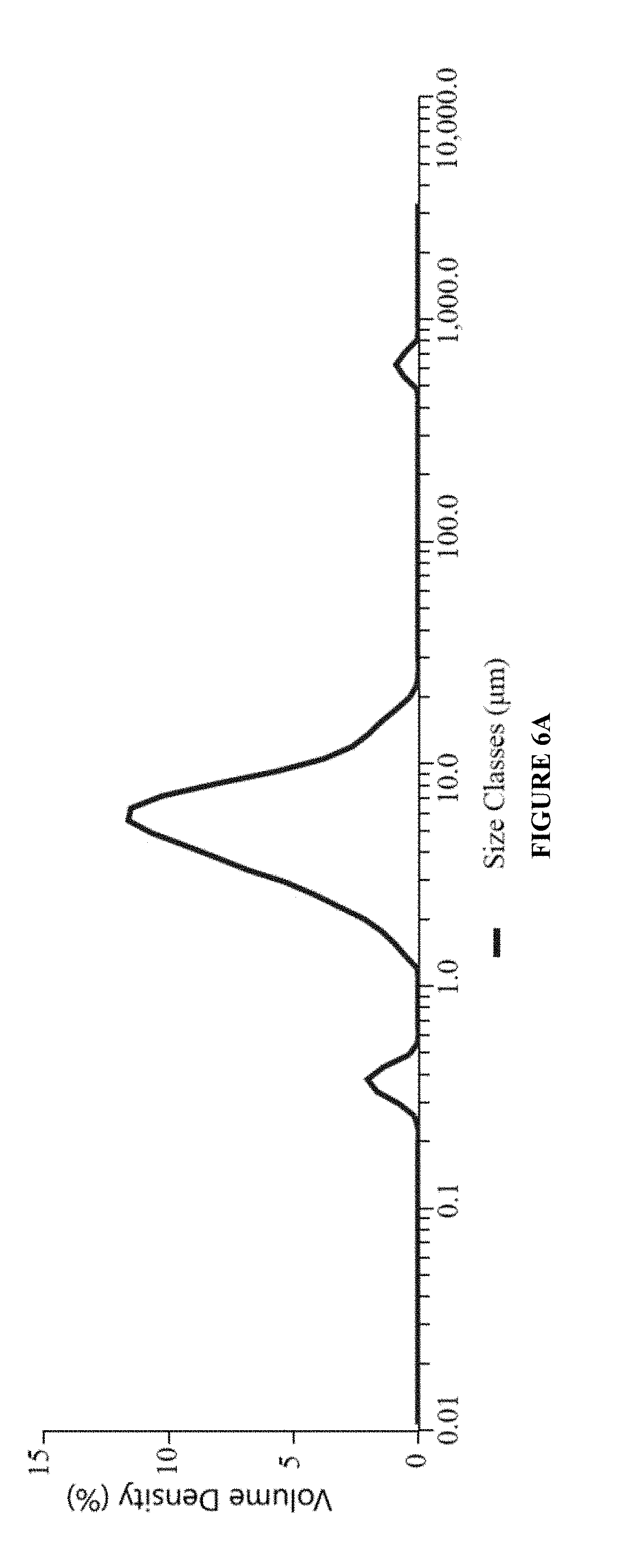
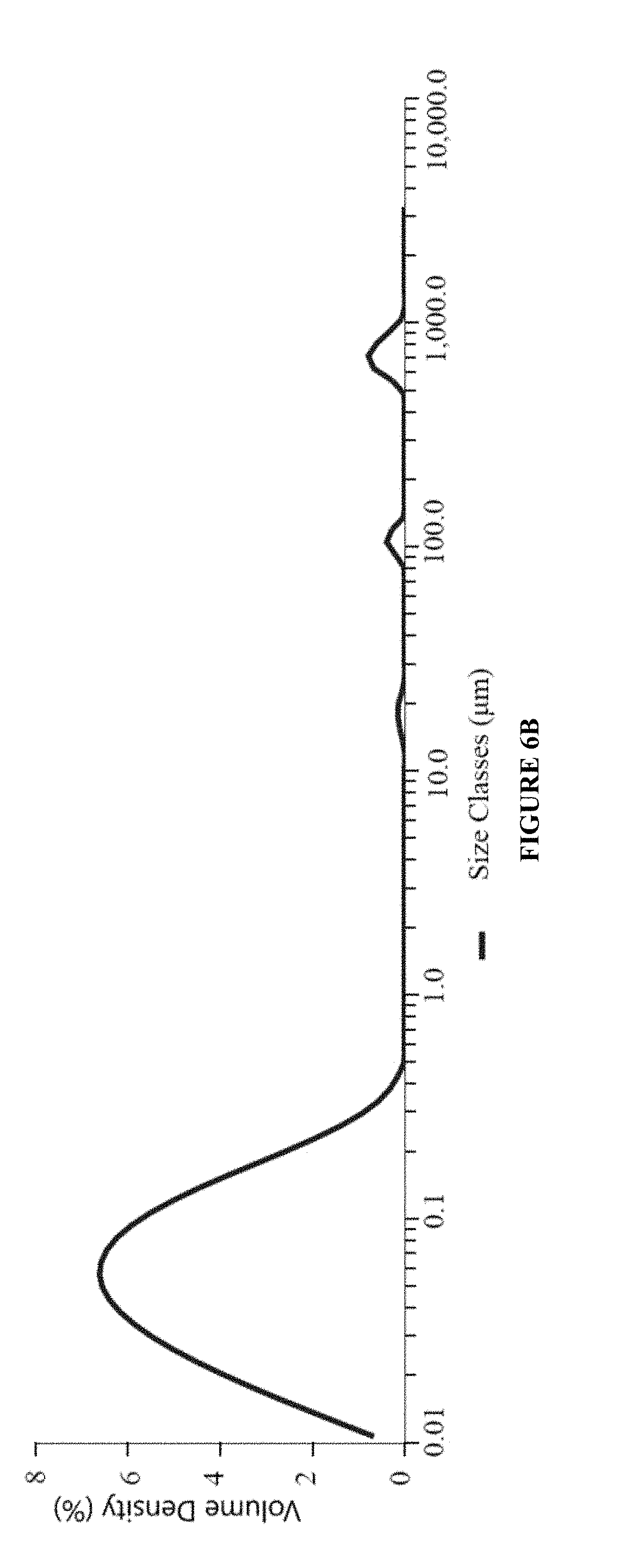
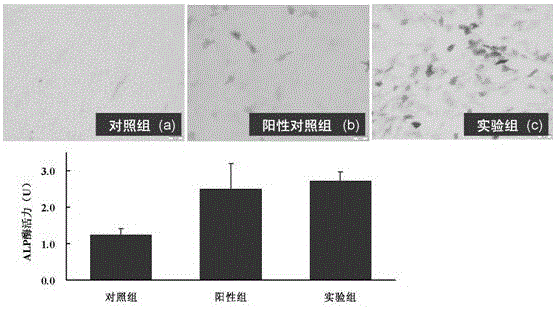
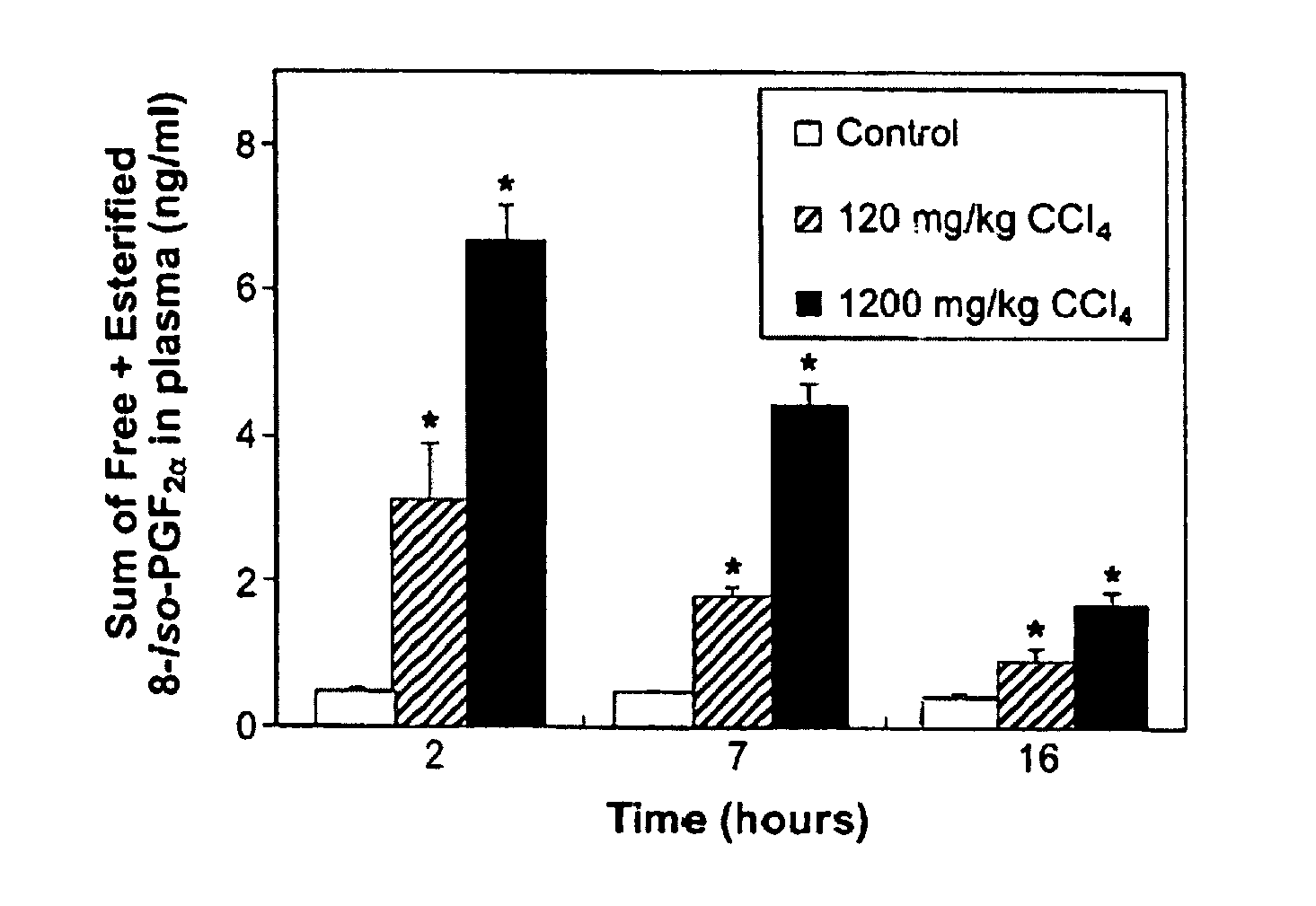
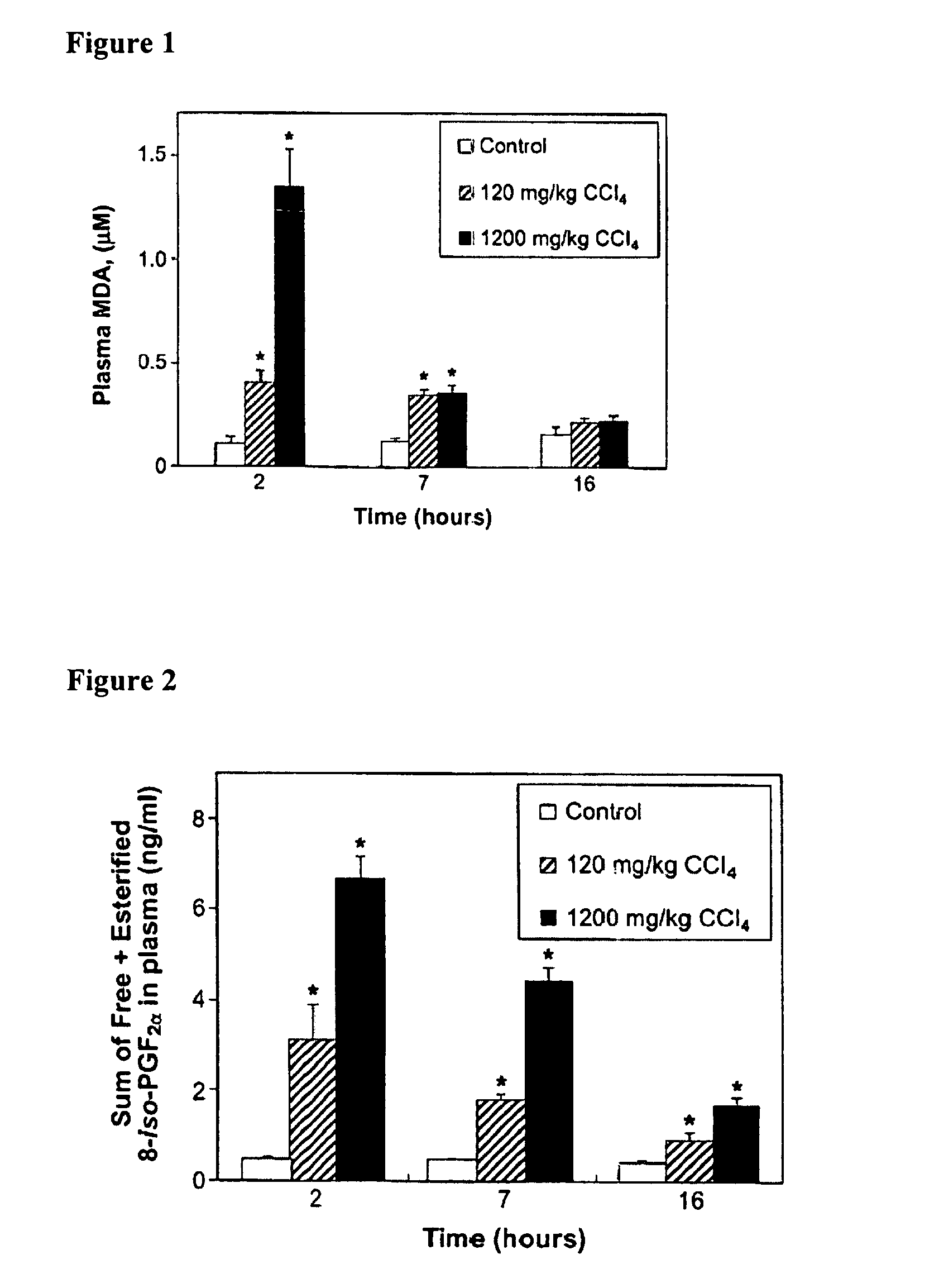
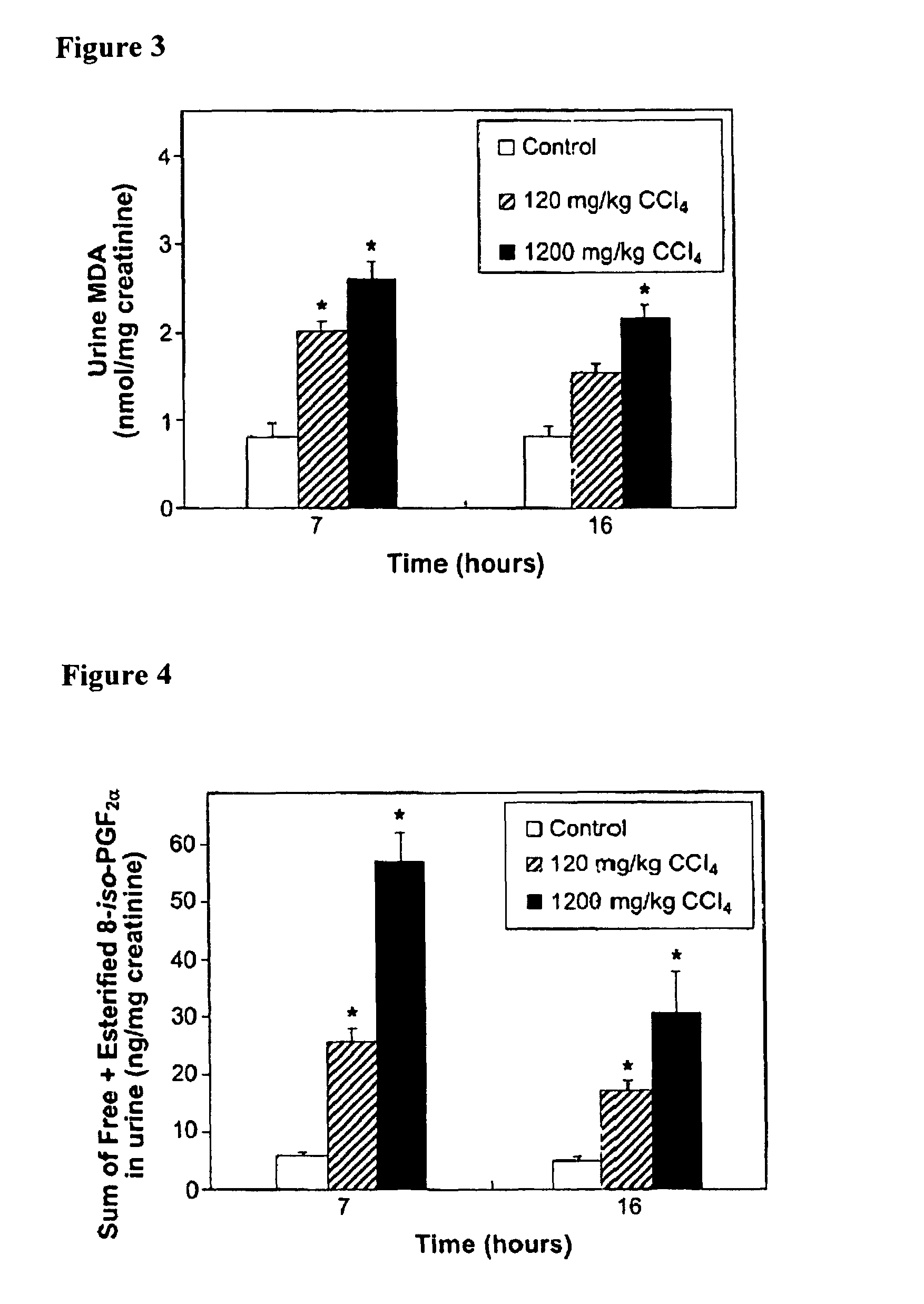
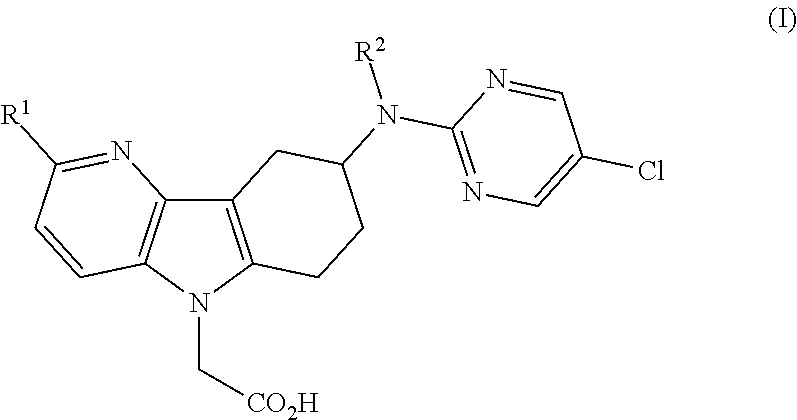
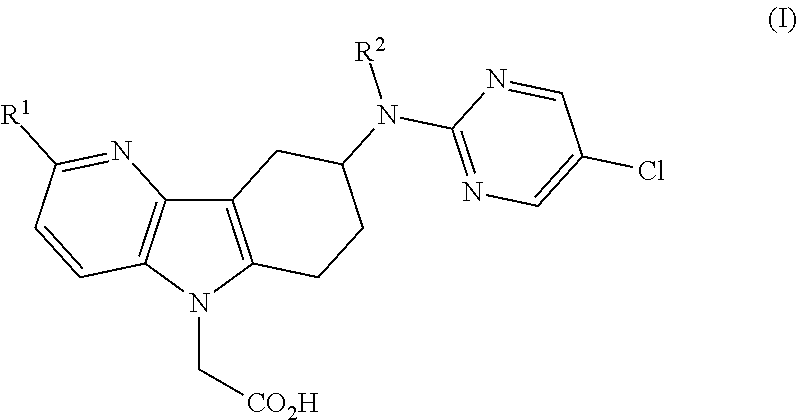
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Prostanoid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prostanoids are a subclass of eicosanoids consisting of the prostaglandins (mediators of inflammatory and anaphylactic reactions), the thromboxanes (mediators of vasoconstriction), and the prostacyclins (active in the resolution phase of inflammation.)
Method of inhibiting prostaglandin synthesis in a human host
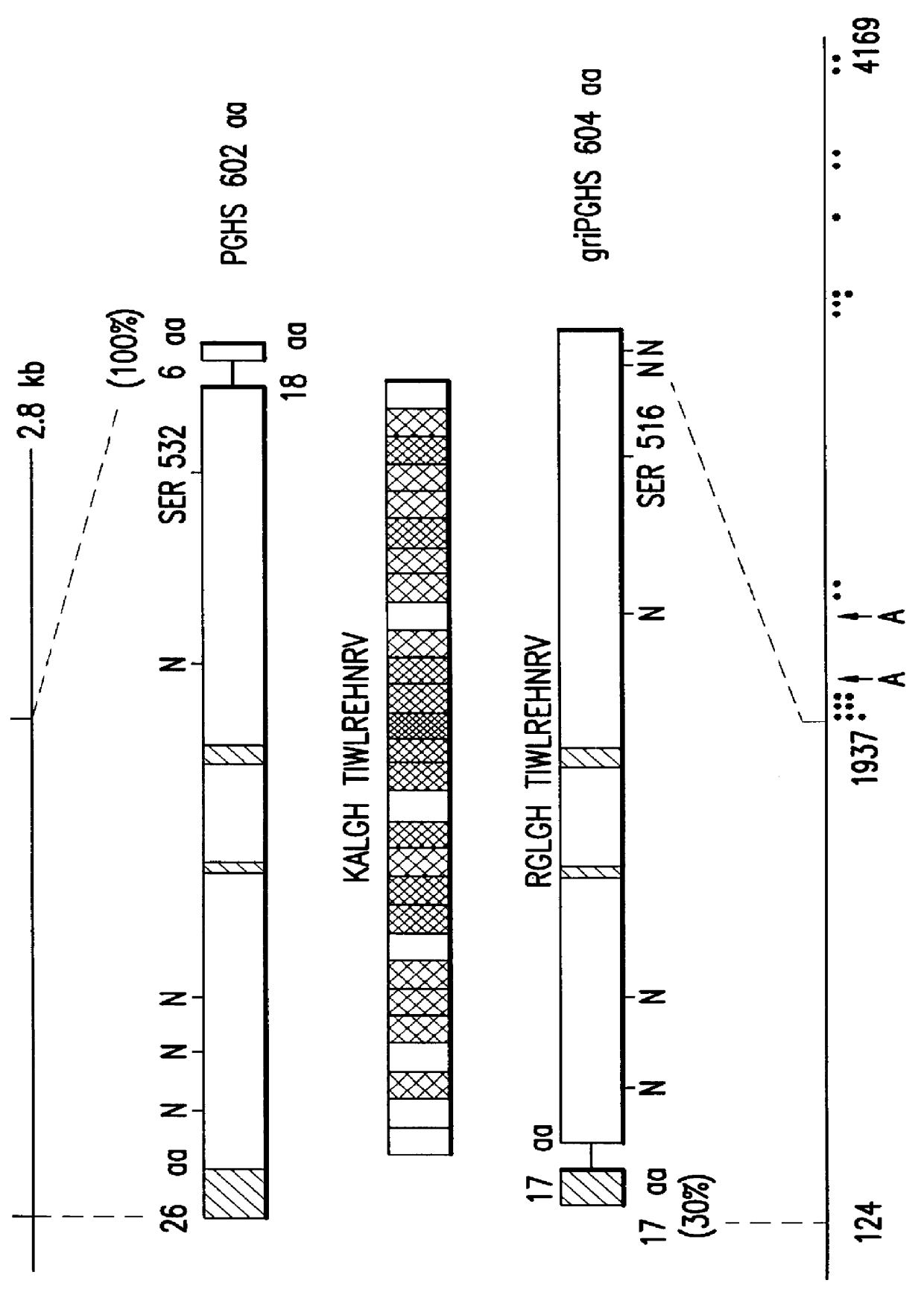
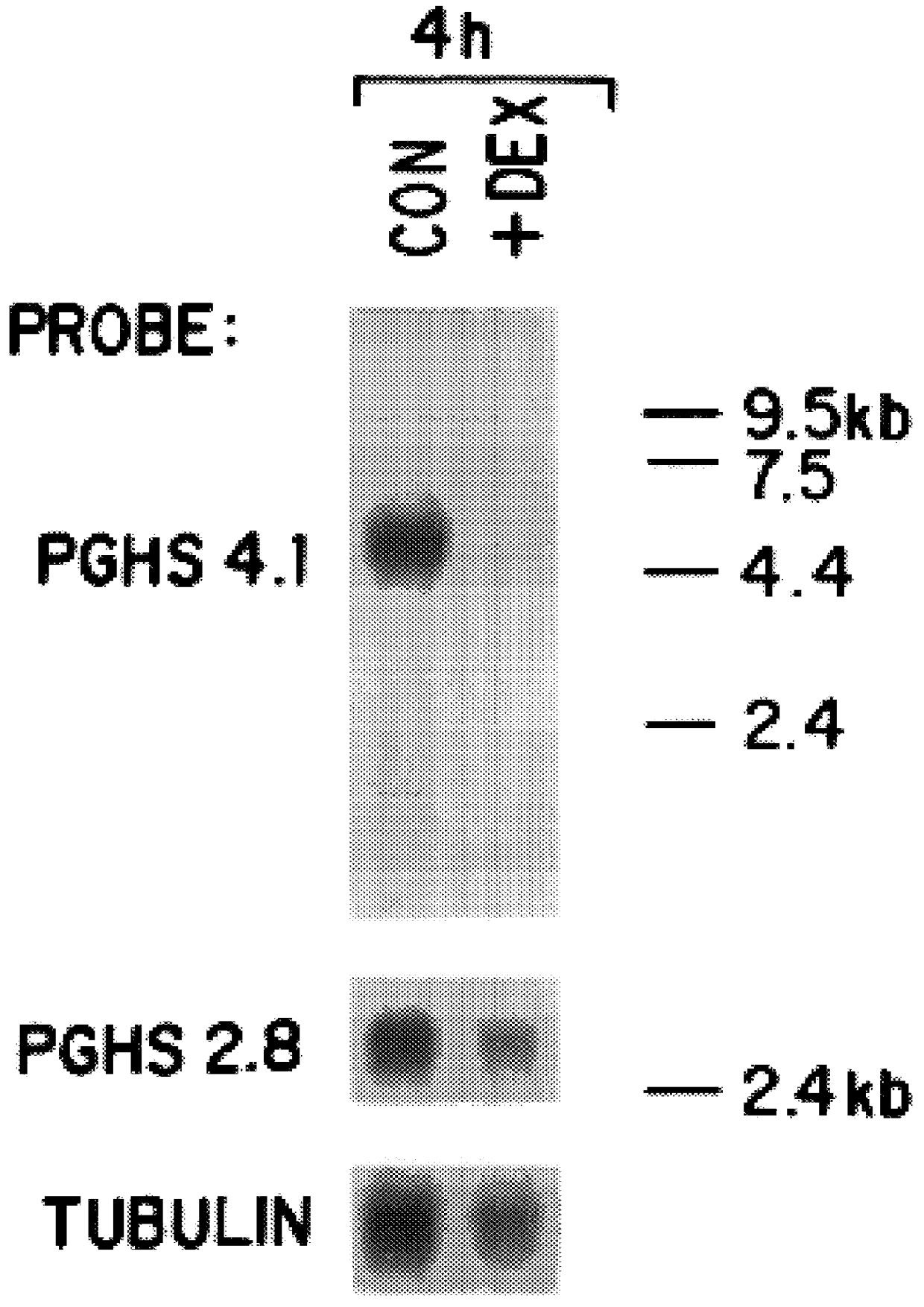
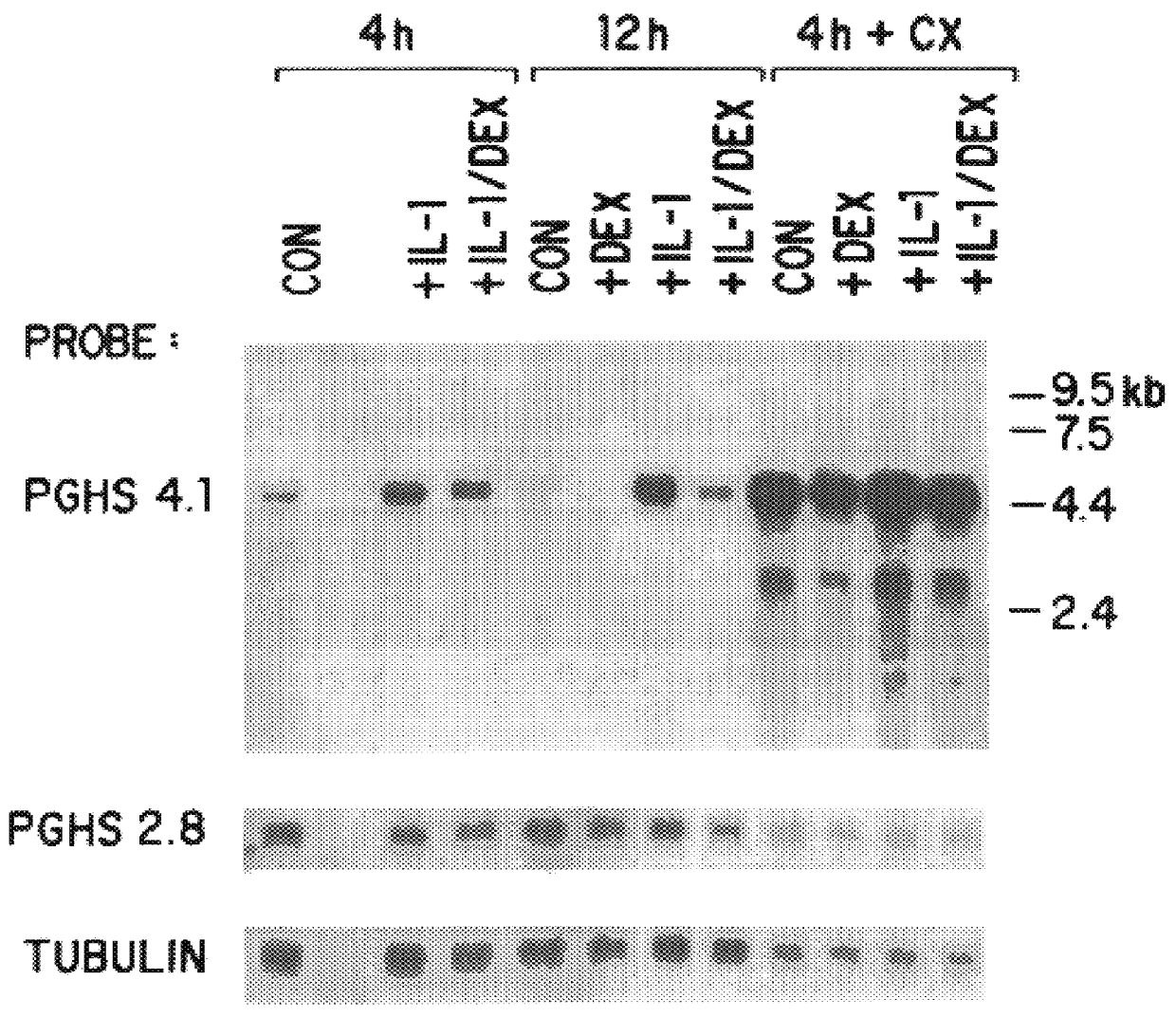
The invention relates to the gene encoding the mammalian prostaglandin H synthase-2 and its product. More specifically, the invention relates to the diagnosis of aberrant PGHS-2 gene or gene product; the identification, production, and use of compounds which modulate PGHS-2 gene expression or the activity of the PGHS-2 gene product including but not limited to nucleic acid encoding PGHS-12 and homologues, analogues, and deletions thereof, as well as antisense, ribozyme, triple helix, antibody, and polypeptide molecules as well as small inorganic molecules; and pharmaceutical formulations and routes of administration for such compounds.
Owner:UNIVERSITY OF ROCHESTER
Assessment of cardiovascular risk
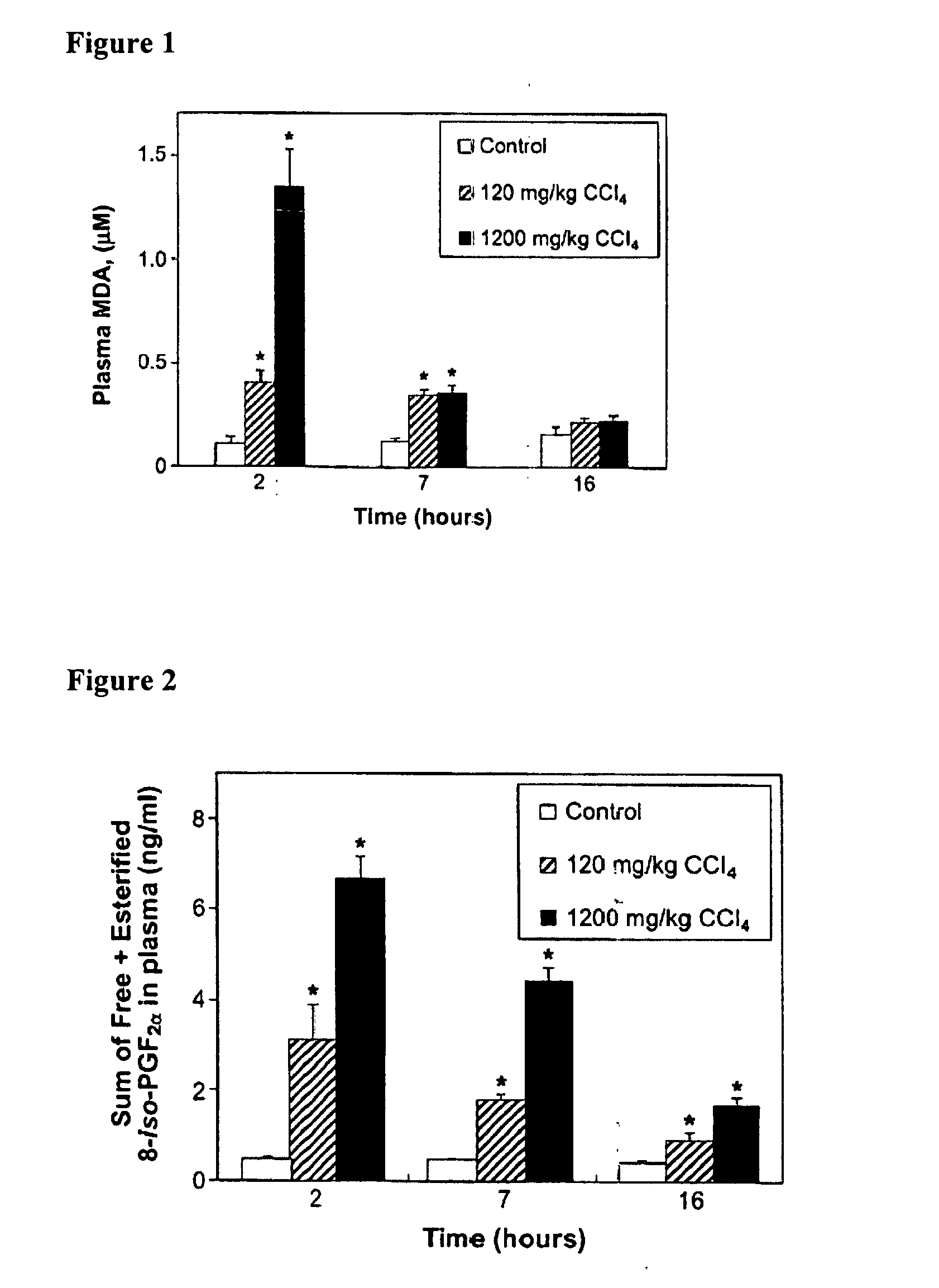
ActiveUS20060051873A1Reduce cardiovascular riskIncreased riskDisease diagnosisBiological testingMetaboliteMammal
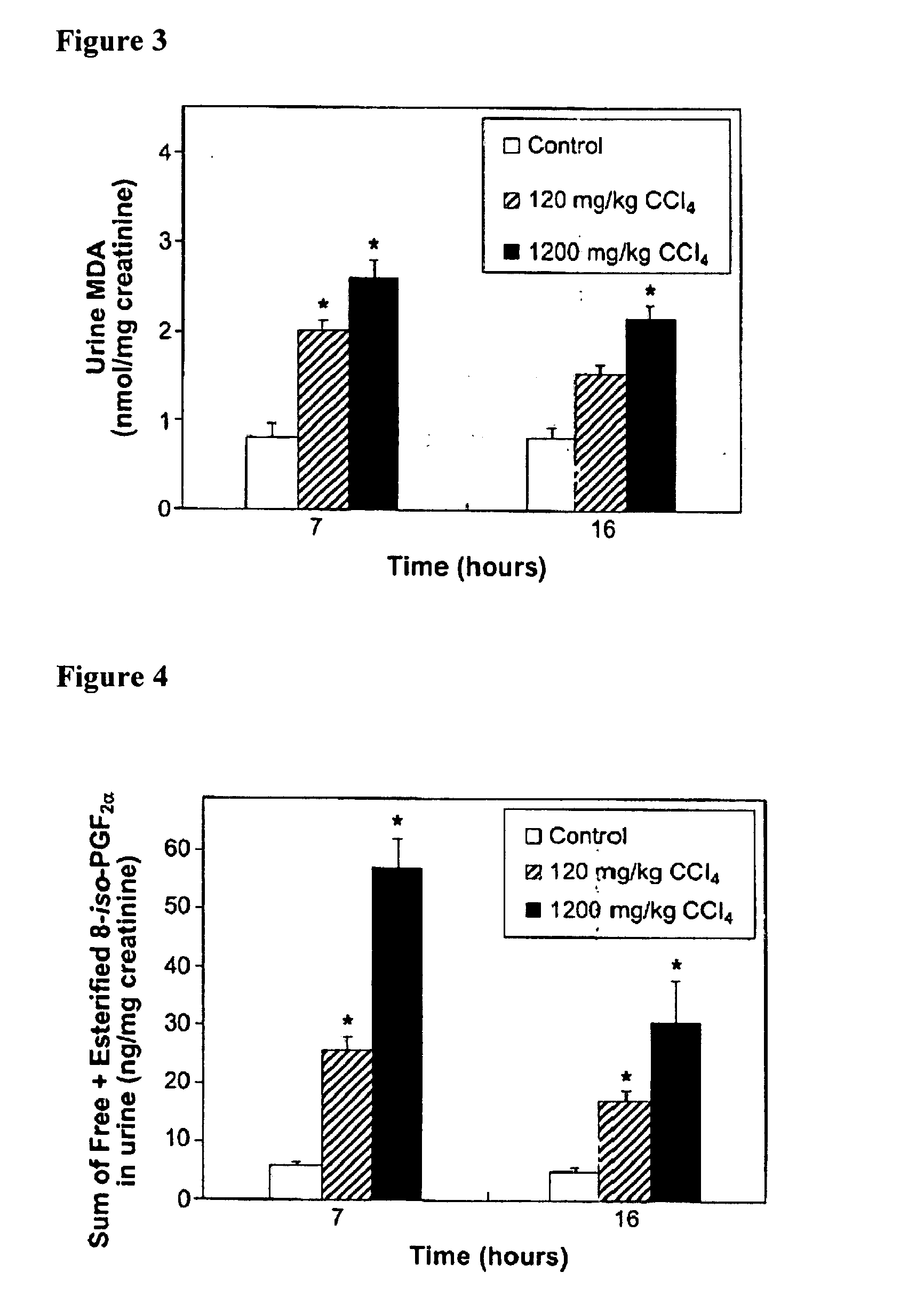
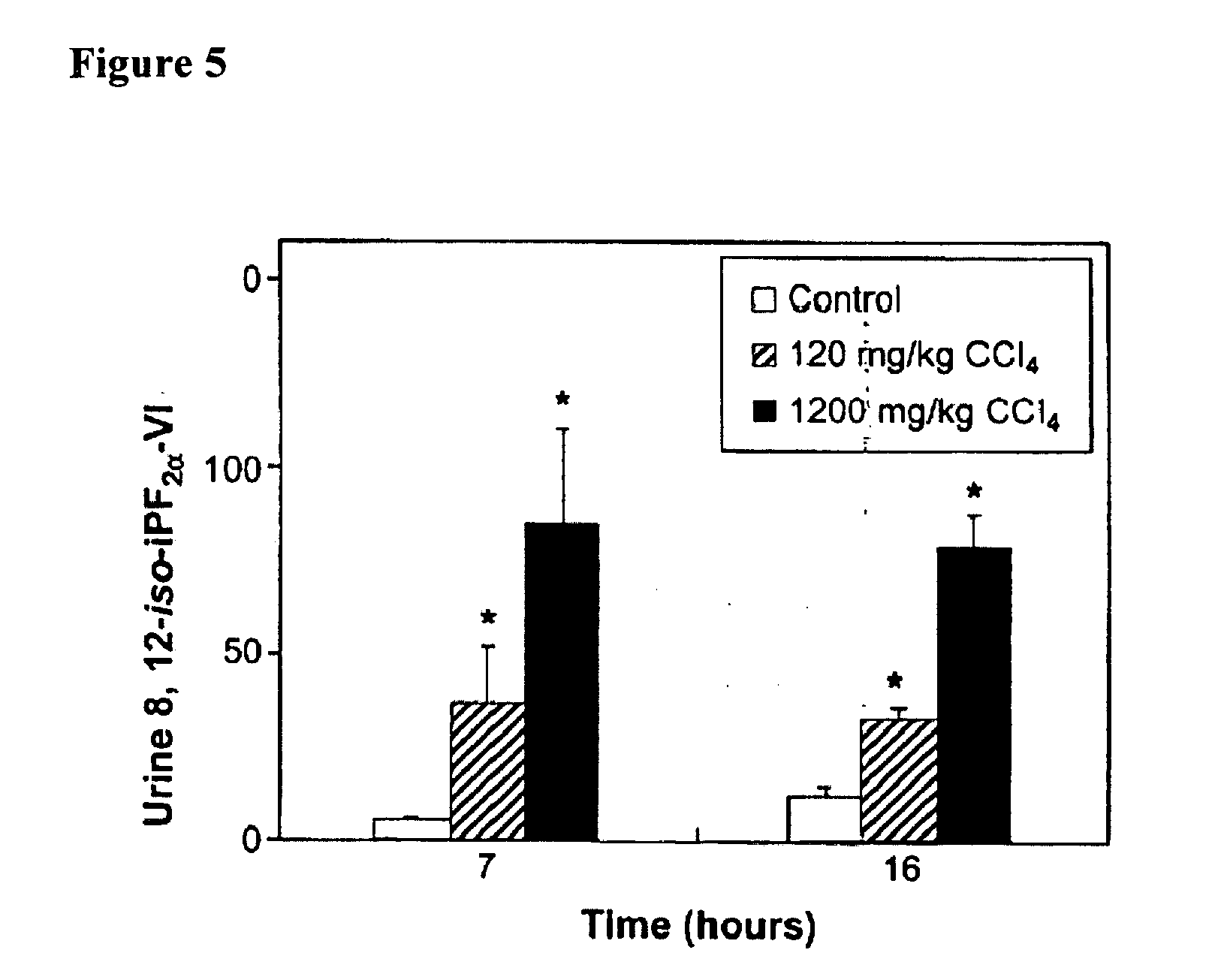
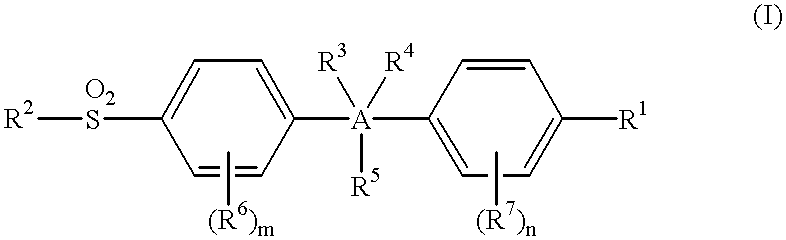
The instant invention is drawn to methods and compositions useful for the assessment of cardiovascular risk in a mammal. The methods utilize biomarkers including prostanoid metabolites and isoprostanes as sensitive and stable markers of cardiovascular risk. The methods are particularly useful in a mammal that is contemplating undergoing coxib therapy, is undergoing coxib therapy, is undergoing antioxidant therapy, has ceased coxib therapy or has never undergone coxib therapy. The invention also includes kits useful for the assessment of cardiovascular risk in a mammal.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
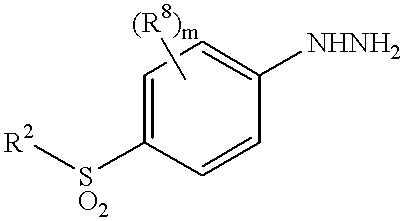
Sulfonylbenzene compounds as anti-inflammatory/analgesic agents
InactiveUS6294558B1Inhibit prostaniod-induced smooth muscle contractionInhibit synthesisBiocideOrganic chemistryArylHydrogen
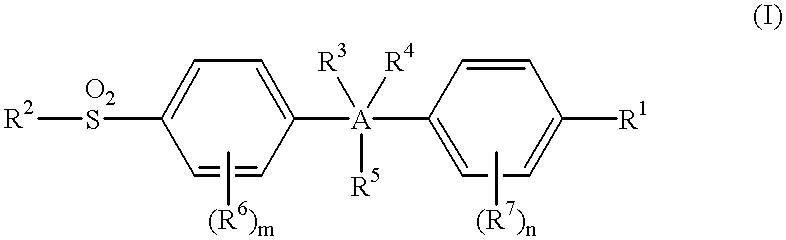
This invention provides a compound of the formula:or its pharmaceutically acceptable salt thereof, wherein A is partially unsaturated or unsaturated five membered heterocyclic, or partially unsaturated or unsaturated five membered carbocyclic, wherein the 4-(sulfonyl)phenyl and the 4-substituted phenyl in the formula (I) are attached to ring atoms of Ring A, which are adjacent to each other; R1 is optionally substituted aryl or heteroaryl, with the proviso that when A is pyrazole, R1 is heteroaryl; R2 is C1-4 alkyl, halo-substituted C1-4 alkyl, C1-4 alkylamino, C1-4 dialkylamino or amino; R3, R4 and R5 are independently hydrogen, halo, C1-4 alkyl, halo-substituted C1-4 alkyl or the like; or two of R3, R4 and R5 are taken together with atoms to which they are attached and form a 4-7 membered ring; R6 and R7 are independently hydrogen, halo, C1-4 alkyl, halo-substituted C1-4 alkyl, C1-4 alkoxy, C1-4 alkylthio, C1-4 alkylamino or N,N-di C1-4 alkylamino; and m and n are independently 1, 2, 3 or 4. This invention also provides a pharmaceutical composition useful for the treatment of a medical condition in which prostaglandins are implicated as pathogens.
Owner:PFIZER INC
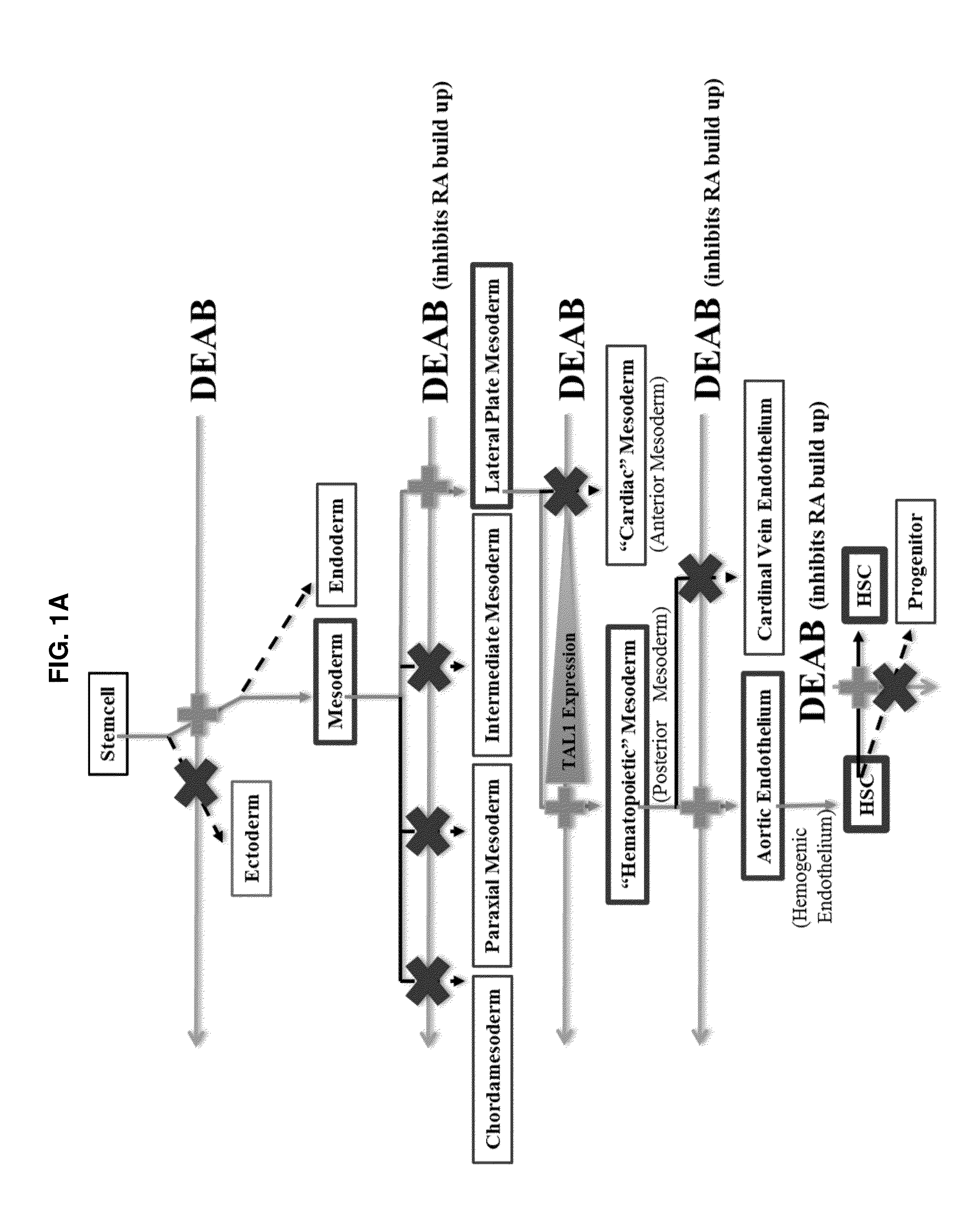
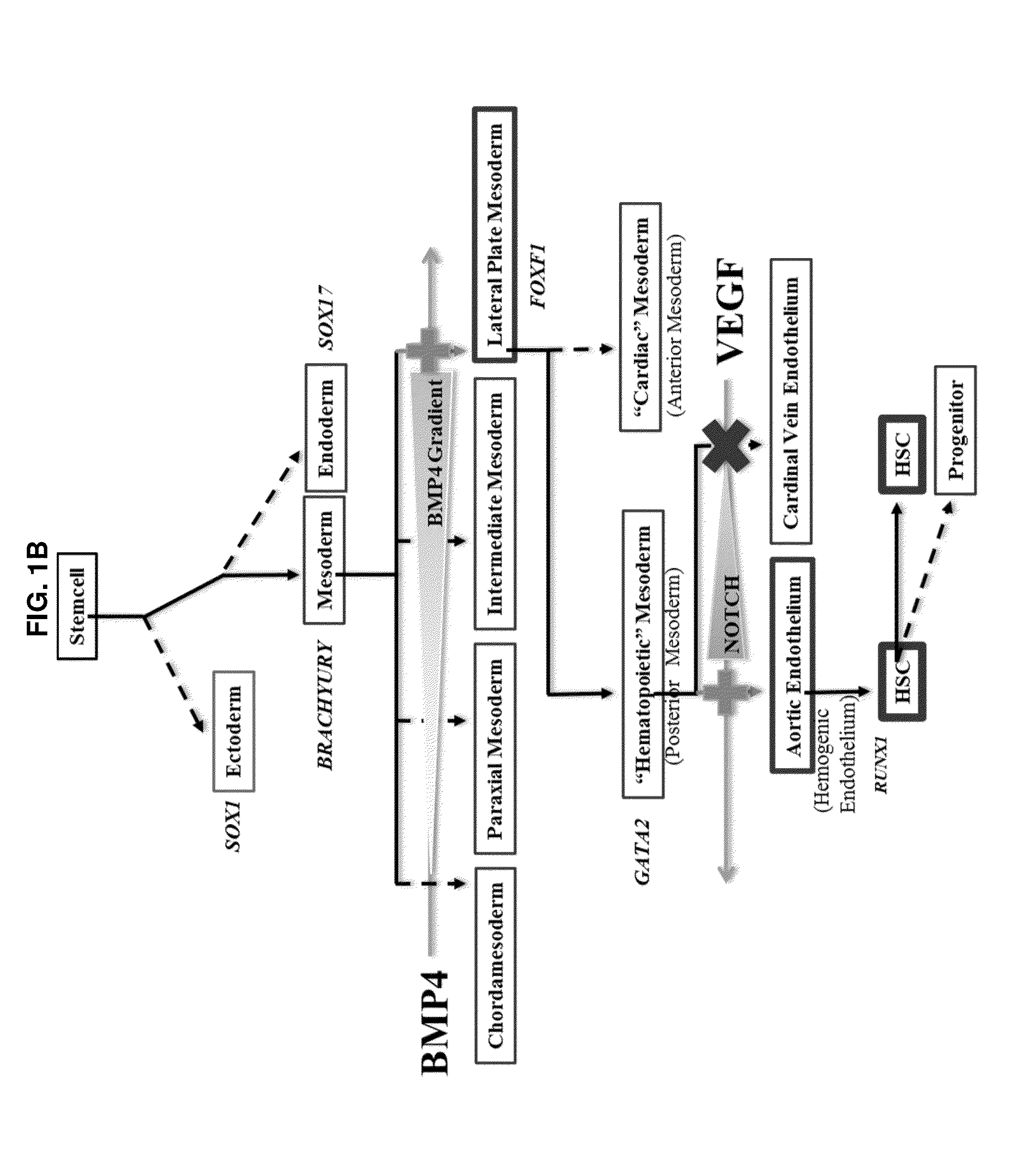
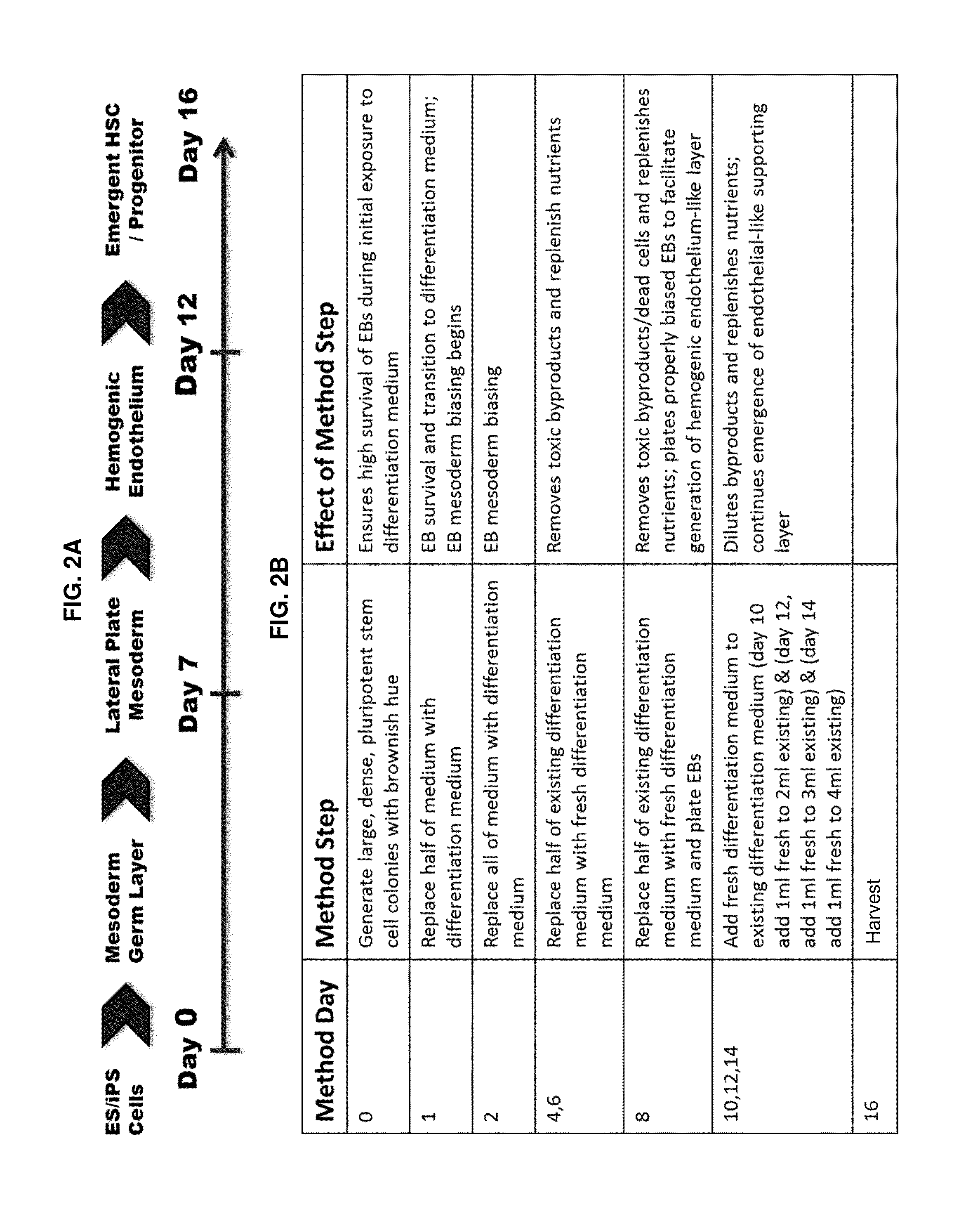
Compositions and methods for differentiating pluripotent stem cells into primitive blood cells and uses thereof
InactiveUS20130171110A1High expressionAccelerate self-renewalBiocideCulture processInduced pluripotent stem cellAntioxidant
Compositions and methods that employ various combinations of such factors as retinoic acid signaling inhibitors, antioxidants, BMP4, VEGF, prostaglandin E2 pathway stimulants, TPO, SCF, FLT-3, EPO, TGFβ1, p38 MAPK inhibitors, beta adrenergic receptor agonists, cell cycle inhibitors, RXR agonists, Cripto, and chromatin remodelers to drive differentiation of pluripotent stem cells towards primitive blood cells. Uses of such primitive blood cells are provided.
Owner:NUCLEUS BIOLOGICS LLC
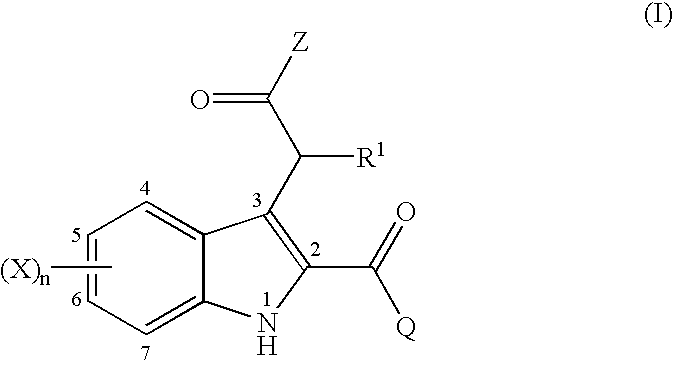
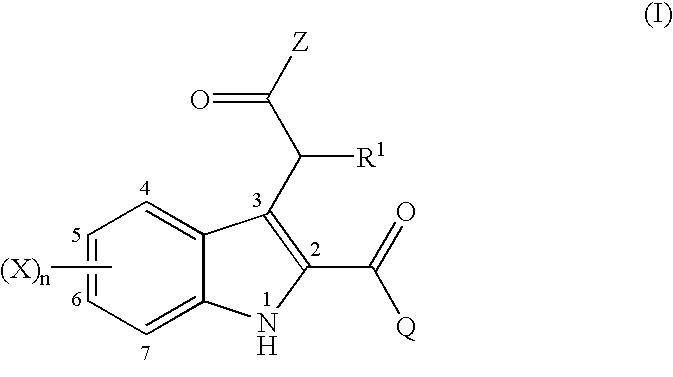
2,3-substituted indole compounds as anti-inflammatory and analgesic agents
InactiveUS6608070B1Inhibit prostaniod-induced smooth muscle contractionInhibit synthesisBiocideNervous disorderPharmaceutical medicinePhenyl group
This invention provides a compound of the following formula:or the pharmaceutically acceptable salts thereof wherein Z is OH, C1-6 alkoxy, -NR2R3 or heterocycle; Q is selected from the following: (a) an optionally substituted phenyl, (b) an optionally substituted 6-membered monocyclic aromatic group containing one, two, three or four nitrogen atom(s), (c) an optionally substituted 5-membered monocyclic aromatic group containing one heteroatom selected from O, S and N and optionally containing one, two or three nitrogen atom(s) in addition to said heteroatom, (d) an optionally substituted C3-7 cycloalkyl and (e) an optionally substituted benzo-fuzed heterocycle; R1 is hydrogen, C1-4 alkyl or halo; R2 and R3 are independently hydrogen, OH, C1-4 alkoxy, C1-4 alkyl or C1-4 alkyl substituted with halo, OH, C1-4 alkoxy or CN; X is independently selected from H, halo, C1-4 alkyl, halo-substituted C1-4 alkyl, OH, C1-4 alkoxy, halo-substituted C1-4 alkoxy, C1-4 alkylthio, NO2, NH2, di-(C1-4 alkyl)amino and CN; and n is 0, 1, 2, 3 and 4.This invention also provides a pharmaceutical composition useful for the treatment of a medical condition in which prostaglandins are implicated as pathogens.
Owner:NAKAO KAZUNARI +5
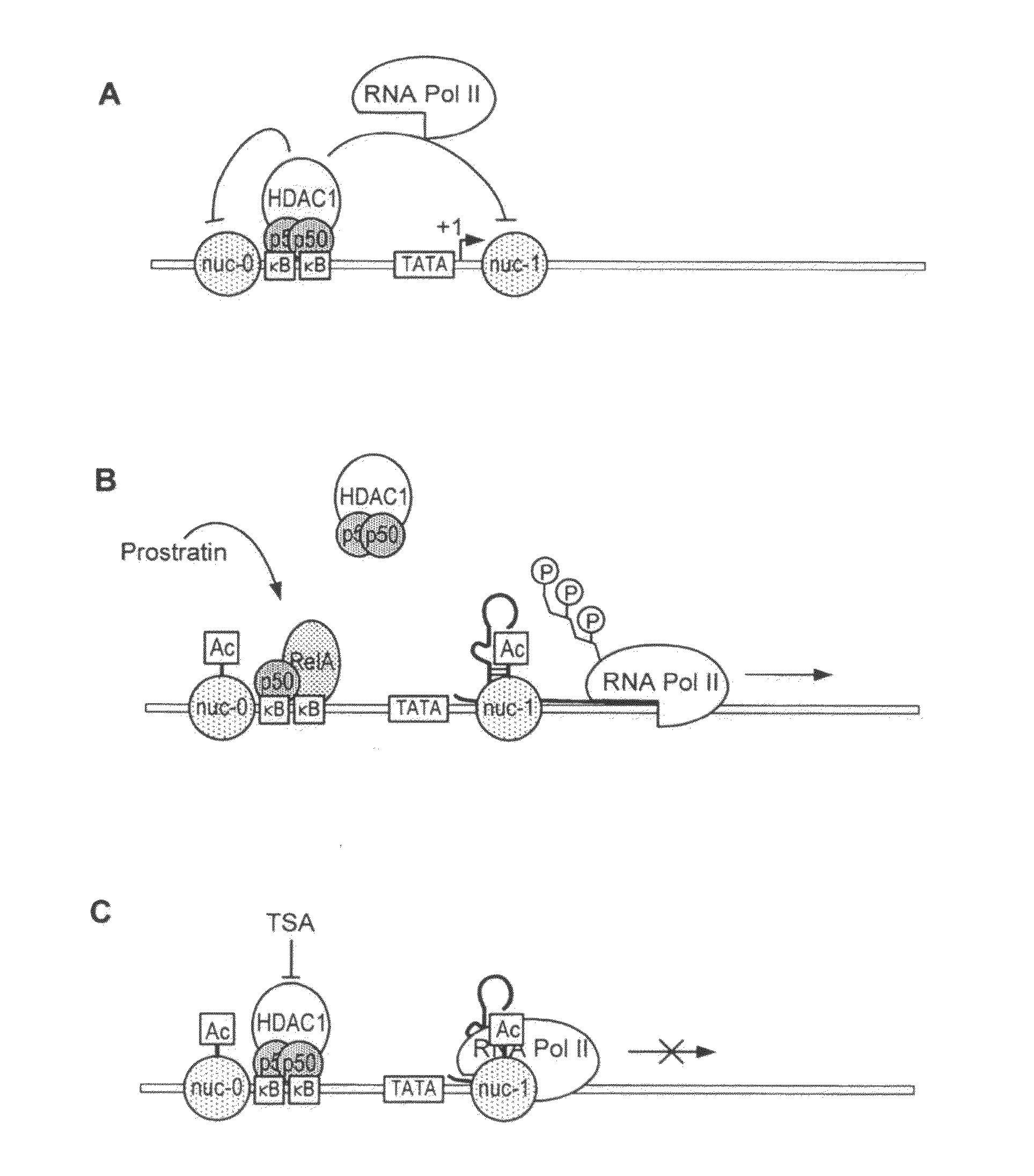
Methods and compositions for the synergistic activation of latent HIV
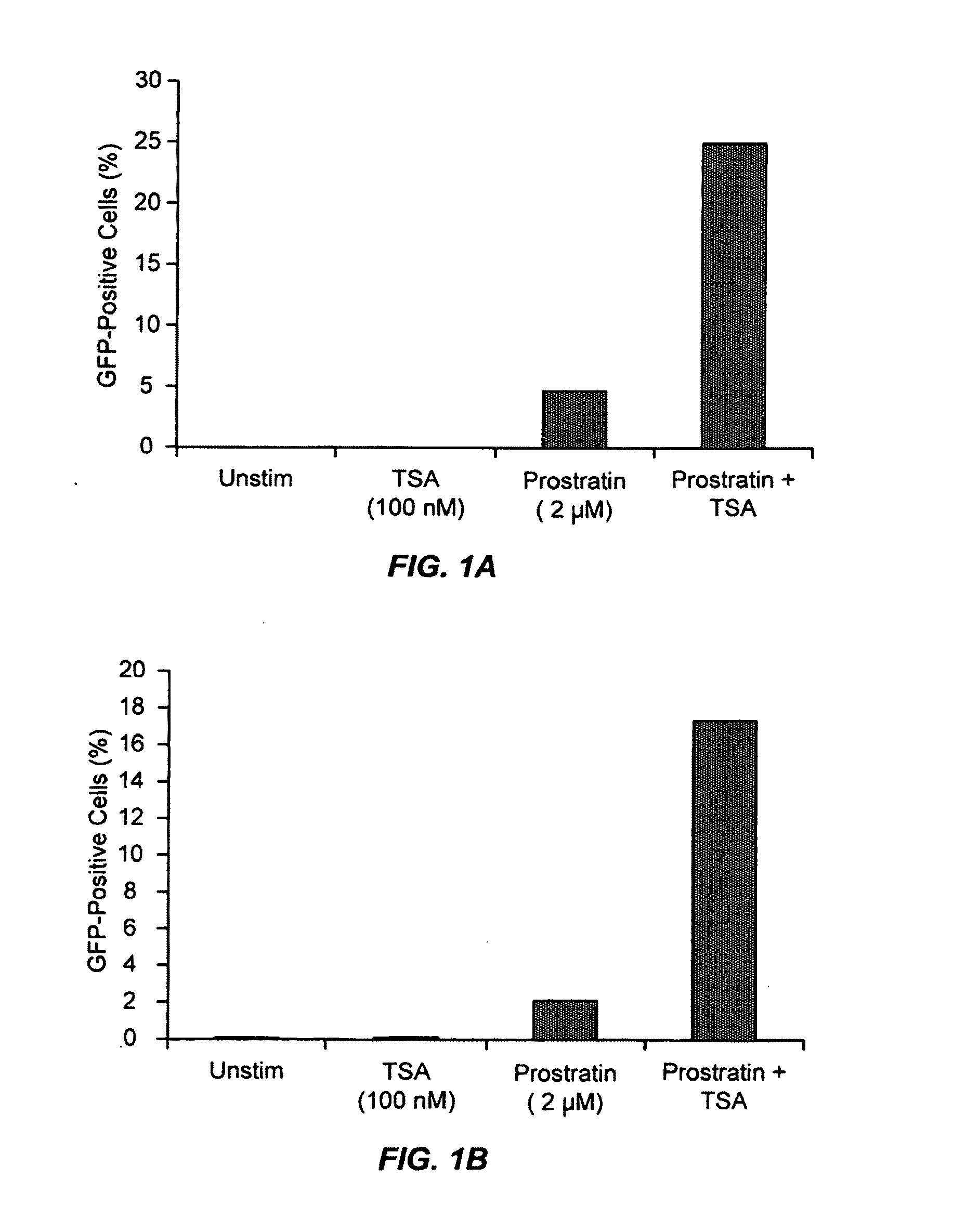
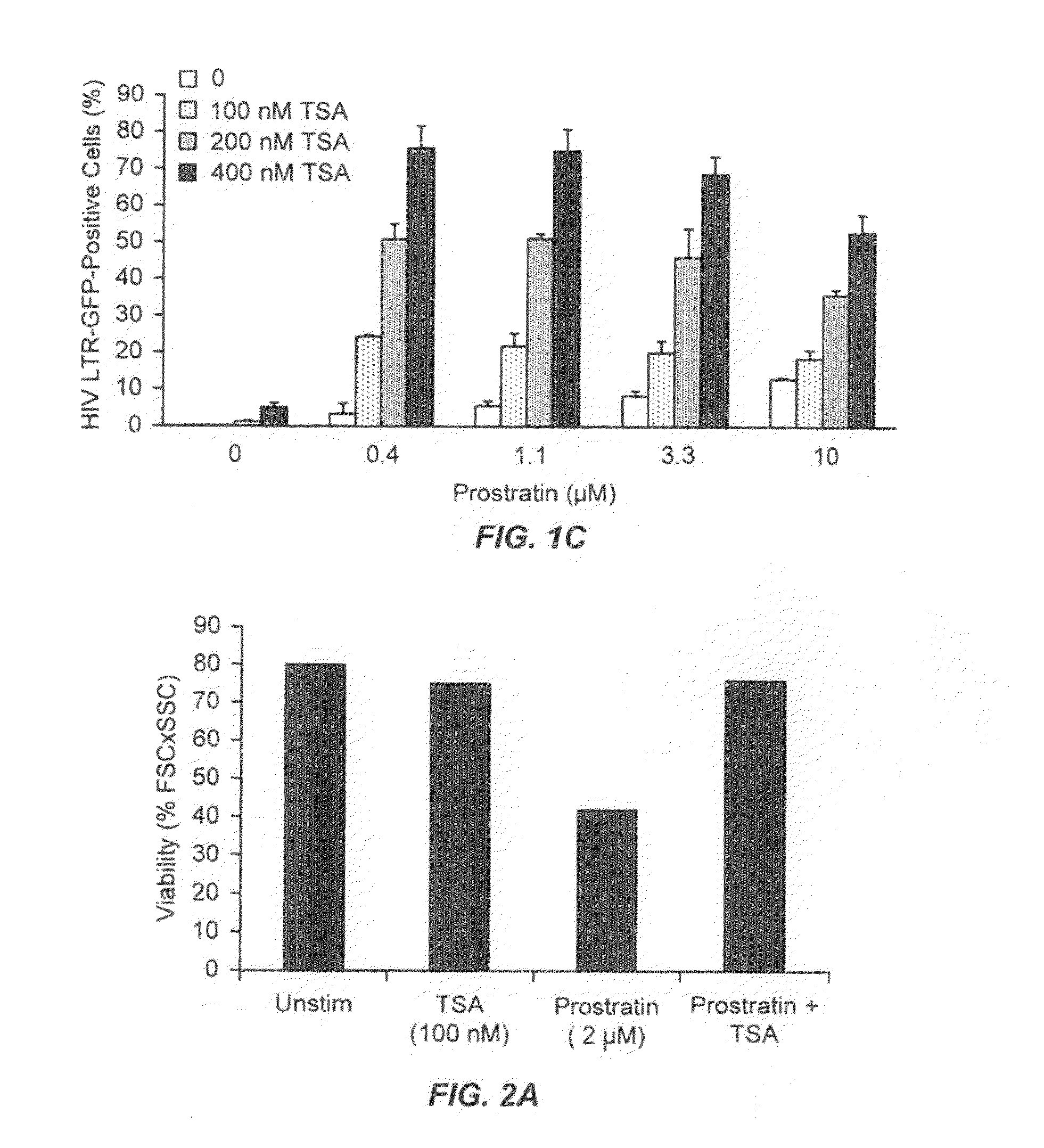
The present invention provides methods and compositions useful for the elimination of latent HIV reservoirs that persist despite HAART. The methods and compositions overcome this latent barrier by inducing the replication of HIV in latently infected T cells while preventing the spread of the newly produced virions to uninfected cells by providing HAART simultaneously. Compositions of the invention comprise an activator of latent HIV expression, such as prostratin, and an inhibitor of histone deacetylase, such as TSA. A surprising finding of this invention is that the inhibitor of the histone deacetylase synergizes the effect of prostratin thus, allowing administering to a patient a lower, non-toxic dose of prostratin.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
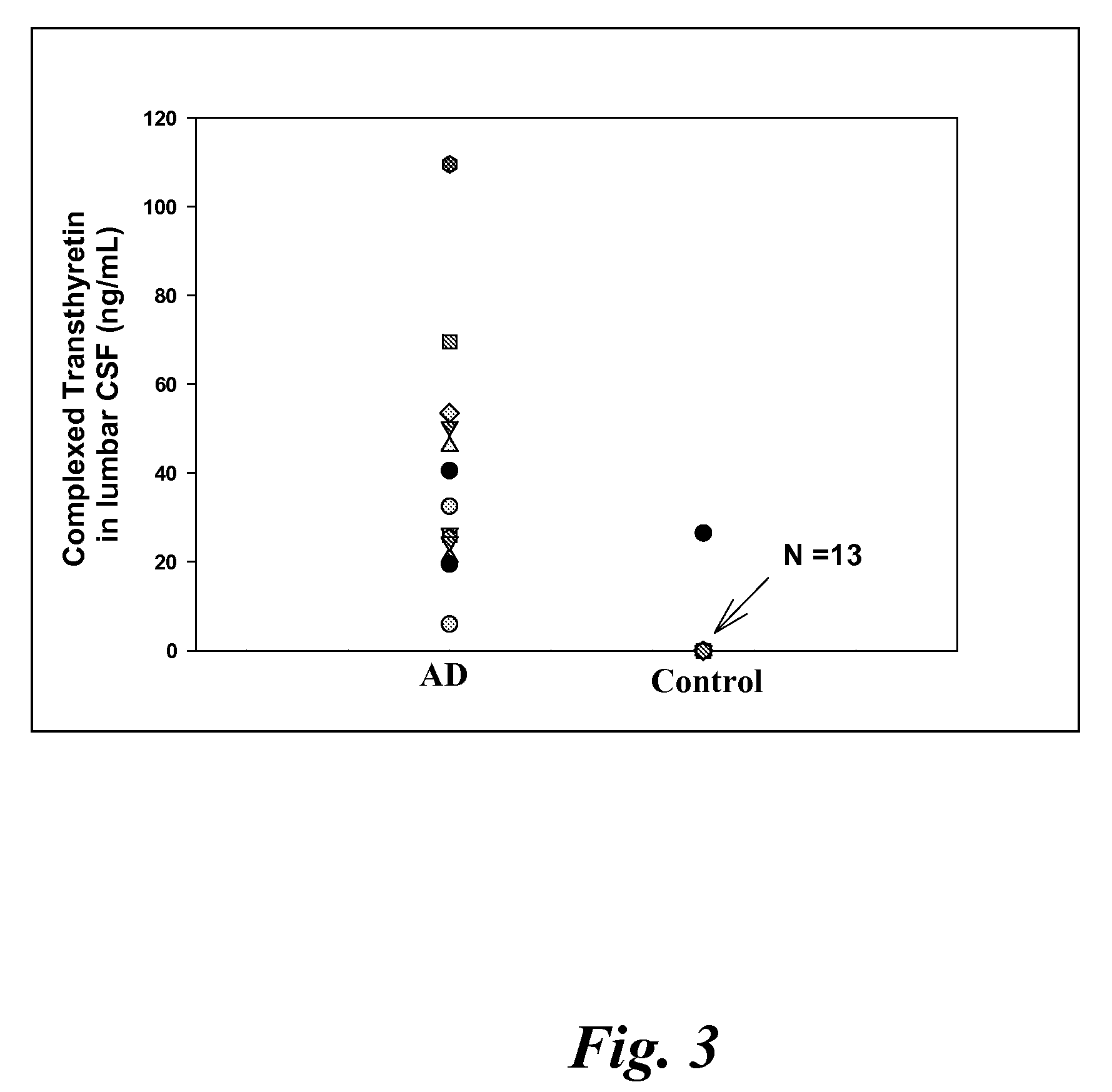
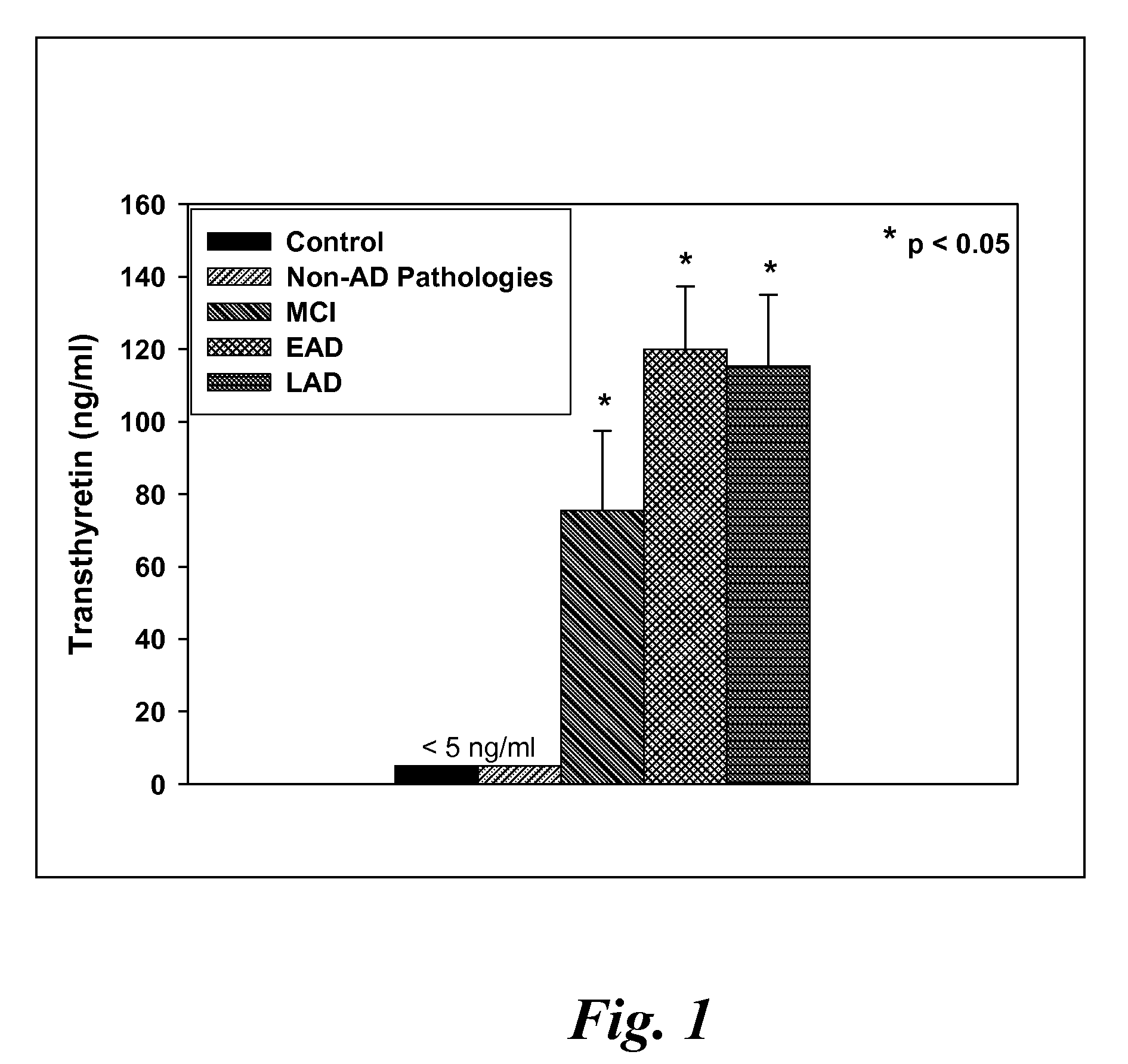
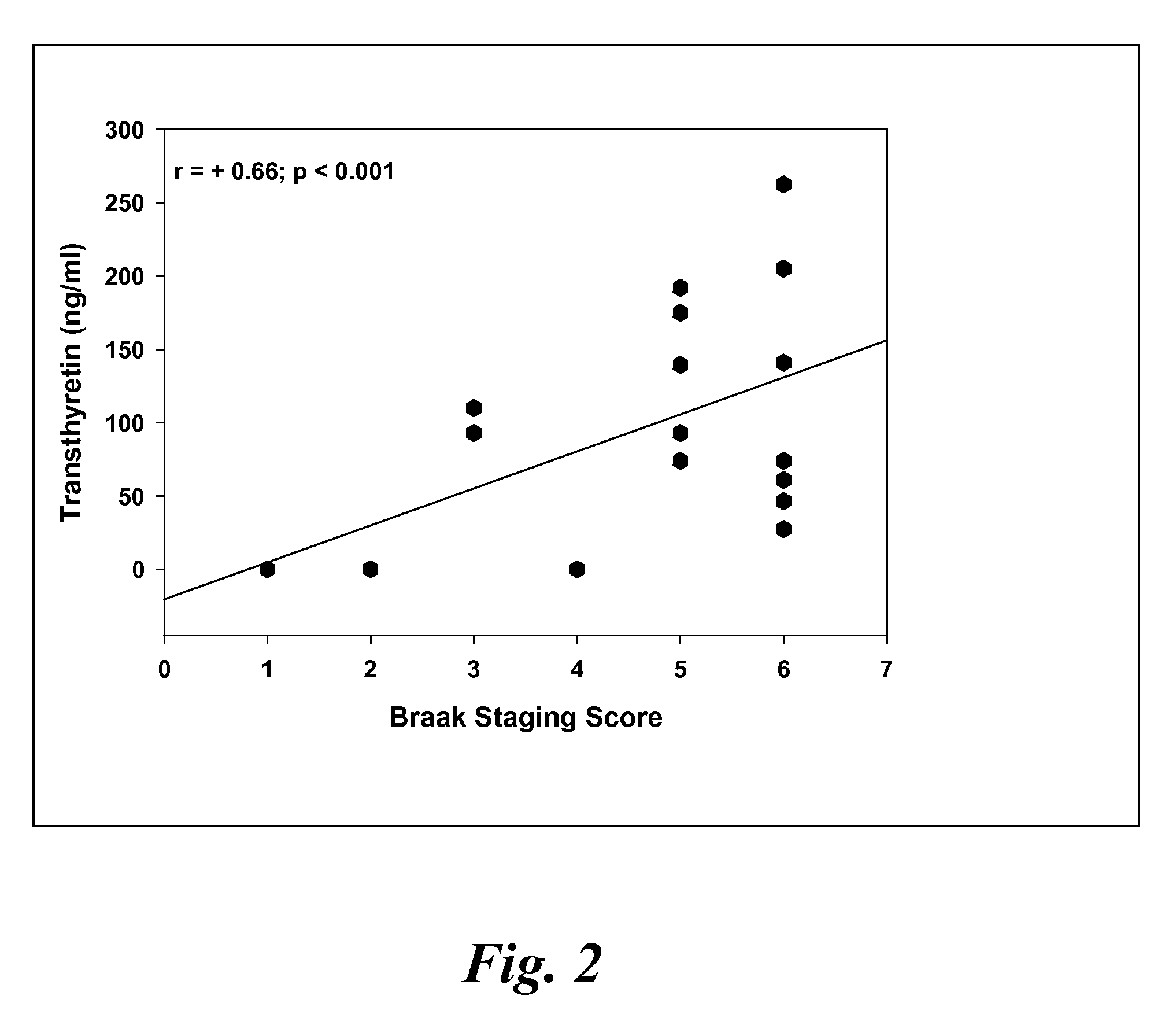
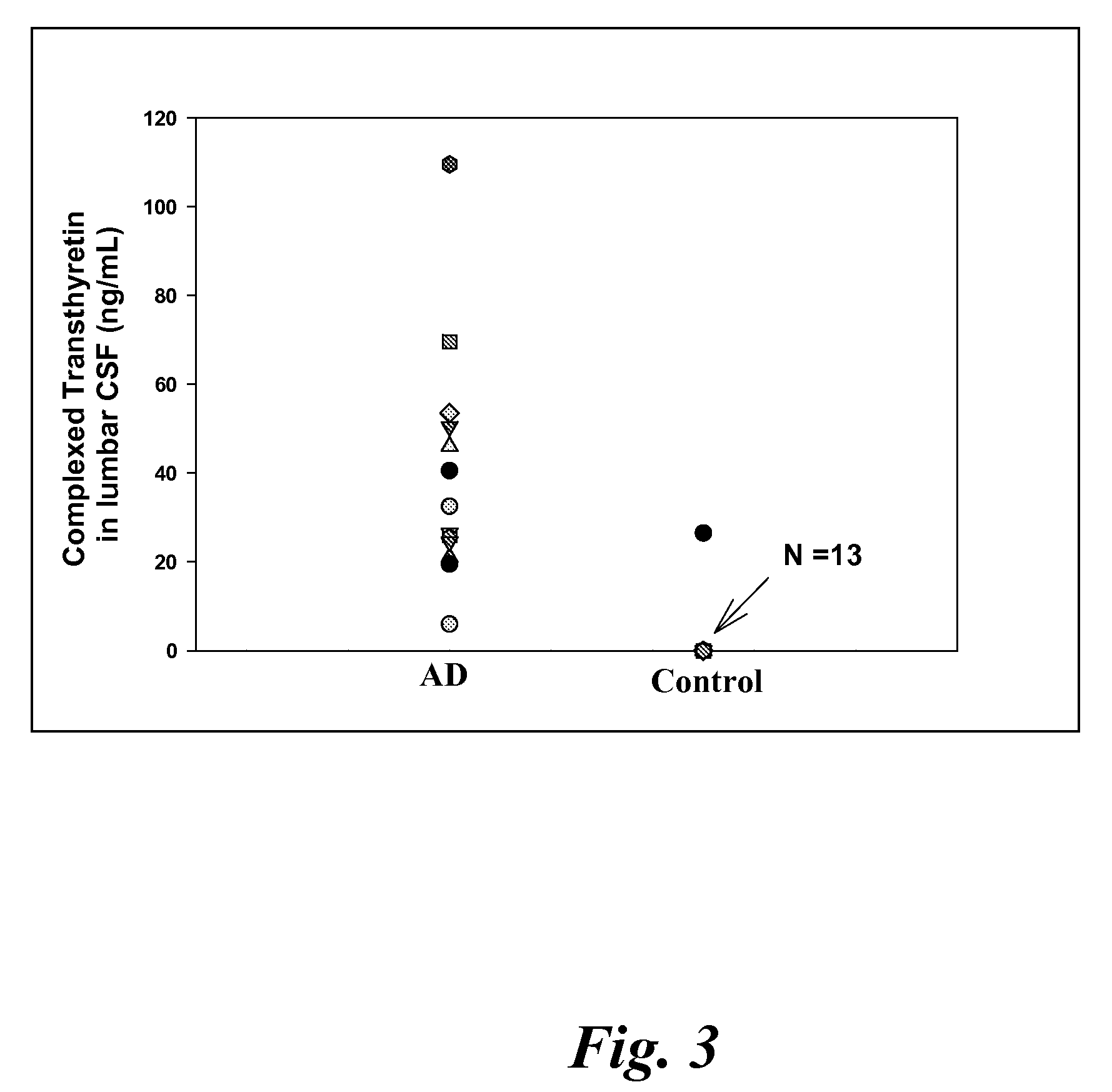
Biomarkers of mild cognitive impairment and alzheimer's disease
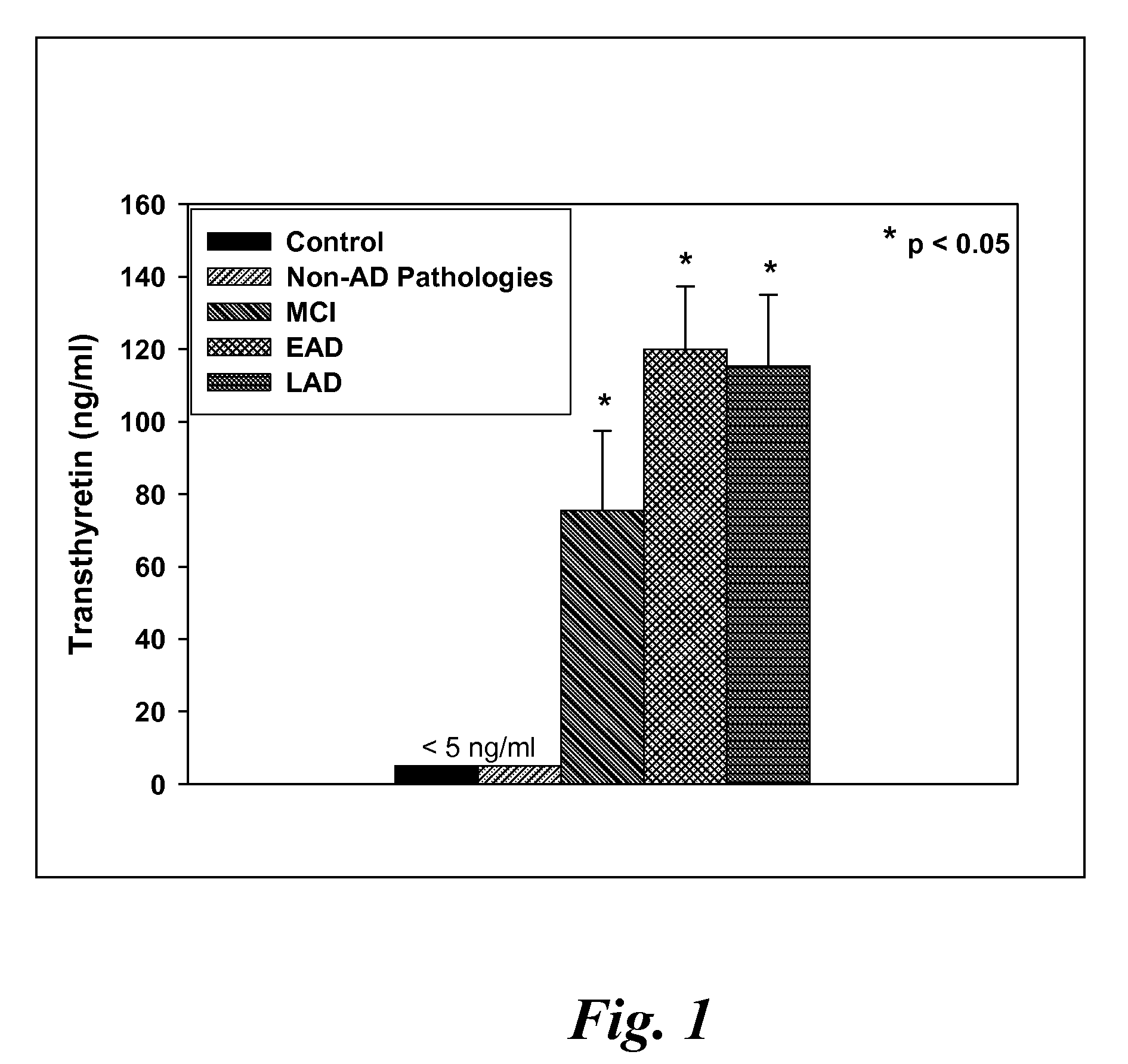
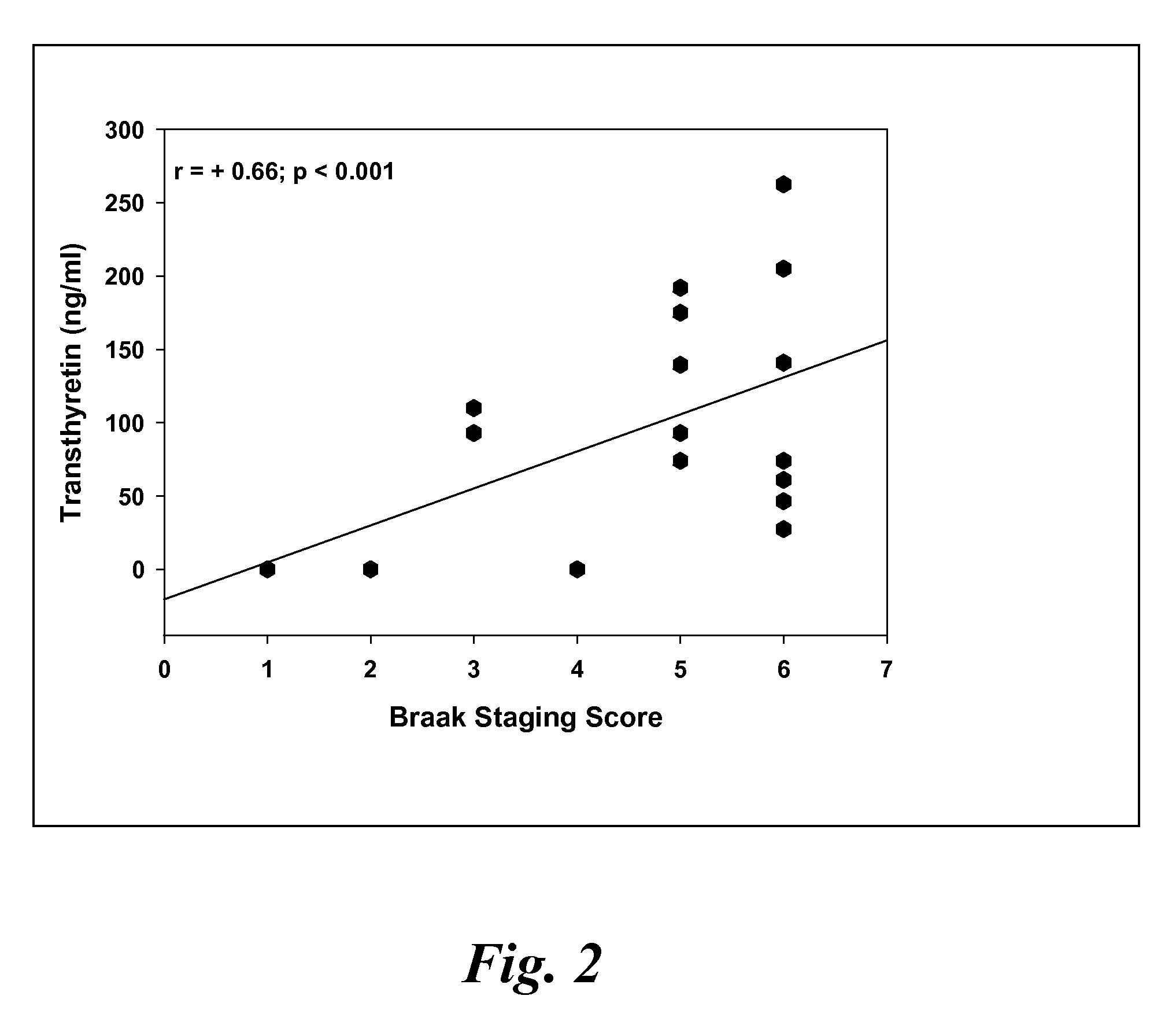
A method for quantifying a neurodegenerative disorder in a patient that includes obtaining a fluid sample from the subject; measuring a protein biomarker complex in said fluid sample and correlating the measurement with mild cognitive impairment or Alzheimer's disease status. The biomarkers include those that comprise at least one of a transthyretin protein and / or a prostaglandin-H2 D-isomerase protein, and at least one second, different protein selected from a transthyretin, prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide dismutase [Cu—Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein, carbonic anhydrase 2, prostaglandin-H2 D-isomerase, and / or serotransferrin protein.
Owner:UNIV OF KENTUCKY RES FOUND
Prostaglandin agonists
This invention relates to prostaglandin agonists, methods of using such prostaglandin agonists, pharmaceutical compositions containing such prostaglandin agonists and kits containing such prostaglandin agonists. The prostaglandin agonists are useful for the treatment of bone disorders including osteoporosis.
Owner:PFIZER INC
10-Hydroxy-11-dihydroprostaglandin analogs as selective EP4 agonists
A compound comprising or a pharmaceutically acceptable salt or a prodrug thereof, wherein the dashed line represents the presence or absence of a double bond; J is C═O or CHOH; A is —(CH2)6—, or cis —CH2CH═CH—(CH2)3—, wherein 1 or 2 carbons may be substituted with S or O; B is CO2H, or CO2R, CONR2, CONHCH2CH2OH, CON(CH2CH2OH)2, CH2OR, P(O)(OR)2, CONRSO2R, SONR2, or R is H, C1-6 alkyl; D is —(CH2)n—, —X(CH2)n, or —(CH2)nX—, wherein n is from 0 to 3 and X is S or O; and E is an aromatic or heteroaromatic moiety having from 0 to 4 substituents, said substituents each comprising from 1 to 6 non-hydrogen atoms is disclosed herein. Methods, compositions, and medicaments related thereto, as well as experimental results showing prostaglandin EP4 selective agonist activity for certain compounds disclosed herein, are also disclosed.
Owner:ALLERGAN INC
Biomarkers of mild cognitive impairment and alzheimer's disease
A method for quantifying a neurodegenerative disorder in a patient that includes obtaining a fluid sample from the subject; measuring a protein biomarker complex in said fluid sample and correlating the measurement with mild cognitive impairment or Alzheimer's disease status. The biomarkers include those that comprise at least one of a transthyretin protein and / or a prostaglandin-H2 D-isomerase protein, and at least one second, different protein selected from a transthyretin, prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide dismutase [Cu—Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein, carbonic anhydrase 2, prostaglandin-H2 D-isomerase, and / or serotransferrin protein;
Owner:UNIV OF KENTUCKY RES FOUND
Drug composition antagonistic to both PGD2/TXA2 receptors
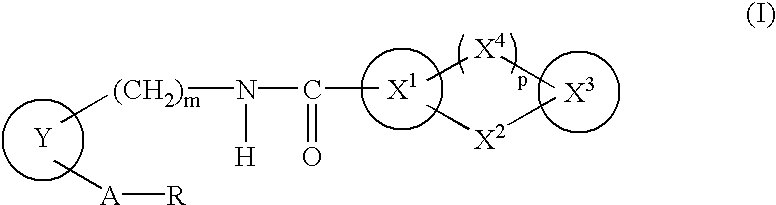
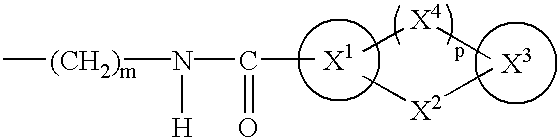
A compound of the formula (I):wherein A is alkylene optionally having an unsaturated bond; R is —C(═O)—R1; R1 is hydroxy or the like; m is 0 or 1; p is 0 or 1; X1 and X3 are each independently optionally substituted aryl or optionally substituted heteroaryl or the like; X2 is a bond, —CH2—, —S—, —SO2—, —CH2—O—, —O—CH2—, —CH2—S—, —S—CH2—, or the like; X4 is —CH2—, —CH2—CH2—, —C(═O)—, or the like, having a dual antagonistic activity against both a thromboxane A2 receptor and a prostaglandin D2 receptor is found.
Owner:SHIONOGI & CO LTD
Prostaglandin agonists
This invention relates to prostaglandin agonists, methods of using such prostaglandin agonists, pharmaceutical compositions containing such prostaglandin agonists and kits containing such prostaglandin agonists. The prostaglandin agonists are useful for the treatment of bone disorders including osteoporosis.
Owner:PFIZER INC
Quick-releasing talbet in vagina and its preparing method
InactiveCN1473561AImprove complianceLittle discomfortOrganic active ingredientsPill deliveryTreatment effectWater insoluble
The quick releasing vagina tablet includes various active medicinal components, such as antibiotic, antifungal, antiviral, hormone, prostanoid, etc.; and medicinal supplementary material stuffing, adhesive and disintegrating agent. The stuffing includes water soluble stuffing and water insoluble stuffing. The quick releasing vagina tablet, after contacting with vagina secretion, can disintegrate to disperse or dissolve so that it is absorbed via vagina mucous membrane to produce local treatment effect and made to enter body circulation to produce bodily treatment effect. It is convenient and accommodative.
Owner:NINGBO LIWAH PHARM CO LTD
Prostaglandin agonists
InactiveUS20030078261A1Reduce the chance of fractureIncrease bone formationBiocideSenses disorderAgonistOsteoporosis
Owner:PFIZER INC
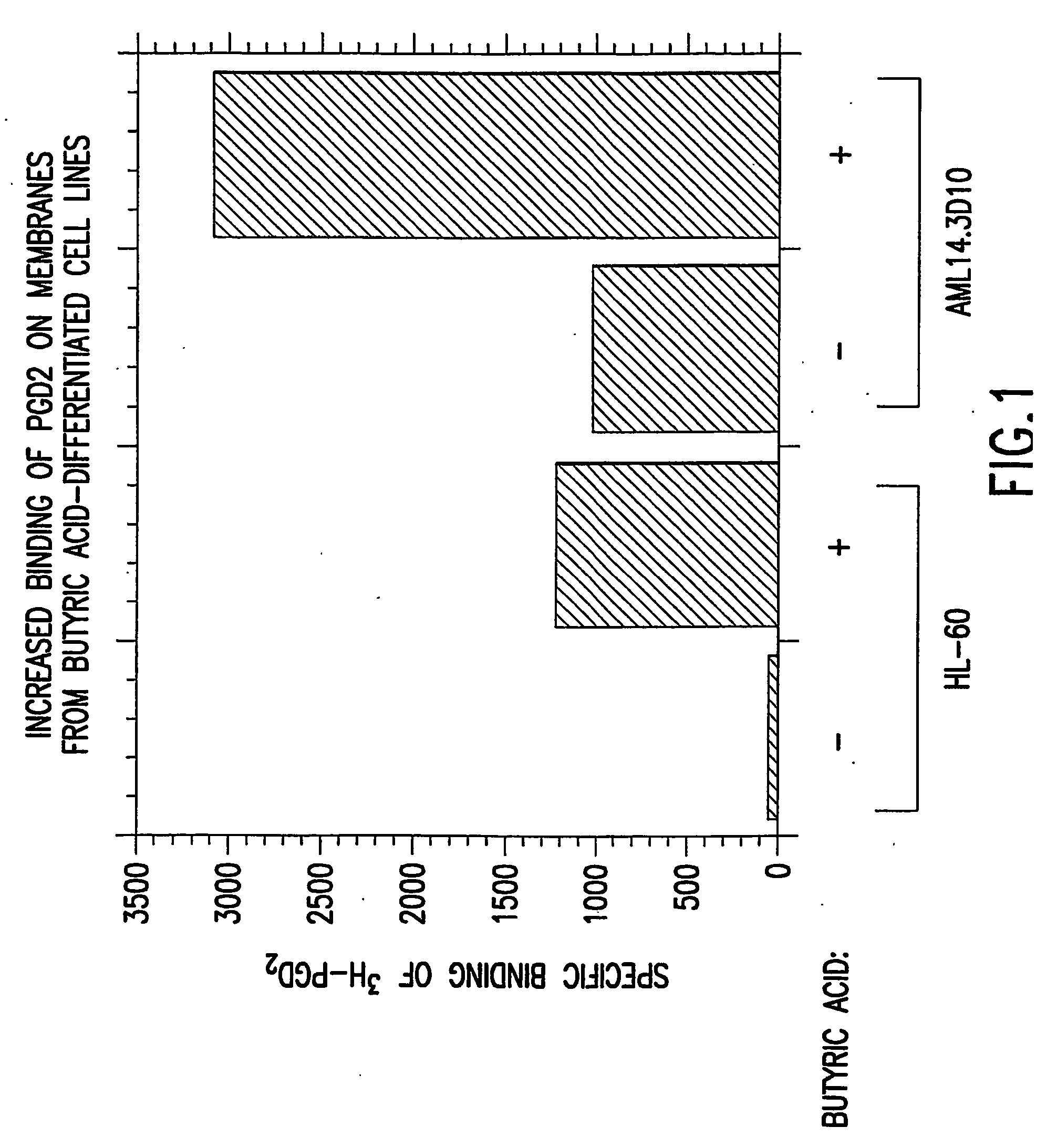
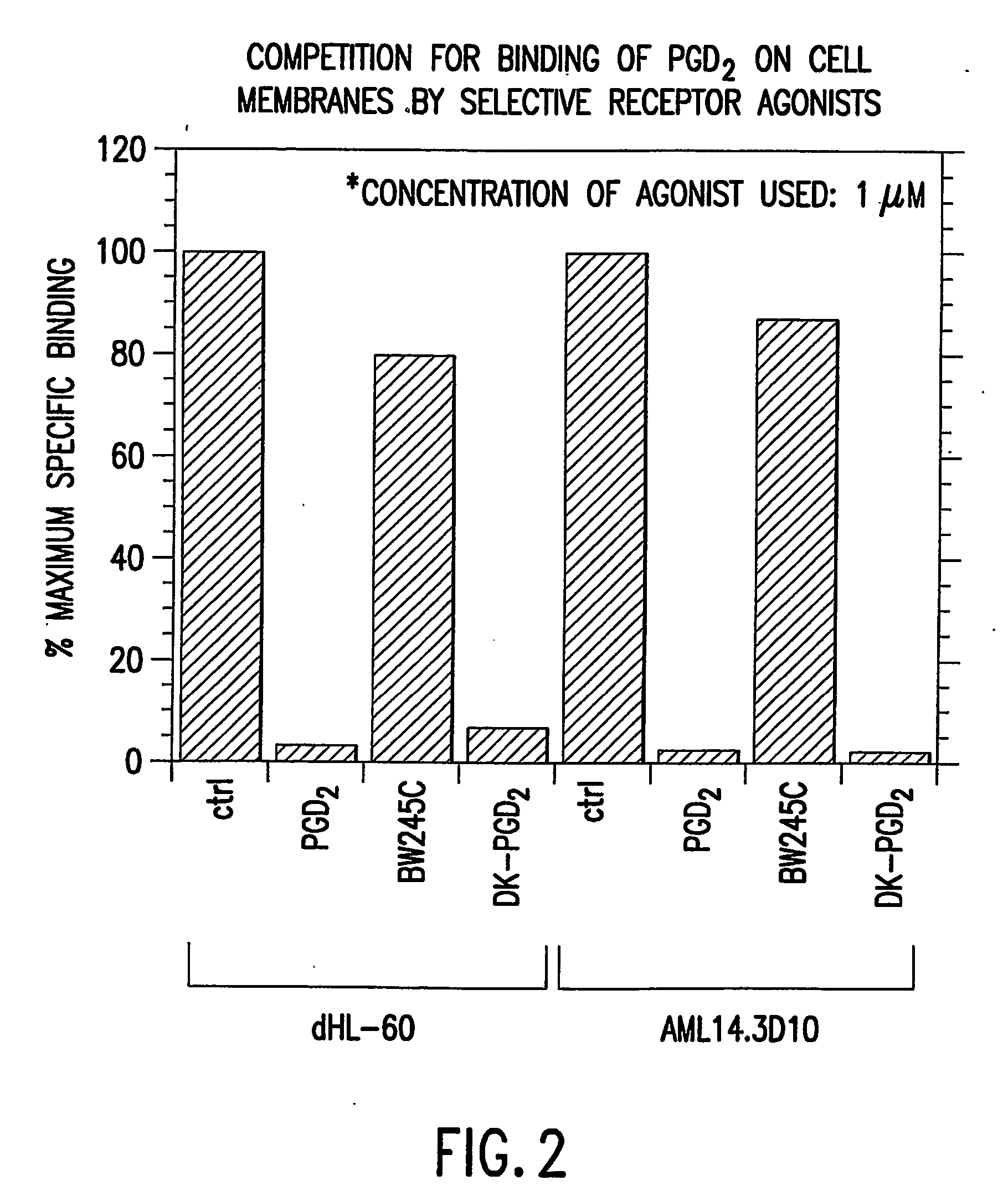
Method to increase expression of pgd2 receptors and assays for identifying modulators of prostaglandin d2 receptors
InactiveUS20040197834A1Increase in cAMPIncrease blockingBiological testingProstaglandins DReceptor for activated C kinase 1
The present invention provides cell lines expressing endogenous PGD2-specific receptors, methods for increasing expression of the receptors and assays utilizing the hereindisclosed cell lines for identifying modulators of the PGD2-specific receptors. Increasing expression of PGD2-specific receptors is achieved by treating the disclosed cell lines with an agent that induces differentiation.
Owner:MERCK FROSST CANADA INC +1
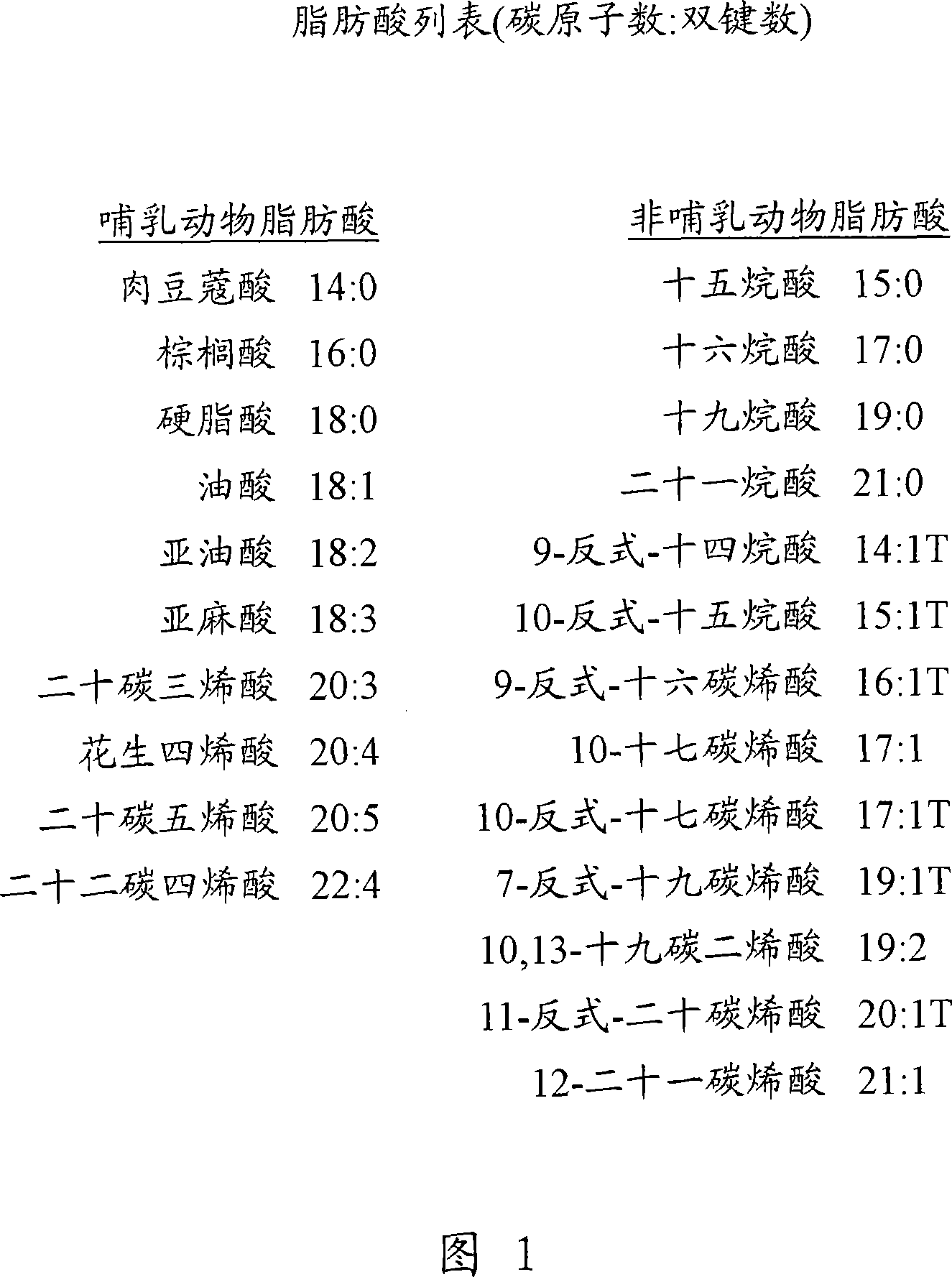
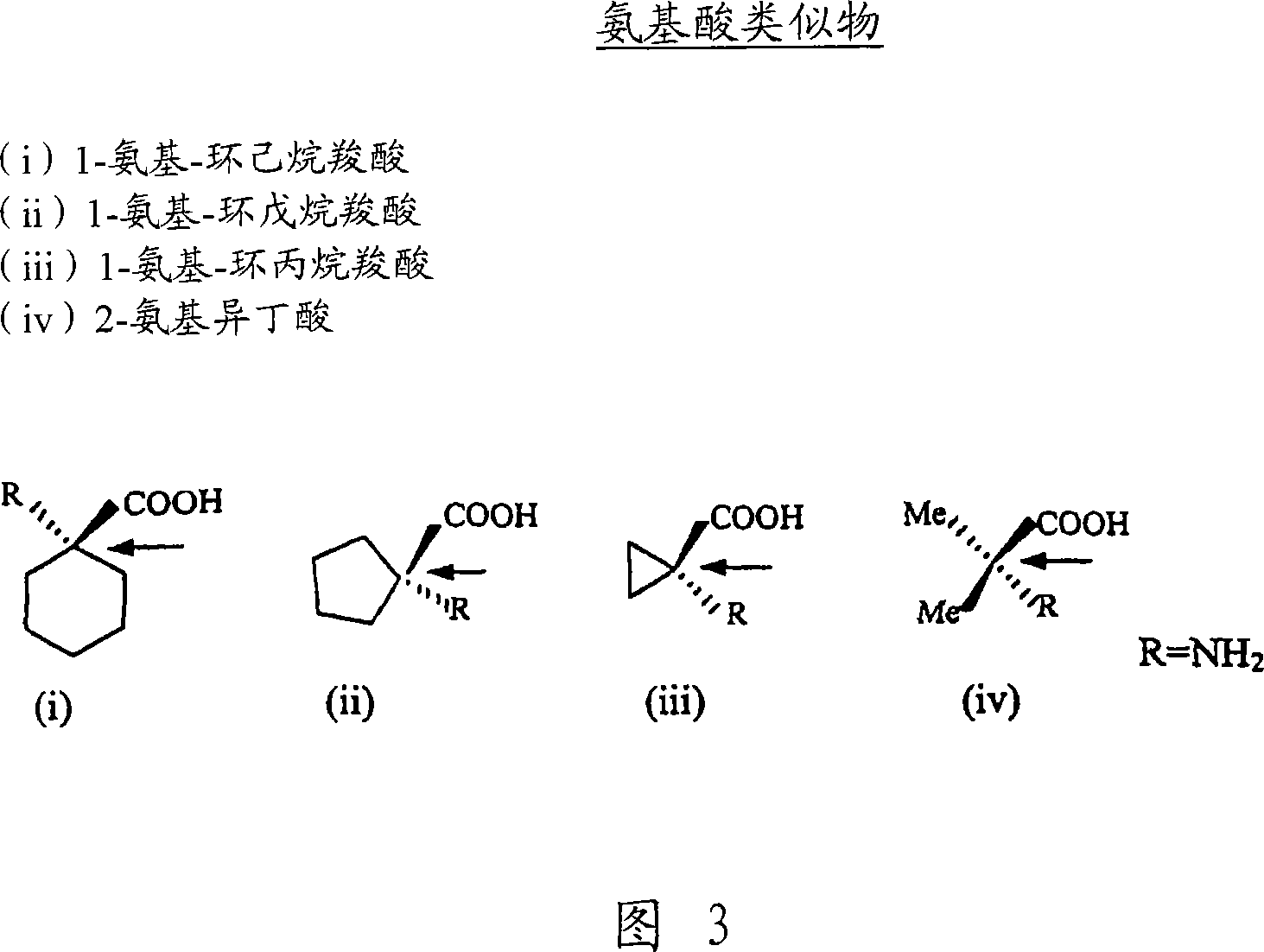
Lipid-amino acid conjugates and methods of use
N-fatty acid-amino acid conjugates and J2 prostanoid-amino acid conjugates are disclosed along with methods for making such conjugates and methods of using these conjugates in the treatment of conditions that involved dysfunctional lipid metabolism, insulin sensitivity, glucose homeostasis, and / or inflammation.
Owner:UNIV OF MASSACHUSETTS
Intravenous composition, process for producing the same and preparation thereof
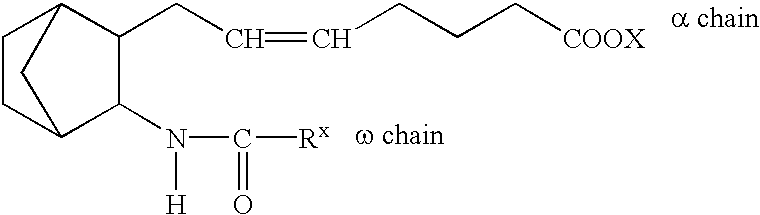
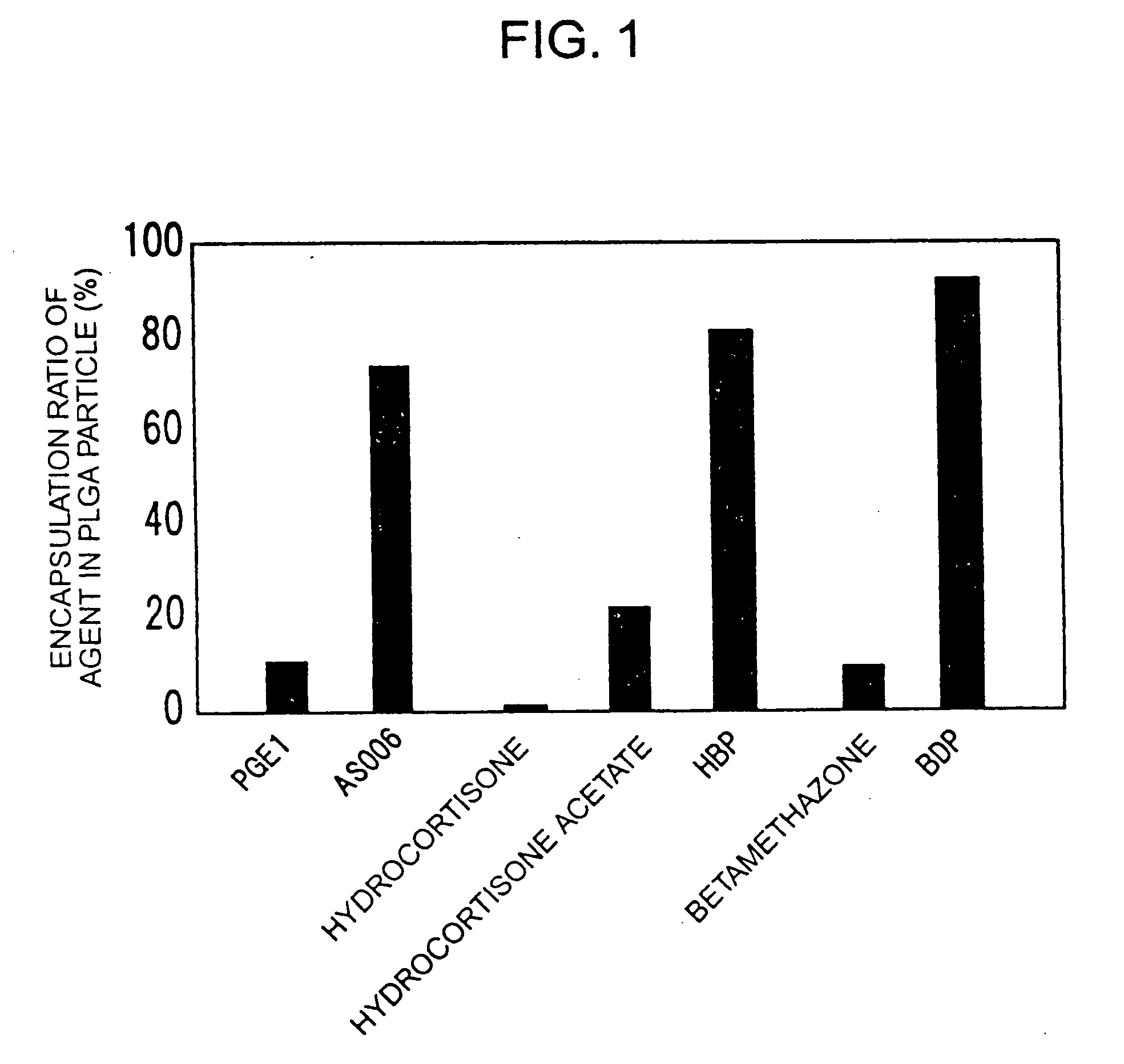
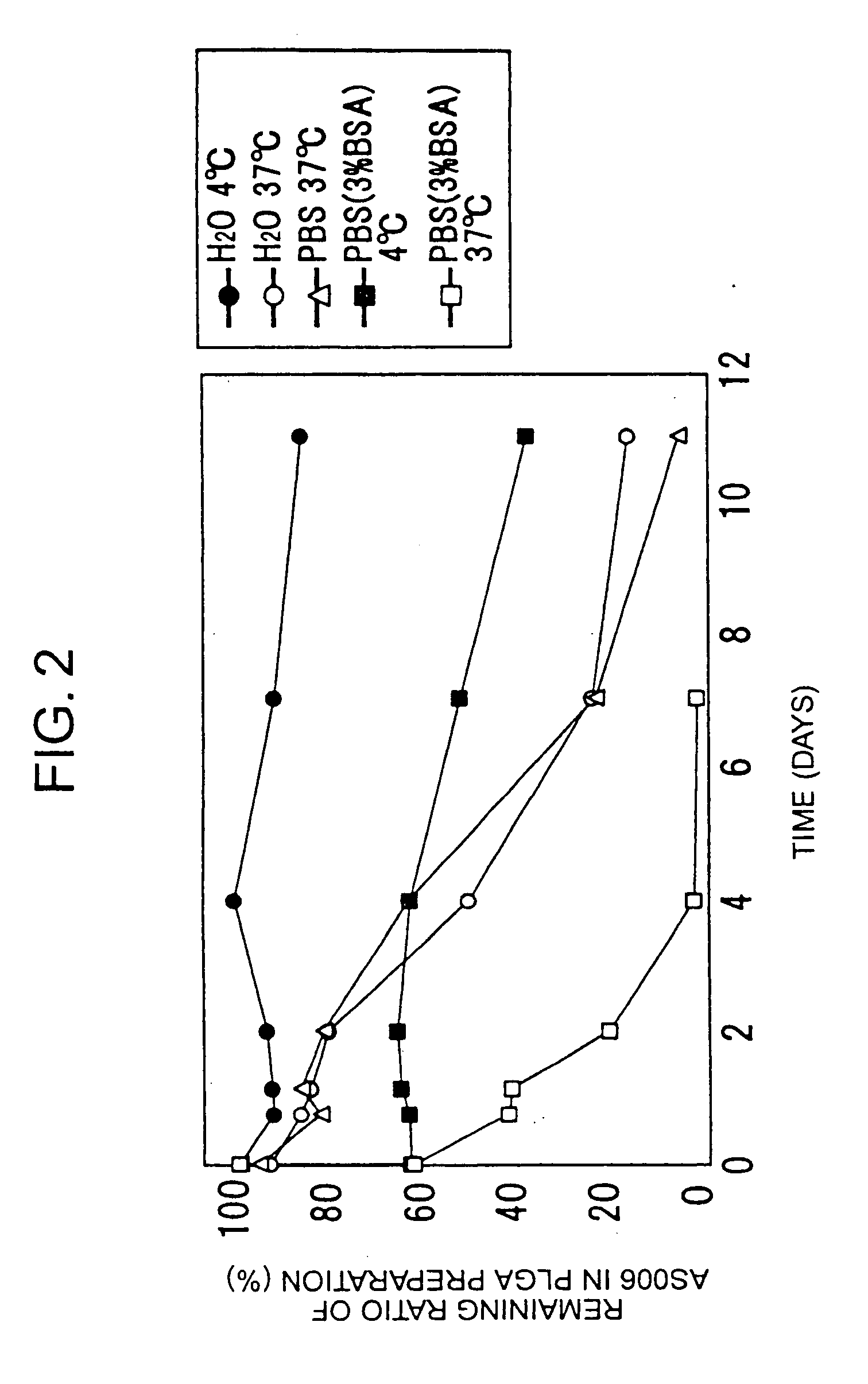
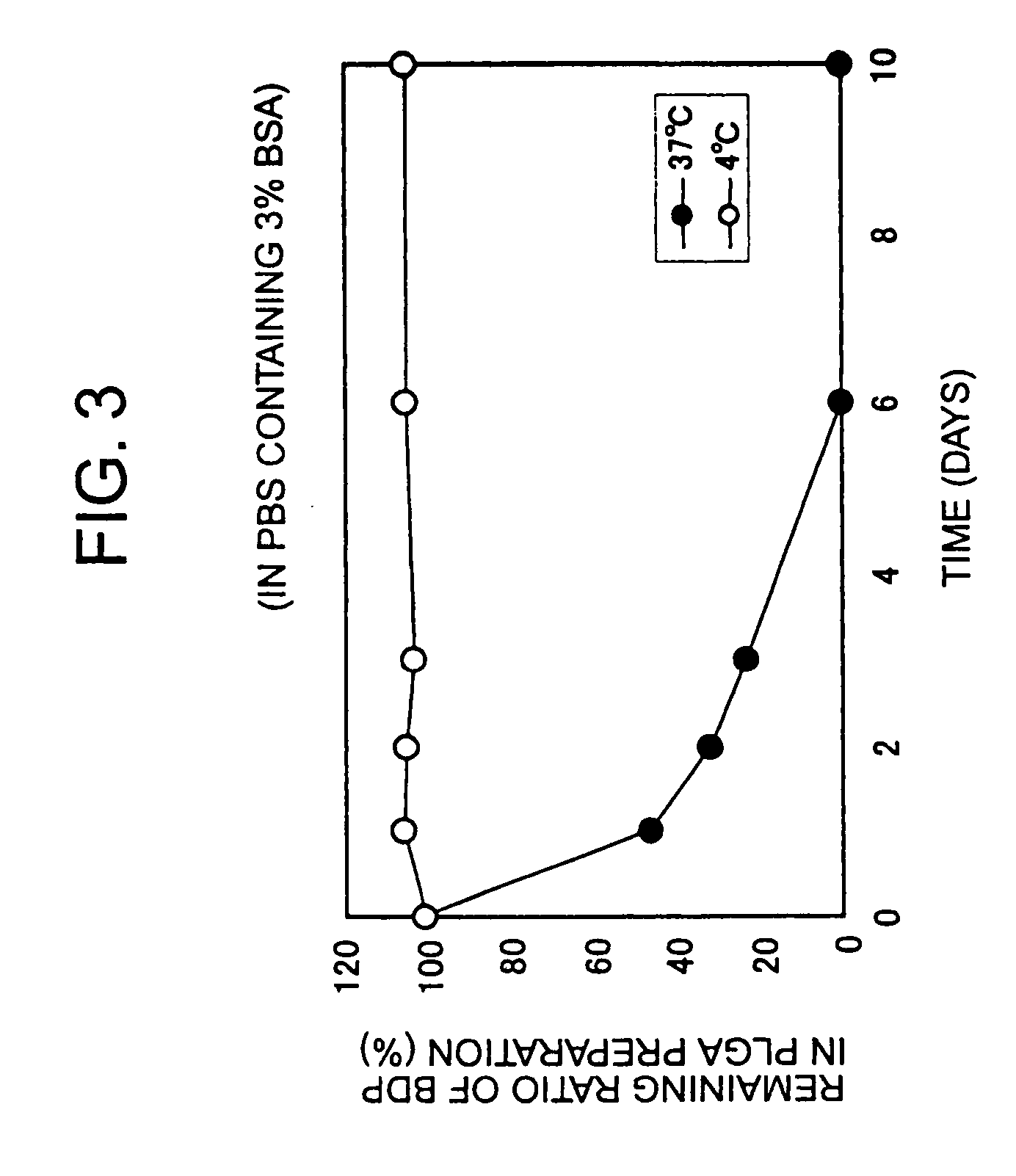
InactiveUS20060088598A1Sufficient sustained release effectGood sustained release effectBiocidePowder deliveryMicroparticleProstanoic acid
A composition for intravenous injection, which gradually decomposed instead of fat particles, has sufficient sustained release effects, has an excellent encapsulation ratio of lipid-soluble agents, and has such sustained release effects at lesion sites; a production method thereof; and a preparation containing the composition. The composition for intravenous injection is produced by encapsulating a prostanoid or steroid in a poly(lactic-co-glycolic acid) or poly(lactic acid) microparticle, and allowing lecithin or similar surfactant to be adsorbed on the surface or the above poly(lactic-co-glycolic acid) or poly(lactic acid) microparticle. A method for producing the composition comprises: dissolving esterified prostanoic acid or esterified steroid and a poly(lactic-co-glycolic acid) or poly(lactic acid) in an organic solvent such as dichloromethane or dimethylsulfoxide; and emulsilfying the obtained mixture in water, using the lecithin or similar surfactant capable of adjusting the diameter of the copolymer or particle to between 50 and 500 nm, employing an ultrasonic generator, a Polytron Homogenizer, or the like.
Owner:LTT BIO PHARMA
Prostaglandin transporter inhibitors
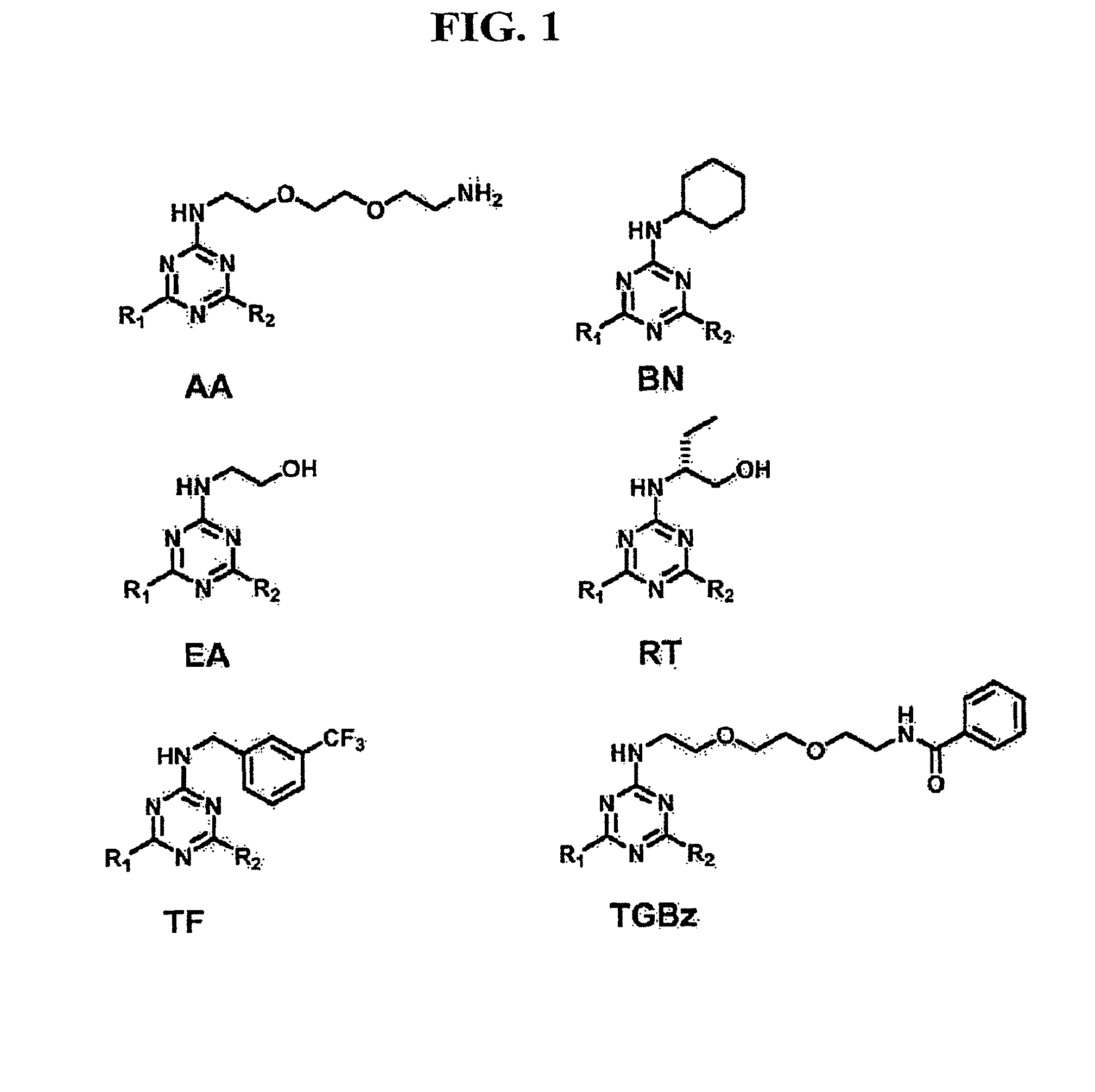
Provided are methods of inhibiting prostaglandin transporter (PGT) activity in mammals. Also provided are methods of determining whether a test compound is an inhibitor of a prostaglandin transporter. Additionally provided are compounds that inhibit prostaglandin transporter activity, and pharmaceutical compositions of those compounds. Methods of inhibiting COX-2 in a mammal are also provided. Additionally, methods of treating pain or inflammation in a mammal are provided.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV +1
Inhibition of neovascularization by inhibition of prostanoid IP receptors
ActiveUS20140275238A1Reduce neovascularizationBiocideSenses disorderMedicineCorneal neovascularization
There are provided inter alia methods and compounds useful for decreasing choroidal neovascularization in a subject in need thereof.
Owner:ALLERGAN INC
Antibodies to human chemokine beta-9
InactiveUS20050244888A1Promote wound healingChronic infectionBacteriaPeptide/protein ingredientsAbnormal tissue growthBone marrow failure
Human Ckβ-9 polypeptides and DNA (RNA) encoding such chemokine polypeptides and a procedure for producing such polypeptides by recombinant techniques is disclosed. Also disclosed are methods for utilizing such Ckβ-9 polypeptides for the treatment of leukemia, tumors, chronic infections, autoimmune disease, fibrotic disorders, wound healing and psoriasis. Antagonists against such polypeptides and their use as a therapeutic to treat rheumatoid arthritis, autoimmune and chronic inflammatory and infective diseases, allergic reactions, prostaglandin-independent fever and bone marrow failure are also disclosed. Diagnostic assays are also disclosed which detect the presence of mutations in the Ckβ-9 coding sequence and over-expression of the Ckβ-9 protein.
Owner:HUMAN GENOME SCI INC
Treatment of inflammation with p20
InactiveUS7074399B2High expressionHigh resolutionBiocidePeptide/protein ingredientsCytokineProstaglandin PGE
The present invention provides compositions, methods, and kits for treating inflammation and regulating inflammatory responses including cytokine, prostanoid, prostaglandin, and growth factor expression.
Owner:VANDERBILT UNIV
Human chemokine beta-10 mutant polypeptides
Human chemokine Beta-10 polypeptides and DNA (RNA) encoding such chemokine polypeptides and a procedure for producing such polypeptides by recombinant techniques is disclosed. Also disclosed are methods for utilizing such chemokine polypeptides for the treatment of leukemia, tumors, chronic infections, autoimmune disease, fibrotic disorders, wound healing and psoriasis. Antagonists against such chemokine polypeptides and their use as a therapeutic to treat rheumatoid arthritis, autoimmune and chronic inflammatory and infective diseases, allergic reactions, prostaglandin-independent fever and bone marrow failure are also disclosed.
Owner:SMITHKLINE BECKMAN CORP +1
Combination therapies using agents that target tumor-associated stroma or tumor cells and tumor vasculature
ActiveUS20170101464A1Treating and ameliorating effectOrganic active ingredientsImmunoglobulins against growth factorsAbnormal tissue growthAntigen
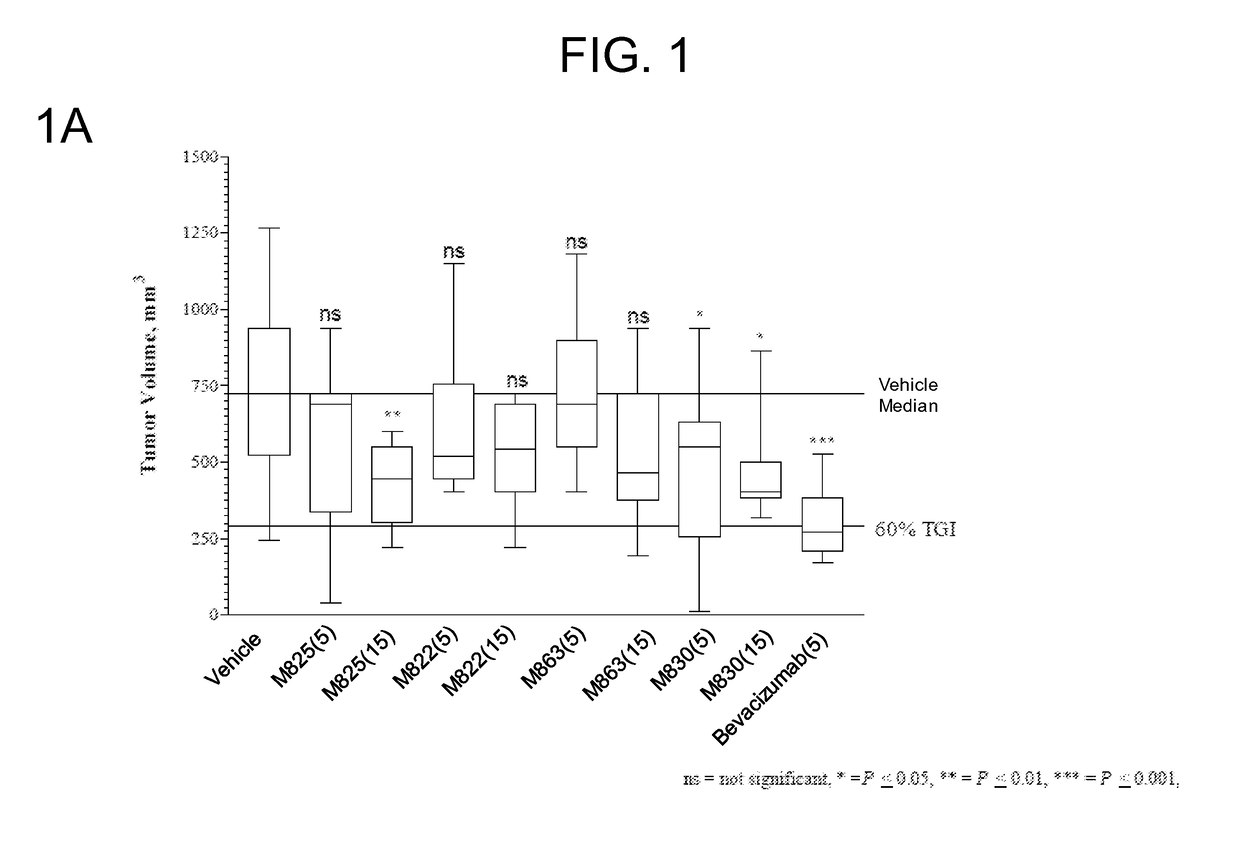
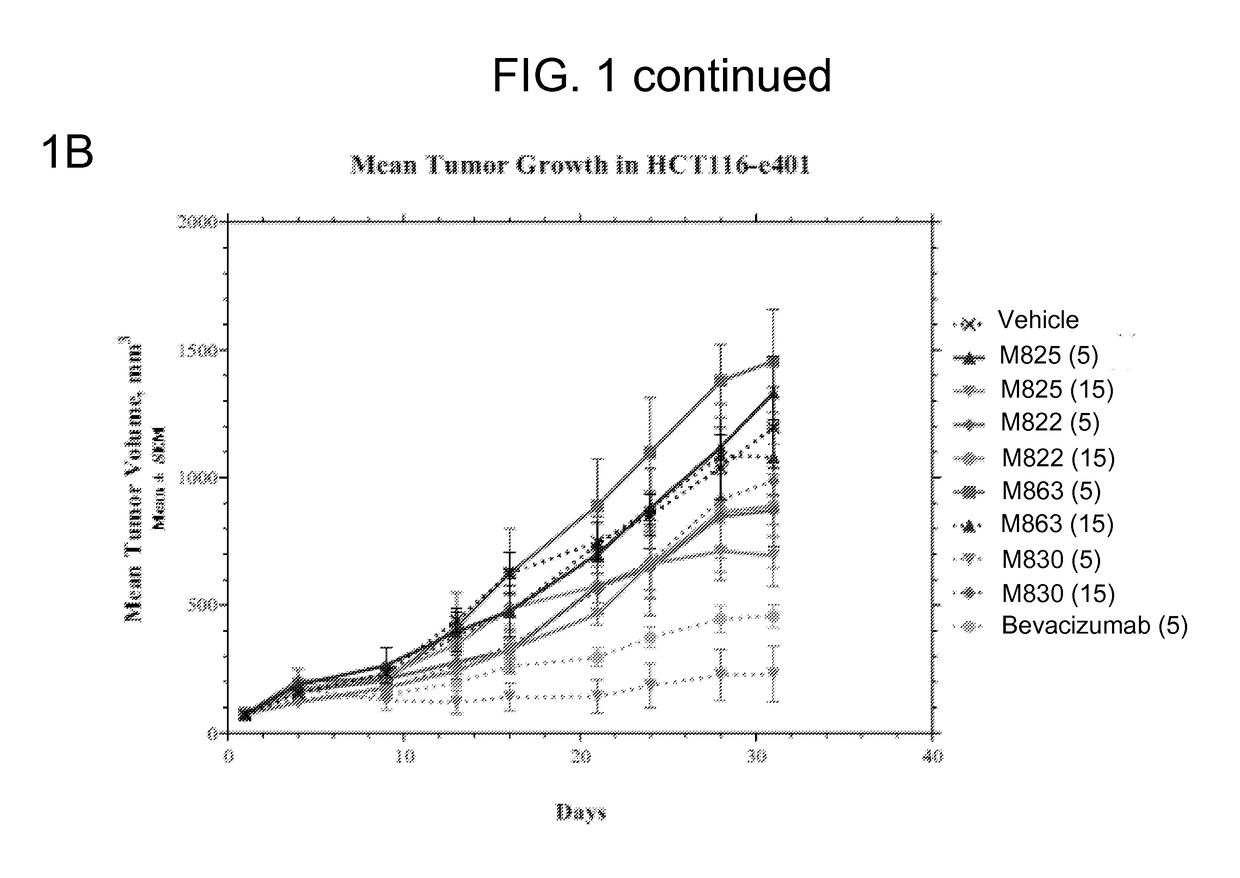
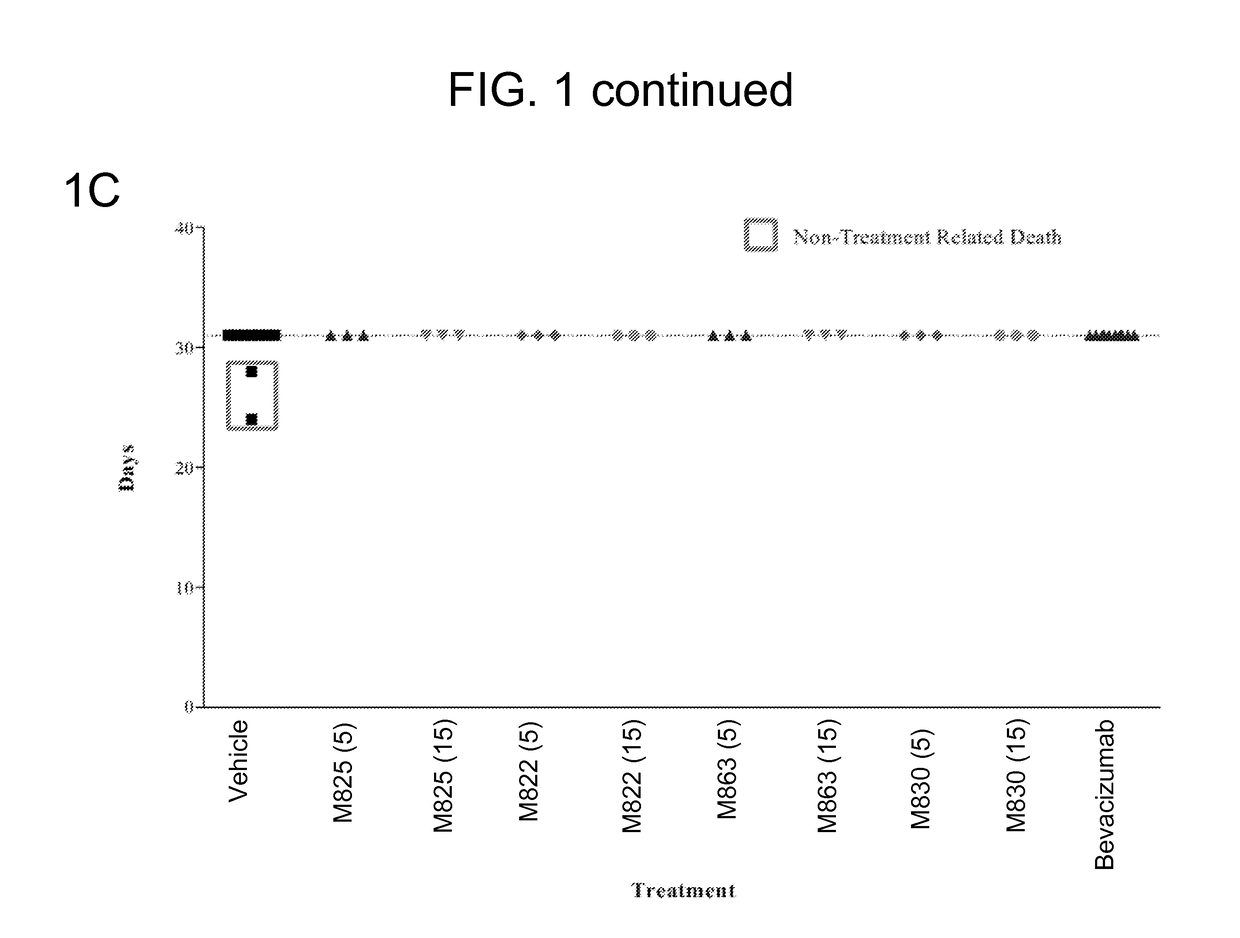
The present invention provides, inter alia, methods for treating or ameliorating the effects of a disease, such as cancer, in a subject. The methods include: administering to a subject in need thereof (a) a therapeutically effective amount of a monoclonal antibody or antigen binding fragment of the present invention, and (b) a therapeutically effective amount of a combination therapy including bevacizumab and at least one additional therapeutic agent; or a therapeutically effective amount of at least one additional therapeutic agent selected from the group consisting of a COX-2 inhibitor (COXIB), a non-steroidal anti-inflammatory drug (NSAID), a prostaglandin E2 (PGE2) synthase inhibitor, and combinations thereof. Compositions, including pharmaceutical compositions, and kits for treating diseases, such as cancer, are also provided herein.
Owner:BIOMED VALLEY DISCOVERIES INC +1
Ophthalmic compositions and methods of use
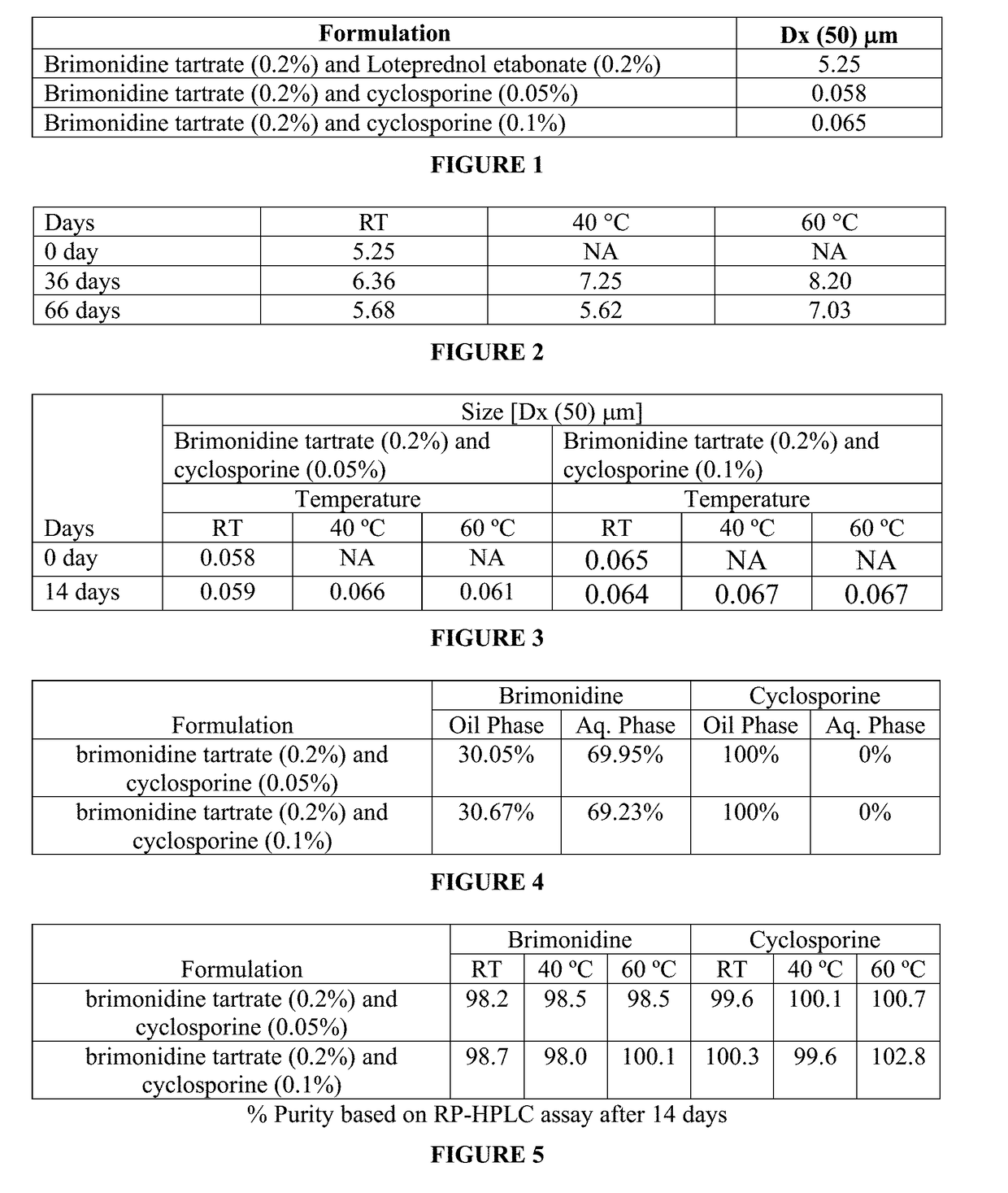
InactiveUS20190008920A1Significant comprehensive benefitsExtended releaseOrganic active ingredientsSenses disorderAntigenBeta blocker
The present invention relates to an ophthalmic composition comprising at least two active pharmaceutical ingredients. In particular, the active pharmaceutical ingredients are selected from the group consisting of: an alpha 2 adrenergic receptor agonist; a beta-adrenergic receptor agonist; an immunosuppressant; a lymphocyte associated antigen antagonist; an anti-inflammatory; a beta-blocker; a prostaglandin analog; a histamine receptor antagonist; a carbonic anhydrase inhibitor; and an antibiotic. In some embodiments, the composition of the invention is a nanoemulsion formulation. In one particular embodiment, the first active pharmaceutical ingredient is an alpha 2 adrenergic receptor agonist. The present invention also provides a method for treating various clinical conditions associated with an eye disorder or eye disease using the composition of the invention.
Owner:OCUGEN INC
Method for improving osteogenic differentiation efficiency of human amniotic mesenchymal stem cells and application thereof
InactiveCN106497871AAvoid High Immunogenicity IssuesImprove the efficiency of osteogenic differentiationCulture processSkeletal/connective tissue cellsBeta-glycerophosphateSodium glycerophosphate
The invention relates to a method for improving osteogenic differentiation efficiency of human amniotic mesenchymal stem cells. The method adds an induction composition in the human amniotic mesenchymal stem cells, wherein the composition comprises 0.1-2g / L of hyaluronic acid, 0-200mg / L of prostaglandin receptor-2 selective agonist, 1-100nmol / L of dexamethasone, 0-10nmol / L of beta-glycerophosphate and 1-100mg / L of ascorbic acid. Compared with a conventional inducing method, the osteogenic differentiation efficiency of the human amniotic mesenchymal stem cells can be significantly improved, expression and activity of alkaline phosphatase are promoted, and expression of osteogenesis-related genes of RunX2, Osx, BSP, Ocn, ALP, Col1a1 and the like and formation of mineralized calcium nodules are enhanced. The method for improving the osteogenic differentiation efficiency of the human amniotic mesenchymal stem cells can be applied in osteogenic differentiation of the human mesenchymal stem cells, and applied in bone diseases of bone defects, bone trauma and the like.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Assessment of cardiovascular risk using isoprostane biomarkers and COX-2 selective inhibitors
The instant invention is drawn to methods and compositions useful for the assessment of cardiovascular risk in a mammal. The methods utilize biomarkers including prostanoid metabolites and isoprostanes as sensitive and stable markers of cardiovascular risk. The methods are particularly useful in a mammal that is contemplating undergoing coxib therapy, is undergoing coxib therapy, is undergoing antioxidant therapy, has ceased coxib therapy or has never undergone coxib therapy. The invention also includes kits useful for the assessment of cardiovascular risk in a mammal.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
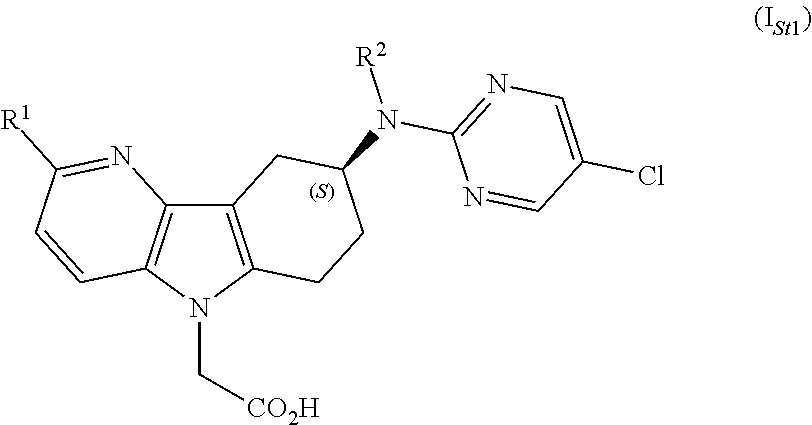
Azaindole acetic acid derivatives and their use as prostaglandin d2 receptor modulators
The present invention relates to azaindole acetic acid derivatives of formula (I),wherein R1 and R2 are as described in the description, and their use as prostaglandin receptor modulators, most particularly as prostaglandin D2 receptor modulators, in the treatment of various prostaglandin-mediated diseases and disorders, to pharmaceutical compositions containing these compounds and to processes for their preparation.
Owner:IDORSIA PHARM LTD
Prostaglandin receptor protein and methods of use thereof
ActiveUS7183062B2Antibody mimetics/scaffoldsReceptors for hormonesADAMTS ProteinsProstaglandin D2 receptor
Described herein is a novel member of the prostanoid receptor family, a guinea pig prostaglandin D2 receptor. Described are the receptor, the nucleic acid that encodes it, and various uses for both.
Owner:AVENTISUB LLC
Treatment and Prevention of Bone and Joint Disorders
ActiveUS20190261668A1Increased apoptosisEffectively treating and/or preventing osteoporosisVitamin food ingredientsNatural extract food ingredientsMedicineDrug biological activity
The invention encompasses compositions and methods for effectively treating and / or preventing the development and / or progression of osteoporosis and related disorders such as osteoarthritis and rheumatoid arthritis, and for promoting overall bone and joint health. This is accomplished by addressing multiple key mechanisms that lead to such disorders. The invention includes compositions comprising a combination of agents having biological activities that effectively suppress, regulate or interfere with the various key biochemical processes and mechanisms that increase the risk for development and / or progression of osteoporosis. The present compositions and methods simultaneously promote bone formation and reduce bone resorption by (a) stimulating osteoblast formation and osteogenesis; (b) suppressing adipocyte differentiation; (c) inhibiting osteoclast formation; and (d) increasing apoptosis of osteoclasts. The inventive compositions used for administration to human and other mammalian subjects having or at risk for development of osteoporosis comprise (1) at least one agent capable of modulating expression and / or activity of one or more of peroxisome activated protein receptor gamma (PPAR-γ), CAAT / enhancer binding protein-α (C / EBPα) and Sterol Regulatory Element-Binding Protein (SREBP-1); (2) at least one agent that activates expression and / or activity of one or more of the osteogenic transcription factors (Runx2 / Cbfα1, Dlx5, Osterix, Msx2); (3) at least one agent that activates expression and / or activity of one or more of bone morphogenetic proteins (BMPs: BMP 2 and 4), alkaline phosphatase (ALP), and osteocalcin; (4) at least one agent capable of activating Wnt / β-catenin signaling pathway; (5) at least one agent that inhibits the activity of pro-oxidants including reactive nitrogen species and reactive oxygen species (ROS); (6) at least one agent that suppresses one or more of inflammatory mediators including interleukins IL-1α, IL-1β, IL-6, NF-κB, TNF-α, matrix metalloproteinases (MMPs) and prostaglandin E2 (PGE2); and (7) at least one agent that induces the expression of and / or activates one or more of adenosine monophosphate-activated protein kinase (AMPK), sirtuin (SIRT1) and adiponectin (AP).
Owner:SUMMIT INNOVATION LABS LLC
DNA encoding a prostaglandin F2alpha receptor, a host cell transformed therewith and an expression product thereof
Molecular cloning and expression of a prostaglandin F2alpha receptor which is linked to the signal transduction pathways via guanine nucleotide binding regulatory (G) proteins and measured by, for example, cAMP, IP3 or intracellular calcium. By constructing cell lines that express a prostaglandin F2alpha receptor, the affinities and efficacies of agonist and antagonist drugs with the receptor can be assessed. A recombinant DNA construct includes a vector and a DNA fragment encoding a prostaglandin F2alpha receptor. A host cell is transformed with a recombinant DNA construct, so that the DNA fragment is expressed and a prostaglandin F2alpha receptor is produced. Suitable host systems include eukaryotic and prokaryotic cells, especially mamalian cells such as rat or human. Additionally, for diagnostic purposes, antibodies to a prostaglandin F2alpha receptor can be prepared by producing all or a portion of the receptor protein and injecting these into various types of mammals. Using the resulting antibodies, expression of an F2alpha receptor cDNA, i.e. receptor protein in tissue and cells can be measured.
Owner:PHARMACIA & UPJOHN AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com