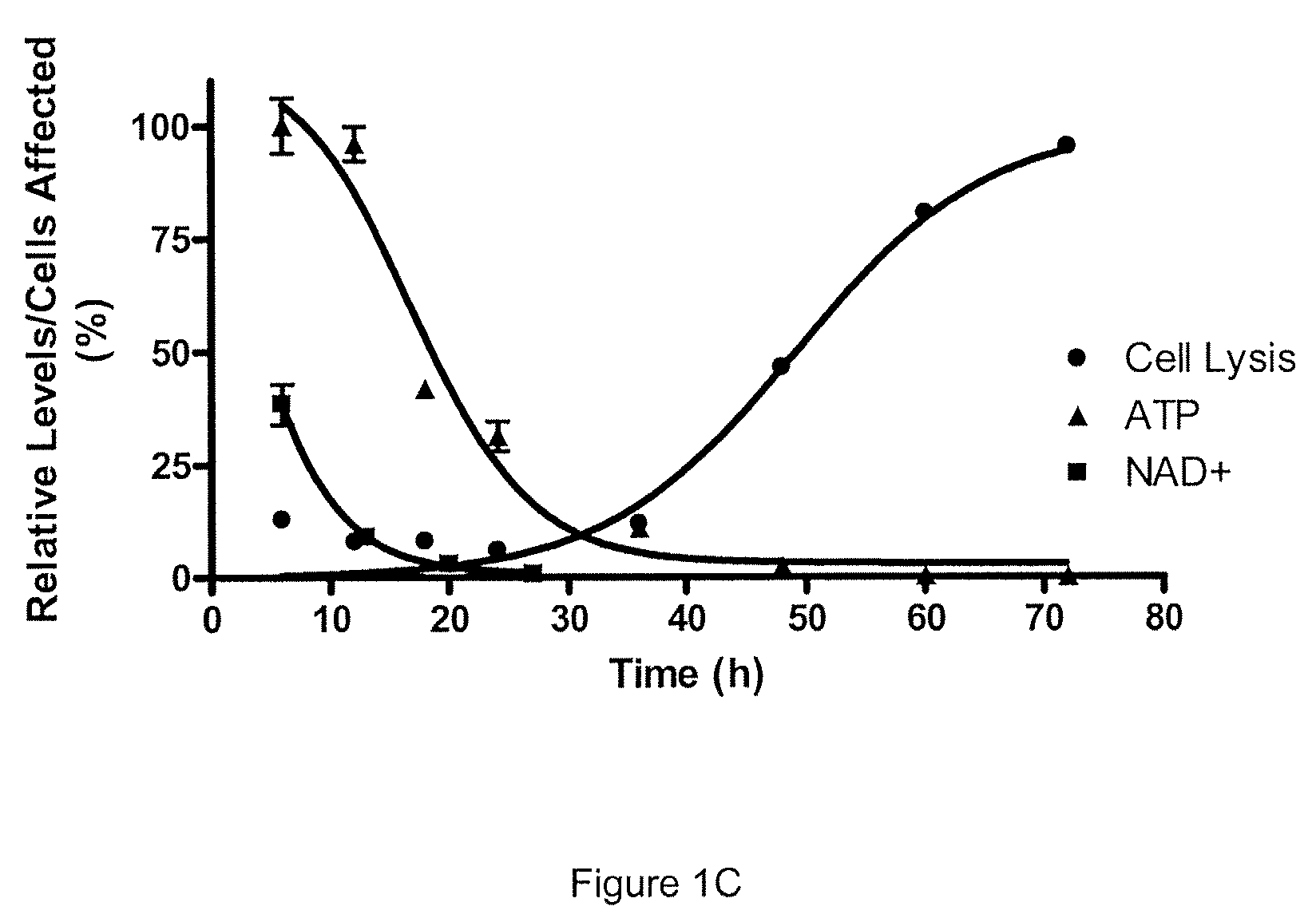
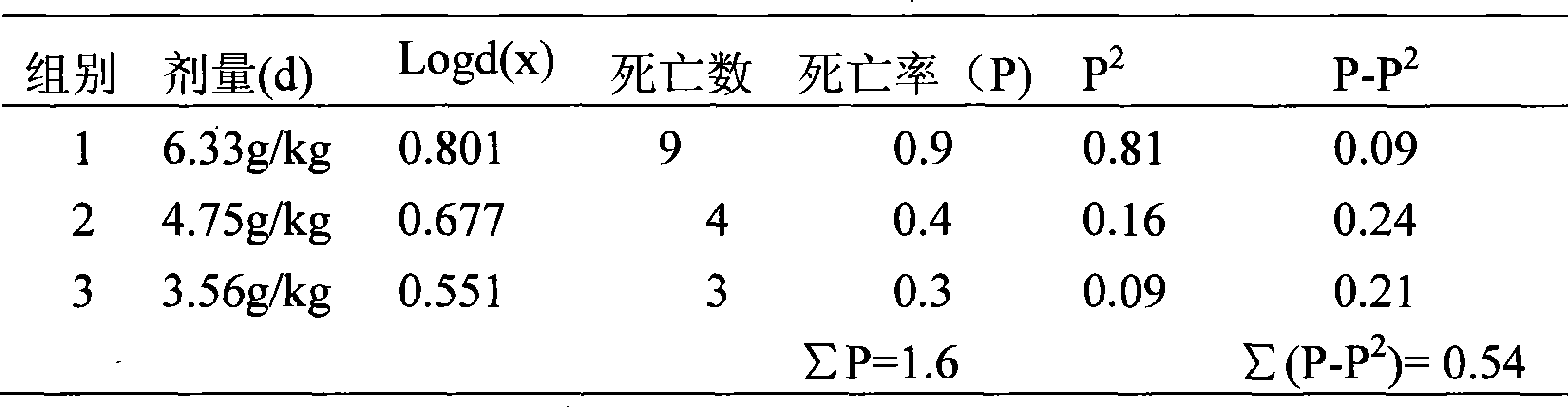
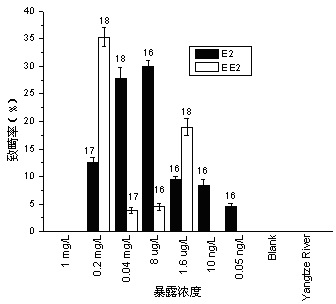
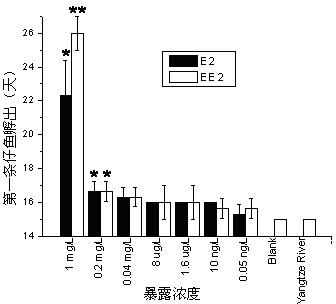
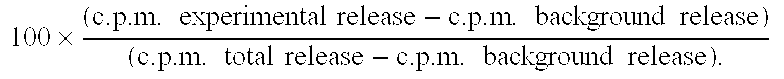Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Toxic dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
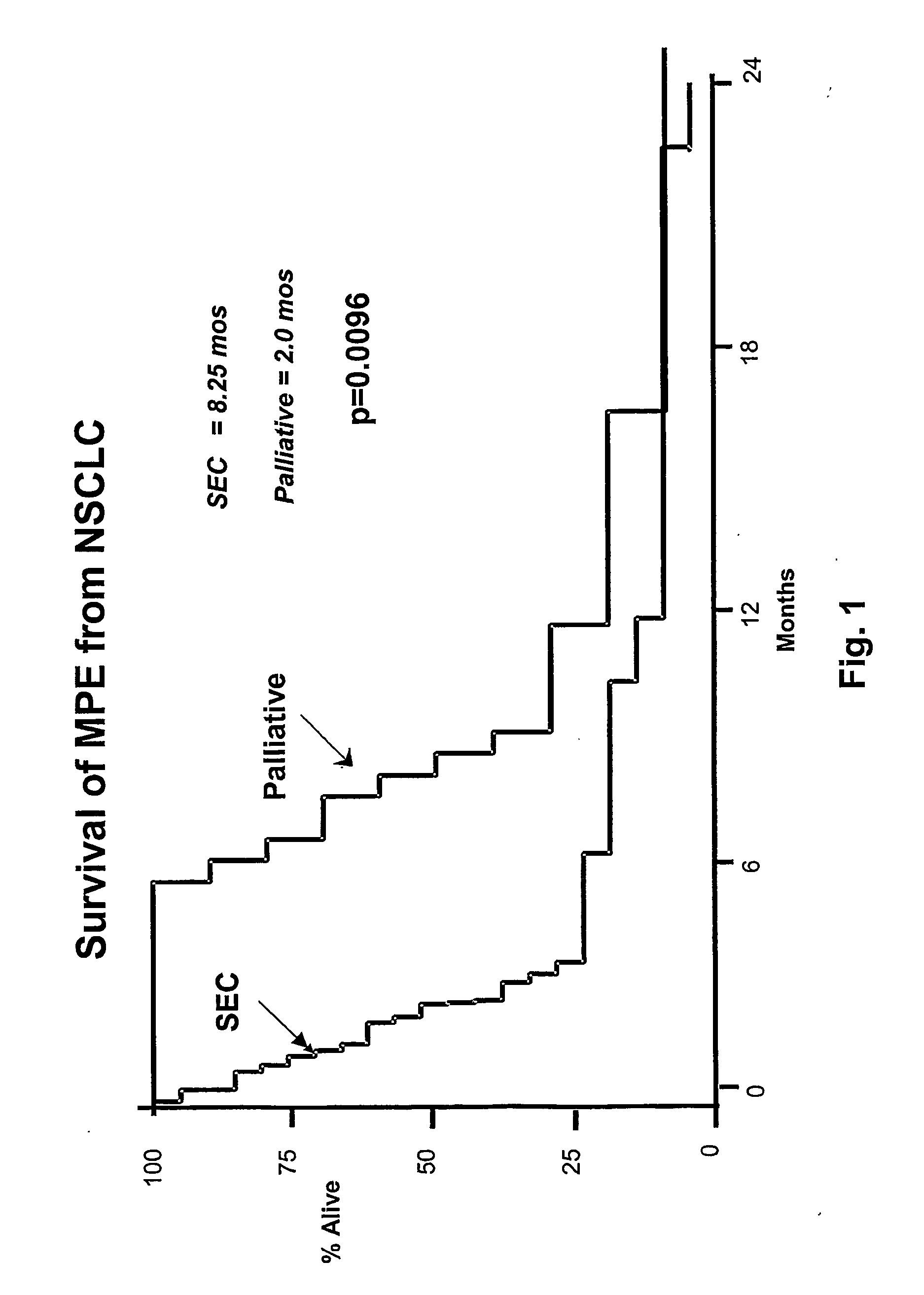
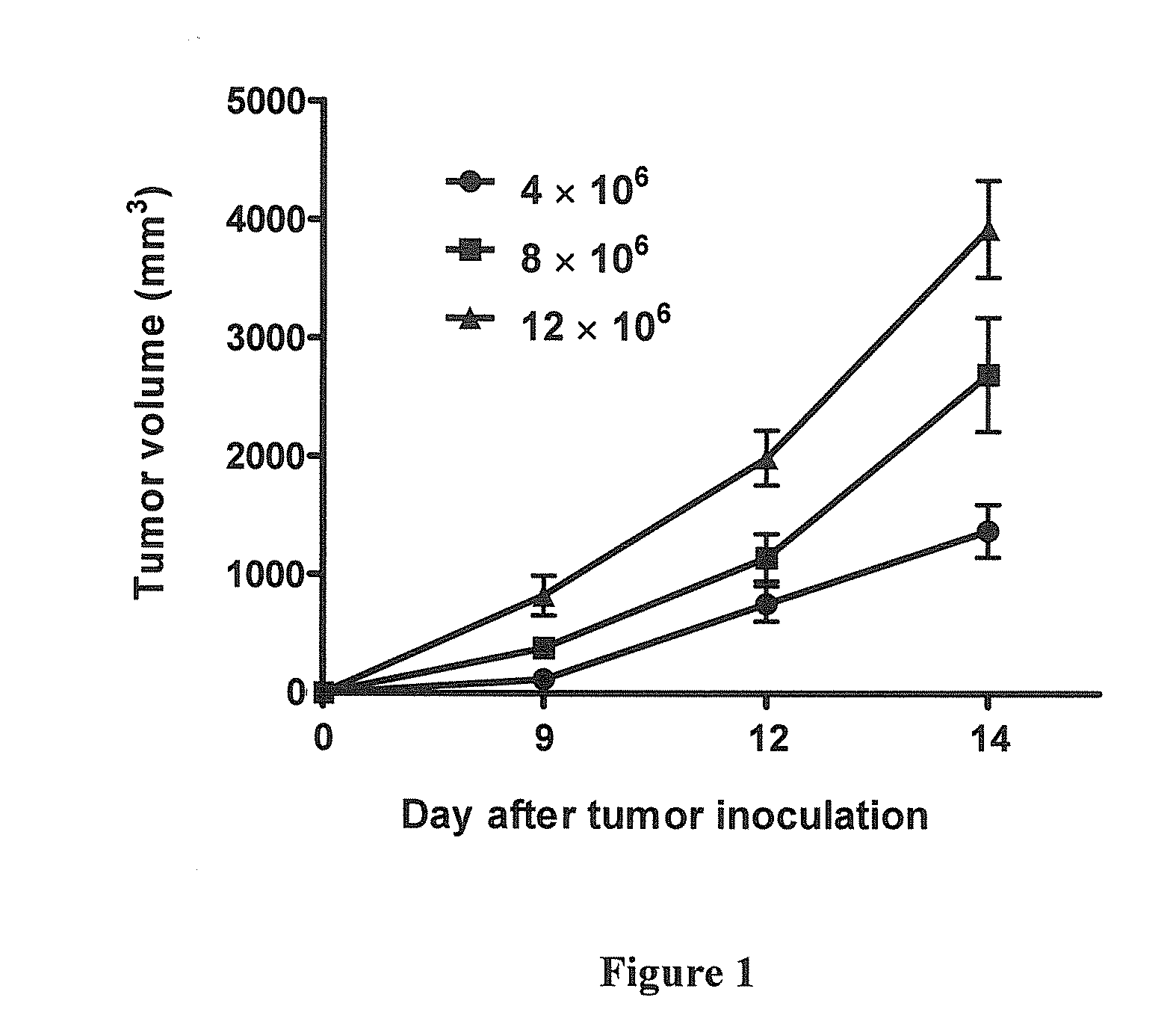
Intrathecal and intratumoral superantigens to treat malignant disease
InactiveUS20060052295A1Improve effectivenessStrong specificityPeptide/protein ingredientsSnake antigen ingredientsDiseaseAbnormal tissue growth
The presence of tumor nodules in organs often results in serious clinical manifestations and the permeation by cancer cells of sheaths surrounding organs often produces clinical manifestations of pleural effusion, ascites or cerebral edema. The present invention addresses this problem by providing a method for treating tumors comprising (a) intratumoral administration of a superantigen and / or (b) intrathecal or intracavitary administration of a superantigen directly into the sheath. Intratumoral superantigen results in significant and sustained reduction of the tumor size. Intrathecal administration produces significant sustained reduction of the fluid accumulation associated with clinical improvement and prolonged survival. Useful superantigen compositions for intrathecal and intratumoral injection include tumoricidally effective homologues, fragments and fusion proteins of native superantigens. Also disclosed is combined therapy that includes intratumoral or intrathecal superantigen compositions in combination with (i) intratumoral low, non-toxic doses of one or more chemotherapeutic drugs or (ii) systemic chemotherapy at reduced and non-toxic doses of chemotherapeutic drugs.
Owner:JENQUEST
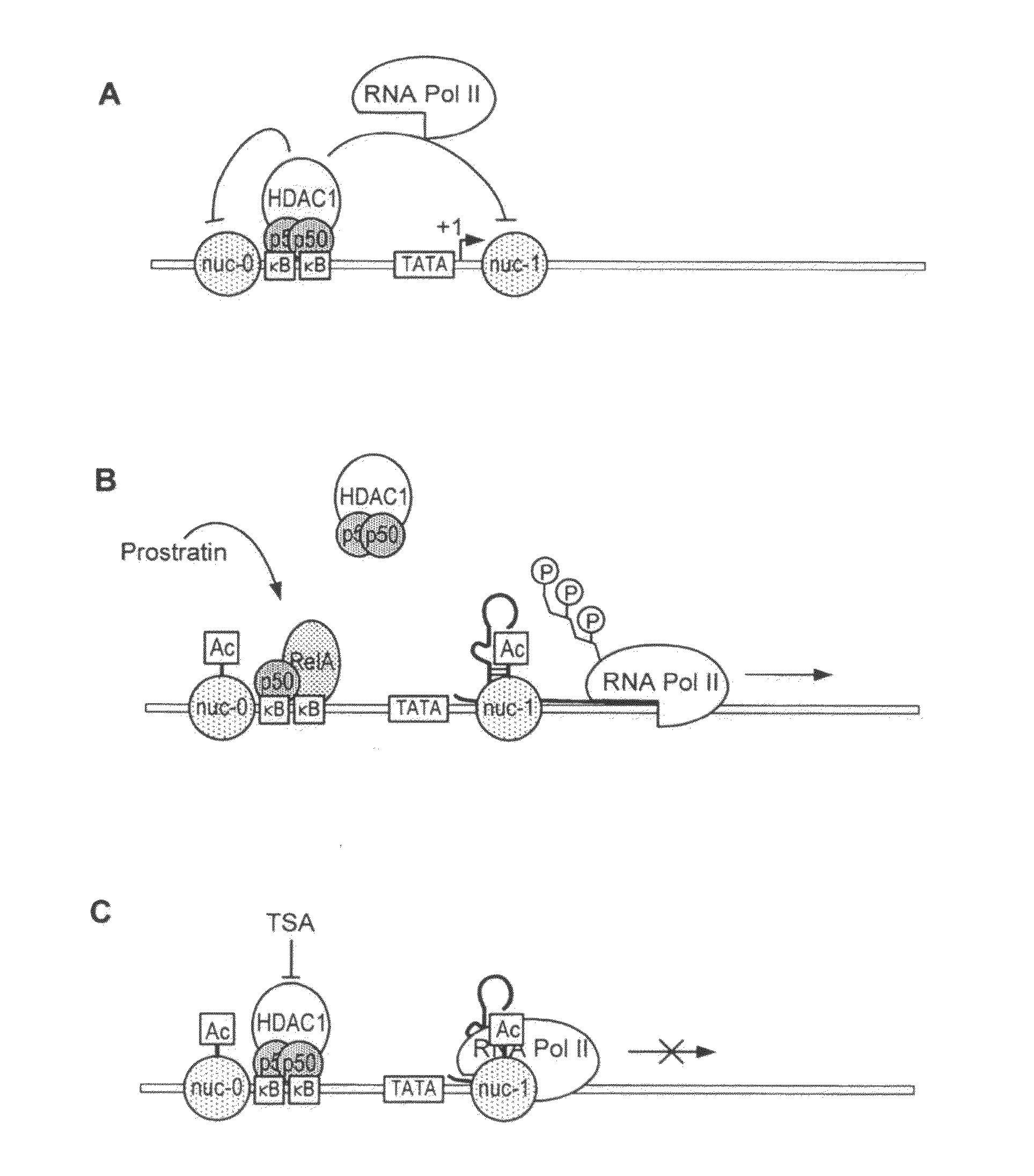
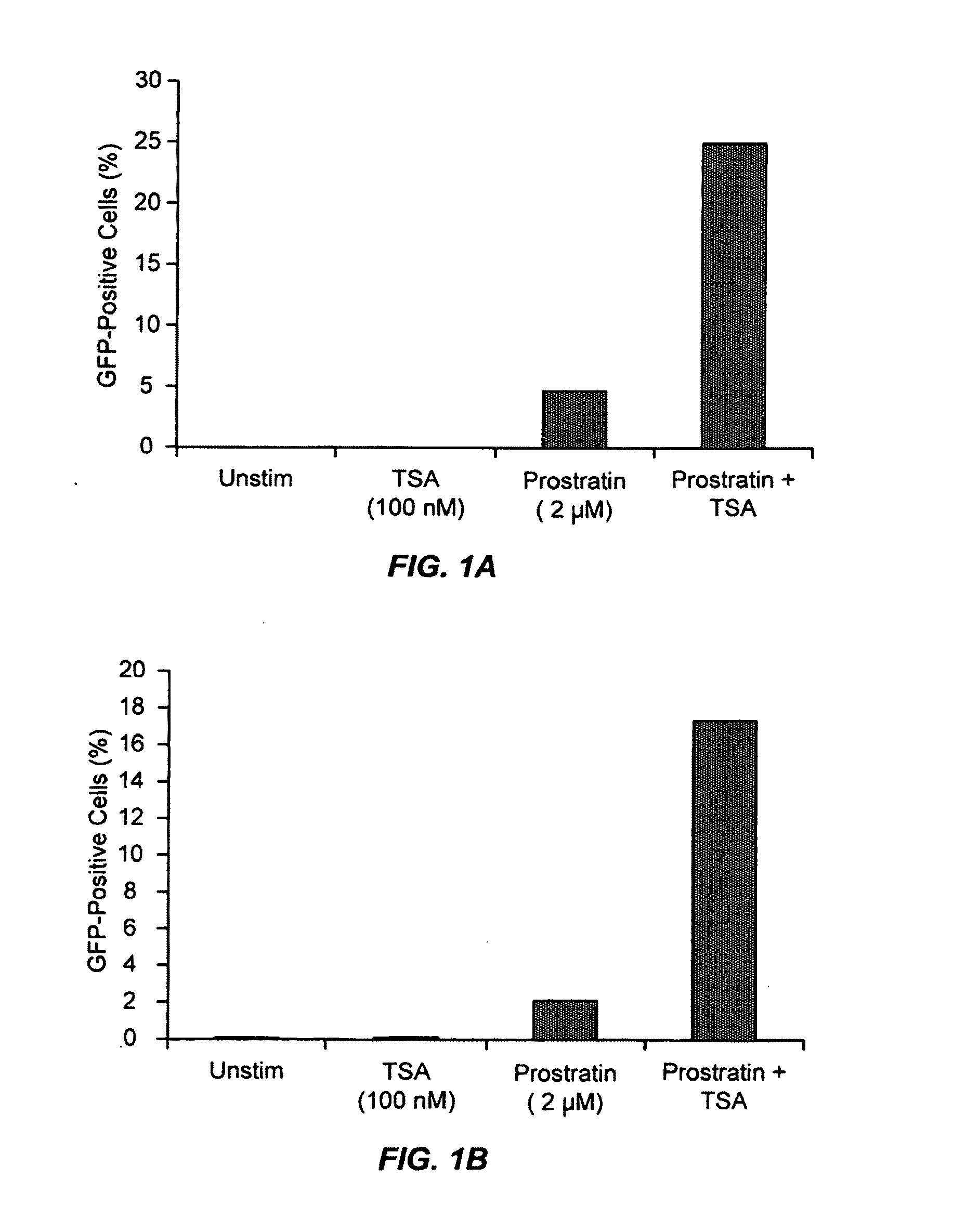
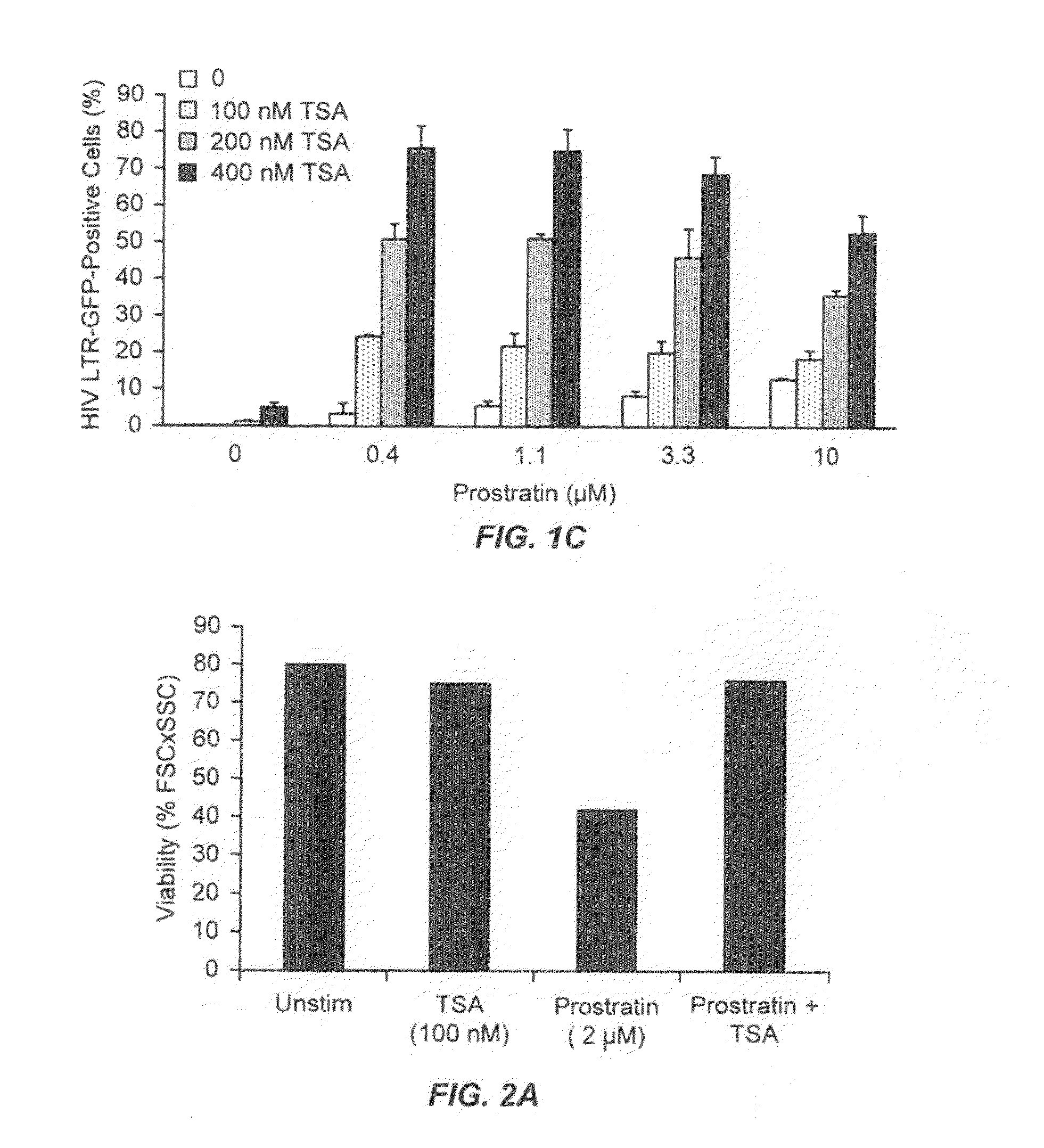
Methods and compositions for the synergistic activation of latent HIV
The present invention provides methods and compositions useful for the elimination of latent HIV reservoirs that persist despite HAART. The methods and compositions overcome this latent barrier by inducing the replication of HIV in latently infected T cells while preventing the spread of the newly produced virions to uninfected cells by providing HAART simultaneously. Compositions of the invention comprise an activator of latent HIV expression, such as prostratin, and an inhibitor of histone deacetylase, such as TSA. A surprising finding of this invention is that the inhibitor of the histone deacetylase synergizes the effect of prostratin thus, allowing administering to a patient a lower, non-toxic dose of prostratin.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
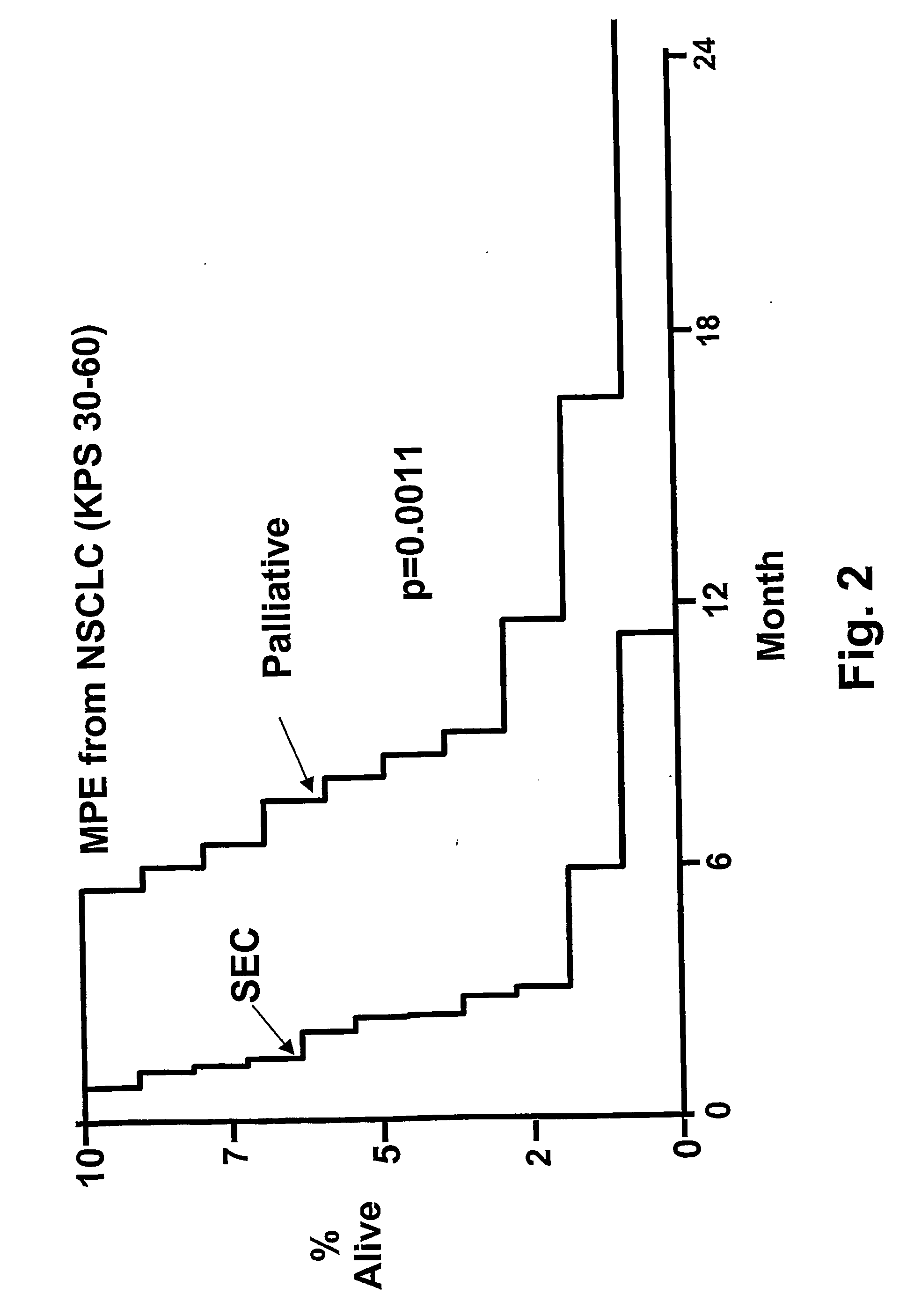
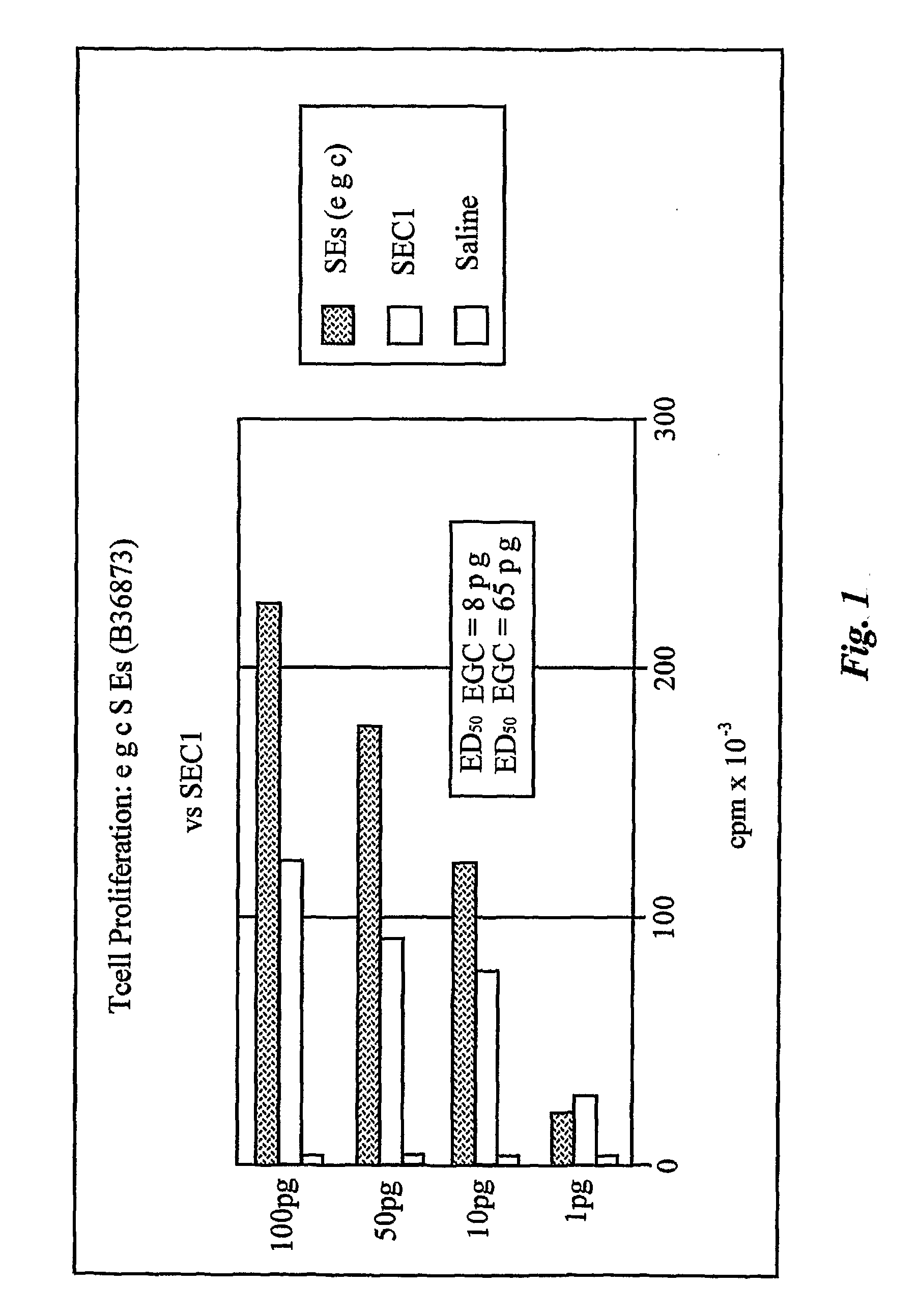
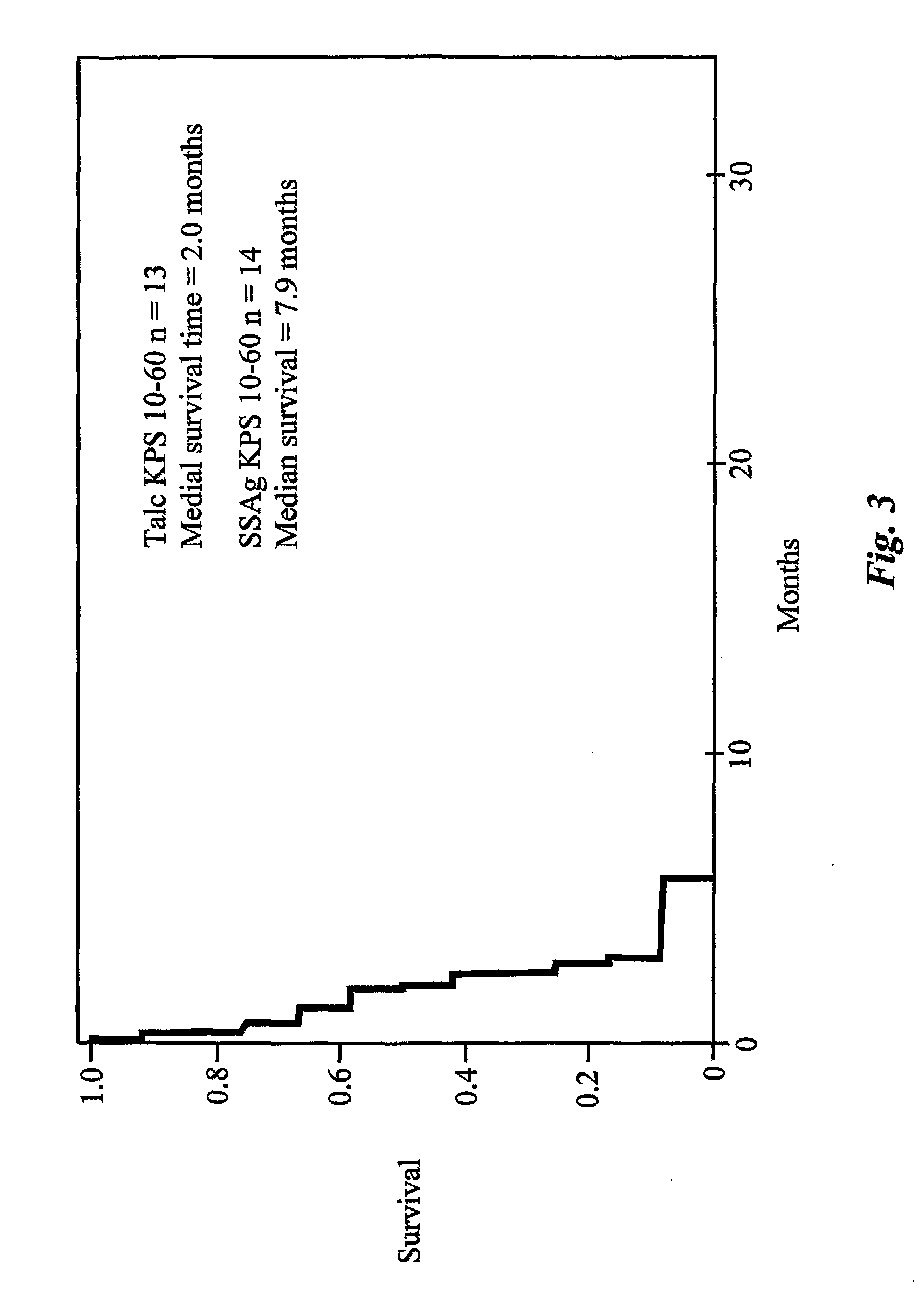
Enterotoxin gene cluster (egc) superantigens to treat malignant disease
InactiveUS20090162315A1Good effectPrevent morbidityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseSystemic chemotherapy
The use of classical superantigens for treatment of cancer has resulted in a low response rates and serious toxicity in humans which is attributable, in part, to the presence of preformed superantigen specific antibodies in the plasma of treated patients. The present invention addresses this problem by providing a method for treating tumors comprising the administration of one or a plurality of egc (enterotoxin gene cluster) staphylococcal enterotoxins comprising staphylococcal enterotoxins G, I, M, N, O. These superantigens in native unmodified form can be administered intrathecally, intratumorally, intravenously to humans with advanced lung cancer while resolving pleural effusions and prolonging survival to 300% above control patients treated with talc pleurodesis. Intratumoral egc superantigens induces a significant and sustained reduction of the tumor size. In contrast to classic Sags, the egc superantigens induced minimal toxicity, are rarely associated with the presence of preformed antibodies and are used as a plurality with a broad T cell Vβ profile. Useful egc superantigen compositions for parenteral administration native egc enterotoxins, homologues, fragments and fusion proteins of native egc enterotoxins capable of activating a broad spectrum of T cells expressing T cell receptor / α motifs. T cell survival-enhancing cytokines IL-7, Il-15, Il-23 are used. together with parenteral egc SE therapy. Also disclosed is combined therapy that includes parenteral, intratumoral or intrathecal superantigen compositions in combination with (i) intratumoral low, non-toxic doses of one or more chemotherapeutic drugs or (ii) systemic chemotherapy at reduced and non-toxic doses of chemotherapeutic drugs or (iii) radiation therapy or (iv) anti-angiogenic and tyrosine kinase inhibitors.
Owner:TERMAN DAVID S +4
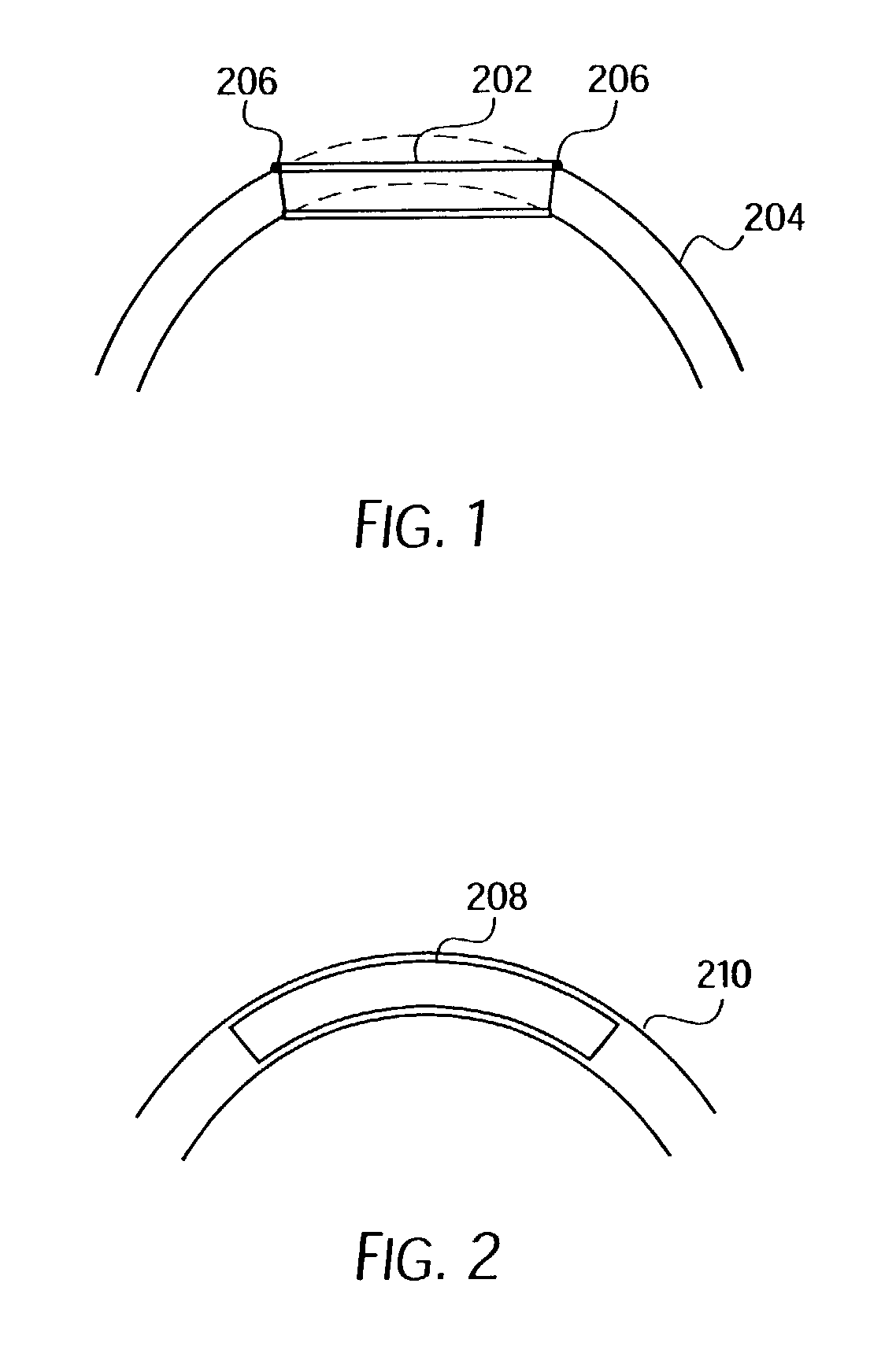
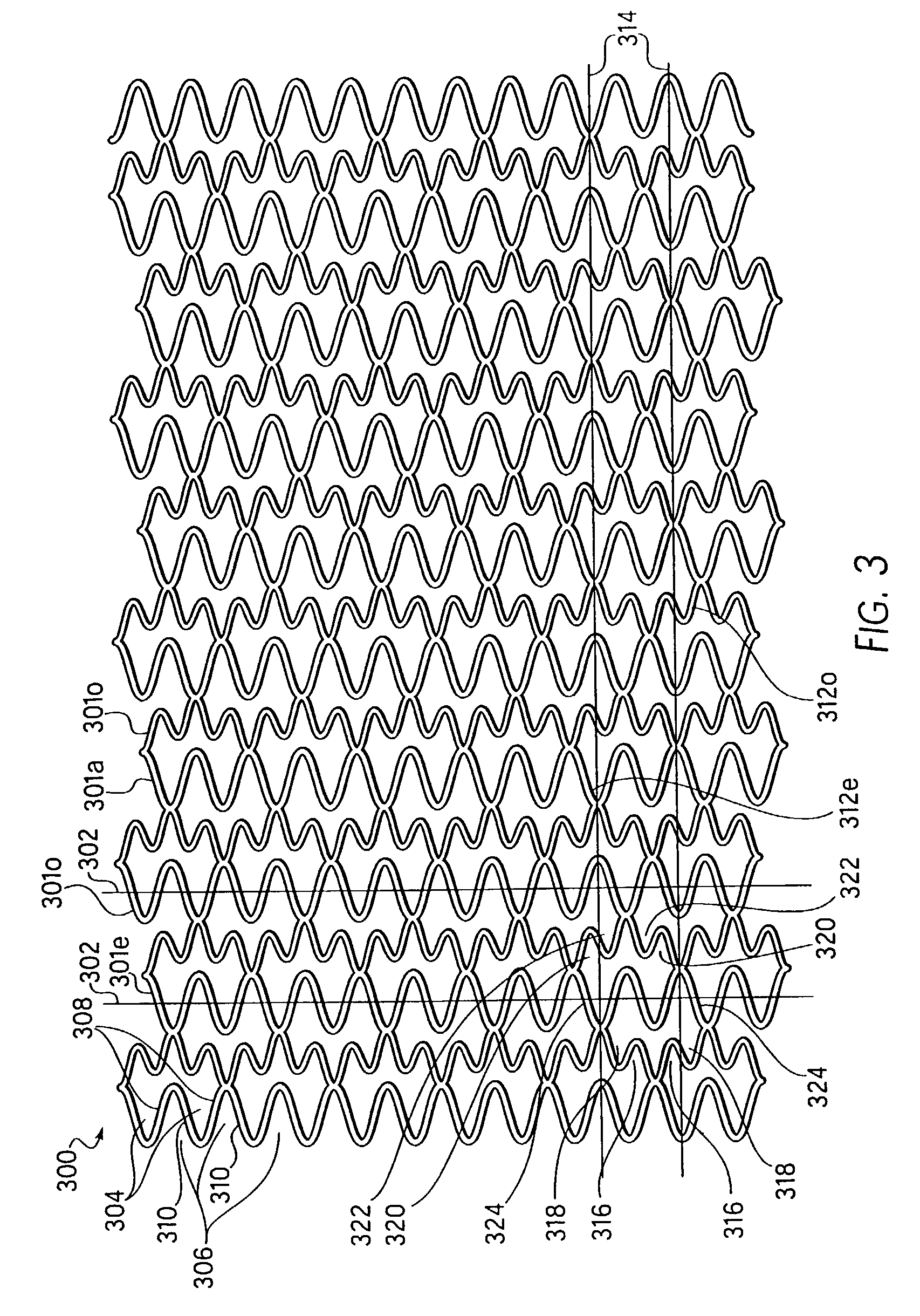
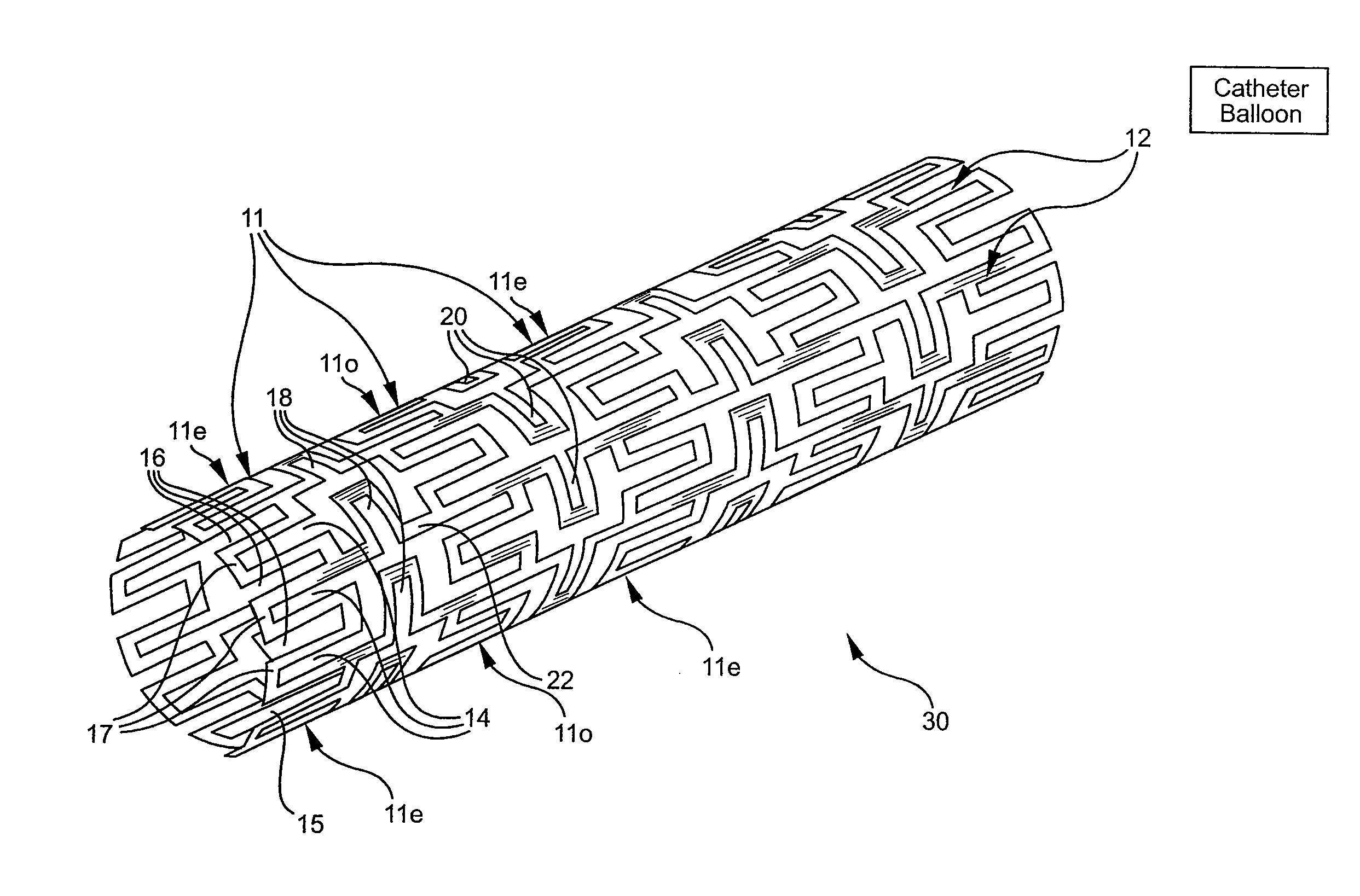
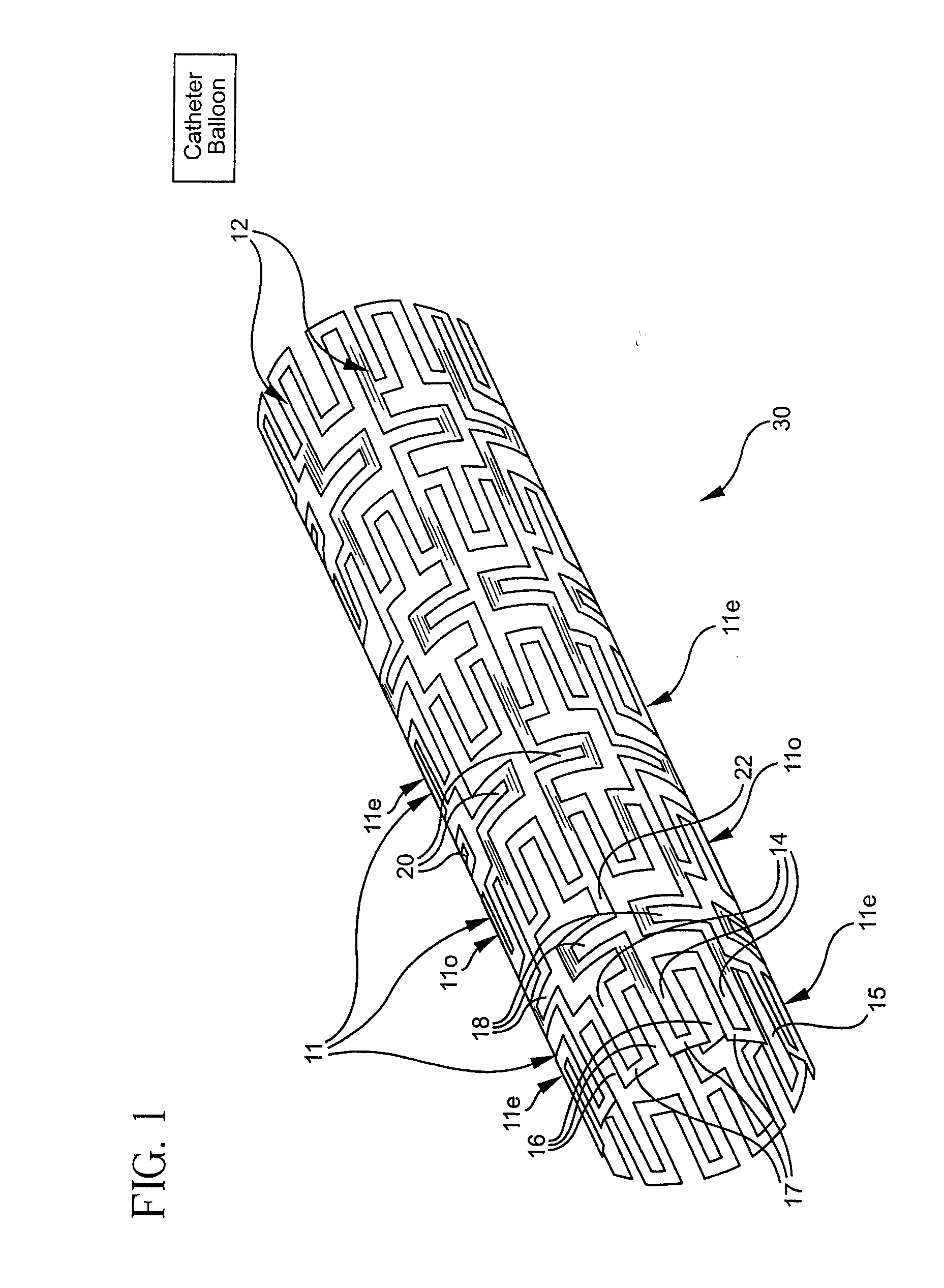
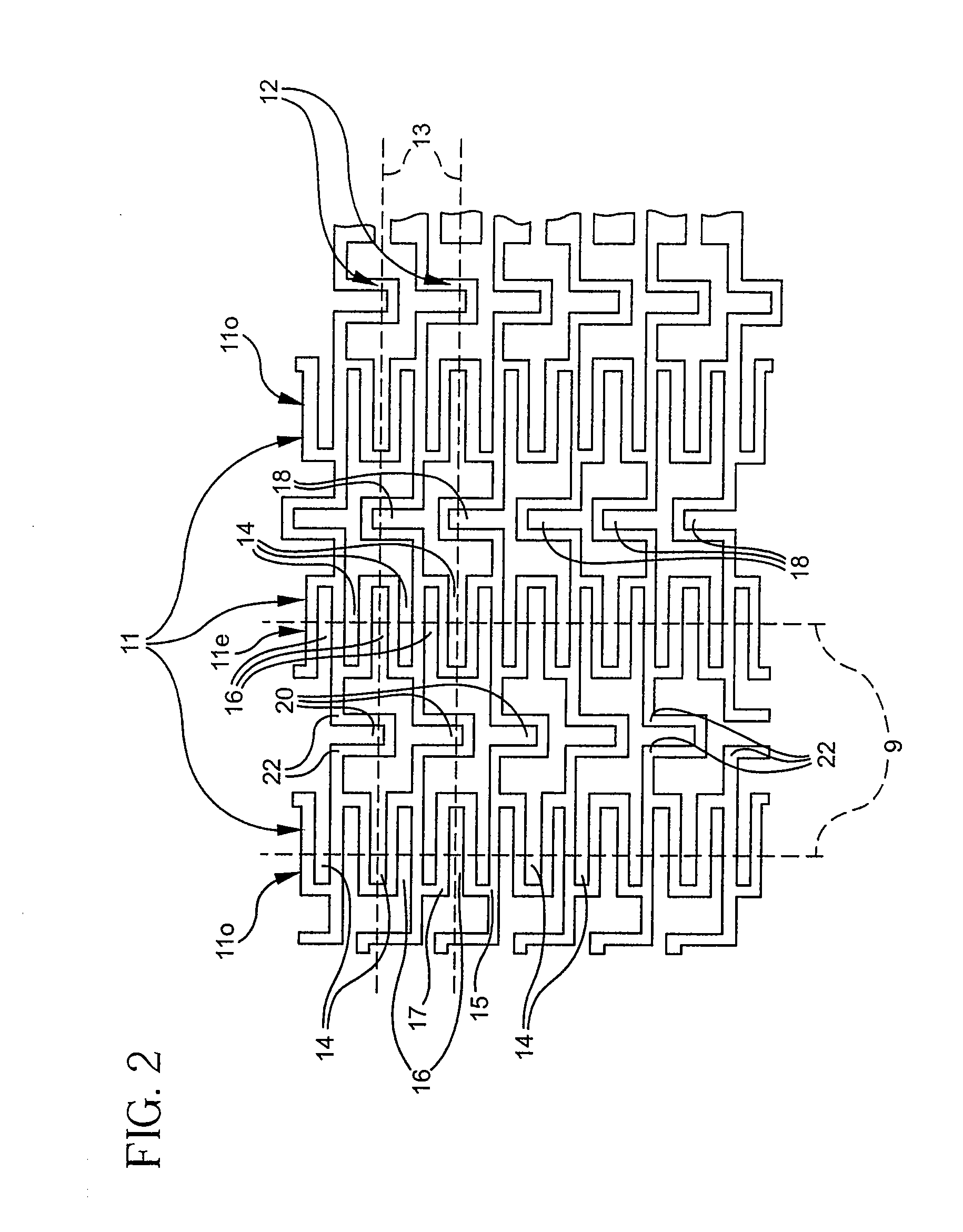
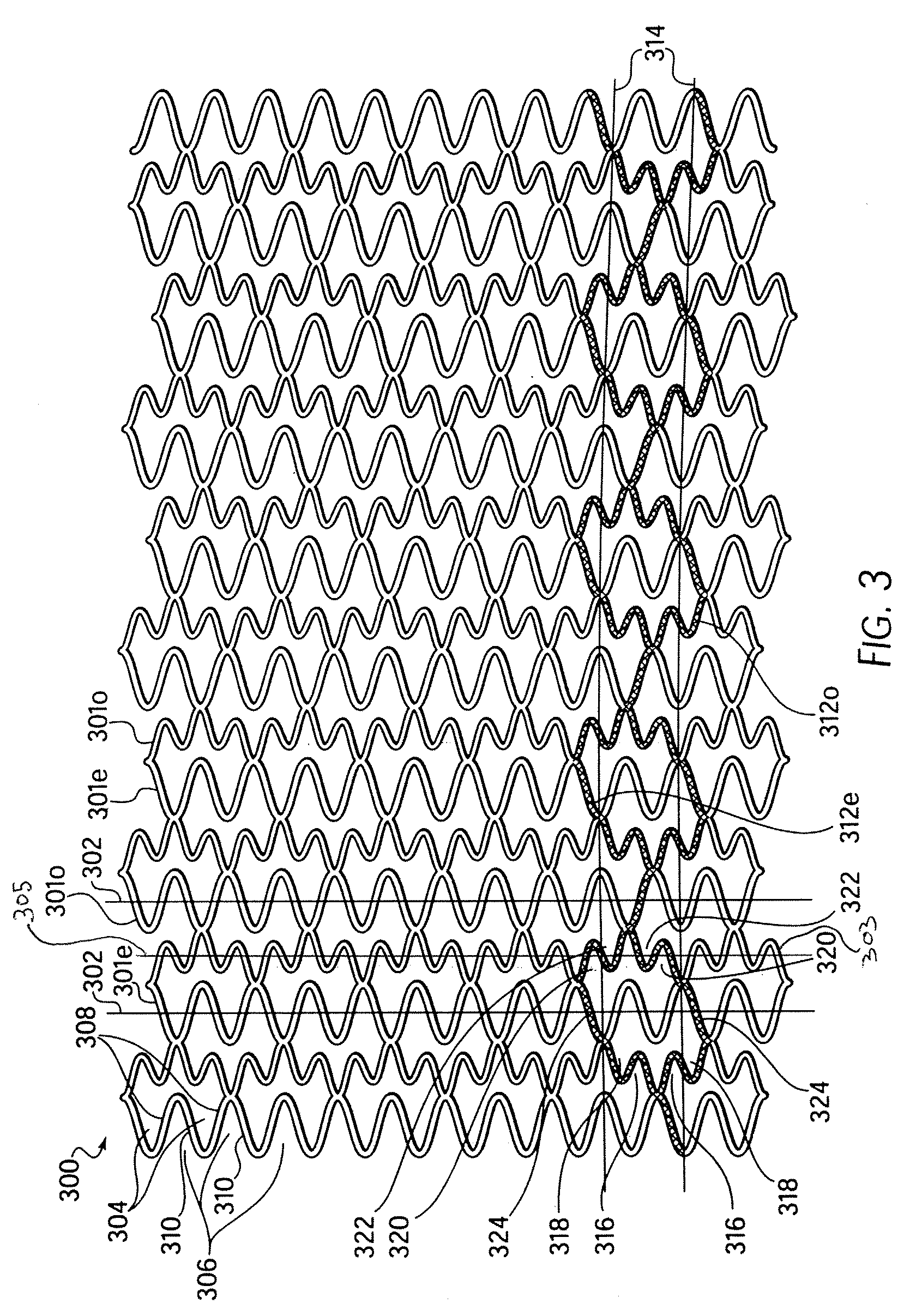
Longitudinally flexible stent
InactiveUS7621947B2Add supportIncreased longitudinal flexibilityStentsEar treatmentMeanderInsertion stent
An intravascular stent especially suited for implanting in curved arterial portions. The stent retains longitudinal flexibility after expansion. The stent is formed of intertwined meander patterns forming triangular cells. The triangular cells are adapted to provide radial support, and also to provide longitudinal flexibility after expansion. The triangular cells provide increased coverage of a vessel wall. The stent can have different portions adapted to optimize radial support or to optimize longitudinal flexibility. Loops in the stent are disposed and adapted to cooperate so that after expansion of said stent within a curved lumen, the stent is curved and cells on the outside of the curve open in length, but narrow in width whereas cells on the inside of the curve shorten in length but thicken in width to maintain a density of stent element area which much more constant than otherwise between the inside and the outside of the curve. As a result, when the stent is coated with a medicine the more constant density of stent elements results in an even dose being applied to the inside wall of the lumen, avoiding the possibility that a toxic dose be supplied at one area while a less than effective dose is applied to another area.
Owner:MEDINOL LTD
Pharmaceutical composition for the management of tumors
InactiveUS20060281793A1Reduce generationAvoid developmentBiocideAnimal repellantsAbnormal tissue growthPositive control
The present invention relates to the effect of naturally occurring compounds on tumor development. As an example of proof, we used low; non-toxic doses of three compound e.g. Calcium D-glucarate, a naturally occurring Ca++ salt of D-glucaric acid; Nicotinamide (NA), a naturally occurring vitamin and butyric acid (BA), a naturally occurring saturated short chain fatty acid. 7,12 dimethylbenzanthracene (DMBA), which is a very potent skin carcinogen and is an environmental pollutant, was used for skin tumor development. Experiment was performed upto 30 weeks. All the above-mentioned compounds were used either alone or concomitantly any two or all the three. In the positive control group 100% tumorigenesis was attained in 28 weeks, use of single compound led to the inhibition of DMBA induced tumorigenesis between 33 to 47%, use of two compounds resulted in the 73 to 80% reduction in tumorigenesis but the concomitant use of three compounds resulted into 100% inhibition of tumor development at the end of 30 weeks. This led us to conclude that the concomitant use of Cag, NA and BA in combination of two is useful for preventing skin tumor develoment for a sort or long period of time. But the concomitant use of all the three compounds, as described, exhibited the perfect synergistic effect in preventing the tumor development completely. This strategy should be equally effective in the management of benign and possibly malignant tumor in any organ caused by any mean.
Owner:COUNCIL OF SCI & IND RES
Synergistic combination and method thereof
Owner:INDIAN INSTITUTE OF SCIENCE
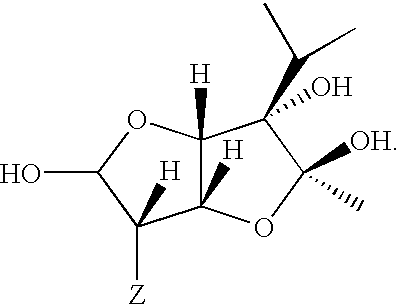
Medicament for the treatment of diabetes
InactiveUS6531461B1Effective treatmentReturn to normal activitiesOrganic active ingredientsEndocrine system disorderDiabetes mellitusToxic dose
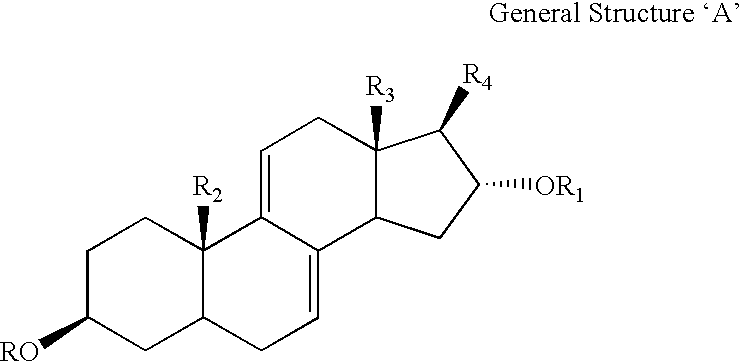
A series of compounds of general structure I and metal salts thereof. These compounds are useful in the treatment of diabetes mellitus and associated conditions when administered in an effective non-toxic dose in the form of a pharmaceutically acceptable composition resulting in cell regeneration.wherein for example,R=H, R1=H, R2=Me, R3=Meand R4=
Owner:NELSON LOUIS OBYO OBYO
Gene therapy of tumors using non-viral delivery system
InactiveUS20060165773A1Reduce lung cancer mortalityReduction in formation of tumorPeptide/protein ingredientsGenetic material ingredientsAbnormal tissue growthLipid formation
The present invention provides a pharmaceutical composition, comprising: (a) cationic lipids, wherein said lipids are a liposomal mixture of a diacyl-ethyl-phosphocholine and 1,2-diacyl-sn-glycero-3-phosphoethanolamine; and (b) a plasmid cDNA sequence encoding a protein having tumor suppressor or pro-apoptotic activity. This composition has a high gene transfection efficiency at non-toxic doses and is designed to transfect human bronchial premalignant lesions and early endo-bronchial malignancies. Also provided is a method of method of treating a cancerous or pre-cancerous condition of the respiratory tract in an individual in need of such treatment, comprising the step of administering to said individual a pharmacologically effective dose of a pharmaceutical composition, comprising: (a) cationic lipids, wherein said lipids are a liposomal mixture of a diacyl-ethyl-phosphocholine and 1,2-diacyl-sn-glycero-3-phosphoethanolamine; and (b) a plasmid cDNA sequence encoding a protein having tumor suppressor or pro-apoptotic activity.
Owner:PEREZ SOLER ROMAN +1
Longitudinally flexible stent
InactiveUS20100100166A1Add supportIncreased longitudinal flexibilityStentsSurgeryMeanderInsertion stent
An intravascular stent especially suited for implanting in curved arterial portions. The stent retains longitudinal flexibility after expansion. The stent is formed of intertwined meander patterns forming triangular cells. The triangular cells are adapted to provide radial support, and also to provide longitudinal flexibility after expansion. The triangular cells provide increased coverage of a vessel wall. The stent can have different portions adapted to optimize radial support or to optimize longitudinal flexibility. Loops in the stent are disposed and adapted to cooperate so that after expansion of said stent within a curved lumen, the stent is curved and cells on the outside of the curve open in length, but narrow in width whereas cells on the inside of the curve shorten in length but thicken in width to maintain a density of stent element area which much more constant than otherwise between the inside and the outside of the curve. As a result, when the stent is coated with a medicine the more constant density of stent elements results in an even dose being applied to the inside wall of the lumen, avoiding the possibility that a toxic dose be supplied at one area while a less than effective dose is applied to another area.
Owner:MEDINOL LTD
Gene therapy of tumors using non-viral delivery system
InactiveUS7157098B1Reduce lung cancer mortalityReduction in formation of tumorIn-vivo radioactive preparationsDispersion deliveryAbnormal tissue growthLipid formation
The present invention provides a pharmaceutical composition, comprising: (a) cationic lipids, wherein said lipids are a liposomal mixture of a diacyl-ethyl-phosphocholine and 1,2-diacyl-sn-glycero-3-phosphoethanolamine; and (b) a plasmid cDNA sequence encoding a protein having tumor suppressor or pro-apoptotic activity. This composition has a high gene transfection efficiency at non-toxic doses and is designed to transfect human bronchial premalignant lesions and early endo-bronchial malignancies. Also provided is a method of method of treating a cancerous or pre-cancerous condition of the respiratory tract in an individual in need of such treatment, comprising the step of administering to said individual a pharmacologically effective dose of a pharmaceutical composition, comprising: (a) cationic lipids, wherein said lipids are a liposomal mixture of a diacyl-ethyl-phosphocholine and 1,2-diacyl-sn-glycero-3-phosphoethanolamine; and (b) a plasmid cDNA sequence encoding a protein having tumor suppressor or pro-apoptotic activity.
Owner:PEREZ SOLER ROMAN +1
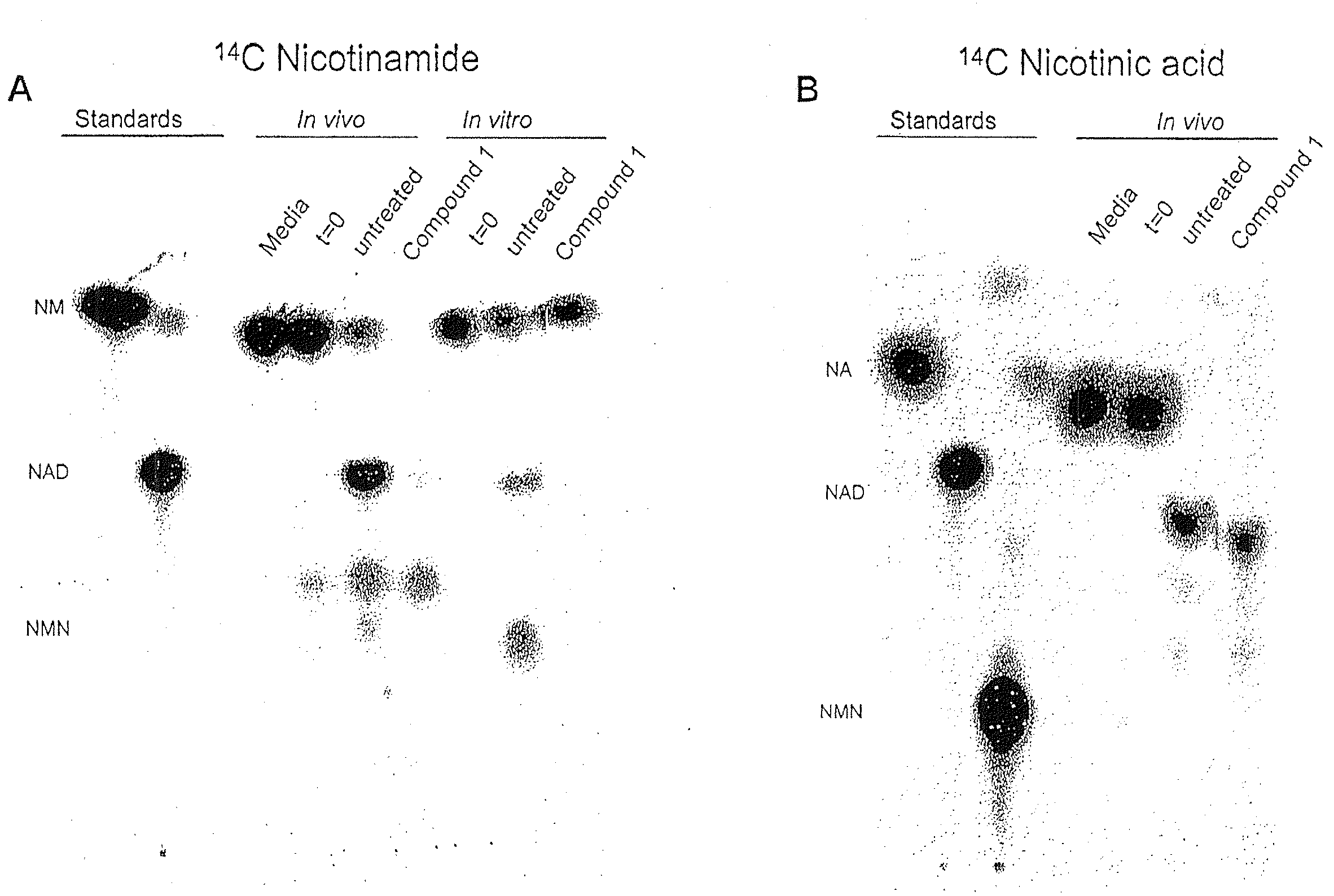
Compositions and methods for effecting NAD+ levels using a nicotinamide phosphoribosyl tranferase inhibitor
ActiveUS8211912B2Reduce repairImprove efficiencyBiocideHeavy metal active ingredientsToxic doseA-DNA
The present invention relates to methods for decreasing cellular DNA repair in a target patient; decreasing cellular NAD+ biosynthesis in a target patient; increasing efficiency of radiation therapy in a target patient; modulating nicotinamide phosphoribosyl transferase activity in a patient; or sensitizing a patient to a DNA damaging therapy. The invention relates to methods for treating a patient who received a toxic dose of an nicotinamide phosphoribosyl transferase inhibitor. The invention also relates to pharmaceutical compositions comprising a physiologically acceptable carrier; an effective amount of a NMPRT inhibitor; and nicotinic acid. The invention also relates to methods for treating a patient diagnosed with or suspected to have a cancer deficient in nicotinic acid pathway.
Owner:GEMIN X PHARMA CANADA INC (CA)
Quality control method for toxin ingredient, yunaconitine in Yuannan Hongyao capsule
InactiveCN101396492AImprove controllabilityMake sure the processing is qualifiedAntipyreticComponent separationAdditive ingredientColumn temperature
The invention discloses a method for controlling the quality of yanaconitine which is toxic ingredient in Yunnan Hongyao capsule, and pertains to the technical field of drugs. The method comprises: controlling the toxicity of the crude drug prepared kusnezoff monkshood root, controlling the content of the yanaconitine in the capsule, controlling the toxicity of the crude drug prepared kusnezoff monkshood root by animal safety test, and detecting the content of the toxic ingredient yanaconitine by high performance liquid chromatography. The toxic dose of the crude drug prepared kusnezoff monkshood root is not more than 2g / kg, the content of the yanaconitine per capsule is not more than 0.01mg, a chromatographic column is U-bondapaKCN with the volume of 7.8 multiplied by 300mm; a mobile phase is methanol-water-ammonia water (35:65:0.5); the column temperature is 30 DEG C; the detection wavelength is 260nm; the flow rate is 2mL / min; and the theoretic number of column plates is not less than 4100. The method has the advantages of comprehensive monitoring means, simple and graspable operations, and high detection accuracy and precision; the method enhances the controllability of the toxic ingredient of the Yunnan Hongyao capsule, and better ensures the processing qualification of the crude drug prepared kusnezoff monkshood root and the drug safety.
Owner:YUNNAN PHYTOPHARML
Method for testing toxicity of environmental estrogen on whitebait embryonic development
The invention discloses a method for testing toxicity of environmental estrogen on whitebait embryonic development. The method of the invention utilizes the sensitivity of embryo to the simulation of environmental pollutant or chemical matter to perform a test under semi-static state on whitebait embryo which is exposed in standard diluted aqueous solution of subject having a series of concentrations. The test lasts about 30 days, begins as exposing a live embryo at the blastula stage in the aqueous solution of subject, and ends with hatching all embryos in a control group and an exposed group. The toxicity endpoint contains death, monstrosity and delayed hatching. The method comprises the steps of confirming the toxicity of the subject on the whitebait embryonic development by observing the toxicity endpoint of the exposed group and comparing with the control group; researching on the influence of two natural estrogens E2 and EE2 on the whitebait embryo-yolk sac stage so as to confirm the toxic dose-effect relationship of the natural estrogens to the whitebait embryonic development, and evaluating the potential risk of this type of fish exposed under the estrogen with environmental concentration.
Owner:SHANGHAI ACADEMY OF ENVIRONMENTAL SCIENCES +1
Longitudinally flexible stent
InactiveUS20110022156A1Add supportIncreased longitudinal flexibilityStentsEar treatmentMeanderInsertion stent
An intravascular stent especially suited for implanting in curved arterial portions. The stent retains longitudinal flexibility after expansion. The stent is formed of intertwined meander patterns forming triangular cells. The triangular cells are adapted to provide radial support, and also to provide longitudinal flexibility after expansion. The triangular cells provide increased coverage of a vessel wall. The stent can have different portions adapted to optimize radial support or to optimize longitudinal flexibility. Loops in the stent are disposed and adapted to cooperate so that after expansion of said stent within a curved lumen, the stent is curved and cells on the outside of the curve open in length, but narrow in width whereas cells on the inside of the curve shorten in length but thicken in width to maintain a density of stent element area which much more constant than otherwise between the inside and the outside of the curve. As a result, when the stent is coated with a medicine the more constant density of stent elements results in an even dose being applied to the inside wall of the lumen, avoiding the possibility that a toxic dose be supplied at one area while a less than effective dose is applied to another area.
Owner:MEDINOL LTD
Compositions and Methods for Effecting NAD+ Levels Using A Nicotinamide Phosphoribosyl Tranferase Inhibitor
ActiveUS20090162454A1Reduce repairImprove efficiencyHeavy metal active ingredientsBiocideToxic doseA-DNA
The present invention relates to methods for decreasing cellular DNA repair in a target patient; decreasing cellular NAD+ biosynthesis in a target patient; increasing efficiency of radiation therapy in a target patient; modulating nicotinamide phosphoribosyl transferase activity in a patient; or sensitizing a patient to a DNA damaging therapy. The invention relates to methods for treating a patient who received a toxic dose of an nicotinamide phosphoribosyl transferase inhibitor. The invention also relates to pharmaceutical compositions comprising a physiologically acceptable carrier; an effective amount of a NMPRT inhibitor; and nicotinic acid. The invention also relates to methods for treating a patient diagnosed with or suspected to have a cancer deficient in nicotinic acid pathway.
Owner:GEMIN X PHARMA CANADA INC (CA)
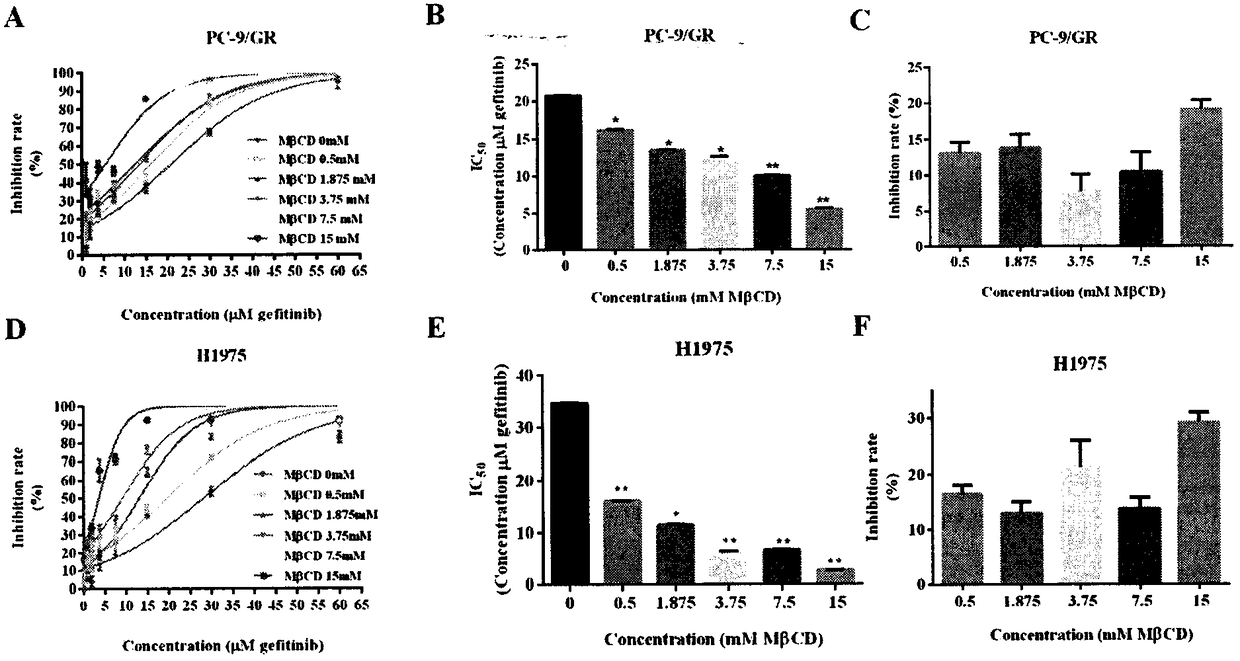
EGFR-TKIs for overcoming drug resistance of non-small cell lung cancer and combined medication scheme
InactiveCN108078979AIncreased sensitivitySynergistic inhibition and even induction of deathRespiratory disorderEster active ingredientsCholesterolWilms' tumor
The invention relates to the field of clinical overcoming of drug resistance of tumors and combined medication, in particular to EGFR-TKIs which can overcome the drug resistance of non-small cell lungcancer by lowering cholesterol. Statins can enhance the antitumor efficacy of EGFR-TKIs. The invention discloses the fact that MbetaCD is used for removing cholesterol from human non-small cell lungcancer cell lines PC-9 / GR and H1975 to enhance the efficacy of gefitinib in resisting the non-small cell lung cancer, enhance the inhibition to signal pathways and enhance the affinity of EGFR and EGFR-TKIs. The statins can synergize with EGFR-TKIs in resisting tumors, inhibit the signal pathways and enhance the affinity of EGFR-TKIs and EGFR without toxic doses, and at the same time, the synergistic anti-tumor efficacy of combined use of the statins and EGFR-TKIs is verified on PC-9 and H1975 nude-mouse transplanted tumor models.
Owner:CHINA PHARM UNIV
Composition for treating lung cancer and application in preparing medicine for treating lung cancer
ActiveCN106728912ASymptoms improvedProlong lifeAnthropod material medical ingredientsAntineoplastic agentsLife qualitySide effect
The invention discloses a composition for treating a lung cancer and an application in preparing a medicine for treating the lung cancer. The composition comprises the following ingredients in effective treatment doses: any one or two of radix ranunculi ternati and selfheal, any one or two of bombyx batryticatus and gecko, any one or two of glabrous sarcandra herb and solani nigri herba, any one or two of edible tulip and sculellaria barbata, any one or two of thunberg fritillary and northern apricot kernel, ganoderma lucidum, any one or two of rhizoma pinellinae praeparata and tangerine peel and any one or two of American ginseng and codonopsis pilosula. The composition is provided in an ingredient proportion lower than a toxic dose. The composition can be further combined with a chemotherapeutant or a target medicine for use. According to the composition and the application, relevant symptoms of the lung cancer can be effectively improved; the tumor progression is retarded; the tumor medicine resistance is antagonized; toxic and side effects of chemotherapy are relieved, so that the life quality of a patient with the advanced lung cancer is improved; life benefits are brought to the patient with the lung cancer; the curative effect is exact and objective.
Owner:林丽珠
Flexible expandable stent
Owner:MEDINOL LTD
Applications of radix salviae miltiorrhizae in prevention or preparation of drugs for treating porcine reproductive respiratory syndrome virus infection
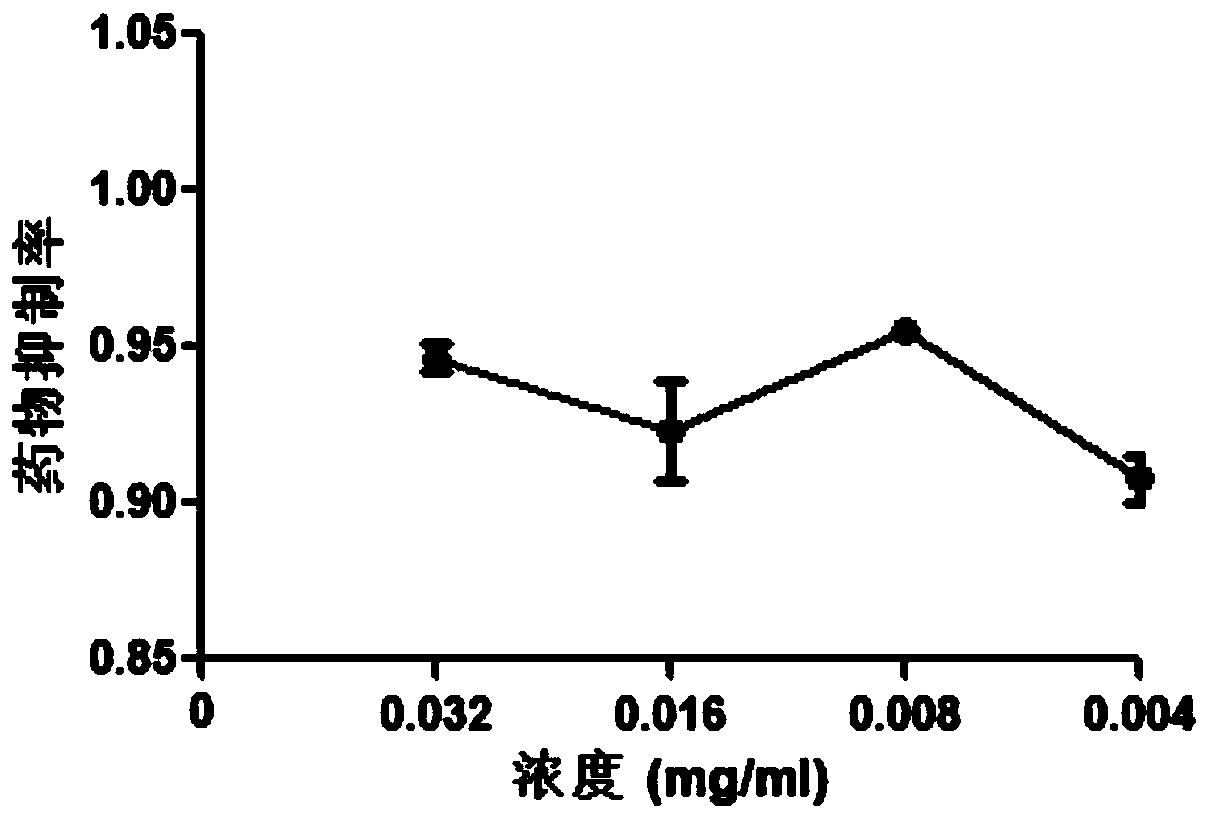
InactiveCN110433198AThe preparation method is scientific and reasonableSimple processAntiviralsPlant ingredientsSalvia miltiorrhizaToxic dose
The invention provides a new purpose of radix salviae miltiorrhizae in the technical field of medicines, and specifically relates to applications of radix salviae miltiorrhizae in the prevention or preparation of drugs for treating porcine reproductive respiratory syndrome virus infection. A radix salviae miltiorrhizae extract product can effectively inhibit the infection of a porcine reproductiverespiratory syndrome virus on a Marc-145 cell within a non-toxic dose range, namely, the inhibition rate of the radix salviae miltiorrhizae extract product on the porcine reproductive respiratory syndrome virus can reach more than 90% when the concentration of the radix salviae miltiorrhizae extract product is 0.004-0.032 mg / mL after being dissolved in dimethyl sulfoxide, and under this concentration, the survival rate of the Marc-145 cell is more than 90%. Thus, the radix salviae miltiorrhizae can be further developed into drugs for treating and preventing the porcine reproductive respiratory syndrome virus.
Owner:武汉百药联科科技有限公司
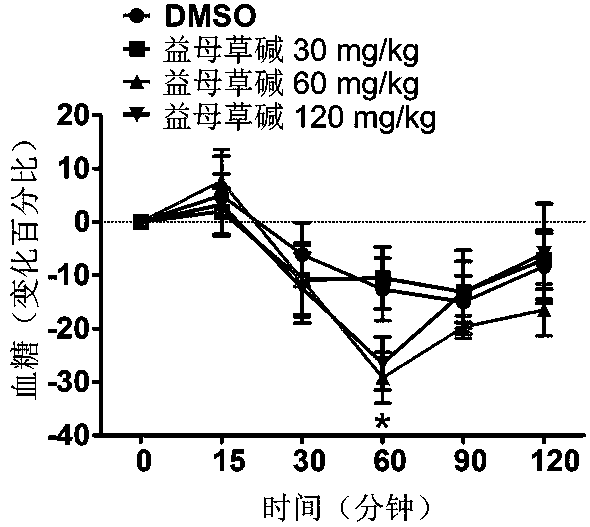
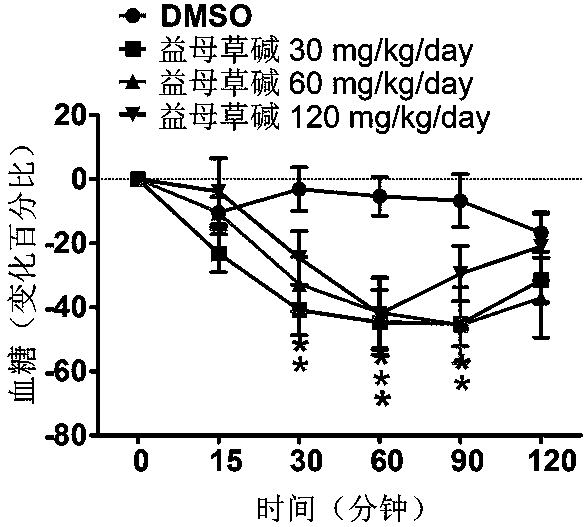
Application of leonurine in preparing euglycemic agent
The invention belongs to the field of modern traditional Chinese medicine production, and relates to Chinese herbal medicine herba leonuri extract leonurine and application of the leonurine in preparing drugs, in particular to application of the leonurine in preparing a euglycemic agent and preventing the obesity. Through the pharmacological study and insulin sensibilization experiments, the results show that the leonurine has the effect of improving the sensibility of insulin in the body, and the leonurine can enhance the reaction of the body to the insulin, enhance the physiological effect of the insulin and increasing the sensibility of an insulin receptor; the experimental results show that under the condition that the leonurine is far lower than the toxic dose, the effect of reducingthe body weight is shown. The leonurine can be used for preparing the euglycemic agent and the drugs for preventing the obesity and is especially suitable for etiological treatment of the insulin resistance syndrome and the secondary disease symptoms caused by reduction of insulin resistance or insulin sensitivity, such as the central obesity, the simple obesity and the obesity with any other disease states.
Owner:ZHUHAI HENGQIN NEW DISTRICT ZHONGZHU ZHENGTAI MEDICAL MANAGEMENT CO LTD
Longitudinally flexible stent
Owner:MEDINOL LTD
Methods and compositions for the synergistic activation of latent HIV
The present invention provides methods and compositions useful for the elimination of latent HIV reservoirs that persist despite HAART. The methods and compositions overcome this latent barrier by inducing the replication of HIV in latently infected T cells while preventing the spread of the newly produced virions to uninfected cells by providing HAART simultaneously. Compositions of the invention comprise an activator of latent HIV expression, such as prostratin, and an inhibitor of histone deacetylase, such as TSA. A surprising finding of this invention is that the inhibitor of the histone deacetylase synergizes the effect of prostratin thus, allowing administering to a patient a lower, non-toxic dose of prostratin.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Pharmaceutical composition for the management of tumors
The present invention relates to the effect of naturally occurring compounds on tumor development. As an example of proof, we used low; non-toxic doses of three compound e.g. Calcium D-glucarate, a naturally occurring Ca++ salt of D-glucaric acid; Nicotinamide (NA), a naturally occurring vitamin and butyric acid (BA), a naturally occurring saturated short chain fatty acid. 7,12 dimethylbenzanthracene (DMBA), which is a very potent skin carcinogen and is an environmental pollutant, was used for skin tumor development. Experiment was performed up to 30 weeks. All the above-mentioned compounds were used either alone or concomitantly any two or all the three. In the positive control group 100% tumorigenesis was attained in 28 weeks, use of single compound led to the inhibition of DMBA induced tumorigenesis between 33 to 47%, use of two compounds resulted in the 73 to 80% reduction in tumorigenesis but the concomitant use of three compounds resulted into 100% inhibition of tumor development at the end of 30 weeks. This led us to conclude that the concomitant use of Cag, NA and BA in combination of two is useful for preventing skin tumor development for a sort or long period of time. But the concomitant use of all the three compounds, as described, exhibited the perfect synergistic effect in preventing the tumor development completely. This strategy should be equally effective in the management of benign and possibly malignant tumor in any organ caused by any mean.
Owner:COUNCIL OF SCI & IND RES
A use of methylene blue to promote wakefulness after anesthesia
ActiveCN103417546BAvoid damageWake up fasterOrganic active ingredientsAntinoxious agentsSaline waterMedicine
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Novel application of methylene blue to post-anesthesia awakening
ActiveCN103417546AAvoid damageWake up fasterOrganic active ingredientsAntinoxious agentsMedicineToxic dose
The invention relates to novel application of methylene blue to post-anesthesia awakening. The novel application is characterized in that after an animal is completely anesthetized, a certain dose of the methylene blue can be injected to the animal, so that awakening of the animal can be accelerated, the anesthesia time is shortened, and overdose anesthesia can be reduced to a certain extent; the novel application of the methylene blue is beneficial to relieving and resisting the overdose anesthesia by the aid of the methylene blue in animal experiments and clinical application; the experimental animal is anesthetized by the aid of pentobarbital sodium with certain concentration, then methylene blue solution with certain concentration is injected to the completely anesthetized animal, and the concentration of the methylene blue solution is lower than a toxic dose of the methylene blue; then the awakening time of the animal is observed, and an awakening effect of the methylene blue is judged after the awakening effect of the methylene blue is compared with an awakening effect of normal saline; as shown by experiment results, the required time for awakening the animal after the animal is anesthetized by the aid of the pentobarbital sodium can be obviously shortened by the aid of the methylene blue solution, an awakening latency stage can be shortened, and the overdose anesthesia can be reduced. The novel application of the methylene blue has the advantages that a safe, convenient and speedy awakening measure is hopefully provided for clinical application, and possible injury to patients due to the overdose anesthesia can be reduced.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Directed metabolism of compounds by glucuronidation and sulfonation donors to decrease toxicity
InactiveUS20080188439A1Prevention and treatment of toxicityReduce incidenceBiocideDigestive systemGlucuronide metabolismUridine Diphosphoglucose
Disclosed are compositions and methods of treatment with uridine-based and phosphoadenosine-based cofactors for the prevention and treatment of toxicity associated with compounds such as acetaminophen, which undergo Phase II glucuronidation and sulfonation in the liver. Uridine diphosphoglucose, phosphoadenosine-phosphosulfate, and their derivatives can be administered exogenously either to prevent their depletion in the liver during treatment with acetaminophen, or to replace depleted substrates of UDPGA-transferase or PAPS-transferase in order to treat an individual after a toxic dose of acetaminophen has been ingested.
Owner:SALHANICK STEVEN D +1
Synergistic combination and method thereof
A method for the treatment of myeloma and thymoma by administering a therapeutically effective dose of Mycobacterium indicus pranii with Cyclophosphamide. This disclosure generally relates to the field of cancer biology. More specifically, this disclosure relates to the immunotherapeutic treatment of myeloma and thymoma, using a combination of heat killed Mycobacterium indicus pranii and the widely administered chemotherapeutic drug, Cyclophosphamide. Mycobacterium indicus pranii has already shown its efficacy as an immunomodulator and has been safely administered to humans. The most common method of cancer management is the application of chemotherapeutic drugs which results in side-effects. At lower non-toxic doses Cyclophosphamide is not effective. The present disclosure discloses a method of improving efficacy of non-toxic doses of Cyclophosphamide by administrating it together with Mycobacterium indicus pranii. This disclosure is relevant for the treatment of other lymphomas as well.
Owner:INDIAN INSTITUTE OF SCIENCE
A kind of rapeseed meal and its processing method, a kind of animal feed
ActiveCN102273545ADetoxificationAchieve decolorizationFood processingAnimal feeding stuffSodium dithioniteToxic dose
The invention relates to an animal feed, and provides a processing method of rapeseed meal, the method comprises the following steps: detoxificating and decolouring of the rapeseed meal, mixing sodium dithionite, rapeseed meal with water and heating. The invention overcomes the defects of incomplete detoxification, large restriction of geography, influenced nutrition value of rapeseed meal, high cost or difficult popularization of equipments in the present detoxification reaction of rapeseed meal, and when the rapeseed meal content in feed exceeds 2-5%, the feed turns black and is unbeneficial to the sale and usage, so that the invention provides the rapeseed meal and its processing method, and the animal feed containing the rapeseed meal. The processing method of the rapeseed meal realizes a continuous processing of detoxificating and decolouring of the rapeseed meal at same time, and has the advantages of low cost for decolouring, good detoxificating efficiency with residual toxic dose less than 0.2 mg / kg, high safety performance and simple technology, the rapeseed meal and its processing method are suitable for industrial application, and the adding proportion of the processed rapeseed meal can reach 5-15%.
Owner:上海红马饲料有限公司
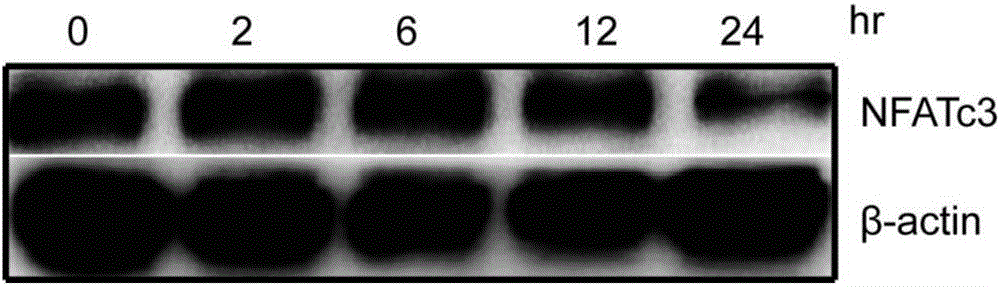
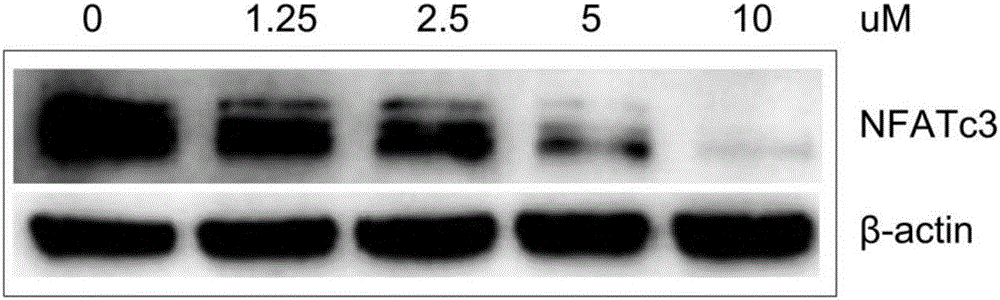
Expression regulation reagent of NFATc3 and screening kit based on arsenical-sensitive cells of NFATc3
ActiveCN106405114ALow toxicityWide variety of sourcesInorganic active ingredientsMicrobiological testing/measurementAcute toxicity testingToxic dose
The invention provides an expression regulation reagent of NFATc3 and a screening kit based on the arsenical-sensitive cells of the NFATc3. Compared with existing reagents using gene knockout, RNAi and the like to lower gene expression, As4S4 serving as the expression regulation reagent of the NFATc3 is low in toxicity. An acute toxicity test shows that LD50 calculated by a Bliss method is 19.3+ / -1.1g / kg, the confidential interval of 95% is 18.3-20.4g / kg, and toxic dose recorded in the Pharmacopoeia is exceeded greatly.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Application of Danjie emodin in preparation of medicine for preventing and treating African swine fever virus
ActiveCN113797184ASignificant anti-African swine fever virus effectAvoid infectionOrganic active ingredientsAntiviralsAfrican swine feverToxic dose
The invention discloses an application of Danjie emodin in preparation of a medicine for preventing and treating African swine fever virus. The Danjie emodin disclosed by the invention has a remarkable effect of resisting the African swine fever virus. The maximum non-toxic dose of the Danjie emodin is 40 mu M, and the Danjie emodin can effectively inhibit infection and proliferation of the African swine fever viruses, can be used as the medicine for preventing and treating African swine fever, and has a very good application prospect in the aspect of preventing and treating the African swine fever.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com