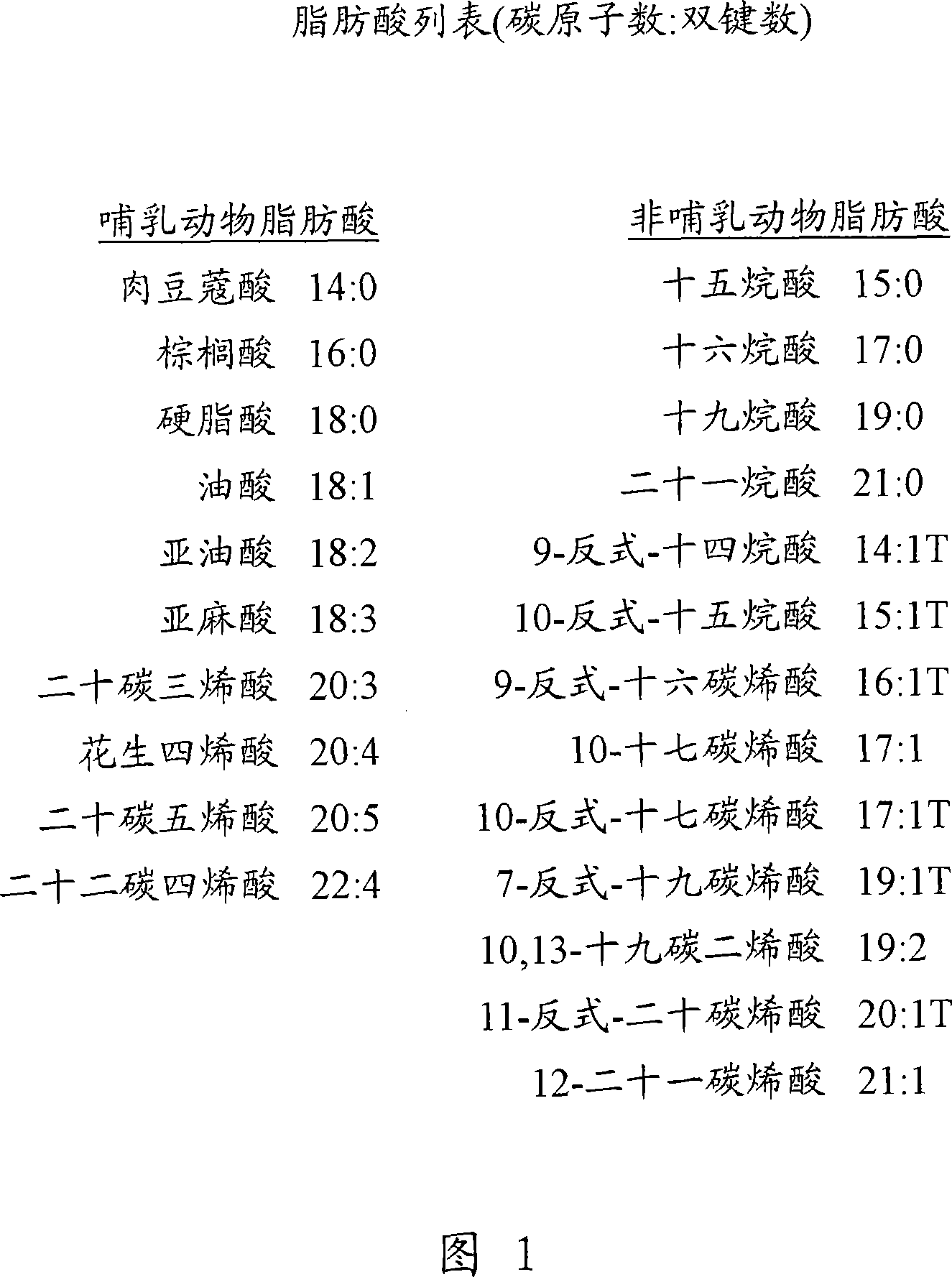
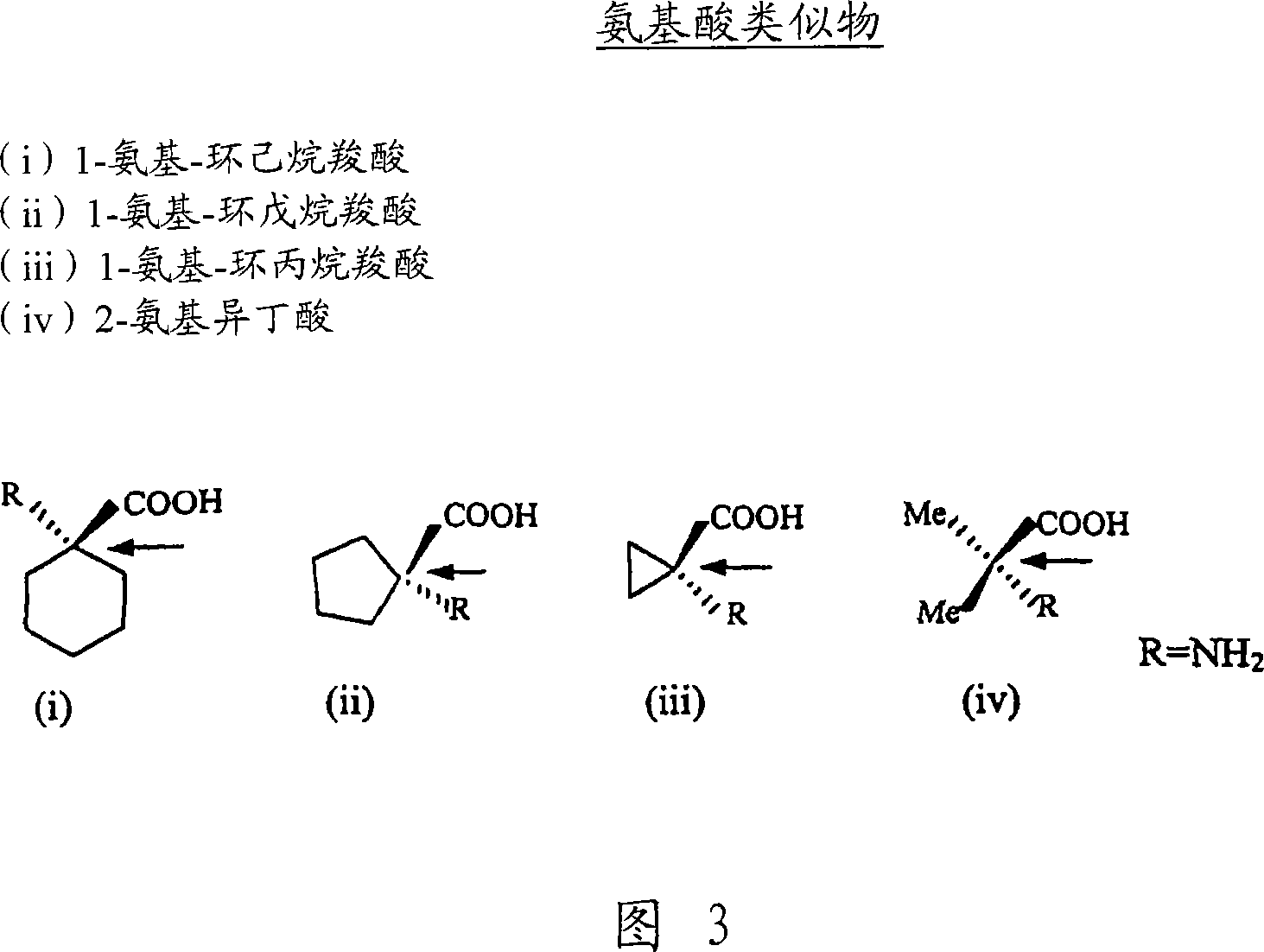
Lipid-amino acid conjugates and methods of use
A technology of amino acids and fatty acids, applied in the field of treatment of diseases, can solve problems such as lack of affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0145] Starch microspheres can be prepared by adding a warm water-soluble starch solution such as potato starch to a hot aqueous polyethylene glycol solution and stirring to form an emulsion. After the formation of the two-phase system (starch solution as the internal phase), the mixture is cooled to room temperature with continuous stirring, whereupon the internal phase transforms into gel particles. The particles are then filtered off at room temperature and slurried in a solvent such as ethanol, after which the particles are filtered off again and left to air dry.
[0146] Microspheres can be hardened by well known crosslinking methods such as heat treatment or use of chemical crosslinking agents. Suitable reagents include dialdehydes, including glyoxal, malondialdehyde, succinaldehyde, adipaldehyde, glutaraldehyde and o-phthalaldehyde, diketones such as diacetyl, epichlorohydrin, polyphosphates, and borates. Dialdehydes are used to cross-link proteins such as albumin by ...
Embodiment 1
[0153] Embodiment 1. the synthesis of arachidonoyl-D-alanine
[0154] N-Fatty acid-D-alanine conjugates were prepared from chirally pure D-amino acid methyl esters (Aldrich Chemicals). Arachidonic acid chloride (Nucheck) in dichloromethane was reacted with alanine methyl ester in dichloromethane containing 5% triethylamine for 4 hours at room temperature. The mixture was partitioned between ethyl acetate and dilute HCl, washed, dried and evaporated to an oily residue. The residue was dissolved in THF, then stirred with 1N LiOH under nitrogen, and saponified at room temperature for 5 hours. The product was extracted as above and subjected to thin-layer chromatography. The main product was identified as N-arachidonic acid-D-alanine by mass spectrometry, and the mass spectrometry showed an MH of 376.1 + . Circular dichroism measurements showed a mirror image corresponding spectrum between 220 and 240 nm when compared to the L isomer. The [α] value of the D isomer is -16.6×10...
Embodiment 2
[0155] Embodiment 2. Synthesis of other Elmiric acid
[0156]The synthetic method of Example 1 was used to prepare the conjugates of arachidonyl and palmityl and 1-aminocyclohexanecarboxylic acid, 1-aminocyclopentanecarboxylic acid, and 2-aminoisobutyric acid. The synthesized compounds are N-palmitoyl-1-aminocyclohexane-COOH, N-palmitoyl-1-aminocyclopentane-COOH, N-palmitoyl-2-aminoisobutyric acid, N-arachidonoyl -1-aminocyclohexane-COOH, N-arachidonoyl-1-aminocyclopentane-COOH and N-arachidonoyl-2-aminoisobutyric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com