Clinical TSCM (T-memory stem cells) induction culture and quality control identification kit and application
A kit and clinical technology, applied in the field of product quality control and identification, can solve problems such as easy differentiation into TEM, and achieve the effects of removing residual lesions, improving proliferation ability, and preventing recurrence and metastasis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Isolation of peripheral blood PBMC or umbilical cord blood UBMC and purification of T cells:
[0053] 100ml of sodium citrate anticoagulated blood was collected, PBMC and UBMC were separated by lymphocyte separation medium, T cells were purified, and cryopreserved.
[0054] 2. Recovery of T cells after freezing: Take out the frozen T cells, quickly put them into a 37 ℃ water bath, and shake gently constantly to ensure that the liquid in the tube is 80% dissolved within 1 min, and absorb the cells into a certain amount of DMEM washing solution , and wash the cryovial once, transfer it to a 50 mL centrifuge tube, and centrifuge at 233 g for 5 min. Discard the supernatant, add an appropriate amount of medium, mix gently, and retain 500 microliters for counting and measuring cell viability.
[0055] 3. Cell counting and trypan blue exclusion method to measure cell viability
[0056] The Countstar measures the cell sample and reads the average value of the cells display...
Embodiment 2
[0060] On the basis of Example 1, use the TSCM induction kit to induce TSCM:
[0061] The invention provides a TSCM induction culture kit, comprising a container and a clinically used TSCM cell serum-free expansion complete culture medium TSCM-I, a TSCM cell expansion coating liquid TSCM-II and an induction factor composition TSCM packed in the container -III:
[0062] 1) The present invention provides a serum-free TSCM cell expansion complete culture medium TSCM-I for clinical use, which contains carbohydrates, growth-promoting factors and hormones, vitamins, human albumin, amino acids, inorganic salt ions, Trace elements, water and human interleukin 7, human interleukin 15 and human interleukin 21. The present invention does not exclude the use of media other than the above serum-free complete media for culturing.
[0063] 2) The present invention provides a TSCM cell expansion coating solution TSCM-II, which contains recombinant human fibrin fragments and PBS, and the wor...
Embodiment 3
[0066] Example 3 Comparison of TSCM induction efficiency between T cells and PBMCs
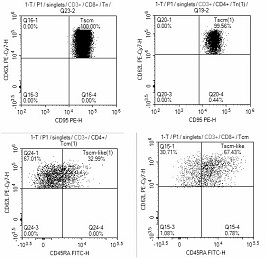
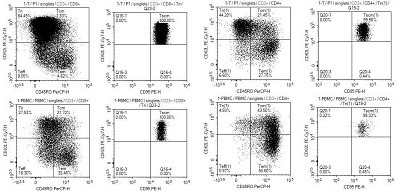
[0067] On D0 day, TSCM-II was made into 12.5ug / ml working solution, coated into a culture bottle, and placed flat in a 37°C incubator for more than 2 hours. PBMC cells according to the cell density 1×10 6 / mL was resuspended with TSCM-III, in which 8ng / mL human interleukin 7, 8ng / mL human interleukin 15, 80ng / mL CD3 monoclonal antibody, and 80ng / mL CD28 were added. After the next day, TSCM cells were expanded and cultured with TSCM-I culture medium. After 12-15 days of expansion and culture, TSCM cells that reached clinical application standards were harvested, and the endotoxin, activity and phenotype of the cell preparations were tested. , the results of cell identification showed that the induction rate of TSCM directly induced by PBMC cells was lower than that of T cells, see image 3 At the same time, it can be seen that after 22 days of induction culture, CD8TCM accounted for 0.92%, CD...
PUM
| Property | Measurement | Unit |
|---|---|---|
| induction rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com