Method for preparing liver organoid from peripheral blood mononuclear cells
An organoid and liver technology, applied in the field of preparation of liver organoids from peripheral blood mononuclear cells, can solve the problems of worsening liver function, limited access to liver tissue, and increased bleeding risk.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
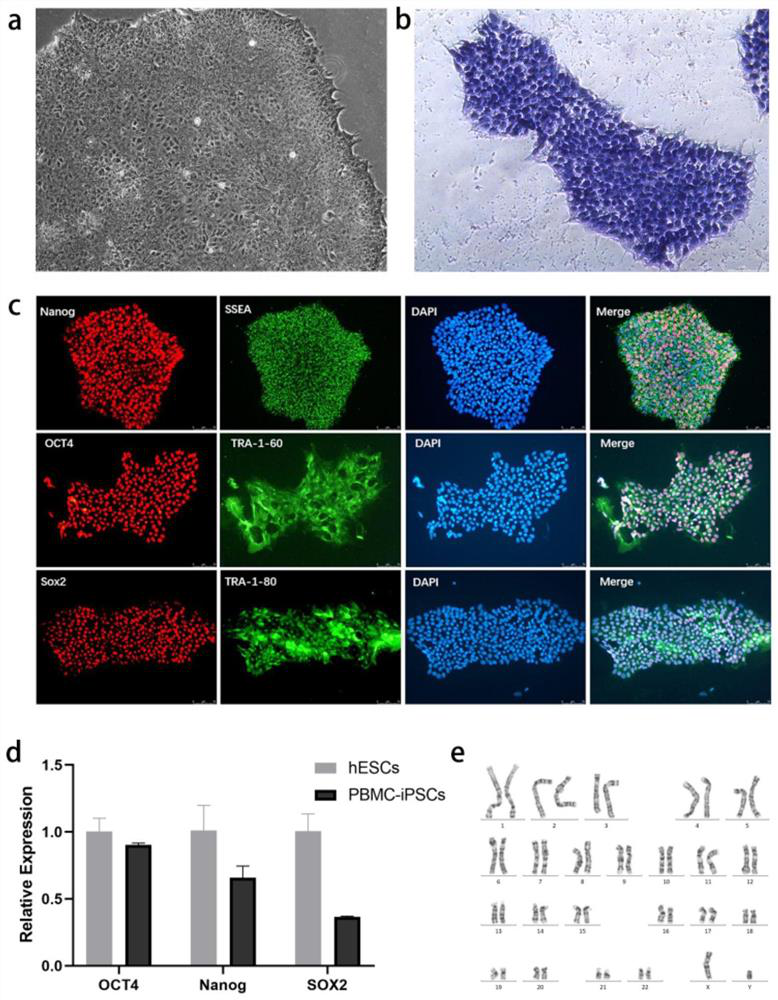
[0091] Example 1 Induction of PBMCs into iPSCs with Multiple Differentiation Potentials
[0092] In this example, PBMCs were isolated and extracted from the peripheral blood of the donor (the collection of the specimen was approved by the ethics committee of the hospital).
[0093] 1. Experimental method
[0094] 1. Extraction of PBMCs by Ficoll-Paque gradient centrifugation
[0095] Use heparin anticoagulant tubes to extract 3ml of peripheral blood from the donor, and place it on ice for later use. Add 6ml of lymphocyte separation medium to a 15ml centrifuge tube, and place the tube at an angle of 45°. After mixing the anticoagulant blood with a pipette tip, slowly add it over the lymphocyte separation solution along the wall of the test tube, so that the lymphocyte separation solution and the anticoagulant blood form a clear dividing line. Centrifuge the centrifuge tube at 12 degrees Celsius, 2500rpm, increase speed to 1, decrease speed to 0, and centrifuge for 20 minutes...
Embodiment 2
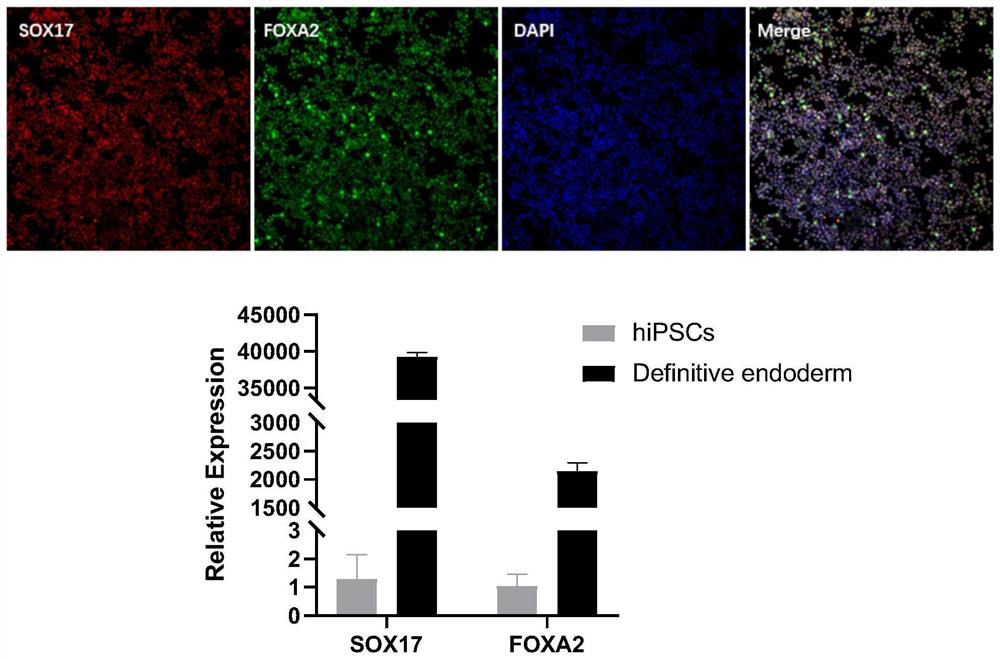
[0116] Example 2 Inducing iPSCs with multilineage differentiation potential into terminal foregut endoderm cells (Posterior Foregut) through committed endoderm cells
[0117] 1. Experimental method
[0118] The iPSCs cells with multilineage differentiation potential prepared in the above Example 1 were incubated with mTeSR medium at 37°C and 5% CO 2 Under the condition of culture, the culture medium was changed every 2 days to expand iPSCs. When the cell density reached 80%, it was digested with Accutase for 5 minutes, culture medium was added to stop the digestion, and centrifuged at 1100 rpm for 4 minutes. Cell count, according to 0.5~1×10 5 piece / cm 2 Inoculated in cell plates and cultured overnight using mTeSH+10μM Y27632 for the next step of endoderm induction.
[0119] Afterwards, on the first day (D1), replace endoderm induction medium 1: RPMI 1640 medium + 1% (v / v) B27 + 50ng / ml WNT3A protein + 100ng / ml Activin A protein, culture for one day, second to third Day (...
Embodiment 3
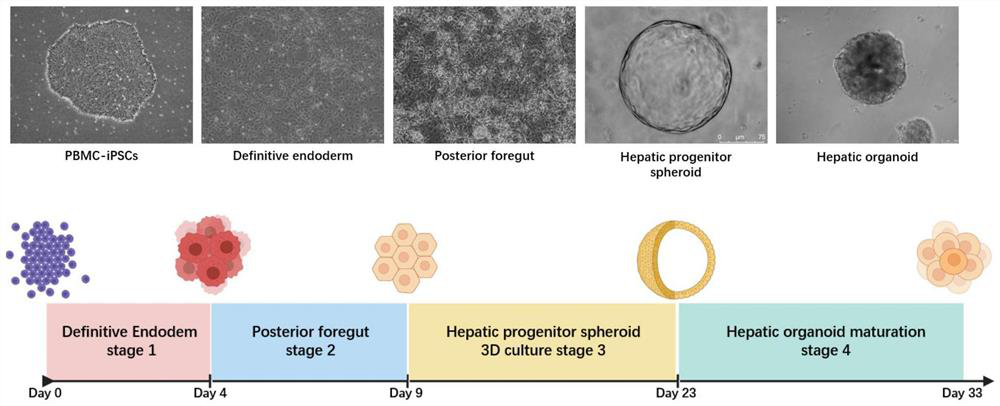
[0124] Example 3 Resuspension culture of terminal foregut endoderm cells in Matrigel to induce hepatic progenitor spheres
[0125] 1. Experimental method
[0126] 1. Terminal foregut endoderm cells were resuspended in Matrigel
[0127] On the ninth day (D9), the flat-cultured terminal foregut endoderm cells were washed twice with PBS, digested into single cells by adding Accutace, and then culture medium was added to terminate the digestion, collected in a centrifuge tube, centrifuged at 1100 rpm for 4 minutes. Count the cells, collect 200,000 to 400,000 cells in a 1.5ml centrifuge tube at a cell density of 10,000 to 20,000 cells / 50 μl Matrigel, discard the supernatant after centrifugation, and place the centrifuge tube on ice for later use.
[0128] Take 1ml of Matrigel matrix collagen solution and put it on ice to melt into a liquid state, and place the pipette tip in a refrigerator at 4°C to pre-cool. Add 1ml of Matrigel into the centrifuge tube containing the cell pellet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com