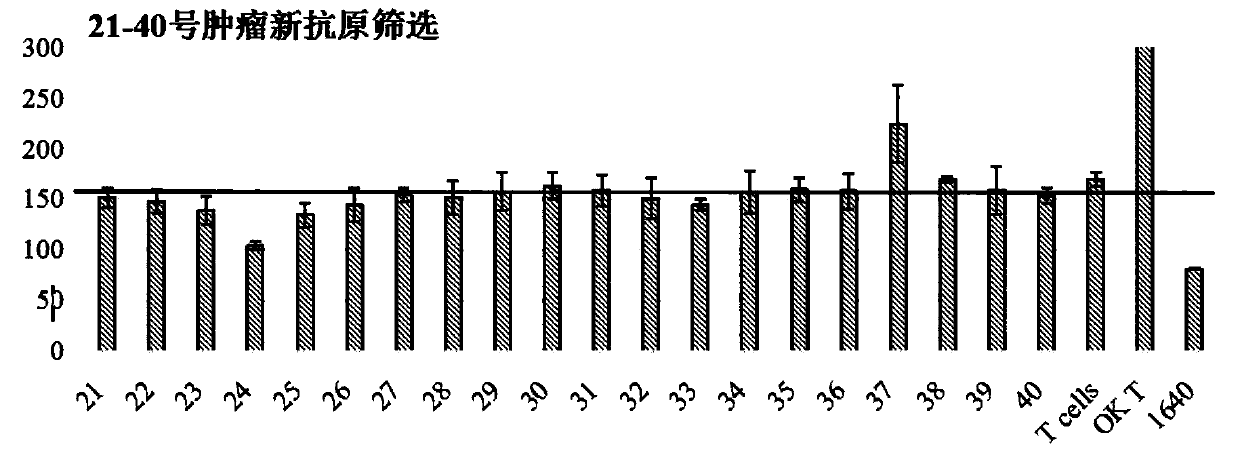
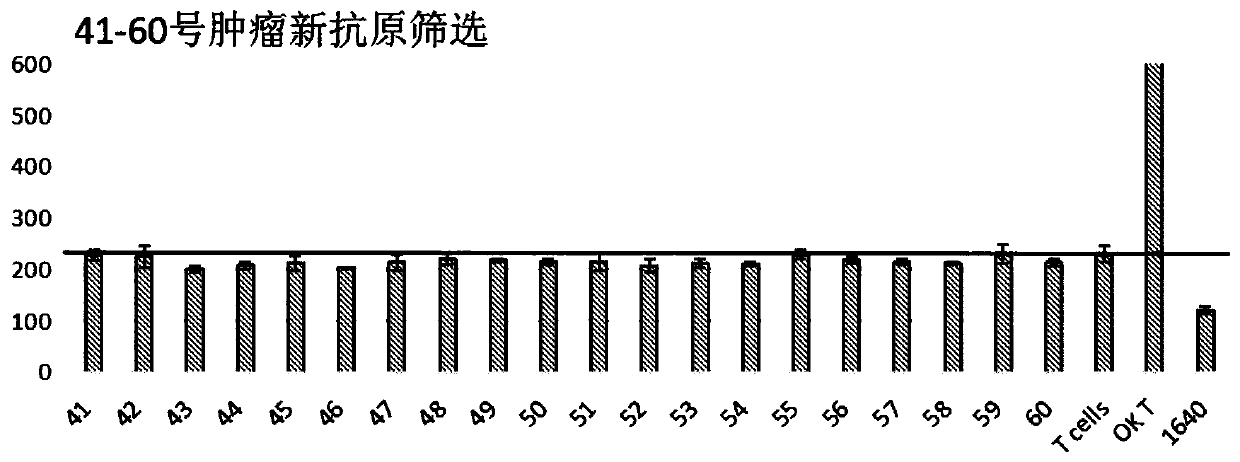
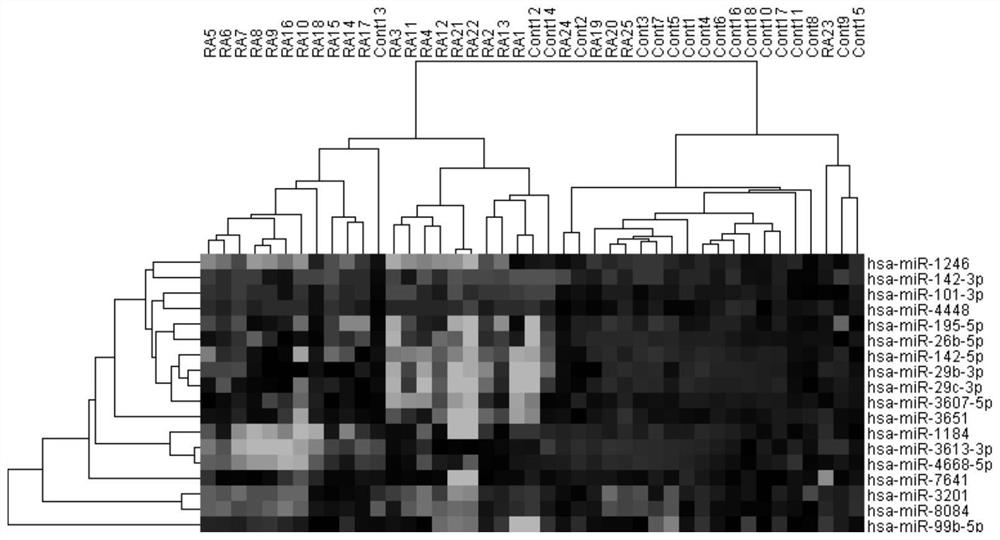
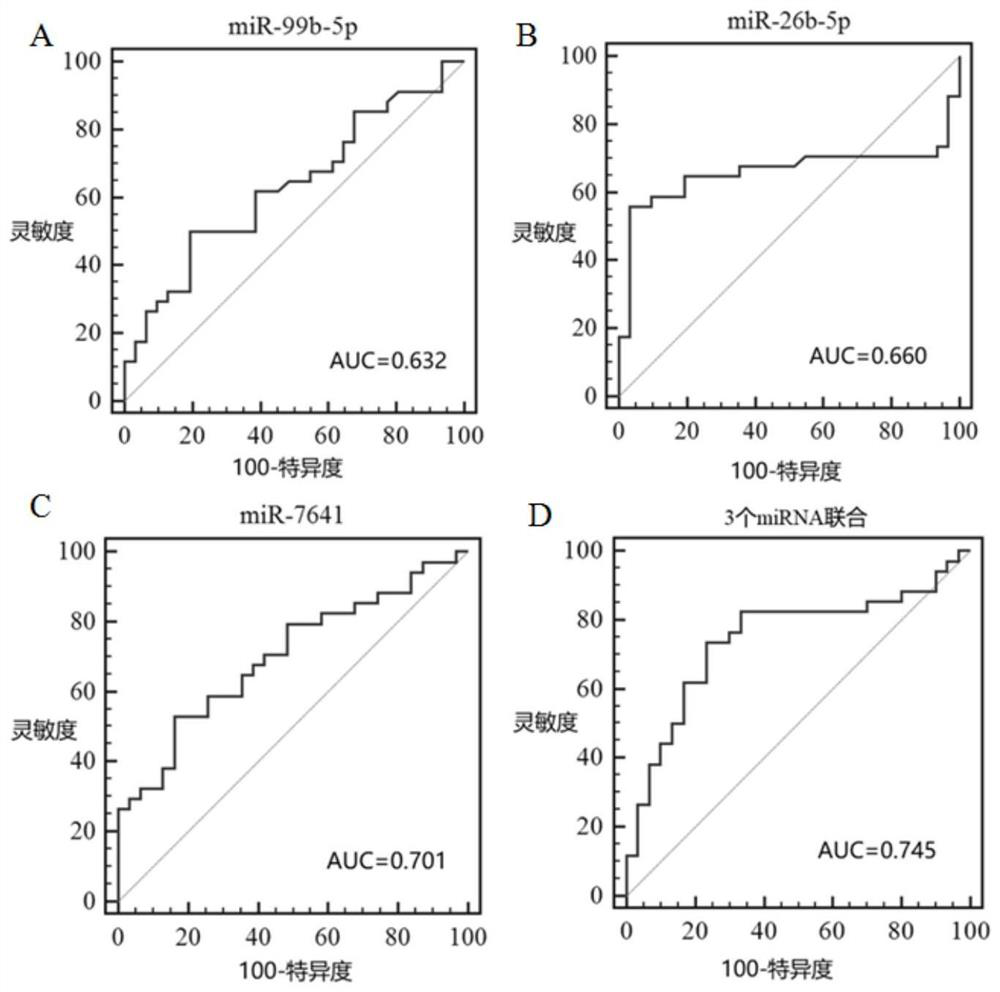
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Mononuclear Blood Cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
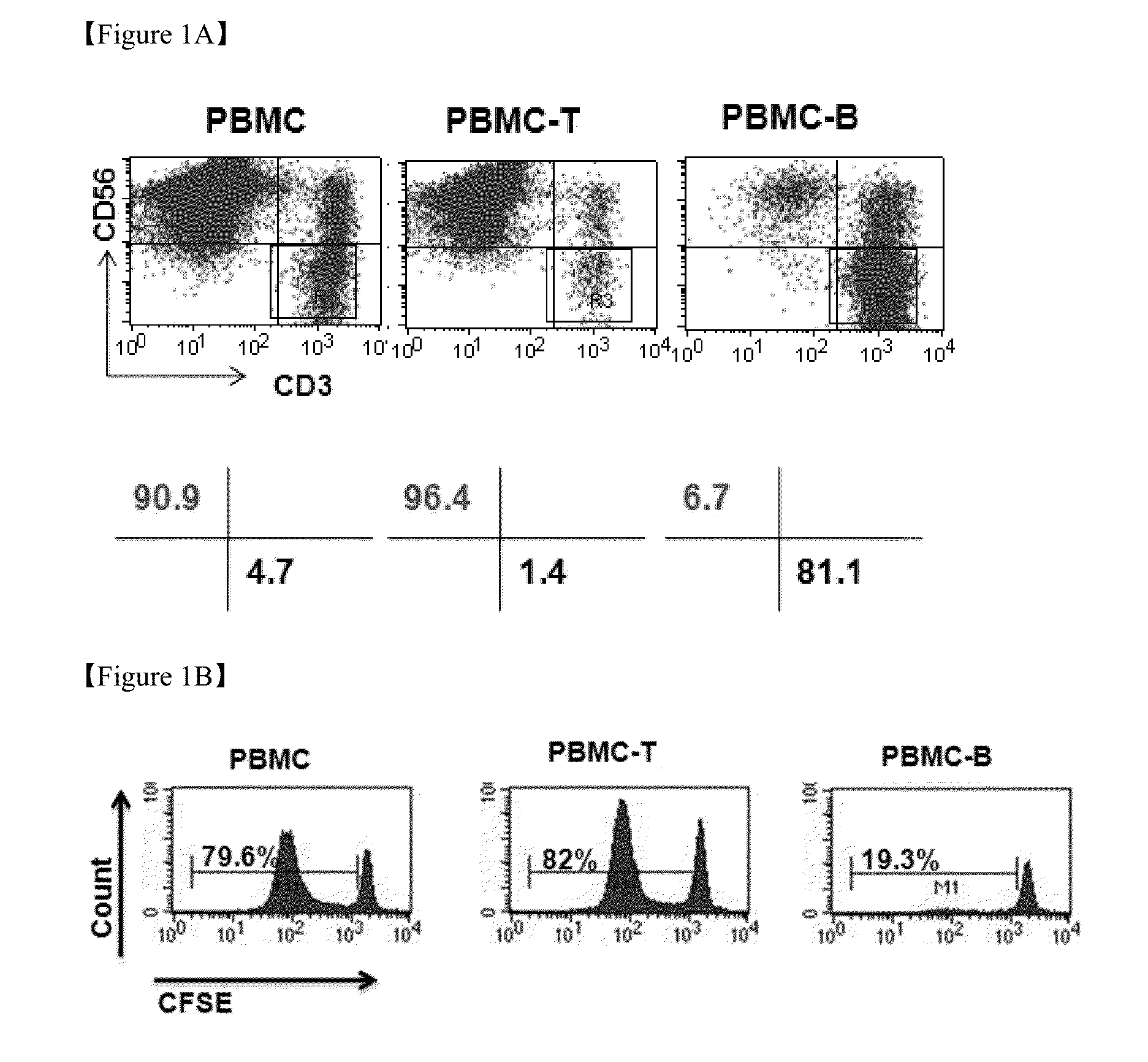
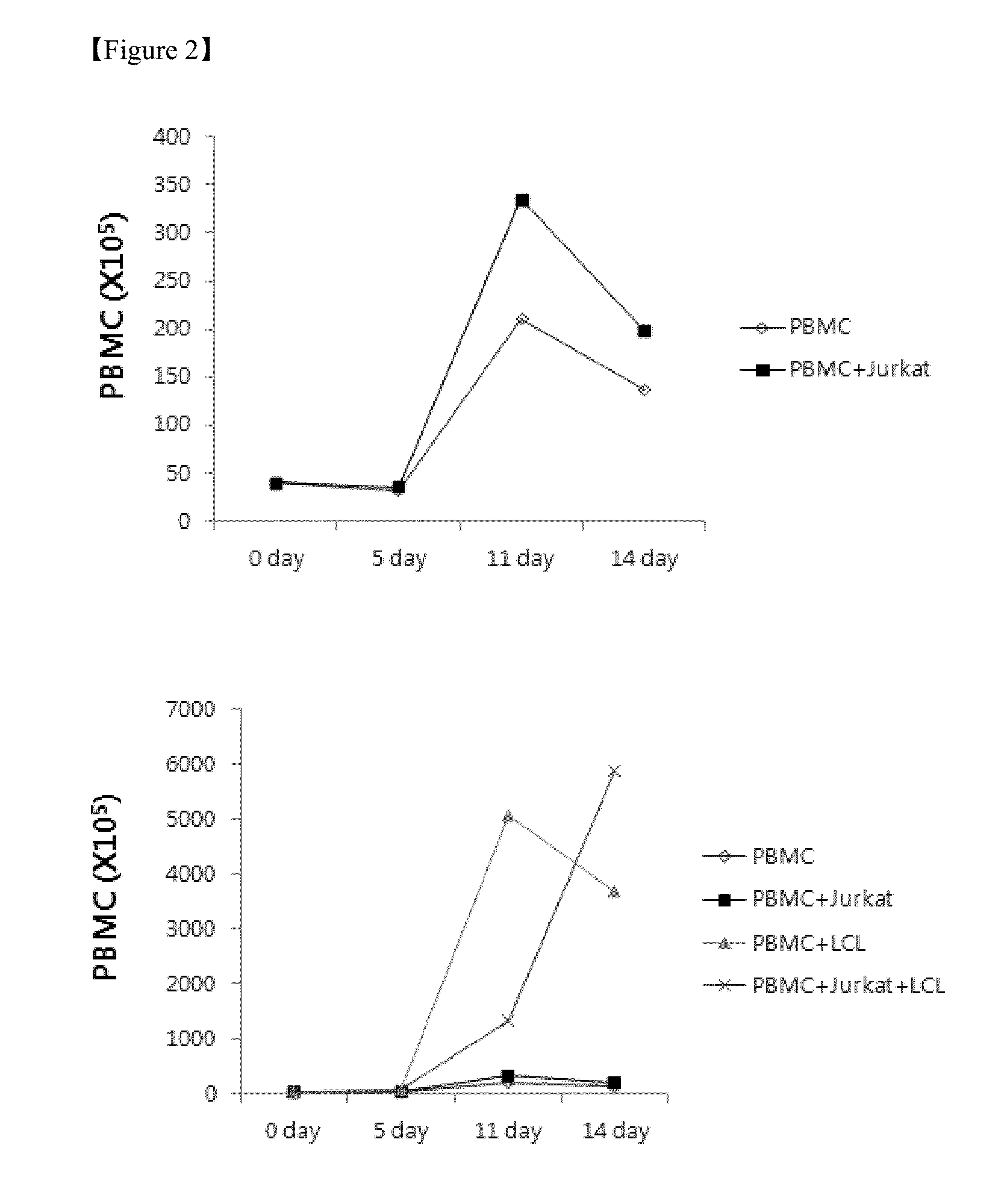
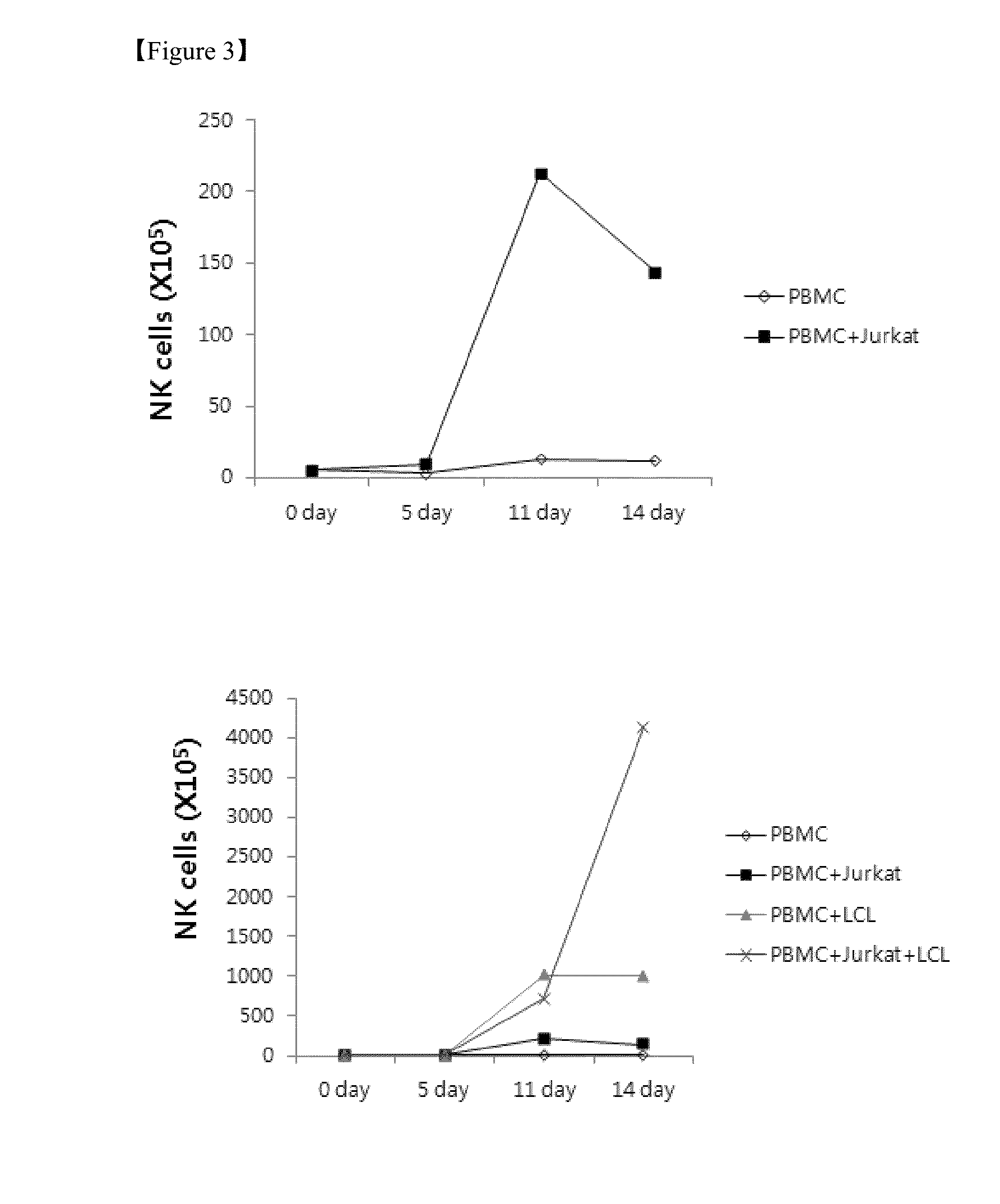
Method for the induction and expansion of natural killer cells derived from peripheral blood mononuclear cells
ActiveUS20150152387A1Improve efficiencyImprove efficacyBiocideMammal material medical ingredientsNatural Killer Cell FunctionNatural killer cell
The present invention relates to a method for inducing and expanding natural killer cells derived from peripheral blood mononuclear cells, which comprises co-culturing, as feeder cells, irradiated Jurkat cells and irradiated Epstein-Barr virus transformed lymphocyte continuous line (EBV-LCL) cells in the presence of cytokines, along with peripheral blood mononuclear cells. According to the present invention, a large quantity of natural killer cells can be induced and proliferated from a small quantity of peripheral blood mononuclear cells even without the use of high-cost equipment or various kinds of expensive cytokines, thereby making it possible to significantly improve the efficiency and efficacy of the prevention and treatment of cancer using the natural killer cells.
Owner:NKMAX CO LTD
Diagnostic microarray for inflammatory bowel disease, crohn's disease and ulcerative colitis
InactiveUS20040077020A1Compound screeningApoptosis detectionIntestinal tract diseasesUlcerative colitis
Using RNA samples from mononuclear blood cells, gene sequences were identified that can be used to identify patients with IBD, and then distinguish patients with Crohn's disease from those with ulcerative colitis. Sequences were identified whose overexpression was distinct to patients with IBD, Crohn's disease, and ulcerative colitis when compared to patients with non-IBD intestinal disorders. Additionally, cluster analysis was used to identify twenty-five sequences that are IBD-related, and whose transcription pattern can be used in a microarray analysis to identify patients with IBD with a sensitivity of 84% and a specificity of 100%. Cluster analysis also identified thirty-six genes that could be used to distinguish patients with Crohn's disease from those with ulcerative colitis with a sensitivity of 89% and a specificity of 80%.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
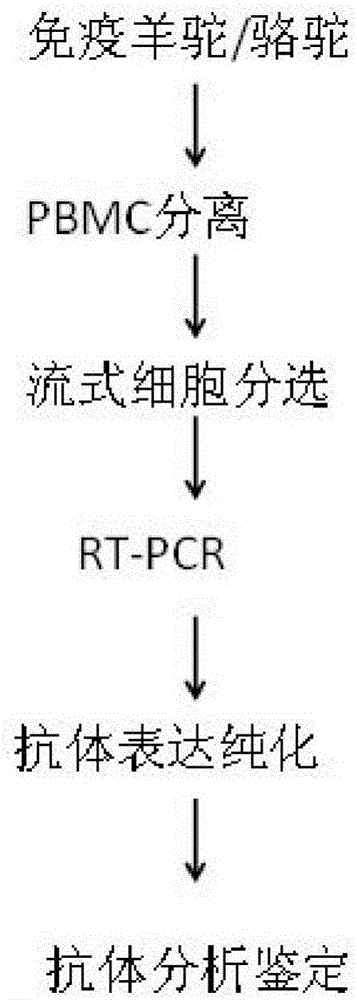
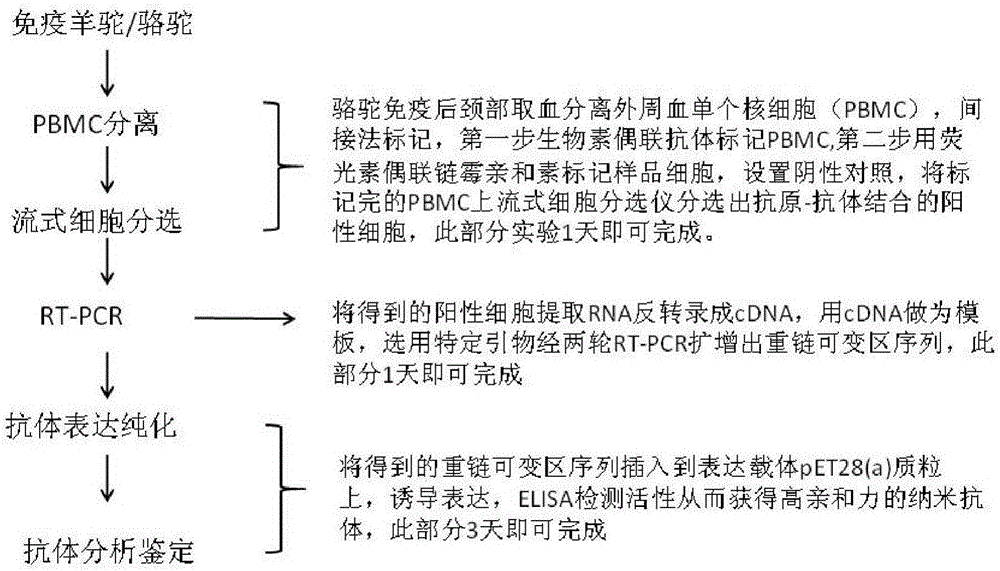
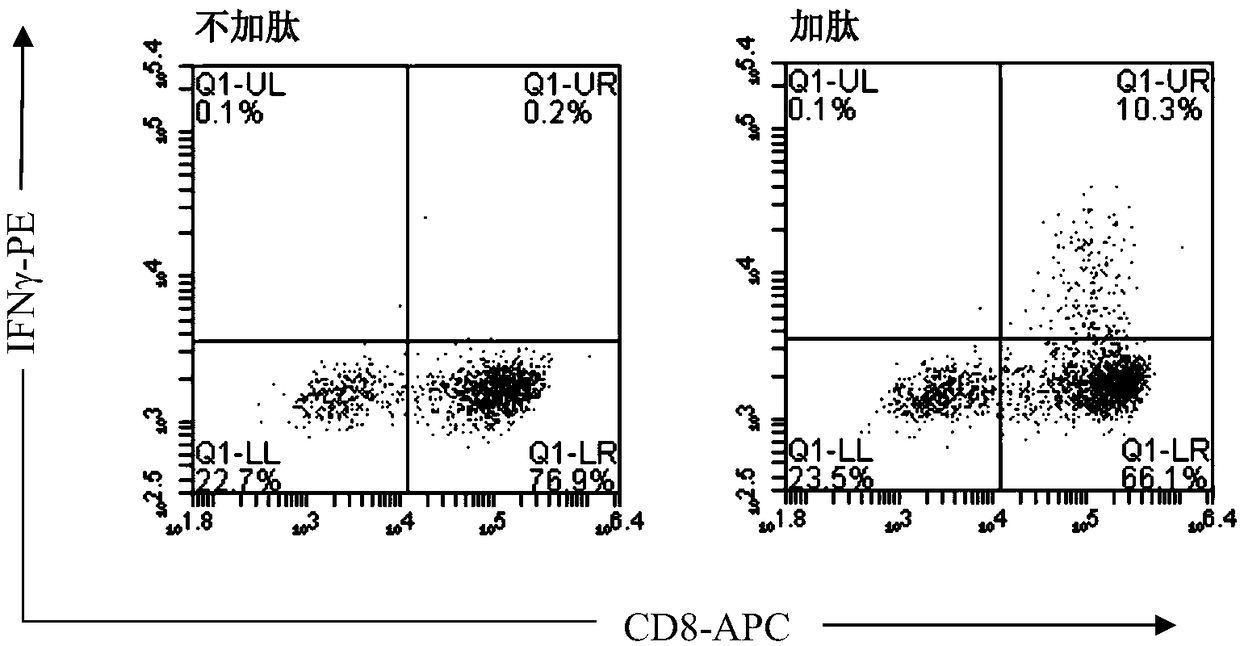
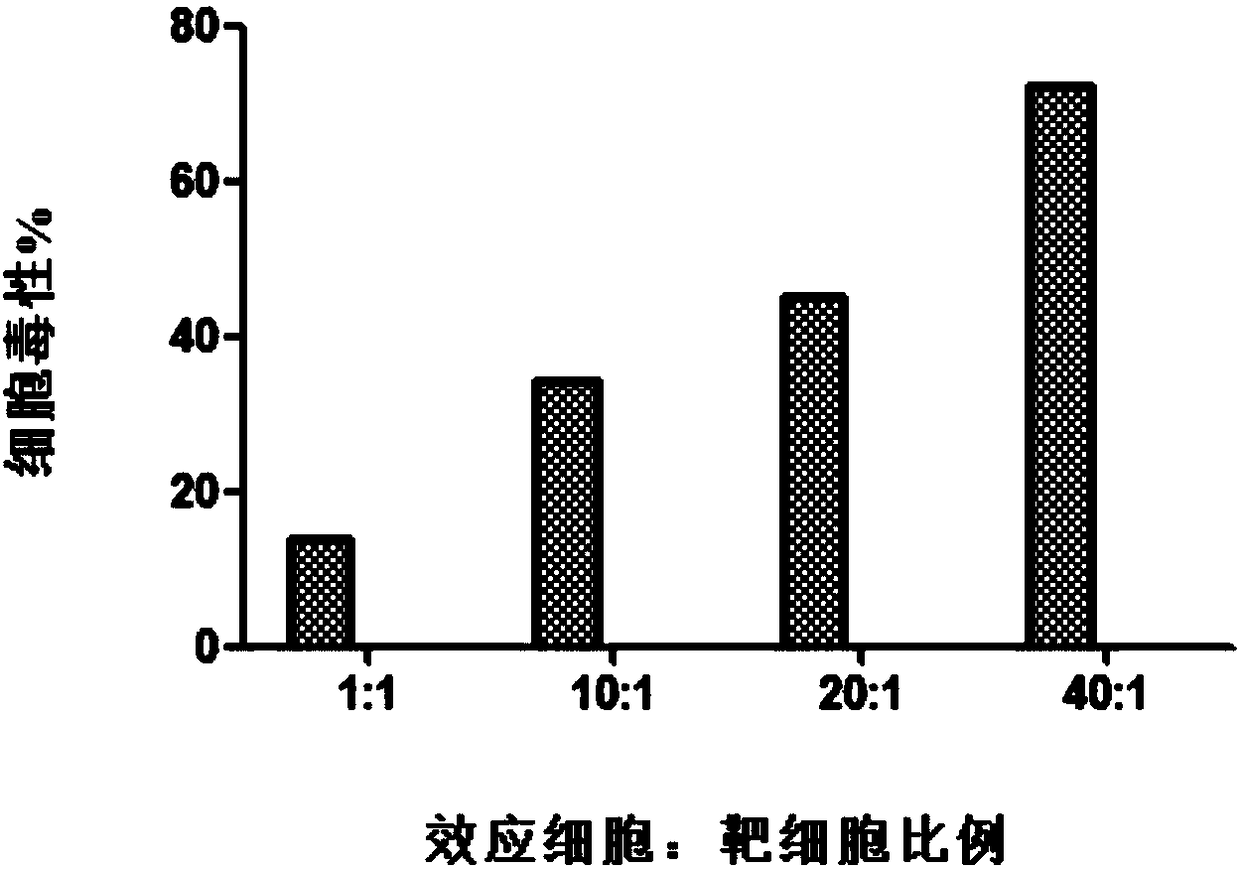
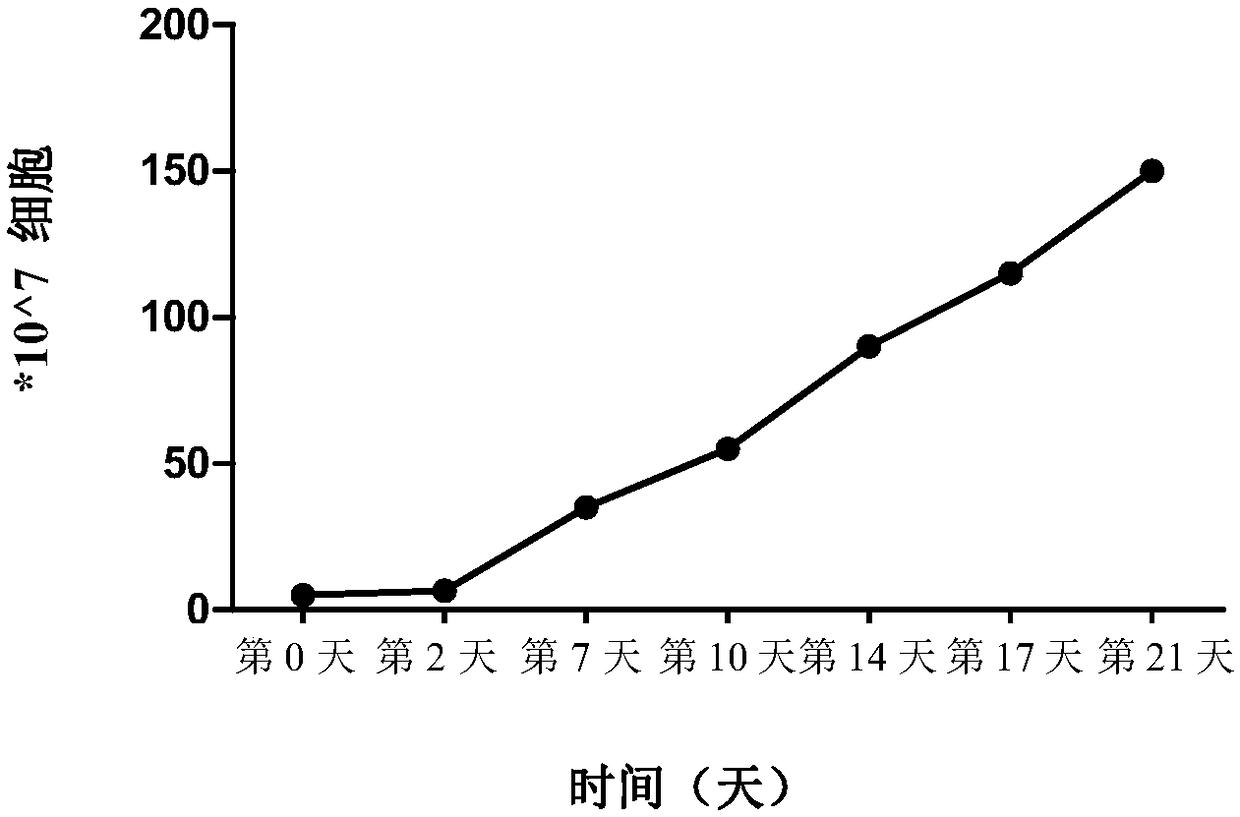
Method for rapidly acquiring nano-antibodies and application of method
InactiveCN106282214ALarge amount of analysisAvoid churnImmunoglobulins against cell receptors/antigens/surface-determinantsVector-based foreign material introductionProkaryotic expressionCell sorting
The invention discloses a method for rapidly acquiring nano-antibodies. The method comprises the following steps: (1) acquiring a negative control; (2) separating peripheral blood mononuclear cells (PBMC) of an alpaca; (3) carrying out flow cytometry sorting; (4) extracting RNA of positive cells, and carrying out inverse transcription to form cDNA; (5) amplifying VHH segments; (6) linking the VHH segments into an expression vector; (7) carrying out PCR identification on a recombinant prokaryotic expression vector; (8) carrying out expression of pet28a-CD19-VHH; and (9) carrying out ELISA activity identification on the expression vector.
Owner:康众(北京)生物科技有限公司
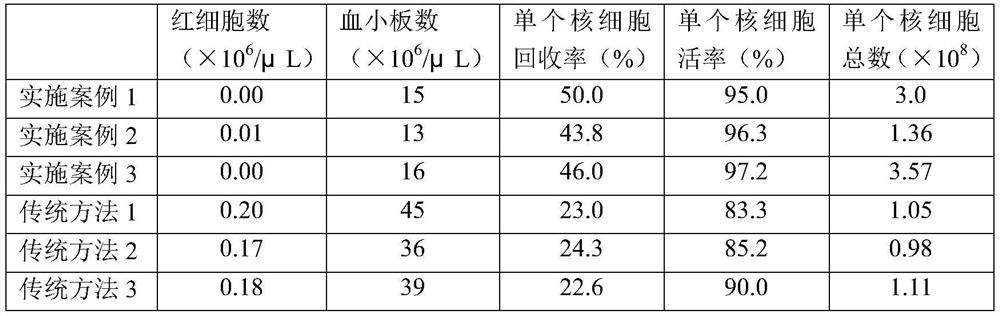
Kit for allogeneic peripheral blood mononuclear cell separation in vitro and application method of kit
InactiveCN104789525AResidue reductionAvoid pollutionBlood/immune system cellsSeparation technologyCryopreservation
The invention relates to a kit for high-purity high-recovery separation in vitro of allogeneic peripheral blood mononuclear cells and an application method of the kit. The allogeneic mononuclear cells separated by the kit can be used for immunotherapy of unexplained habitual abortion. The kit comprises a reagent I, namely a peripheral blood thinner, a reagent II, namely an erythrocyte sedimentation solution, a reagent III, namely mononuclear cell separating solution and a reagent IV, namely a mononuclear cell washing solution. The method for separating the allogeneic peripheral blood mononuclear cells by using the kit comprises the following steps: anticoagulation pre-centrifugation; erythrocyte initial reaction; erythrocyte sedimentation; two-step separation of the mononuclear cell separating solution; secondary purification of mononuclear cells; bacteria detection; mononuclear cell cryopreservation. According to the technology, the mononuclear cell recovery rate can achieve more than 90 percent, the purity can achieve more than 95 percent, the survival rate can achieve more than 98 percent, and the service efficiency of the peripheral blood mononuclear cells is greatly improved. Plasma is removed through the step of anticoagulation pre-centrifugation. Compared with the conventional separation technology, the dose of the separation reagent is reduced, and the separation cost is effectively reduced.
Owner:王盛
Method for preparing HPV (human papillomavirus) antigen specific CTL (cytotoxic T lymphocyte)
ActiveCN108300692AEnhance antigen presentationInduced proliferationMammal material medical ingredientsBlood/immune system cellsHPV AntigenDisease
The invention discloses a method for preparing HPV (human papillomavirus) antigen specific CTL (cytotoxic T lymphocyte) and particularly discloses a preparation method of HLA-A2402 restrictive anti-HPV antigen specific CTL. According to the method, a peripheral blood mononuclear cell is collected through single blood collection or venous blood collection, the antigen presentation function of B cells is enhanced with CpG ODN 2395, the B cells supporting HLA-A2402 restrictive HPV antigen peptide are used for stimulating the peripheral blood mononuclear cell, rhIL-2, rhIL-7, rhIL-15 and rhIL-21 are jointly used to promote growth of the T cell. The target CTL prepared with the method has the characteristics that preparation is simple, the preparation cycle is short, the cost is low, the multiplication capacity and the killing activity are high, the cell viability is high and the like, and can be used for immunotherapy of HPV infection related diseases including cervical cancer.
Owner:JILIN TUO HUA BIOTECH
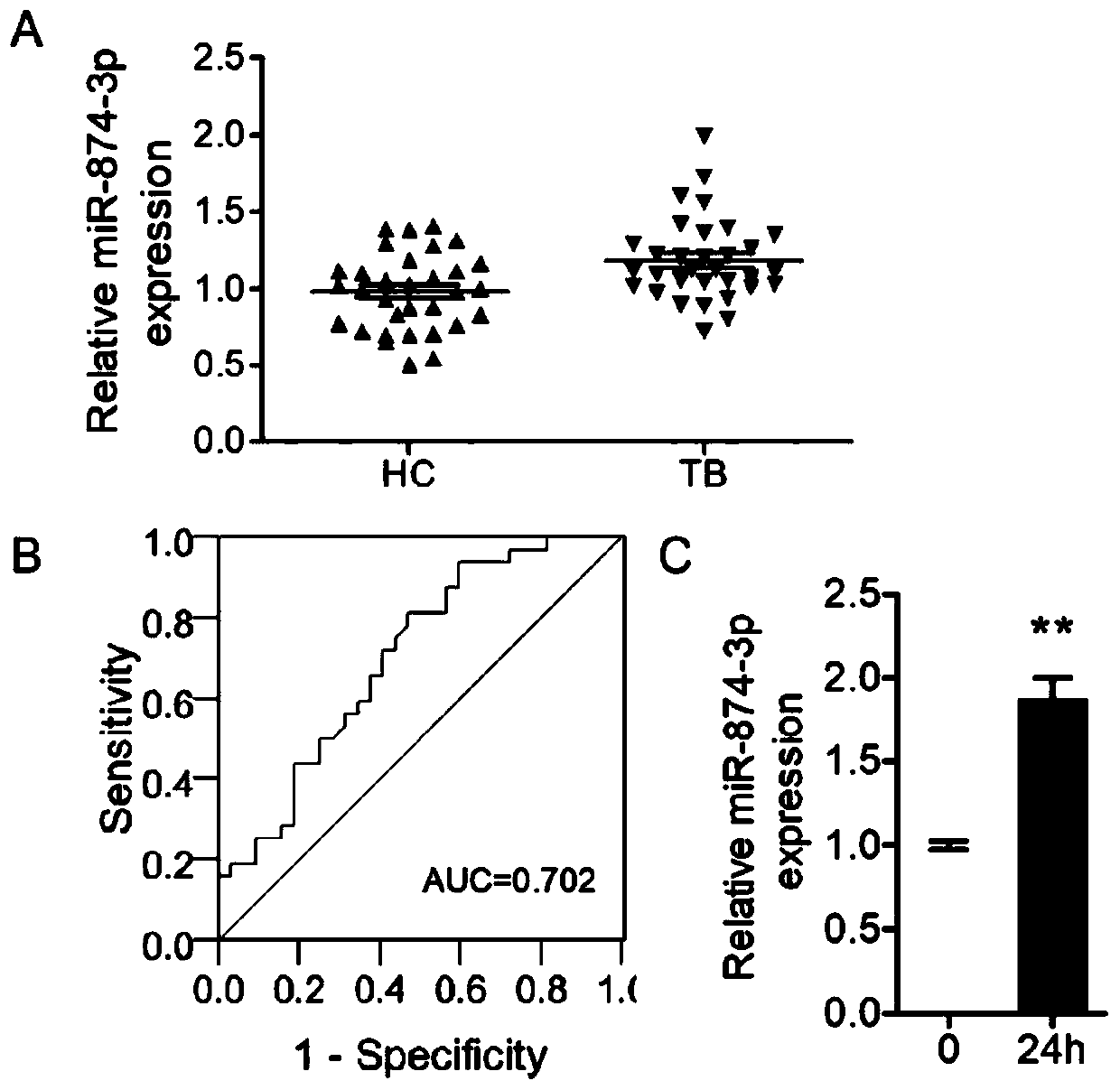
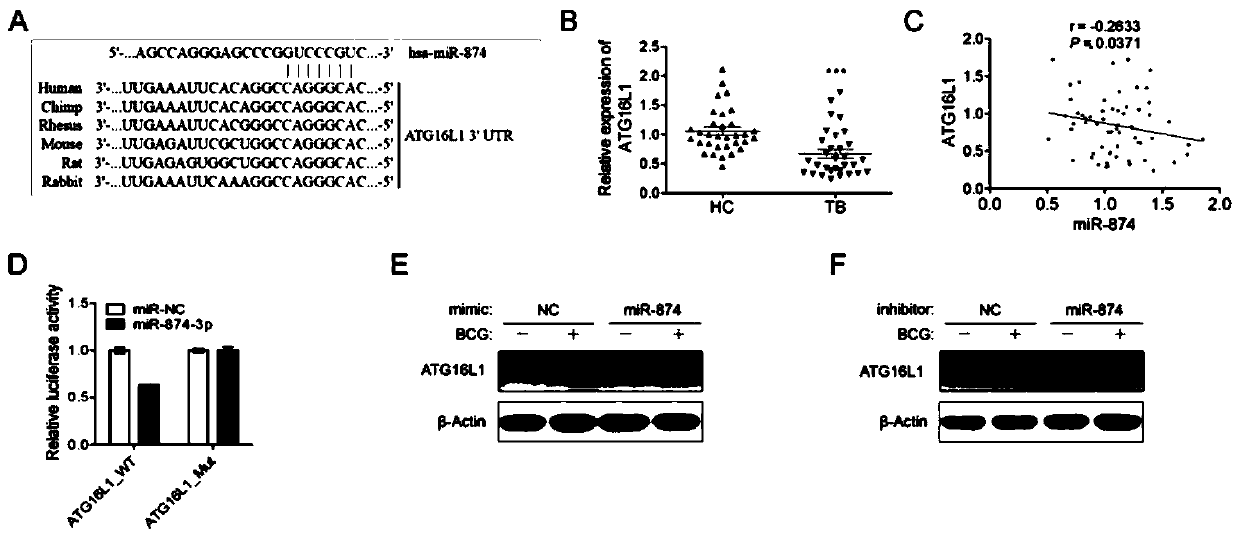
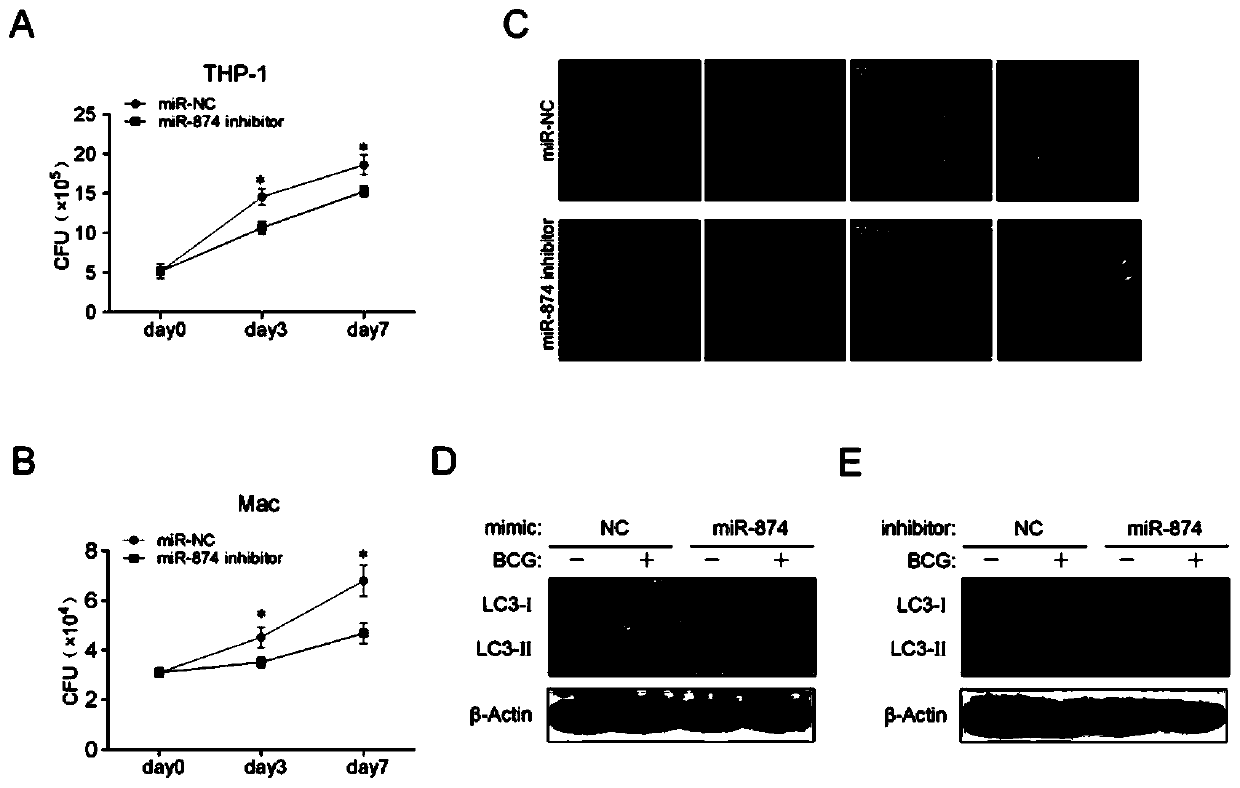
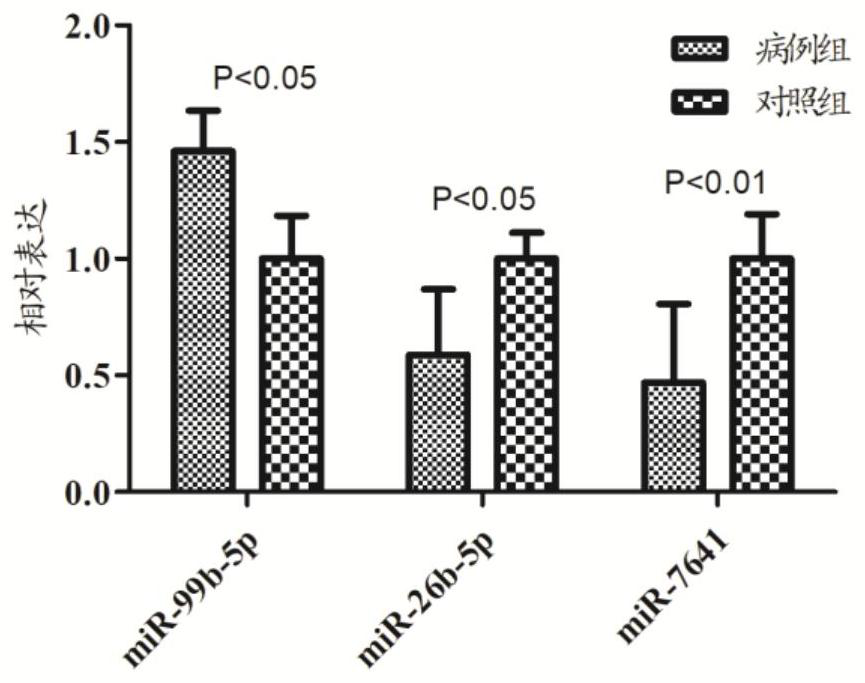
Application of peripheral blood mononuclear cells hsa-miR-8774-3p as marker of active tuberculosis
PendingCN110643697AImprove developmentEasy to collectMicrobiological testing/measurementDNA/RNA fragmentationDiseaseMononuclear Blood Cell
The invention relates to the technical field of biomarkers, in particular to application of a peripheral blood mononuclear cell hsa-miR-874-3p as a marker of active tuberculosis. The hsa-miR-874-3p issignificantly up-regulated in peripheral blood mononuclear cells from patients with active tuberculosis and a human monocyte cell line THP-1 infected with tuberculosis, and the up-regulated hsa-miR-874-3p possibly acts on ATG16L1 mRNA in mononuclear macrophages to regulate macrophage autophagy and participate in the infection process of Mtb. The up-regulated hsa-miR-874 has certain significance for the early diagnosis of APTB patients. The detection of the expression condition of hsa-miR-874 in patients with APTB using a qRT-PCR method can diagnose the disease. A new molecular target for clinical diagnosis and treatment is provided, and the development of new drugs is facilitated.
Owner:GUANGDONG MEDICAL UNIV
Method for extracting and collecting peripheral blood mononuclear cells
PendingCN112458051AHigh yieldHigh activityCell dissociation methodsBlood/immune system cellsFicollCell extraction
The invention discloses a method for extracting and collecting peripheral blood mononuclear cells. The method comprises the following steps: 1) collecting peripheral blood; 2) pretreating peripheral blood; 3) performing centrifuging; 4) extracting plasma; 5) performing plasma inactivation; 6) collecting mononuclear cell layers; 7) centrifuging the mononuclear cell layers; 8) performing cleaning; and 9) extracting mononuclear cells. The invention belongs to the technical field of cell extraction, and particularly provides a method for separating peripheral blood mononuclear cells, which is usedfor obtaining the peripheral blood mononuclear cells by centrifugal extraction of Ficoll lymphocyte separation liquid and is high in cell yield and high in activity.
Owner:海南优尼科尔生物科技有限公司
Cryopreservatio solution and cryopreservation method for human peripheral blood mononuclear cells
The invention discloses a cryopreservation solution and a cryopreservation method for human peripheral blood mononuclear cells, and belongs to the technical field of cell biology. The cryopreservationsolution comprises the following components: 8% of dimethyl sulfoxide, 70% of autologous plasma, 10% of hydroxyethyl starch and 12% of human serum albumin. The specific cryopreservation procedure comprises the following steps: a, waiting at 4 DEG C until the product is put in; b, cooling to 0 DEG C at a speed of 1 DEG C / min, and keeping for 5 minutes; c, cooling to -10 DEG C at a speed of 1 DEG C / min, and keeping for 5 minutes; d, cooling to -45 DEG C at a speed of 1 DEG C / min, and keeping for 20 minutes; e, cooling to -90 DEG C at a speed of 5 DEG C / min, and keeping for 5 minutes; and f, ending. According to the cryopreservation solution and the cryopreservation method, the cell activity and the recovery rate after cryopreservation recovery can be remarkably increased, high-quality NK cells with high purity, high cell activity and high amplification factor are induced and cultured, and a favorable guarantee is provided for clinical treatment of the NK cells.
Owner:FUJIAN YINFENG STEM CELL ENG +1
Separation method for peripheral blood mononuclear cell
InactiveCN107254438AAvoid damageHigh yieldCell dissociation methodsBlood/immune system cellsLymphocytic cellCell layer
The invention discloses a method for isolating peripheral blood mononuclear cells, which comprises the following steps: (1) mixing the peripheral blood with Duchenne's phosphate buffer to obtain diluted peripheral blood; placing the lymphocyte separation solution in a centrifuge tube and Store in shading; (2) Tilt the centrifuge tube containing the lymphocyte separation solution so that the liquid level of the lymphocyte separation solution is at an angle of 50°-70° to the horizontal plane; add the diluted peripheral blood to the lymphocyte along the side wall of the centrifuge tube. The mixed solution is obtained from the cell separation solution; the volume ratio of the diluted peripheral blood to the lymphocyte separation solution is 1-2:1; (3) centrifuge the centrifuge tube containing the mixed solution and take the buffy coat to obtain the peripheral blood mononuclear cell layer. The separation method is easy to operate and is suitable for the separation of a large number of samples; it can realize aseptic operation, and the obtained peripheral blood mononuclear cells are suitable for clinical application; the damage to the isolated peripheral blood mononuclear cells is small, and the cell yield is high. will not be disturbed.
Owner:MIAOSHUN SHANGHAI BIOTECH CO LTD
Method for preparing liver organoid from peripheral blood mononuclear cells
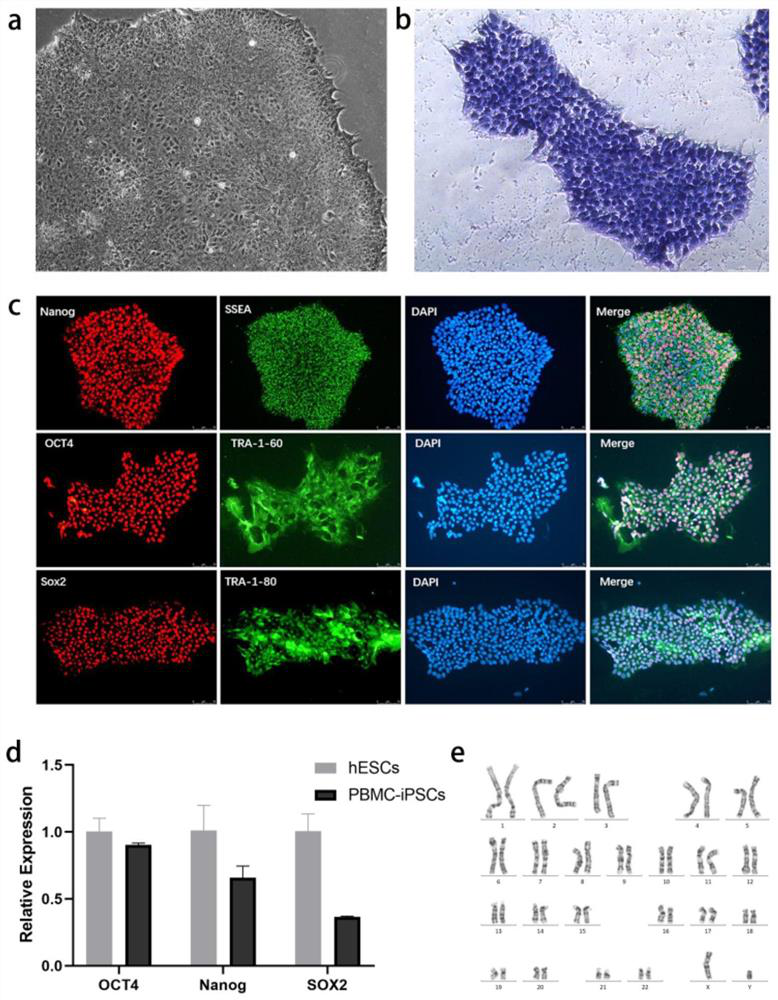
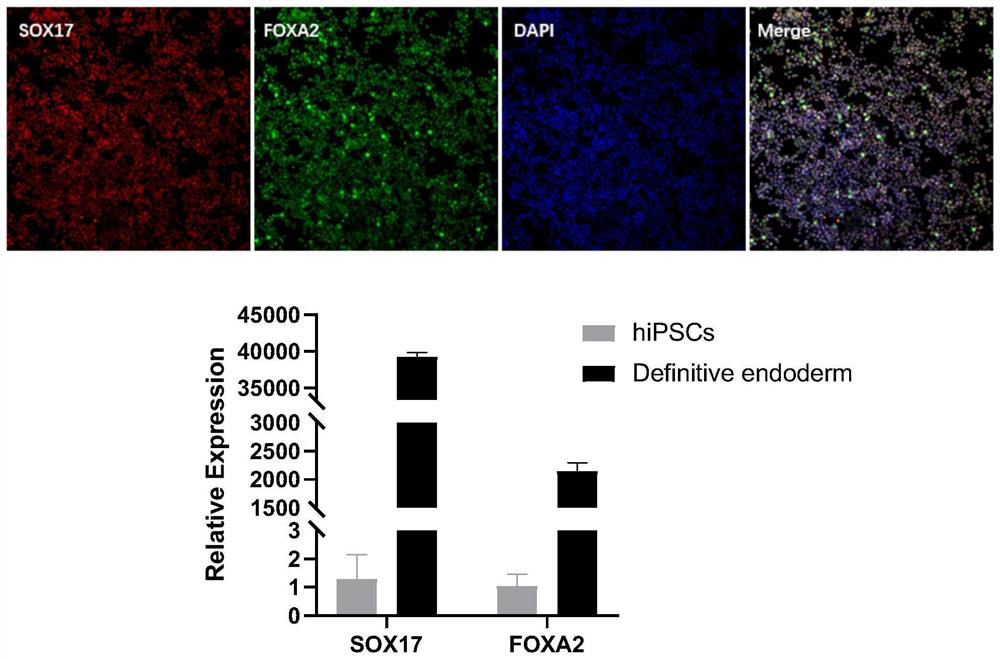
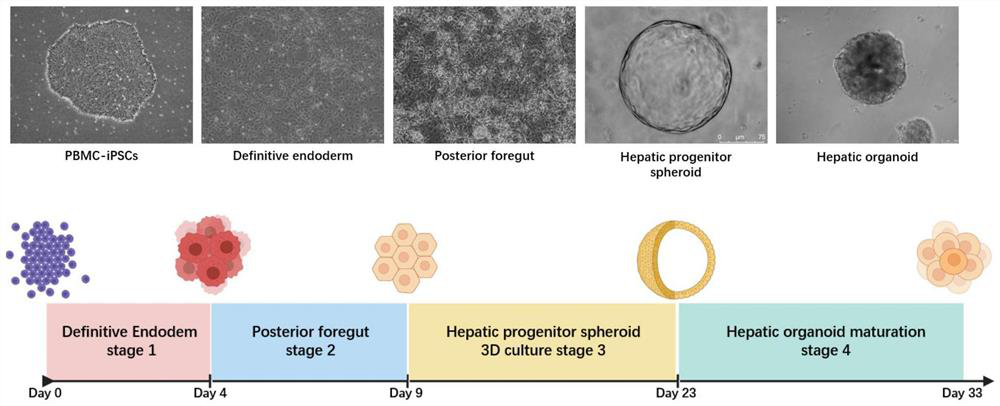
The invention discloses a method for preparing liver organoid from peripheral blood mononuclear cells (PBMCs). A liver organoid model which has individual genetic information and fully simulates liver development and pathological and physiological processes of liver-related diseases in vitro is obtained in a relatively noninvasive manner. According to the method, the collected PBMCs are reprogrammed into induced pluripotent stem cells (iPSCs), the iPSCs are further induced and differentiated into the liver organs, the target is achieved through the two steps, the iPSCs can be infinitely amplified and stored in vitro, and a basis is provided for large-scale production of the liver organs.
Owner:SUN YAT SEN UNIV
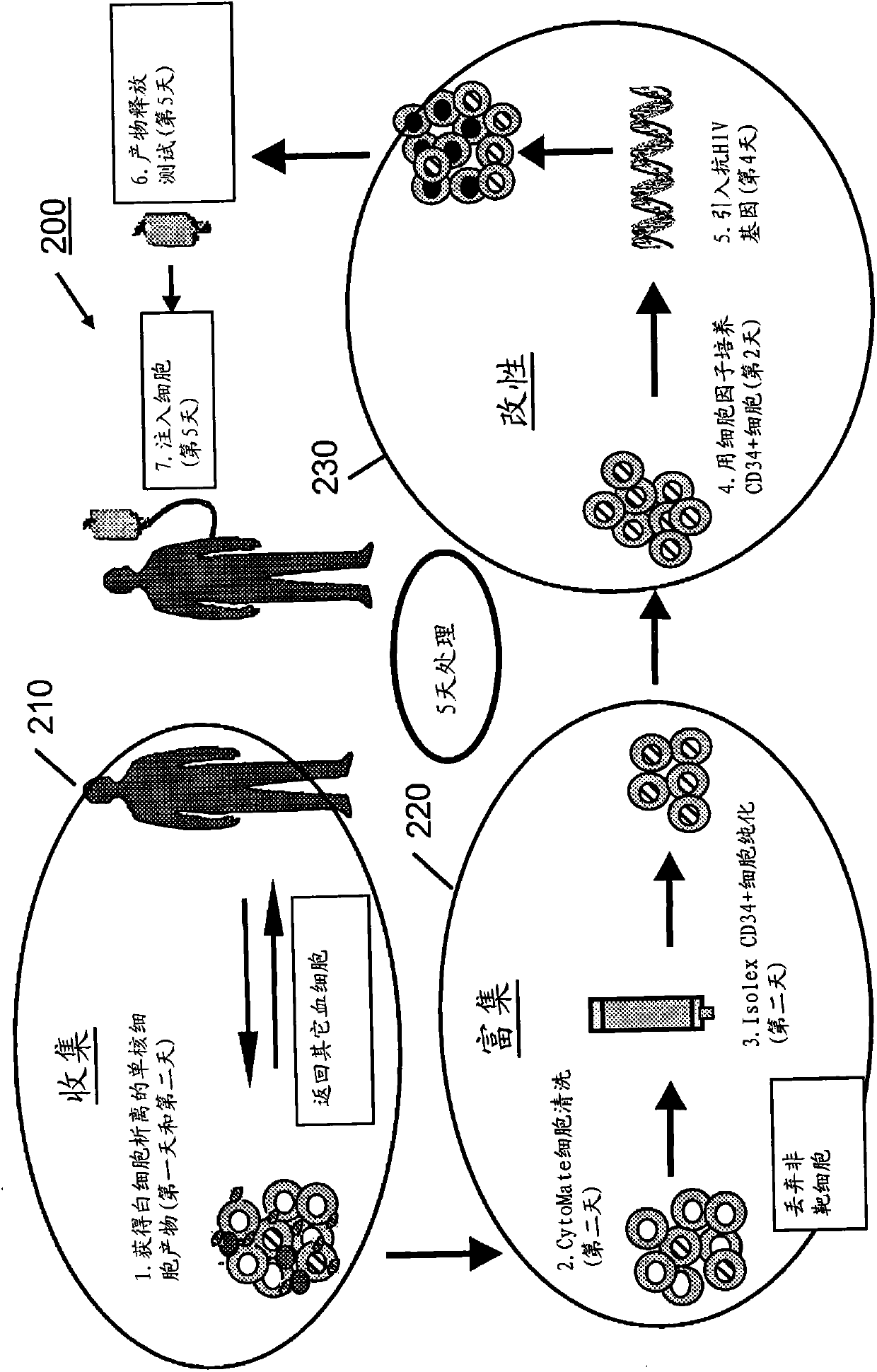
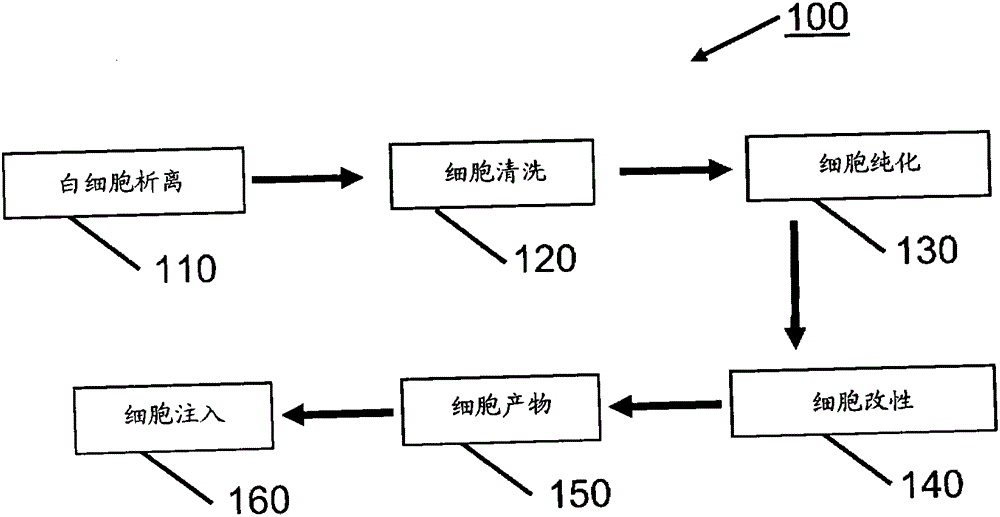
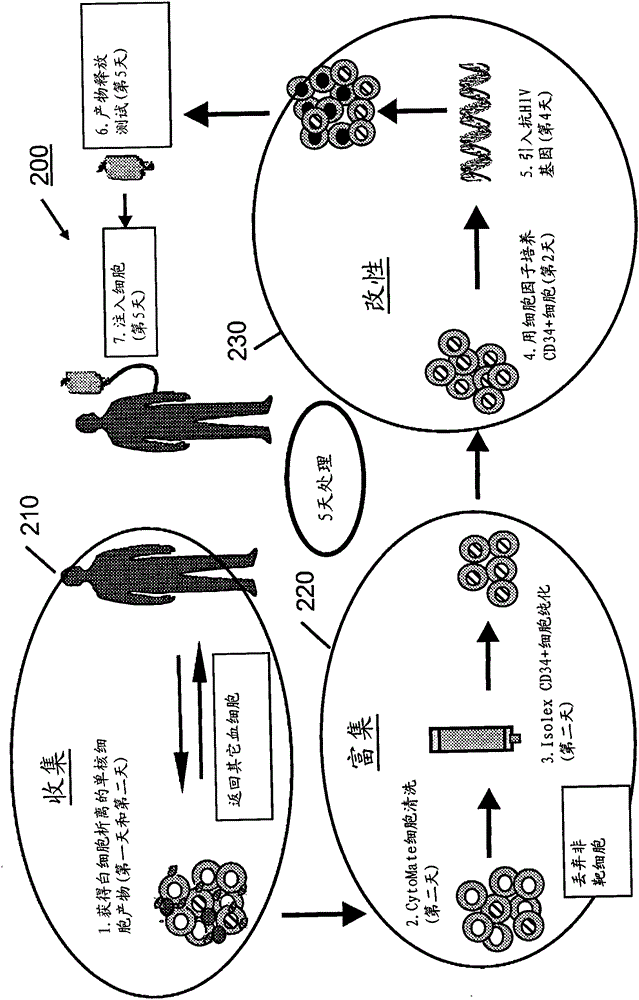
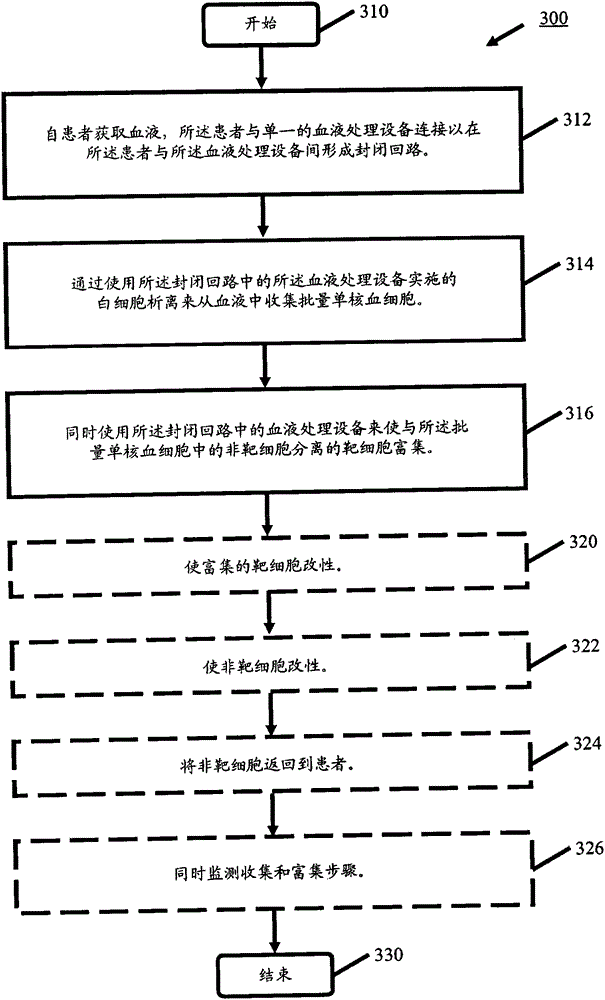
processing blood
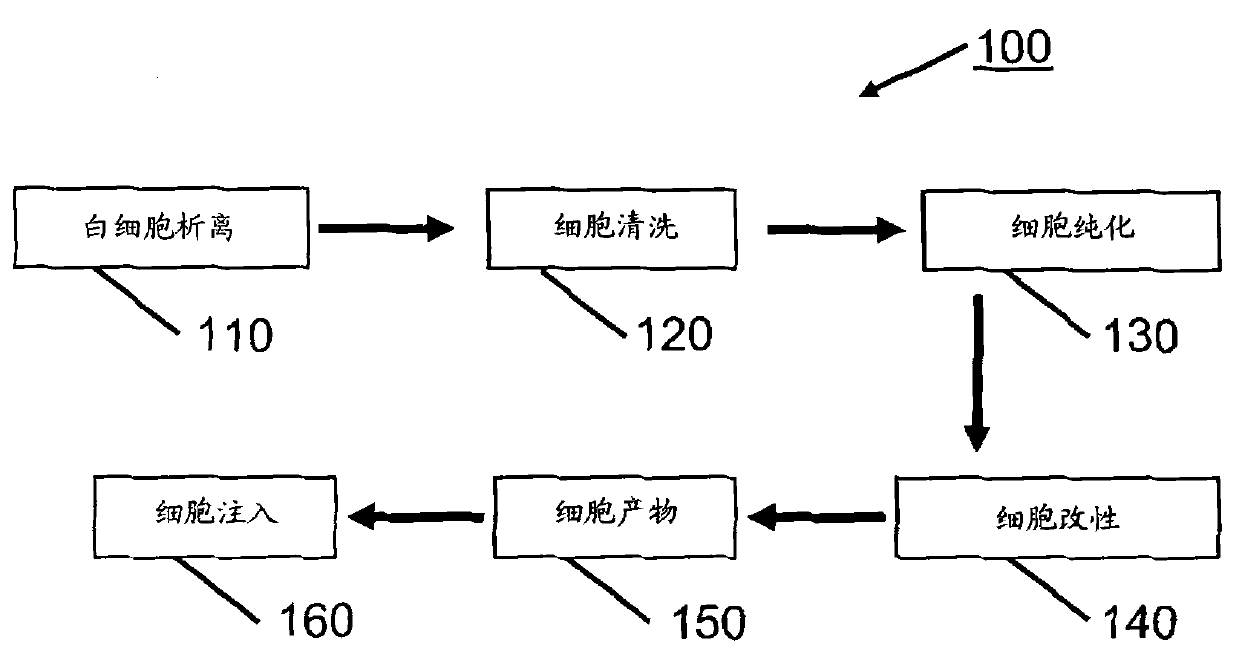
Methods (300), devices, and systems of processing blood are describes. The method (300) comprises the steps of: obtaining (312) blood from a patient coupled to a single blood processing device to form a closed loop between the patient and the blood processing device; collection (314) bulk mononuclear blood cells from the blood by leukapheresis using the blood processing device in the closed loop; and enriching (316) concurrently target cells separated from non-target cells in the bulk mononuclear blood cells using the blood processing device in the closed loop.
Owner:THERAKOS INC
Method for separating and culturing peripheral blood mononuclear cells
InactiveCN111849890AInhibition of lesionsPrevent hyperactivationCell dissociation methodsCulture processCell culture mediaCultured cell
The invention provides a method for separating and culturing peripheral blood mononuclear cells, and relates to the field of biotechnology cell culture. The separation and culture method of the peripheral blood mononuclear cells comprises the following steps: S1, collecting peripheral blood, S2, carrying out centrifugal separation, S3, extracting peripheral blood mononuclear cells, S4, purifying the peripheral blood mononuclear cells, S5, preparing a cell culture medium, S6, carrying out cell culture, and S7, performing preservation. An ALK inhibitor and a GSK-3 inhibitor are arranged, cytopathy in a cell culture process can be inhibited, excessive activation of pathological cells is prevented and rapid proliferation is carried out, animal serum is not added in the culture process, so thatthe possibility of obtaining viruses from animals in the culture process is reduced, the safety in the culture process is ensured, and peripheral blood mononuclear cells are cleaned and purified formultiple times after separation is completed, so that the cultured cells basically do not contain other impurity cells, and the cultured cells are relatively pure.
Owner:KUNMING MEDICAL UNIVERSITY
Method for preparing cord blood mononuclear cells
PendingCN110229788AHigh activityAvoid contamination riskCell dissociation methodsBlood/immune system cellsHydroxyethyl starchFicoll
The invention provides a method for preparing cord blood mononuclear cells. The method comprises the following steps: (1) transferring collected cord blood into a sterile triple bag; (2) carrying outcentrifugal separation on the transferred cord blood; and (3) placing the centrifuged blood bag on a plasma separation clamp to remove plasma supernatant, and acquiring precipitate, namely a mononuclear cell preparation. The preparation method disclosed by the invention does not use hydroxyethyl starch and ficoll lymphocyte separation liquid, the prepared mononuclear cell preparation is safe and has no side effect, the recovery rate of mononuclear cells reaches more than 95%, and the cell activity is more than 93%. The invention also provides a cord blood mononuclear cell preparation. The preparation has small volume of just 10ml, and is convenient to infusion and use for newborn.
Owner:广州市天河诺亚生物工程有限公司
Separation method for separating peripheral blood mononuclear cells
InactiveCN111117957AEasy to manufactureHigh purityCell dissociation methodsBlood/immune system cellsFicollMeglumine diatrizoate
The invention provides a separation method for separating peripheral blood mononuclear cells, and particularly relates to the field of biological medicines. The separation method comprises the following steps: S1, preparation of a separation tube: firstly, adding 2-6 ml of Percoll or polysucrose or meglumine diatrizoate cell separation liquid with the density of 1.075-1.0796 g / ml into a centrifugal tube, sucking 0.5-1.5 ml of separation gel with the density of 1.06-1.07 g / ml, adding the separation gel into a tube opening of the centrifugal tube, and carrying out horizontal centrifuging for 1-3minutes at room temperature under the centrifugal force of 800-1200 g, so that the separation gel forms an isolation layer on the liquid surface of the Percoll or polysucrose or meglumine diatrizoatecell separation liquid, and preparation of the separation tube is finished; and S2, separation of the peripheral blood mononuclear cells: adding 2-6 ml of anticoagulant whole blood into the preparedseparation tube, and carrying out horizontal centrifuging for 8-12 minutes at room temperature under the centrifugal force of 800-1200 g; sucking and discarding the uppermost liquid to remove cell fragments and platelets, directly pouring the liquid above the isolation layer into a collection tube, carrying out centrifuging for 4-6 minutes at room temperature under the centrifugal force of 600-1000 g, and resuspending the cells by using PBS to obtain the peripheral blood mononuclear cells. According to the separation method, it can be ensured that the anticoagulant whole blood can be absolutely not mixed with a cell separation medium after being added, and other cells are not polluted when the peripheral blood mononuclear cells are harvested.
Owner:JIANGSU TAIZHOU PEOPLES HOSPITAL +1
Initiative immune cell activation and amplification system and application thereof
ActiveCN113088489ABinding blockAlleviate shortagesDead animal preservationBlood/immune system cellsAntigenCell therapy
The invention relates to the technical field of immune cell therapy, in particular to an initial immune cell activation and amplification system and application thereof. The invention provides an initial immune cell activation and amplification system. A TGF-beta antagonist is added into an activation culture medium, TGF-beta R on the surface of a T cell in a peripheral blood mono-nuclear cell is competitively combined, the combination of TGF-beta and TGF-beta R is blocked, a TGF-beta mediated signal channel is inhibited, the quantity and function of abnormally increased Treg cells are reduced, the functional activity and amplification capacity of T effector cells and antigen presenting cells are up-regulated, the insufficient number and function decline of initial immune cells caused by negative immune regulation mechanisms such as TGF-beta up-regulated Treg cells are avoided, and high-quality and high-quantity excellent seed cells are provided for later successful preparation of targeted immune cells. The invention also provides a method for activating and amplifying initial immune cells and a method for preparing targeted immune cells.
Owner:THE SECOND AFFILIATED HOSPITAL OF ANHUI MEDICAL UNIV +1
Separation method of peripheral blood mononuclear cells
PendingCN112251404AHigh activityHigh purityCell dissociation methodsBlood/immune system cellsFicollHydroxyethyl starch
The invention discloses a separation method of peripheral blood mononuclear cells. According to characteristics of separating a large number of blood samples, the method comprises steps of removing most of red blood cells by using a hydroxyethyl starch precipitation method, conducting natural sedimentation in the air, and further purifying the mononuclear cells by using a Ficoll density gradient centrifugation method to obtain PBMC with more than 95% of purity and more than 90% of activity. Compared with methods without natural sedimentation in the air, the cells obtained by the separation method of the present invention are higher in activity and purity.
Owner:贵州北科生物科技有限公司
Sika deer specific CpG oligodeoxynucleotide and application thereof
ActiveCN109234280AImprove disease resistanceGood immune protectionSsRNA viruses positive-senseViral antigen ingredientsMucosal diseasePeripheral blood mononuclear cell
The invention provides a sika deer specific CpG oligodeoxynucleotide and application thereof, and relates to the field of immunopotentiator for deer and vaccine adjuvant for deer. To boost the sika deer's immunity, the immune efficacy of sika deer vaccine and the antibody level of deer-derived bovine viral diarrhea / mucosal disease inactivated vaccine were increased, A CpG oligomeric deoxynucleotide specific to sika deer, As shown in SEQ ID NO. 3, A Chinese medicinal preparation is use for preparing immunopotentiator for sika deer, immunizing adjuvant for sika deer vaccine, and preparation of antiviral preparation against deer-derived bovine viral diarrhea / mucosal disease virus, and preparation of induce cells to produce antiviral substance against deer-derived bovine viral diarrhea / mucosaldisease virus, so as to improve antibody level of deer-derived bovine viral diarrhea / mucosal disease inactivated vaccine. As prove by experiments, that artificially synthesize sika deer specific CpGoligonucleotide can be used for sika deer peripheral blood mononuclear cells to exhibit antiviral activity, and at the same time, the deer-derived bovine viral diarrhea / mucosal disease inactivated vaccine immunity can be enhance. The sika deer specific CpG oligonucleotide can be used for sika deer peripheral blood mononuclear cells to show antiviral activity.
Owner:JILIN AGRICULTURAL UNIV
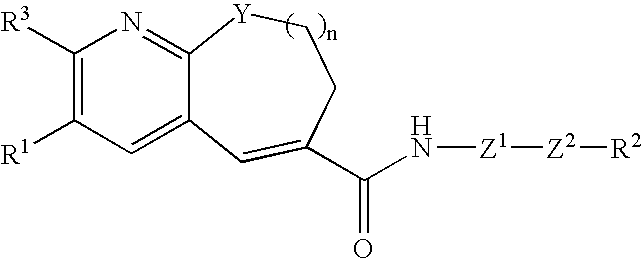
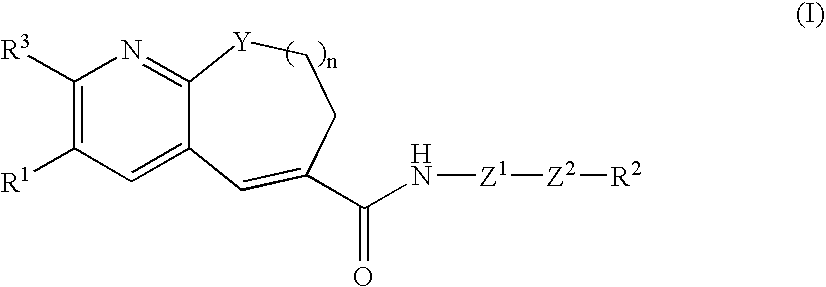
Fused-ring pyridine derivative, process for producing the same, and use
InactiveUS20060100197A1Excellent CCR antagonist activityGood effectBiocideOrganic chemistryChemokine receptor CCR5Antagonist
The present invention provides a compound represented by the formula: wherein R1 is a 5- or 6-membered ring which may be substituted; R3 is a hydrogen atom, a lower alkyl group or a lower alkoxy group; Z1 is a 5- or 6-membered aromatic ring; Z2 is a group represented by -Z2a-W2-Z2b wherein Z2a and Z2b are each O, S(O)m (wherein m is 0, 1 or 2), an imino group, or a bond; and W2 is an alkylene chain which may be substituted; n is an integer of 0 to 4; Y is O, S(O)p (wherein p is 0, 1 or 2), CH2 or NR4 (wherein R4 is a hydrogen atom, a hydrocarbon group, a heterocyclic group, or an acyl group); and R2 is (1) an amino group, in which the nitrogen atom is converted to quaternary ammonium or oxide, (2) a nitrogen-containing heterocyclic group which may contain a sulfur atom or an oxygen atom as a ring-constituting atom, in which the nitrogen atom may be converted to a quaternary ammonium or an oxide, and the like, or a salt thereof. The compound has excellent CCR5 antagonist activity and is useful as a prophylactic and / or therapeutic medicine for HIV infection in human peripheral mononuclear blood cells, especially AIDS.
Owner:TAKEDA PHARMA CO LTD
Clinical TSCM (T-memory stem cells) induction culture and quality control identification kit and application
PendingCN111893094AStandardize clinical applicationHigh induction rateAntibacterial agentsCulture processIn vivoCytoplasm
The invention relates to the technical field of immune cell targeted therapy, and particularly relates to an optimized kit for separating and purifying T cells from fresh or low-temperature cryopreserved human peripheral blood mononuclear cells or umbilical cord blood mononuclear cells and inducing and amplifying TSCM (T-memory stem cells) through the T cells, a product quality control identification kit system and application. The TSCM have higher proliferation, self-updating and survival capability and can be differentiated into TCM (central memory T cell), TEM (effector memory T cell) and effector T cells. Mouse animal experiments show that compared with other differentiated T cell subgroups, the TSCM have higher anti-tumor effect in vivo. In clinical application, more reagents are adopted in the TSCM culture process, the cell quality standard is difficult to control, TSCM with uniform quality are difficult to culture in a short time, and a simple and formatted kit is urgently needed to be developed and has important significance for clinical application.
Owner:路春光
A CTL preparation method for efficient proliferation and targeted killing of tumors
InactiveCN103923880BImprove proliferation efficiencyHigh activityBlood/immune system cellsAntigenMonoclonal antibody agent
The invention discloses an efficient multiplication CTL preparation method killing tumors in a targeted mode. The CTL preparation method comprises the following steps: (a) removing CD4+CD25+Treg cells through immunomagnetic bead negative sorting; (b) arranging mixed cells in a serum-free medium for cultivation, and obtaining suspension cells and adherent cells; (c) adding GM-SCF and IL-4 in the adherent cells, culturing the cells for five days; in the sixth day, adding a tumour cell holoantigen, and in the seventh day, adding TNF-alpha and IL-27; (d) transferring the suspension cells to a culture flask wrapped by a CD3 monoclonal antibody and recombinant human fibronectin, adding IFN-gamma, in the second day, adding IL-2, IL-12 and the IL-27, and culturing the mixture till the eighth day to obtain CIK cells; (e) mixing the CIK cells and mature DC cells, and adding the IL-12, IL-7 and an anti-CD 28 monoclonal antibody for cultivation; in the third day, adding an anti-CTLA-4 monoclonal antibody, and then culturing the mixture for four days. According to the efficient multiplication CTL preparation method killing tumors in the targeted mode, efficiency of in-vitro CTL cell proliferation is improved, activity of killing the tumor cells in the targeted mode is improved, transformation of peripheral blood mononuclear cells to the CD4+CD25+Treg cells is inhibited.
Owner:四川全组生命科技有限公司
Preparation and application of a car-nk cell sustained-release agent for ovarian cancer
ActiveCN109288864BEasy to getLess prone to immune rejectionImmunoglobulin superfamilyPeptide/protein ingredientsCancer cellWhite blood cell
Owner:广东美赛尔细胞生物科技有限公司
A method for preparing HPV antigen-specific cytotoxic T lymphocytes
ActiveCN108300692BEnhance antigen presentationInduced proliferationMammal material medical ingredientsBlood/immune system cellsHPV AntigenDisease
The invention discloses a method for preparing HPV antigen-specific cytotoxic T lymphocytes. Specifically, the present invention discloses a preparation method of HLA-A2402-restricted anti-HPV antigen-specific CTL. In this method, peripheral blood mononuclear cells were collected by apheresis or venous blood sampling, the antigen presentation function of B cells was enhanced with CpG ODN 2395, and peripheral blood mononuclear cells were stimulated with B cells loaded with HLA-A2402-restricted HPV antigen peptides, and rhIL ‑2, rhIL‑7, rhIL‑15, rhIL‑21 combined to promote T cell growth. The target CTL prepared by the method has the characteristics of simple preparation, short preparation cycle, low cost, high proliferation ability, high killing activity, high cell viability and the like, and can be used for immunotherapy of diseases related to HPV infection including cervical cancer.
Owner:JILIN TUO HUA BIOTECH
NK cell culturing solutions and culturing method
ActiveCN111073852AIncrease the number ofHigh activityCulture processBlood/immune system cellsNatural Killer Cell Inhibitory ReceptorsBiochemistry
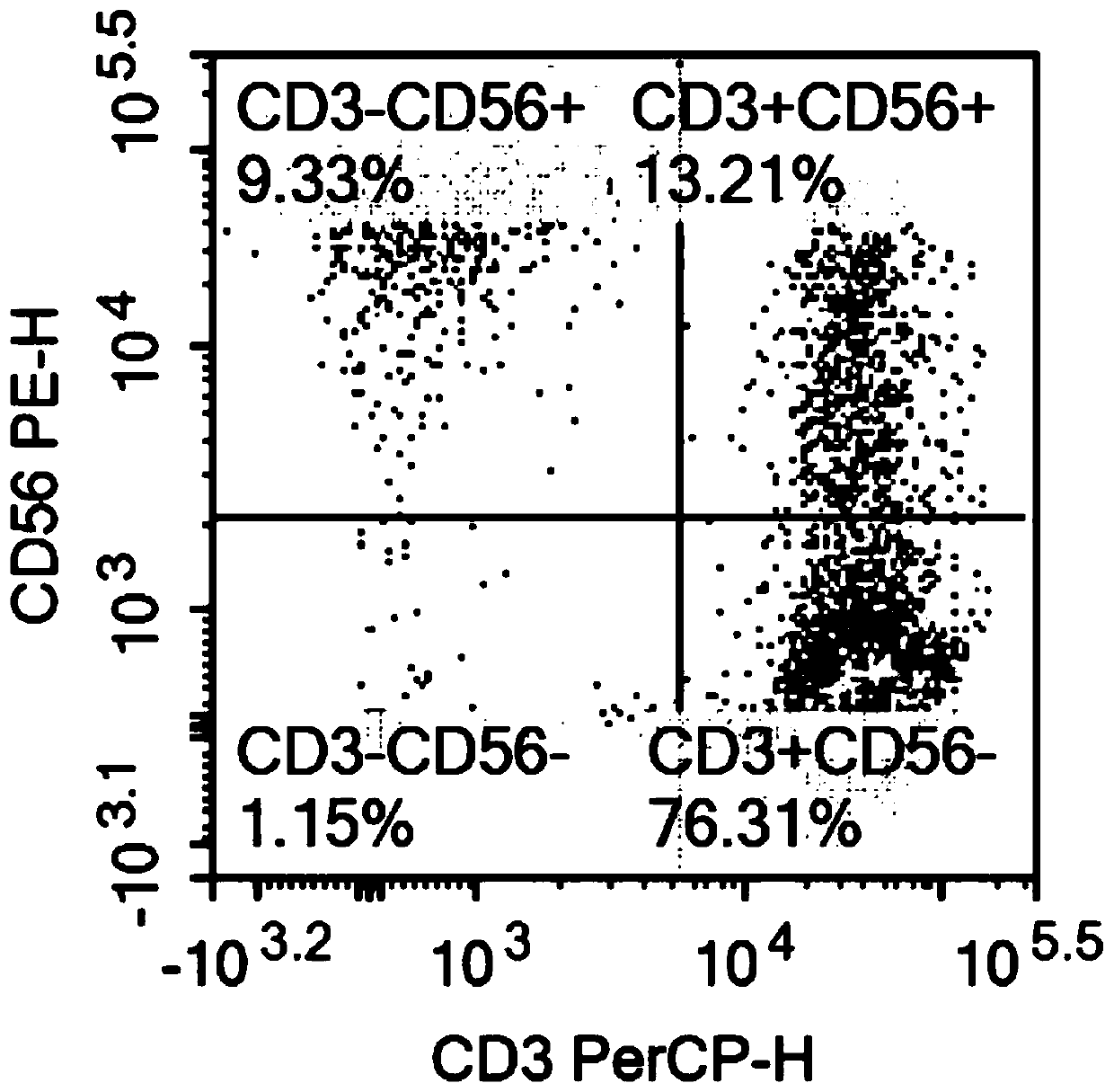
The invention relates to the technical field of cell culturing, and particularly relates to an NK cell culturing solutions and culturing method. The invention discloses the NK cell culturing solutions, and the culturing solutions can perform compounding of IFN-gamma and IL-15 and perform compounding of IL-2, IL-1alpha and CD3 monoclonal antibodies, and based on combined effect, efficient transformation from peripheral blood mononuclear cells to NK cells is stimulated, the multiplying power of the NK cells is increased, and more NK cells are obtained, and moreover, contents of CD3-CD56+ and CD3+CD56+ are high, and the killing activity is high. The NK cell culturing solutions provided by the invention are illustrated that the toxic activity of the cells is obviously improved while the proliferation quantity of the NK cells is increased at the same time.
Owner:广州航华生物医药科技有限公司
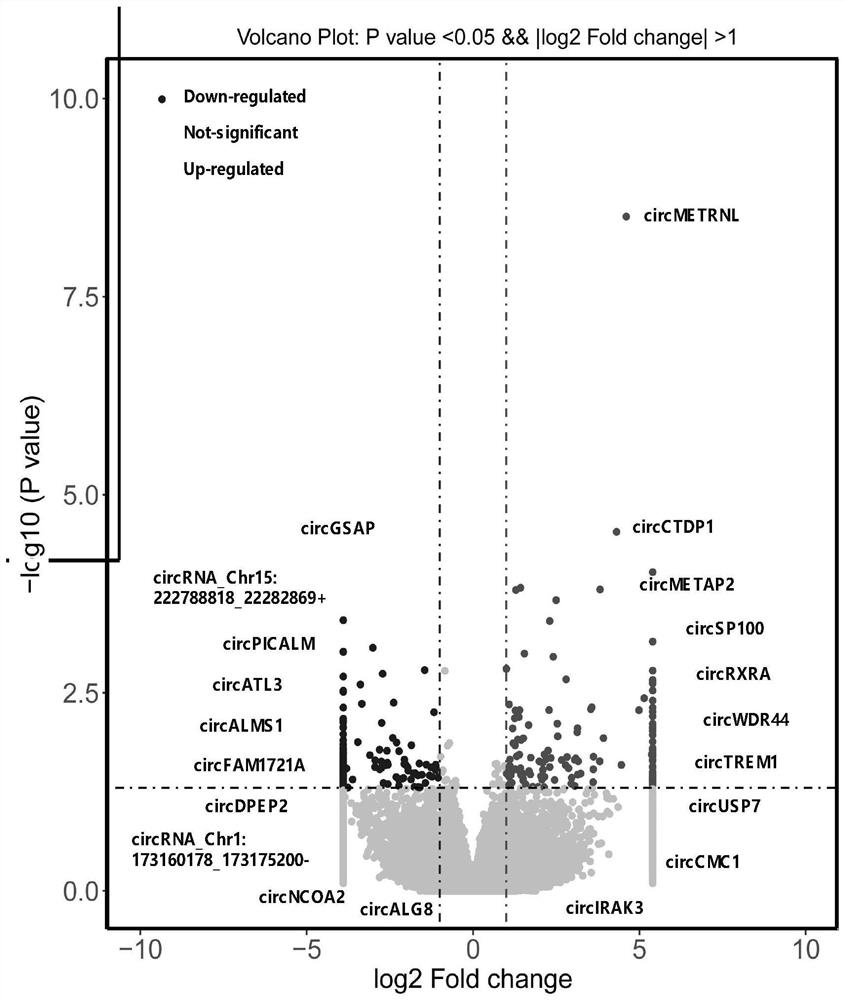
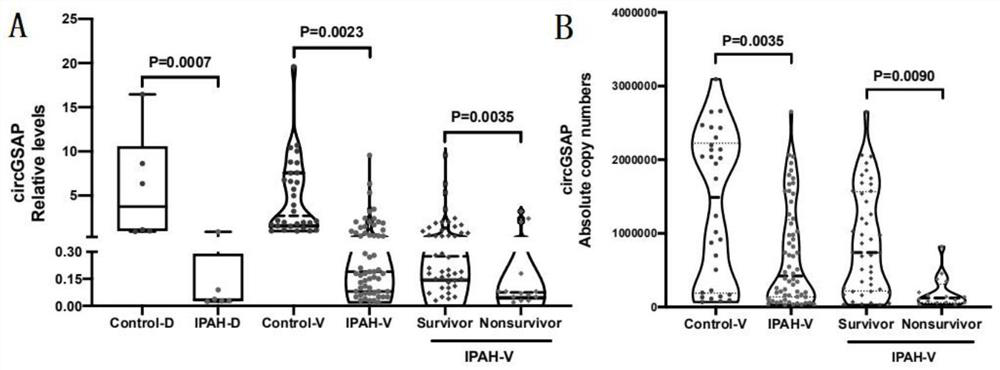
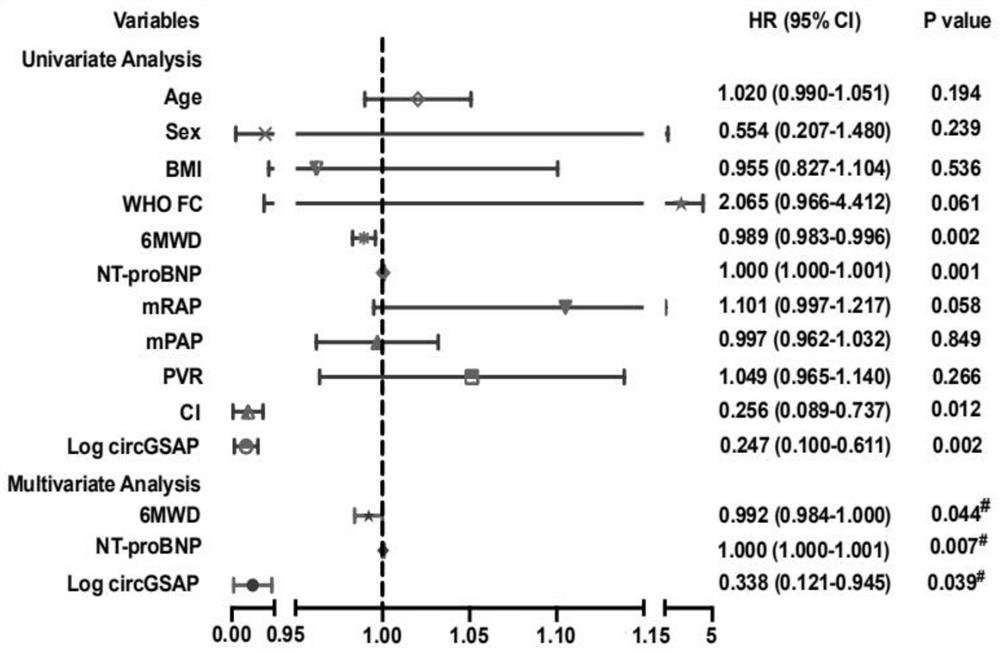
Application of peripheral blood mononuclear cells circGSAP
The invention discloses application of a preparation for detecting peripheral blood mononuclear cells circGSAP in preparation of a kit for predicting the survival rate of patients with idiopathic pulmonary hypertension. It is found for the first time that the expression levels of the peripheral blood mononuclear cells circGSAP of IPAH survivors and non-survivors are different. The peripheral blood mononuclear cells circGSAP can be used as an independent survival rate predictor, and provides a reference for evaluating the severity and clinical prognosis of the disease progress of the IPAH patient.
Owner:SHANGHAI PULMONARY HOSPITAL
Detection method and application of human peripheral blood mononuclear cell membrane surface transporter
ActiveCN114002347AImprove throughputComponent separationAgainst vector-borne diseasesCell membraneDisease patient
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
A combination of lung cancer antigens and its application, cytotoxic T lymphocytes
ActiveCN110551198BEfficient killingTumor rejection antigen precursorsMammal material medical ingredientsCancer antigenT lymphocyte
The invention provides a lung cancer antigen combination and an application thereof, and cytotoxic T lymphocytes, and belongs to the technical field of biological medicines. The amino acid sequences of lung cancer antigens are as shown in SEQ ID NO.1-10. The lung cancer antigen combination is used for co-culturing peripheral blood mononuclear cells; and the obtained cytotoxic T lymphocytes can effectively kill cells expressing lung cancer associated antigens.
Owner:BEIJING DCTY BIOTECH CO LTD
A kind of microrna biomarker and its application for the diagnosis of rheumatoid arthritis
ActiveCN109022568BHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationMicroRNABiologic marker
The invention discloses a microRNA biomarker for diagnosing rheumatoid arthritis and an application thereof, belonging to the technical field of biological detection. These 3 microRNA biomarkers provided by the present invention and their corresponding primer sets and probes can be used to prepare diagnostic kits, and they have excellent performance when applied to the diagnosis of rheumatoid arthritis in peripheral blood mononuclear cell samples. sensitivity and specificity. The combined AUC value of the three microRNAs of the present invention can reach 0.745, and the sensitivity and specificity are 73.5% and 76.7% respectively. These three microRNAs can be used as biomarkers for the diagnosis of human rheumatoid arthritis peripheral blood mononuclear cell samples, which is conducive to promoting the development of early diagnosis, predictive treatment, and monitoring recurrence of rheumatoid arthritis in my country.
Owner:SUZHOU UNIV
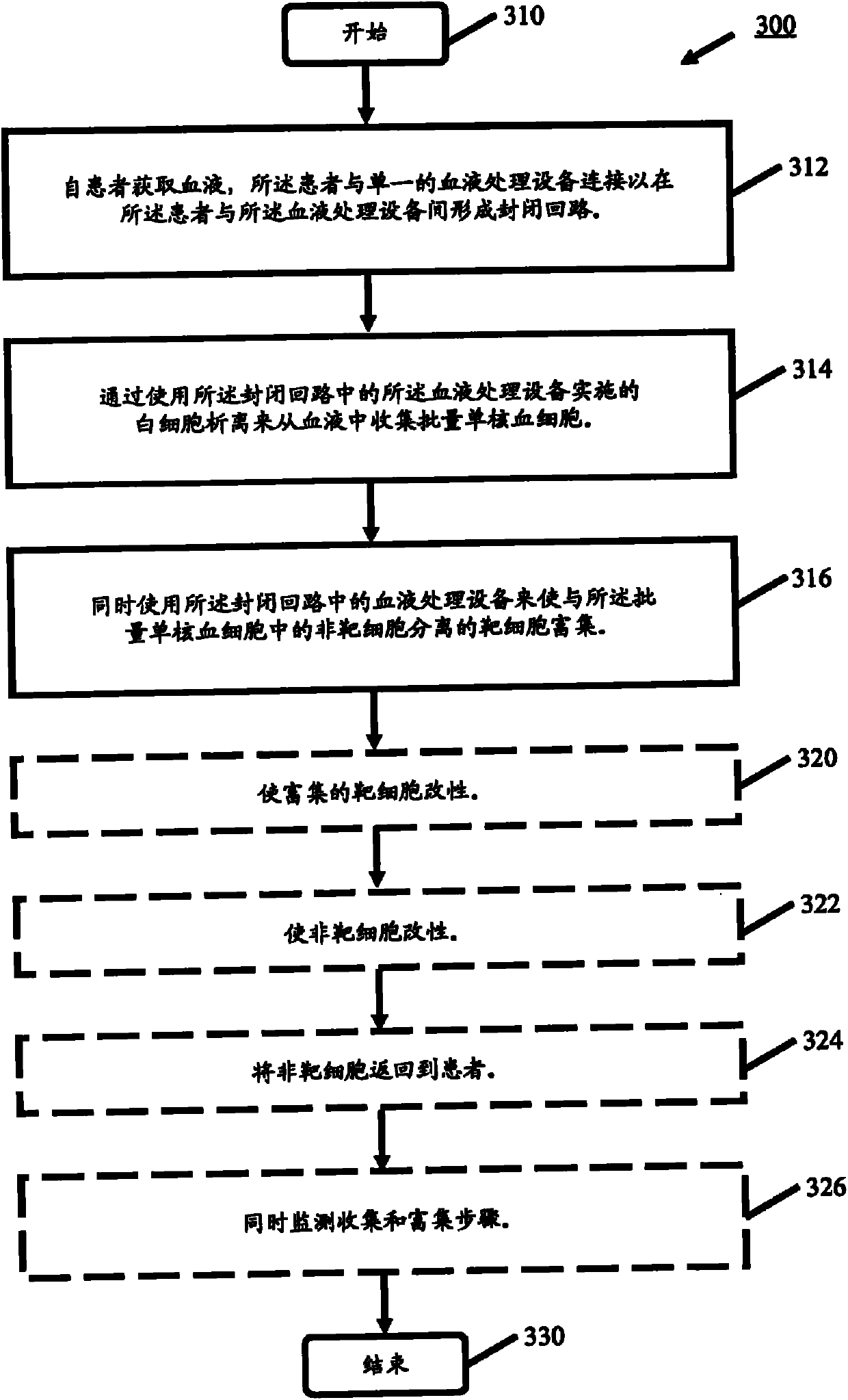
process blood
Methods (300), devices, and systems of processing blood are describes. The method (300) comprises the steps of: obtaining (312) blood from a patient coupled to a single blood processing device to form a closed loop between the patient and the blood processing device; collection (314) bulk mononuclear blood cells from the blood by leukapheresis using the blood processing device in the closed loop; and enriching (316) concurrently target cells separated from non-target cells in the bulk mononuclear blood cells using the blood processing device in the closed loop.
Owner:THERAKOS INC
Application of TLR7 and TLR8 as prophylactic and therapeutic targets for IgA nephropathy and inhibitors of TLR7 and their use
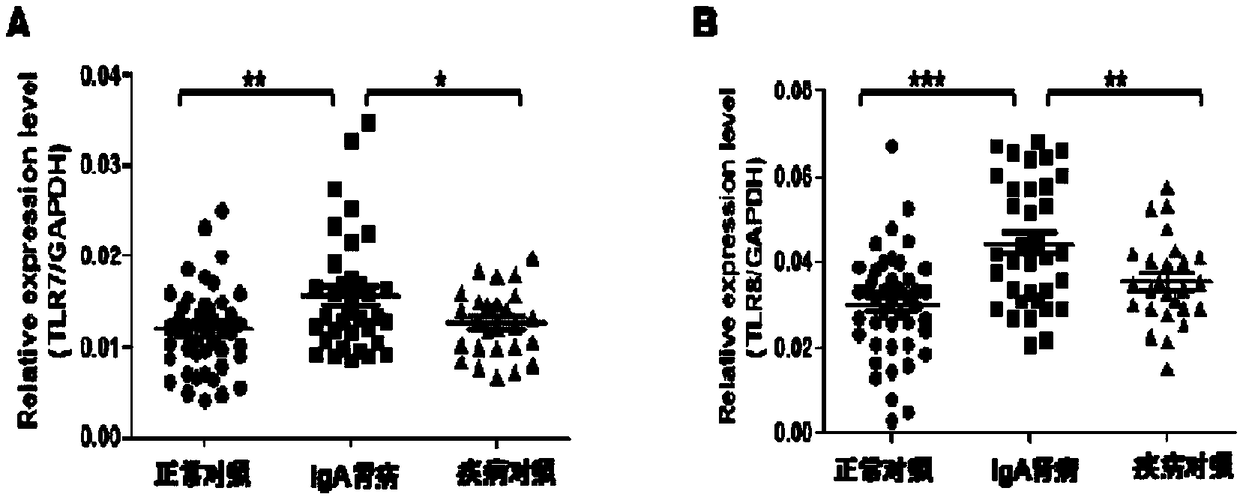
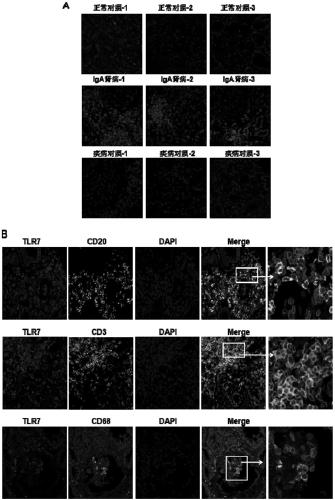
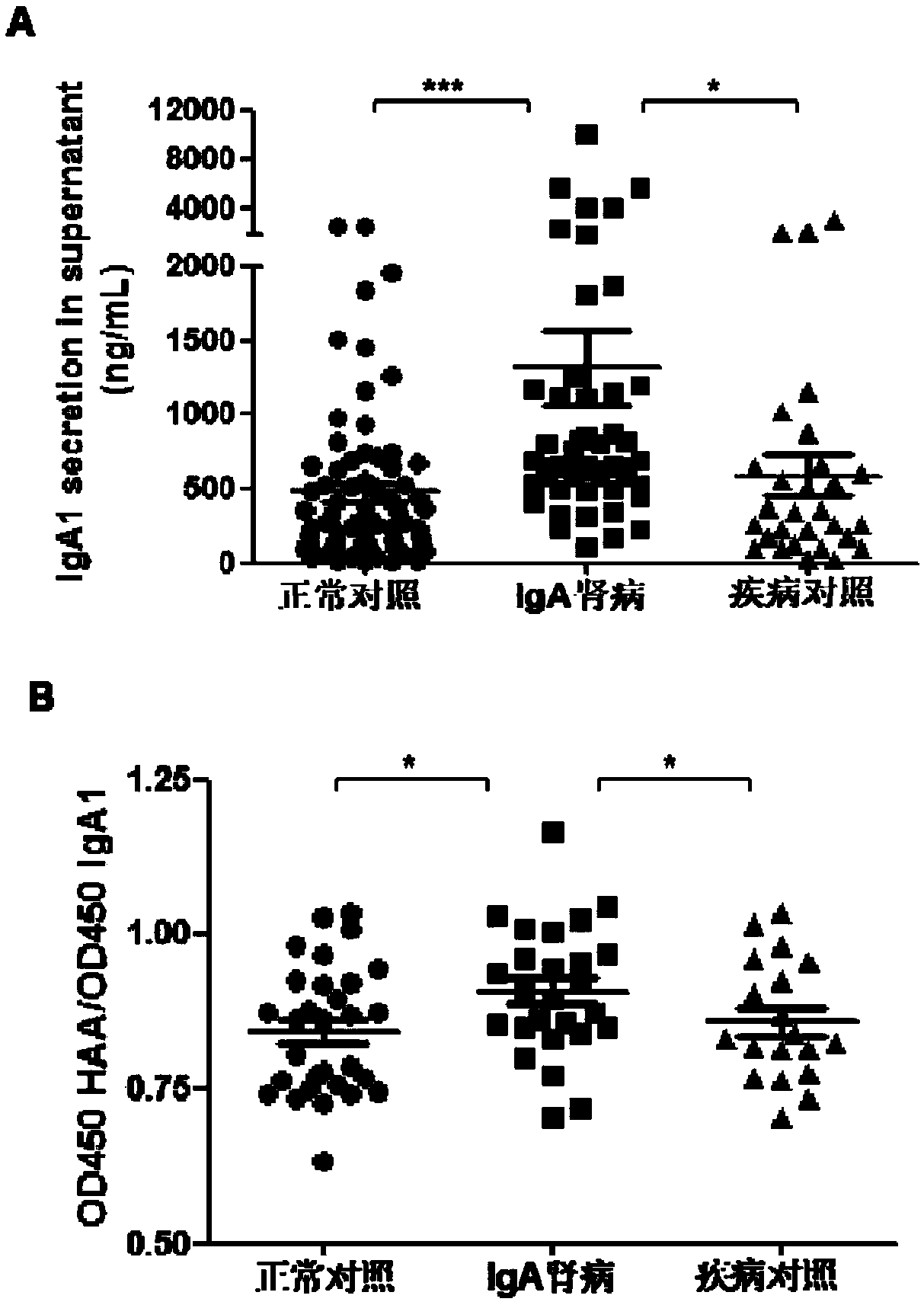
InactiveCN109266732AIncreased O-glycosylation abnormalitiesEasy to synthesizeOrganic chemistryMicrobiological testing/measurementClinical valueTherapeutic treatment
The invention relates to the technical field of biomedical technology, in particular to the application of TLR7 and TLR8 as prophylactic and therapeutic targets of IgA nephropathy and the applicationof inhibitor of TLR7 in prophylactic and therapeutic treatment of IgA nephropathy. The invention proves that the expression level of TLR7 gene and TLR8 gene in peripheral blood mononuclear cells (PBMCs) of IgA nephropathy patients is significantly increased compared with healthy control and disease control, when activated by TLR7 and TLR8 co-ligand R848, more IgA1 antibodies were produced, and abnormal O-glycosylation of the IgA1 antibodies were aggravated. Compounds of formula C<16>H<19>N<3>O<2> act as inhibitors of TLR7, inhibiting the ability of peripheral mononuclear blood cells to synthesize IgA1 and decreasing the abnormal O-glycosylation of these IgA1 molecules. Glycosylation. TLR7 and TLR8 are novel targets for the treatment of IgA nephropathy and have great clinical value.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Method for determining the activity of autoimmune diseases and kit
InactiveUS20190391144A1Preparing sample for investigationBiological material analysisDiseaseAutoimmune disease
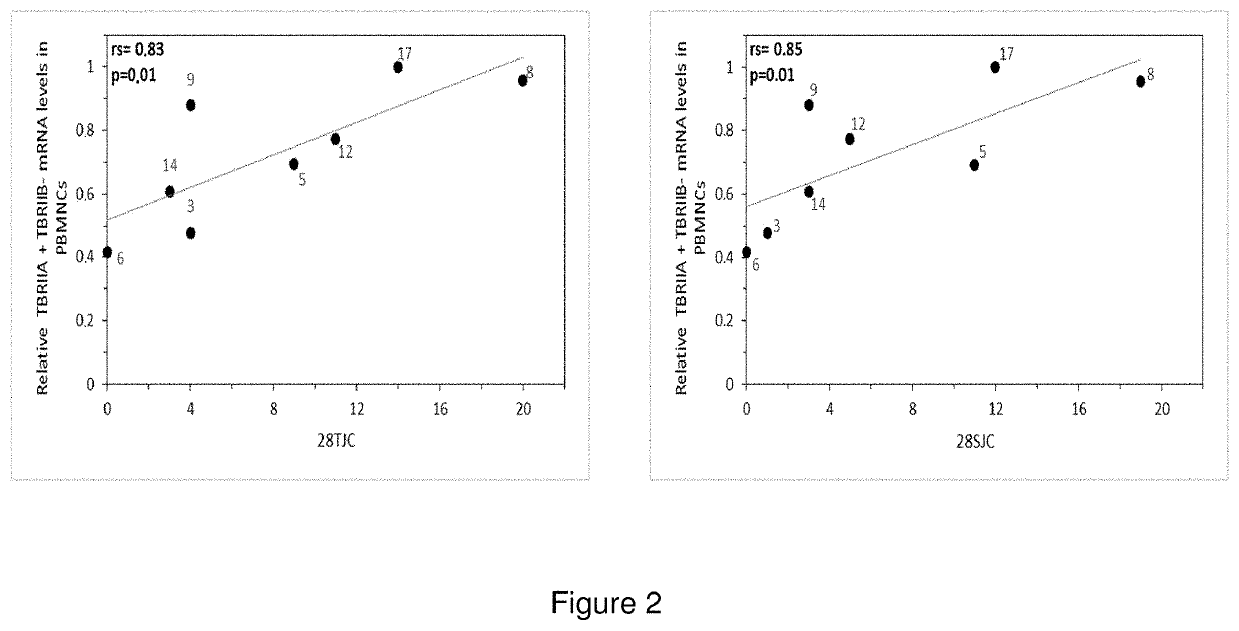
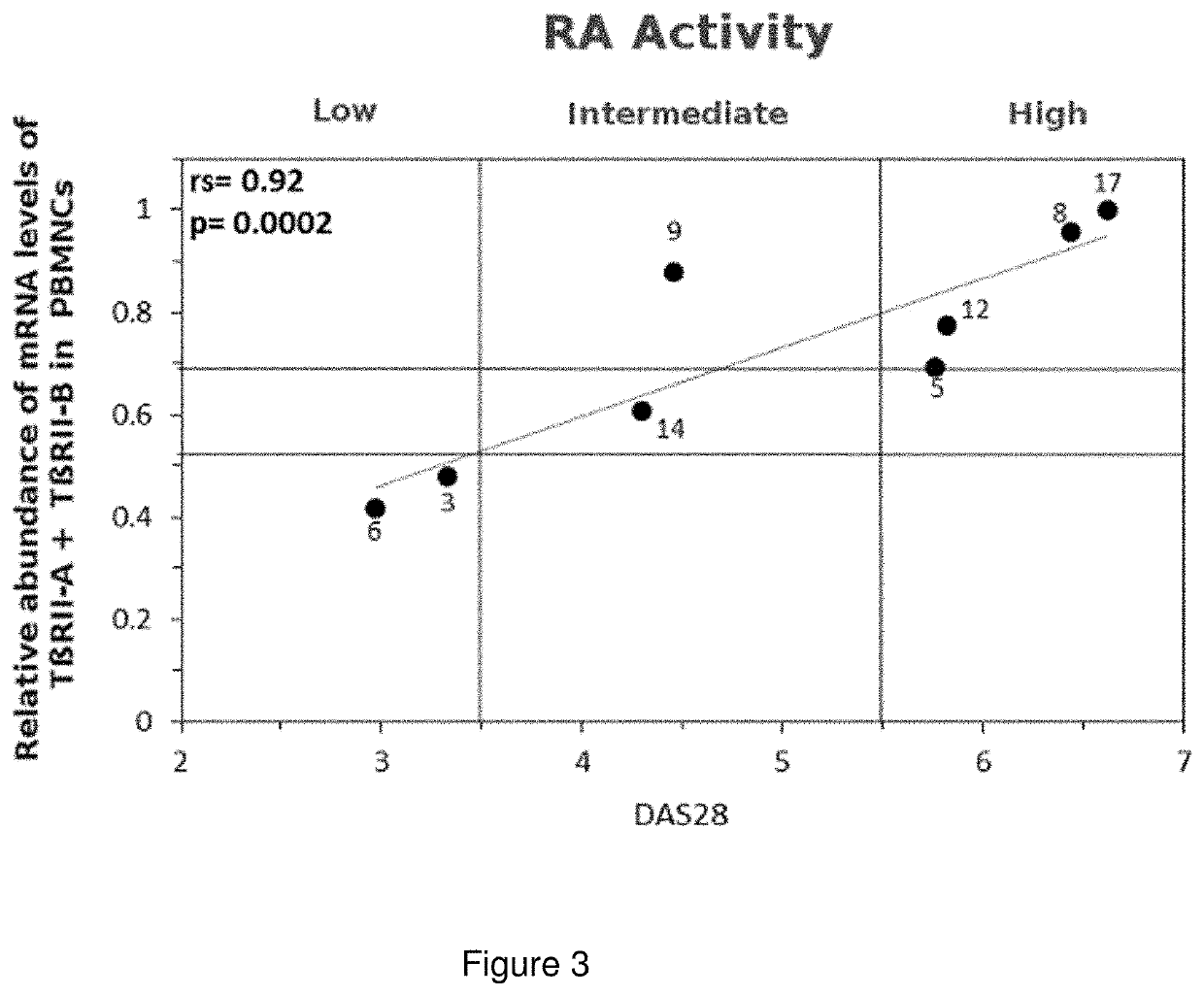
A method to determine the activity level of autoimmune diseases, the method including a) to supply a biological sample; b) to determine the TßRII-A, TßRII-B and TßRII-Se isoforms level of the biological sample; c) calculate the activity level by performing the quotient between the TßRII-Se level and the level of the addition of TßRII-A y TßRII-B. The isoforms level are measured by detecting the polypeptides of the isoforms or the mRNA of the isoforms in the isolated circulating mononuclear blood cells by means of, for instance, RT-qPCR. The ΔCt of each splice variants individually showed a correlation with autoimmune disease activity. Additionally, a similar correlation with autoimmune disease activity was obtained when the ΔCt of TβRII-SE was added to the ΔCt of TβRII-A and the ΔCt of TβRII-A.
Owner:CONSEJO NAT DE INVESTIGACIONES CIENTIFICAS Y TECH +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com