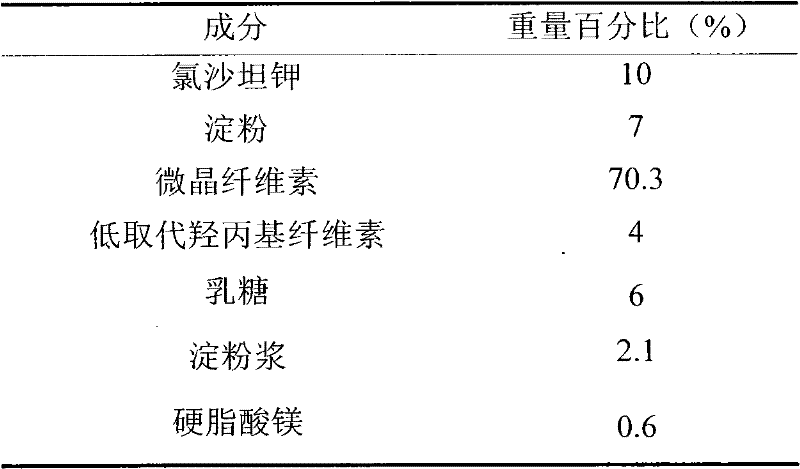
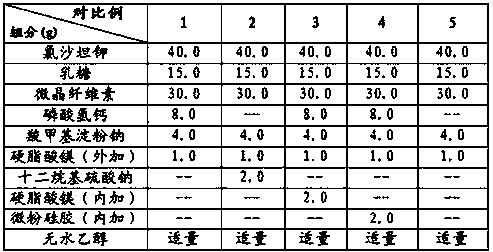
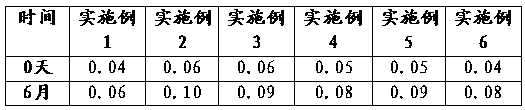
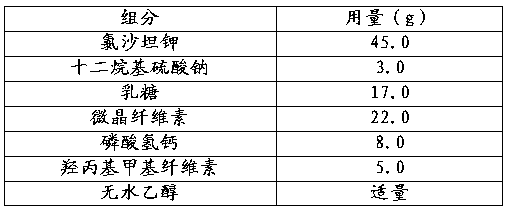
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Losartan Potassium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
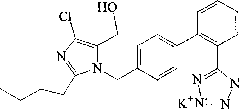
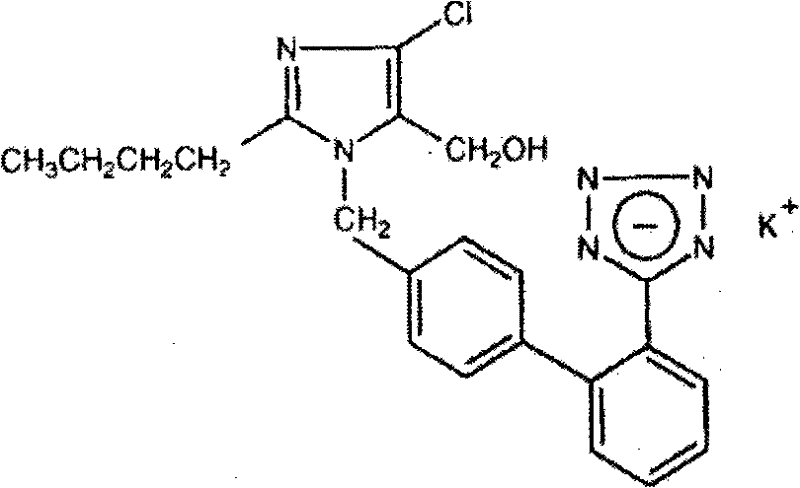
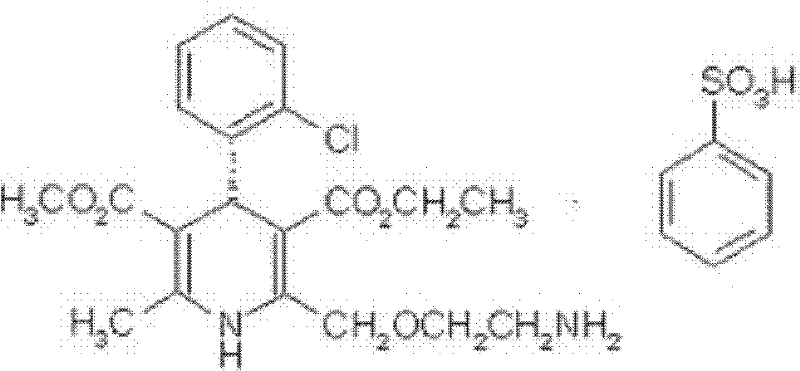
The potassium salt of losartan, a non-peptide angiotensin II receptor antagonist with antihypertensive activity. Losartan selectively and competitively binds to the angiotensin II receptor (type AT1) and blocks the binding of angiotensin II to the receptor, thus promoting vasodilatation and counteracting the effects of aldosterone. Converted from angiotensin I by angiotensin-converting enzyme (ACE), angiotensin II stimulates the adrenal cortex to synthesize and secrete aldosterone, decreasing sodium excretion and increasing potassium excretion, and acts as a vasoconstrictor in vascular smooth muscle.
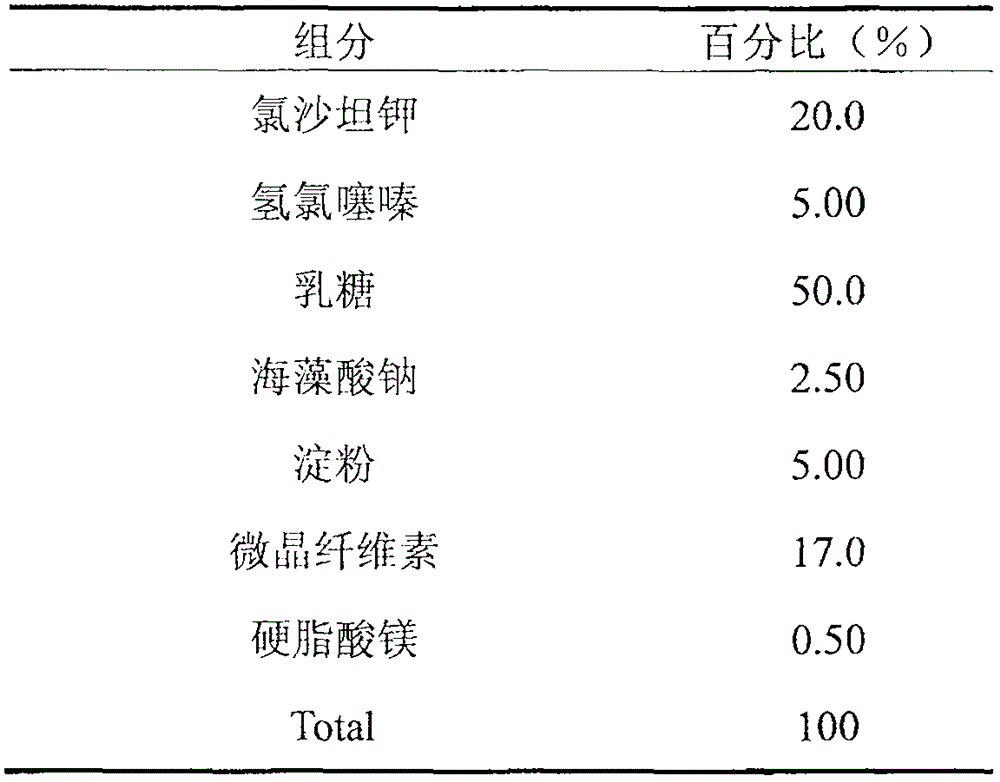
Liposome solid preparation of losartan potassium hydrochlorothiazide pharmaceutical composition
InactiveCN101797230AMitigate counter-regulationGood blood pressure effectOrganic active ingredientsPharmaceutical non-active ingredientsYolkSide effect
The invention discloses a liposome solid preparation of a losartan potassium hydrochlorothiazide pharmaceutical composition and a preparation method thereof. In the invention, active components of losartan potassium and hydrochlorothiazide and specific combined hydrogenated yolk lecithin, cholesterol and poloxamer 188 are prepared into a liposome which is then mixed with other pharmaceutical accessories to prepare the solid preparation, thereby greatly improving the pharmaceutical stability and bioavailability and having stable and lasting effect, small side effect and obvious curative effect.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
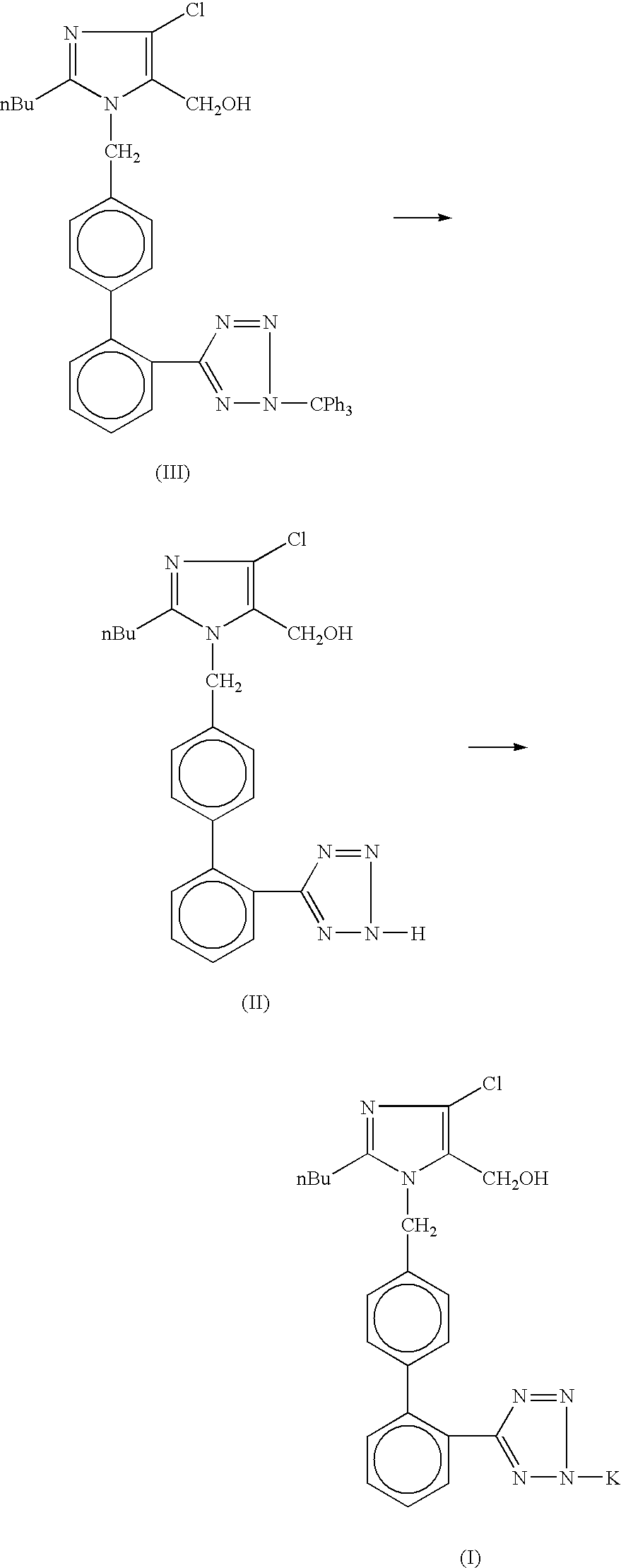
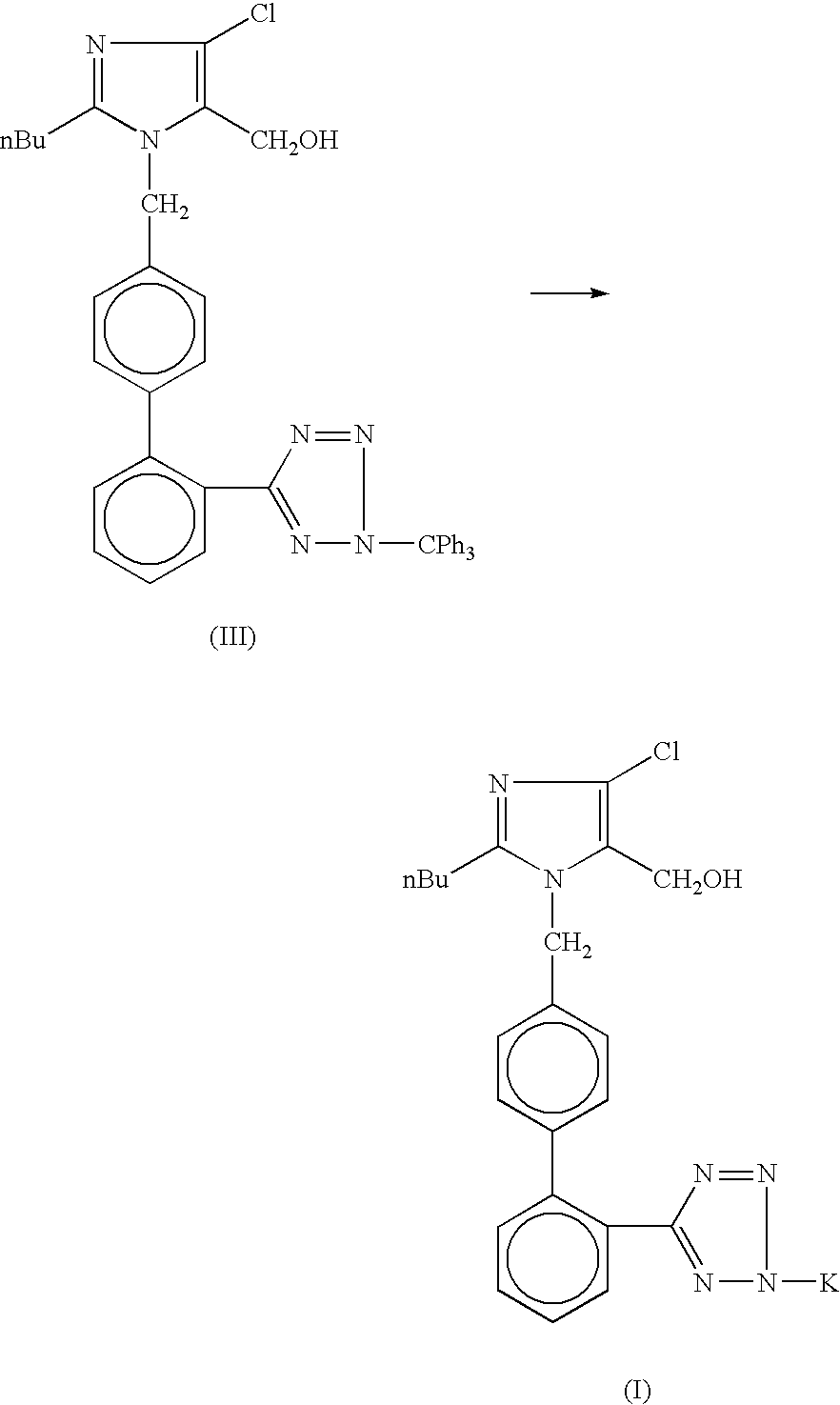
Losartan potassium and preparation method thereof
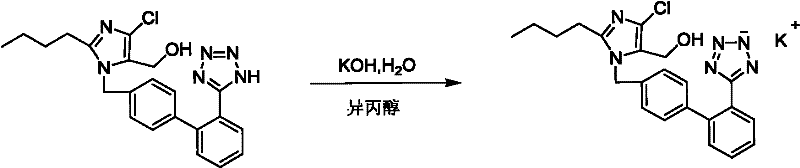
ActiveCN102276586AProduction conditions require peaceEasy to operateOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsTetrazolePotassium hydroxide
The invention discloses preparation methods of losartan potassium and a preparation thereof. The losartan potassium is prepared by the steps of: by taking 2-butyl-5(4)-formoxyl-4(5)-trichlorophenidin (II) and N-(triphenylmethyl)-5-[(4'-brooethyl)-xenyl-2-]tetrazole (III) as raw materials and adopting tetrabutyl ammonium bromide as a phase-transfer catalyst for N-alkylating, performing condensation, reduction and deprotection to synthesize losartan; and salifying the obtained losartan with potassium hydroxide to synthesize raw material losartan potassium. The raw material losartan potassium is mixed with proper auxiliaries to prepare a medicinal preparation. The preparation methods in the invention have the characteristics of mild requirements on production conditions, simplicity in operation and high yield, and are suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +2
Losartan potassium and hydrochrothiazide dropping pills and their preparing methods
InactiveCN1981766ARapid dissolutionHigh dissolution rateOrganic active ingredientsPill deliveryHydrochlorothiazideTreatment hypertension
Owner:陈茜
Lercanidipine hydrochloride and losartan potassium compound preparation and preparation method thereof
ActiveCN102600146AReduce adverse reactionsImprove protectionOrganic active ingredientsMetabolism disorderTolerabilitySodium starch
The invention relates to the field of medicines, and in particular discloses a lercanidipine hydrochloride and losartan potassium compound preparation. Particularly, the invention also provides a compound preparation which takes lercanidipine hydrochloride and losartan potassium as basic remedy, takes lactose monohydrate, microcrystalline cellulose, A-type sodium starch glycollate, povidone K30, magnesium stearate, pregelatinized starch and colloidal silicon dioxide as excipients, and takes white Opadry as a coating to prepare a tablet. Clinical tests prove that compared with the single-component preparation, the lercanidipine hydrochloride and losartan potassium compound preparation provided by the invention is remarkably increased in effective rate in light and moderate blood pressures, is remarkably reduced in occurrence rate of adverse effects, and has better clinical application prospect because patients have good tolerance.
Owner:ZHAOKE PHARMA HEFEI
Compound preparation containing losartan potassium and hydrochlorothiazide and preparation method for compound preparation
InactiveCN102526063ARapid dissolutionImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientLow-substituted hydroxypropylcellulose
The invention relates to a compound preparation containing losartan potassium and hydrochlorothiazide and a preparation method for the compound preparation. The compound preparation containing the losartan potassium and the hydrochlorothiazide comprises a tablet core and a film coating, wherein the tablet core comprises active pharmaceutical ingredients of the losartan potassium and the hydrochlorothiazide, and minor pharmaceutical ingredients such as microcrystalline cellulose, lactose, low substituted hydroxyprepyl cellulose and magnesium stearate. The prepared compound preparation has high dissolubility, is simple in preparation process and can be easily produced in large scale; and moreover, the degradation speed and degree of the hydrochlorothiazide can be remarkably lowered, and impurities 4-amino-6-chloro-1,3-benzenedisulfonamide (DSA) and chlorothiazide can be prevented from increasing.
Owner:CHINA PHARM UNIV
Stable oral solid preparation containing losartan potassium and hydrochlorothiazide
InactiveCN102058602AReduce the degradation rateReduce the degree of degradationOrganic active ingredientsPharmaceutical non-active ingredientsOrganic acidBenzene
The invention relates to a stable oral solid preparation containing losartan potassium and hydrochlorothiazide, comprising 100 parts by weight of hydrochlorothiazide, 400 parts by weight of losartan potassium and 0.5-160 parts by weight of pharmacologically-allowable acid substance, wherein the pharmacologically-allowable acid substance is selected from organic acid, acidic amino acid or a combination of the organic acid and the acidic amino acid. A certain amount of pharmacologically-allowable acid substance is added in the oral solid preparation containing losartan potassium and hydrochlorothiazide to prepare a composition which can remarkably reduce the speed and the degree of degrading the hydrochlorothiazide and prevent an impurity of 4-amino-6-chloro-1,3-benzene disulfonamide (DSA) from raising. Therefore, the composition has better clinical treatment function and clinical medication safety.
Owner:CHINA PHARM UNIV
Amlodipine and losartan potassium medicinal composition and preparation method thereof
ActiveCN102335172ALarge specific surface areaIncrease dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsCarboxymethyl starchPotassium
The invention relates to an amlodipine and losartan potassium medicinal composition, which comprises the following components calculated according to weight part: 2.5-10 parts of amlodipine, 20-100 parts of losartan potassium, 5-20 parts of pregelatinized starch, 20-60 parts of microcrystalline cellulose, 15-40 parts of low substitution hydroxypropyl cellulose, 1-8 parts of carboxymethyl starch sodium and 1-3 parts of magnesium stearate. The amlodipine is an amlodipine maleate hydrate crystal with a molecular formula of C24H29ClN2O9.1.5H2O. The amlodipine maleate in the medicinal composition can be used for stably releasing the medicinal effect within 24 hours and stably and quickly taking effect. The medicinal composition has strong synergism, accumulation and complementary action and high bioavailability.
Owner:HAINAN JINRUI PHARMA
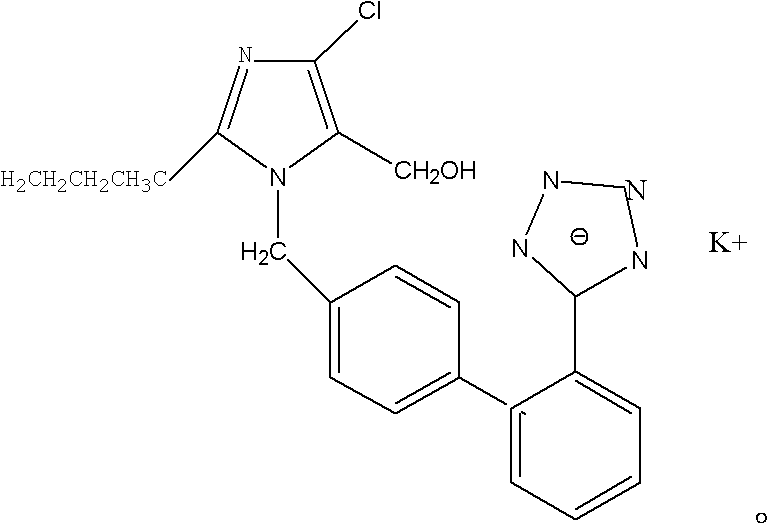
Amorphous and crystalline forms of losartan potassium and process for their preparation
InactiveUS7332612B2Lower the temperature of the solutionOrganic chemistryCardiovascular disorderPotassiumCrystallization
This invention relates to novel amorphous losartan potassium, novel losartan potassium in a crystalline form that is a hydrate, novel crystalline losartan potassium Form IV and solvates thereof, novel crystalline losartan potassium Form V and solvates thereof, to processes for their preparation, to compositions containing them and to their use in medicine. This invention further relates to a novel process for preparing crystalline losartan potassium Form I and Form II.
Owner:TEVA PHARM USA INC
Losartan potassium gastric floating capsule and preparation method thereof
ActiveCN103070848AImprove stabilityQuality improvementOrganic active ingredientsPharmaceutical delivery mechanismAlcoholRoom temperature
The invention relates to a losartan potassium gastric floating capsule, which comprises losartan potassium, PEG6000 (polyethyleneglycol), stearic acid, octodecyl alcohol, pre-gelatinized starch and mannite, wherein the losartan potassium is a powdery substance which is sieved with a 100-mesh sieve. A preparation method of the capsule comprises the following steps of: S1, mixing the PEG6000, the stearic acid and the octodecyl alcohol according to a certain proportion, heating to 64-66 DEG C, and smelting into a liquid; S2, heating the liquid mixture to 69-72 DEG C, and preserving heat for 9-11 minutes; S3, adding a proportional amount of losartan potassium into the mixture obtained in the step S2, keeping the temperature at 69-72 DEG C, and stirring uniformly; S4, adding a proportional amount of the pre-gelatinized starch and the mannite, and stirring uniformly; and S5, after the uniformity is qualified, filling in a hot state, covering a capsule cap, and cooling to the room temperature. The losartan potassium gastric floating capsule has the advantages that the medicament releasing time is long, the medicine taking times of a patient are reduced, the treatment compliance of the patient is enhanced, and the quality in a storage life is stable.
Owner:CHENGDU HENGRUI PHARMA
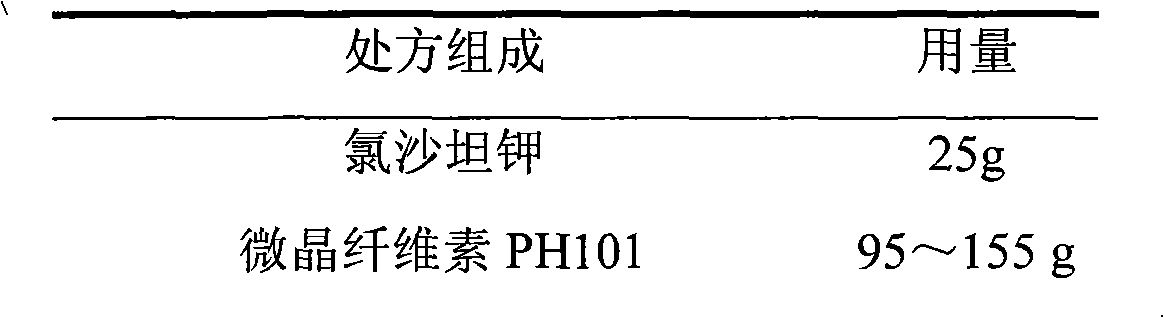
Losartan potassium-containing medicine composition and preparation method thereof
InactiveCN102335149AImprove shielding effectShort operating timeOrganic active ingredientsPharmaceutical non-active ingredientsMedicineMagnesium stearate
The invention belongs to the technical field of medicaments and relates to a losartan potassium troche and a preparation method thereof. In components of the troche, 10-30% of losartan potassium is used as a primary medicine in percentage by weight; magnesium stearate is used as a lubricant, wherein the ratio of the magnesium stearate to the losartan potassium is 1:10-1:25 in percentage by weight. A wet granulation technology and a tabletting technology with the primary medicine externally used are adopted in the preparation process. The invention aims at providing the troche with stable quality and simple and feasible process.
Owner:北京万全阳光医药科技有限公司
Pharmaceutic preparation of losartan potassium
ActiveCN109481437AImprove water absorptionImprove liquidityOrganic active ingredientsInorganic non-active ingredientsPotassiumDissolution
The invention provides a pharmaceutic preparation of iosartan potassium, comprising losartan potassium, lactose, microcrystalline cellulose, calcium hydrophosphate and lauryl sodium sulfate, and prepared by the wet granulation technology. The pharmaceutic preparation has the advantage that high hydroscopicity of iosartan potassium can be improved, and the mixed materials have good flowability. Besides, the pharmaceutic preparation has high dissolution rate and good stability.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Preparation method for compound losartan potassium-hydrochlorothiazide pharmaceutical composition
ActiveCN102475707AMature technologyEasy to operateOrganic active ingredientsPill deliveryCelluloseCross-link
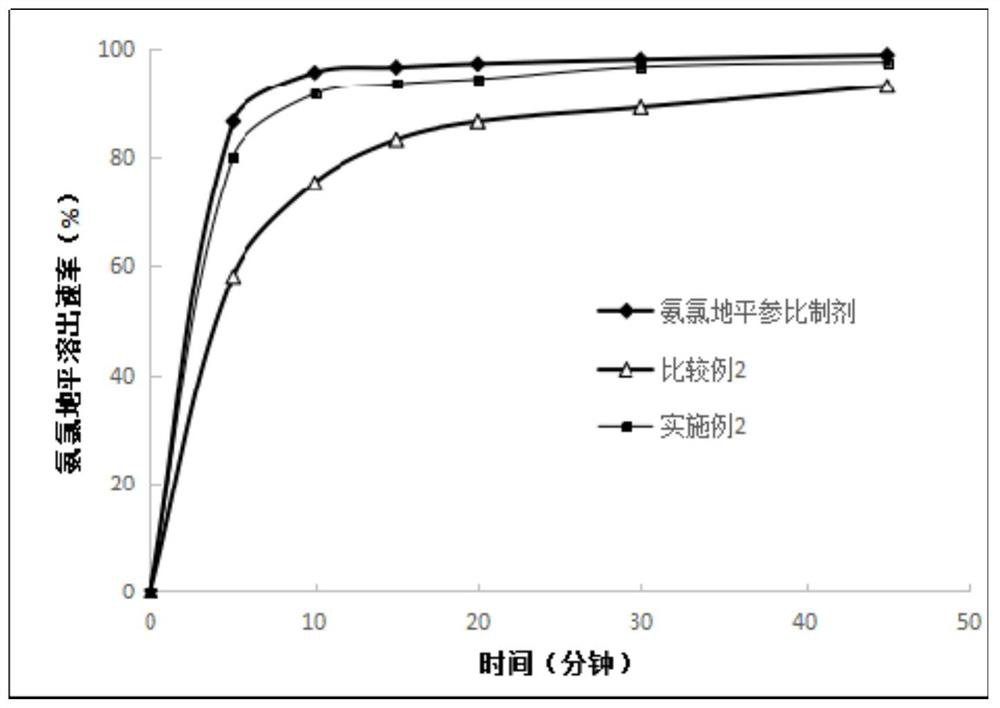
The invention relates to a preparation method for a losartan potassium-hydrochlorothiazide tablet. The losartan potassium-hydrochlorothiazide tablet is characterized in that: after losartan potassium and starch are mixed, 10% of starch slurry is used to prepare particles with a suitable hardness; hydrochlorothiazide, cross linked sodium carboxymethyl cellulose and lactose are mixed, and particles with a proper hardness are prepared from an obtained mixture by using 5% of a polyvinylpyrrolidone K30 solution; the two kinds of particles and magnesium stearate are uniformly mixed and then are subjected to tabletting. According to the invention, a low dissolution rate caused by interaction among drugs in primary granulation is avoided, and an in vitro dissolution rate can be improved greatly, thereby enhancing bioavailability.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Osmotic pump controlled release tablet of losartan potassium and hydrochlorothiazide solid dispersion or inclusion compound
ActiveCN103006566AReduce peak and valley fluctuationsReduce difficultyPowder deliveryOrganic active ingredientsPharmacyHydrochlorothiazide
The invention belongs to the technical field of pharmacy, and in particular relates to a hydrochlorothiazide solid dispersion and hydroxypropyl-beta-cyclodextrin inclusion compound, and a preparation comprising losartan potassium and hydrochlorothiazide. The hydrochlorothiazide solid dispersion is prepared from hydrochlorothiazide and dispersion medium urea or povidone, and the hydrochlorothiazide hydroxypropyl-beta-cyclodextrin inclusion compound is prepared from hydrochlorothiazide and hydroxypropyl-beta-cyclodextrin inclusion. The preparation is an osmotic pump controlled release tablet comprising hydrochlorothiazide solid dispersion and inclusion compound and losartan potassium, consists of a tablet core and a coating for coating the tablet core, and a drug release hole with diameter of between 0.3 and 0.9mm is formed in the center of one side of the coating. The hydrochlorothiazide is compressed in the tablet core in a solid dispersion or inclusion compound mode together with losartan potassium, and is produced into a final product by utilizing an osmotic pump controlled release technology, so that the dissolubility of hydrochlorothiazide is improved, and the losartan potassium and the hydrochlorothiazide can be released at controlled speed in 0 to 24 hours in vitro. Therefore, the osmotic pump controlled release tablet has extremely high application value.
Owner:惠州市九惠药业有限公司
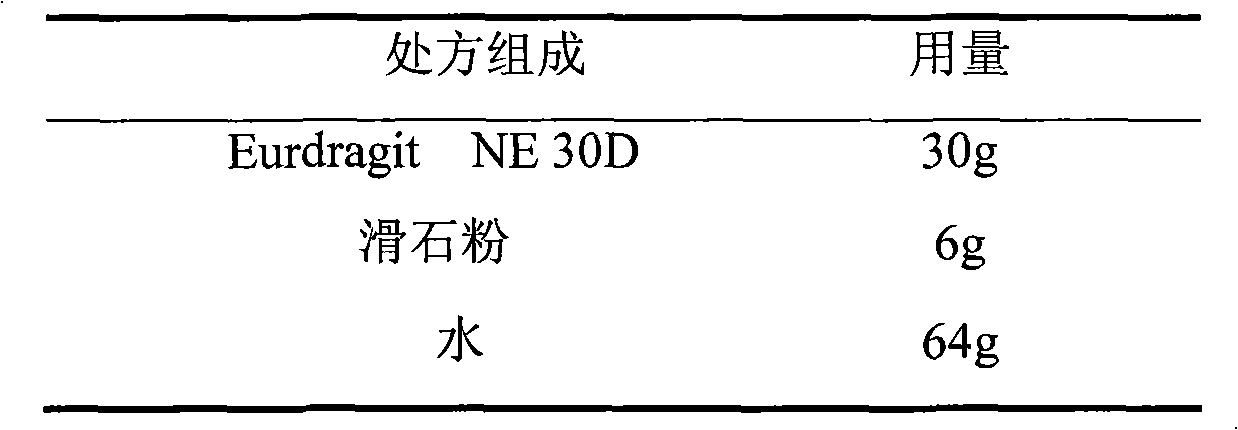
Losartan potassium membrane controlled-release pellet capsule
InactiveCN103211798AReduce permeabilityImprove permeabilityOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseMedicine
The invention relates to a losartan potassium membrane controlled-release pellet capsule. The controlled-release film of the pellet adopts Eurdragit NE 30D as a film forming material, the core of the controlled-release pellet contains high-expansibility low-substituted hydroxypropylcellulose and a pharmaceutically-acceptable excipient commonly used for controlled-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of the low-substituted hydroxypropylcellulose in the core of the controlled-release pellet is 10-40%. The controlled-release film of the controlled-release pellet includes the Eurdragit NE 30D and an anti-adherent talcum powder, the optimal ratio of the Eurdragit NE 30D to the talcum powder is 30:6, and the optimal coating weight gain is 19-36%. The core will obviously expand after absorbing water because of the containment of the low-substituted hydroxypropylcellulose highly expanding after contacting with water, so the controlled-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:内蒙古天衡医院管理有限公司
Losartan potassium synthesis
InactiveUS20040224998A1High purityReduction of solvent usageBiocideOrganic chemistryPotassiumPrimary alcohol
Owner:IPCA LAB LTD
Losartan potassium liposome solid preparation
InactiveCN102579344AImprove stabilityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsSide effectCholesterol
The invention discloses a losartan potassium liposome solid preparation and a preparation method thereof. Excellent quality of losartan potassium liposome is prepared by selecting specific weight proportions of losartan potassium, phosphatidylinositol, dimyristoyl lecithin and cholesterol, and then the losartan potassium liposome is prepared into a solid preparation by a general preparation method. Compared with the existing preparation, the liposome solid preparation provided by the invention has a high encapsulating rate and a uniform grain size, the medicine is maintained in blood circulation for a long time, apparently the stability and the bioavailability of the preparation product are improved, the quality of the preparation product is improved, and toxic and side effects are reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method of amlodipine losartan potassium compound composition
ActiveCN112274490AImprove medication safetyImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsAmlodipine besilateStearic acid
The invention discloses a preparation method of an amlodipine and losartan potassium compound composition. The preparation method comprises the following steps of mixing losartan potassium and hydroxypropyl methylcellulose, adding microcrystalline cellulose and pregelatinized starch, mixing, adding a proper amount of ethanol water solution, granulating, drying to obtain losartan potassium dry particles, and mixing amlodipine besylate and sodium carboxymethyl starch to obtain the amlodipine and losartan potassium compound composition; crushing losartan potassium, adding microcrystalline cellulose and pregelatinized starch, mixing to obtain amlodipine mixed powder, mixing losartan potassium dry particles, amlodipine mixed powder, silicon dioxide and magnesium stearate, tabletting, and coating so that the tablet obtained by the method is stable, and the dissolution rate of the tablet is close to or consistent with that of an original product on the market.
Owner:四川尚锐生物医药有限公司
A new pharmaceutical composition containing levamlodipine and losartan potassium and its preparation method
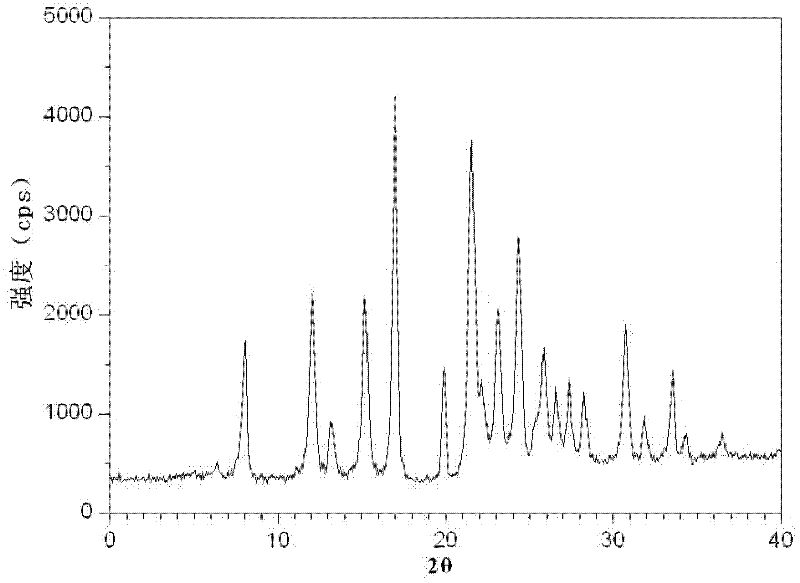
ActiveCN102266331AImprove Medication AdherenceDisintegrates quicklyOrganic active ingredientsCardiovascular disorderSolubilityLevamlodipine
The invention relates to a brand new medicinal composition containing levamlodipine and losartan potassium and a preparation method thereof. The composition comprises the levamlodipine and the losartan potassium serving as active ingredients and pharmaceutically acceptable auxiliary materials, wherein the levamlodipine refers to a levamlodipine besylate crystal; and the characteristic peaks of the crystal in an X-ray powder diffraction pattern obtained by measuring through Cu-K alpha rays are displayed when 2 theta is 8.0 degrees, 12.1 degrees, 15.4 degrees, 17.0 degrees, 19.8 degrees, 21.6 degrees, 23.0 degrees, 24.3 degrees, 25.7 degrees, 27.4 degrees, 30.7 degrees and 33.5 degrees. Since the composition contains the crystal which can improve the solubility of levamlodipine besylate, synchronous release of the levamlodipine besylate and the losartan potassium can be realized, and the synergic action of the levamlodipine besylate and the losartan potassium is brought into fuller play. In the method, a direct powder tableting process is adopted; and the method has a simple process, a short production period and low production cost.
Owner:HAINAN JINRUI PHARMA
Composition with losartan potassium and gliclazide and preparation method thereof
The invention relates to a composition with losartan potassium and gliclazide and a preparation method thereof. The composition is prepared from the following components in parts by weight: 40-60 parts of losartan potassium, 60-100 parts of gliclazide, 40-60 parts of microcrystalline cellulose, 20-40 parts of anhydrous calcium hydrogen phosphate, 60-80 parts of pregelatinized starch, 4-8 parts ofsilicon dioxide and 2-4 parts of magnesium stearate. The preparation method comprises the following steps: taking part of pregelatinized starch, adding water into the part of pregelatinized starch toprepare a pregelatinized starch slurry with the concentration of 8-12%, taking the pregelatinized starch slurry as an adhesive, adding the adhesive into a mixture composed of losartan potassium, microcrystalline cellulose and the part of the pregelatinized starch to prepare a soft material, preparing the soft material into particles through a 20-30-mesh sieve, drying the particles at 40-70 DEG C till the water content of the particles is 1-3%, then neatening the particles through a 20-mesh sieve, then uniformly mixing the particles with gliclazide, anhydrous calcium hydrogen phosphate, silicondioxide and magnesium stearate, then directly tabletting to obtain tablets of the composition with losartan potassium and gliclazide. The composition is capable of exerting the beneficial effect of losartan potassium on treatment of hypertension with diabetes; losartan potassium and gliclazide are capable of synergistically exerting the effect of reducing blood pressure and blood glucose.
Owner:CHENGDU HENGRUI PHARMA
Process for the synthesis of losartan potassium
Improved processes using primary, secondary and tertiary alcohols and with safer mode of introduction of the reagent and reaction conditions are described. Further, the process of manufacture of Losartan potassium by use of alkali metal salt such as Potassium carbonate is disclosed. A process for preparation of the polymorphic Form I of Losartan potassium is also disclosed herein.
Owner:IPCA LAB LTD
Medicament composition containing losartan and hydrochlorothiazidum
InactiveCN101461814BSolve the problem of incomplete dissolutionSolve instabilityOrganic active ingredientsPharmaceutical non-active ingredientsHydrochlorothiazidePharmacology
The invention discloses a medicine composition containing losartan potassium and hydrochlorothiazide and a preparation method thereof. The composition also contains auxiliary materials, namely sodium alginate and lactose. The medicine composition can be prepared into capsules, tablets, granules and so on after being added with other proper pharmaceutic adjuvant.
Owner:BEIJING D VENTUREPHARM TECH DEV
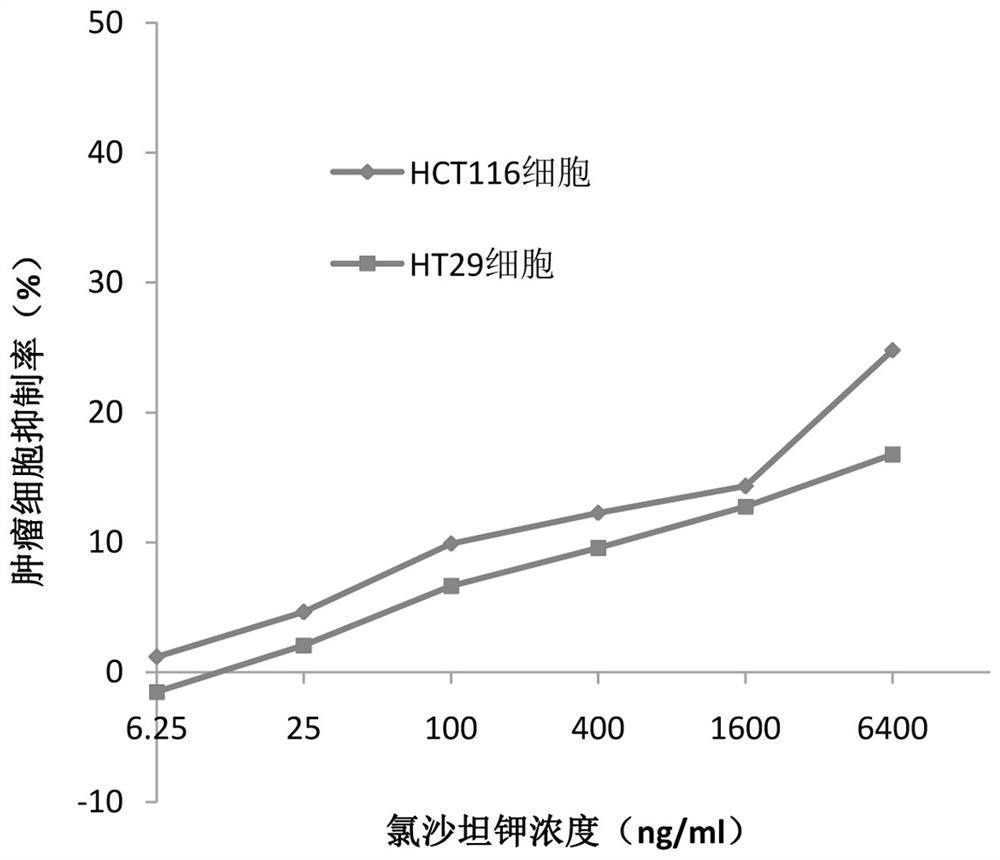
Application of losartan potassium and dacarbazine combined medicine to preparation of medicine for treating intestinal cancer
InactiveCN112438986AIncreased sensitivityReduce dosageOrganic active ingredientsDigestive systemCo medicationUse medication
The invention belongs to the field of medicines, and particularly relates to an application of a losartan potassium and dacarbazine combined medicine to preparation of a medicine for treating intestinal cancer. The application of the losartan potassium and dacarbazine combined medicine to preparation of the medicine for treating intestinal cancer is proposed for the first time, losartan potassiumand the dacarbazine have an obvious synergistic effect, the curative effect is effectively improved, compared with a single component, for the losartan potassium and dacarbazine combined medicine, thecurative effect is more remarkable, and the lethality to tumor cells is improved. The dosage is effectively reduced, so that the toxic and side effects are reduced. The losartan potassium and dacarbazine combined medicine can also save cost, reduce economic burden of patients, provide a new approach for prevention and treatment of intestinal cancer, and have broad application prospects in the field of medicine and pharmacology.
Owner:阿耳法猫(杭州)人工智能生物科技有限公司
Losartan potassium synthesis
Owner:IPCA LAB LTD
Brand new medicinal composition containing levamlodipine and losartan potassium and preparation method thereof
ActiveCN102266331BImprove Medication AdherenceDisintegrates quicklyOrganic active ingredientsPill deliverySolubilityLevamlodipine
The invention relates to a brand new medicinal composition containing levamlodipine and losartan potassium and a preparation method thereof. The composition comprises the levamlodipine and the losartan potassium serving as active ingredients and pharmaceutically acceptable auxiliary materials, wherein the levamlodipine refers to a levamlodipine besylate crystal; and the characteristic peaks of the crystal in an X-ray powder diffraction pattern obtained by measuring through Cu-K alpha rays are displayed when 2 theta is 8.0 degrees, 12.1 degrees, 15.4 degrees, 17.0 degrees, 19.8 degrees, 21.6 degrees, 23.0 degrees, 24.3 degrees, 25.7 degrees, 27.4 degrees, 30.7 degrees and 33.5 degrees. Since the composition contains the crystal which can improve the solubility of levamlodipine besylate, synchronous release of the levamlodipine besylate and the losartan potassium can be realized, and the synergic action of the levamlodipine besylate and the losartan potassium is brought into fuller play. In the method, a direct powder tableting process is adopted; and the method has a simple process, a short production period and low production cost.
Owner:HAINAN JINRUI PHARMA CO LTD
Liposome solid preparation of losartan potassium hydrochlorothiazide pharmaceutical composition
InactiveCN101797230BMitigate counter-regulationGood blood pressure effectOrganic active ingredientsPharmaceutical non-active ingredientsYolkSide effect
The invention discloses a liposome solid preparation of a losartan potassium hydrochlorothiazide pharmaceutical composition and a preparation method thereof. In the invention, active components of losartan potassium and hydrochlorothiazide and specific combined hydrogenated yolk lecithin, cholesterol and poloxamer 188 are prepared into a liposome which is then mixed with other pharmaceutical accessories to prepare the solid preparation, thereby greatly improving the pharmaceutical stability and bioavailability and having stable and lasting effect, small side effect and obvious curative effect.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Brand new oral solid medicinal composition and its preparation method
InactiveCN102342943AHigh content of the main drugEasy to takeOrganic active ingredientsOrganic chemistryLevamlodipineSilicon dioxide
The invention discloses a brand new oral solid medicinal composition and its preparation method, the medicinal composition is an oral preparation prepared by hydrochlorothiazide, levamlodipine, losartan potassium and pharmaceutically-accepted auxiliary materials, the oral preparation is including but not limited to tablets or capsules. The composition comprises the following raw materials by weight part: 5-25 parts of hydrochlorothiazide, 2.5-5 parts of levamlodipine, 20-120 parts of losartan potassium, 30-90 parts of lactose, 20-60 parts of microcrystalline cellulose, 20-60 parts of pregelatinized starch, 2-5 parts of hydroxypropylcellulose, 1-3 parts of silica and 1-2 parts of magnesium stearate. The medicinal composition has the advantages of scientific and reasonable prescription, lowauxiliary materials content and high biological availability, and is a preferred medicine for treating hypertension.
Owner:HAINAN JINRUI PHARMA CO LTD
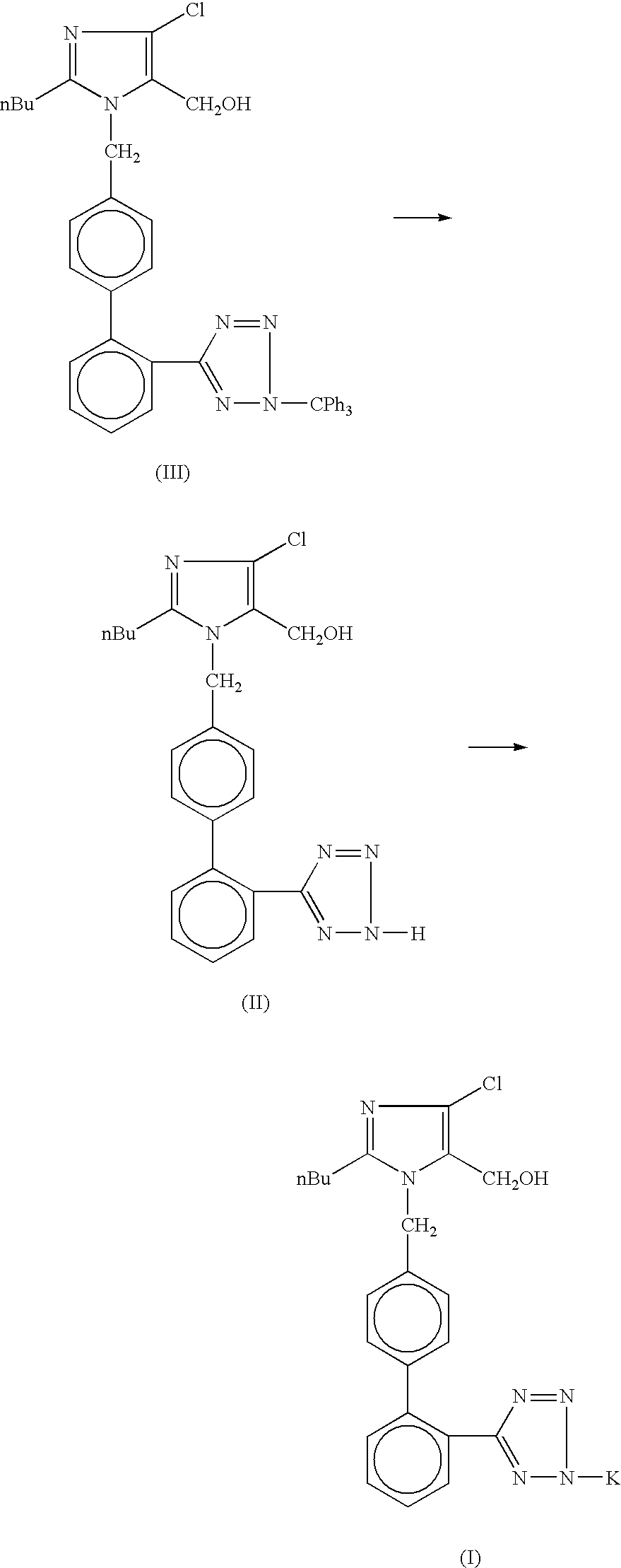
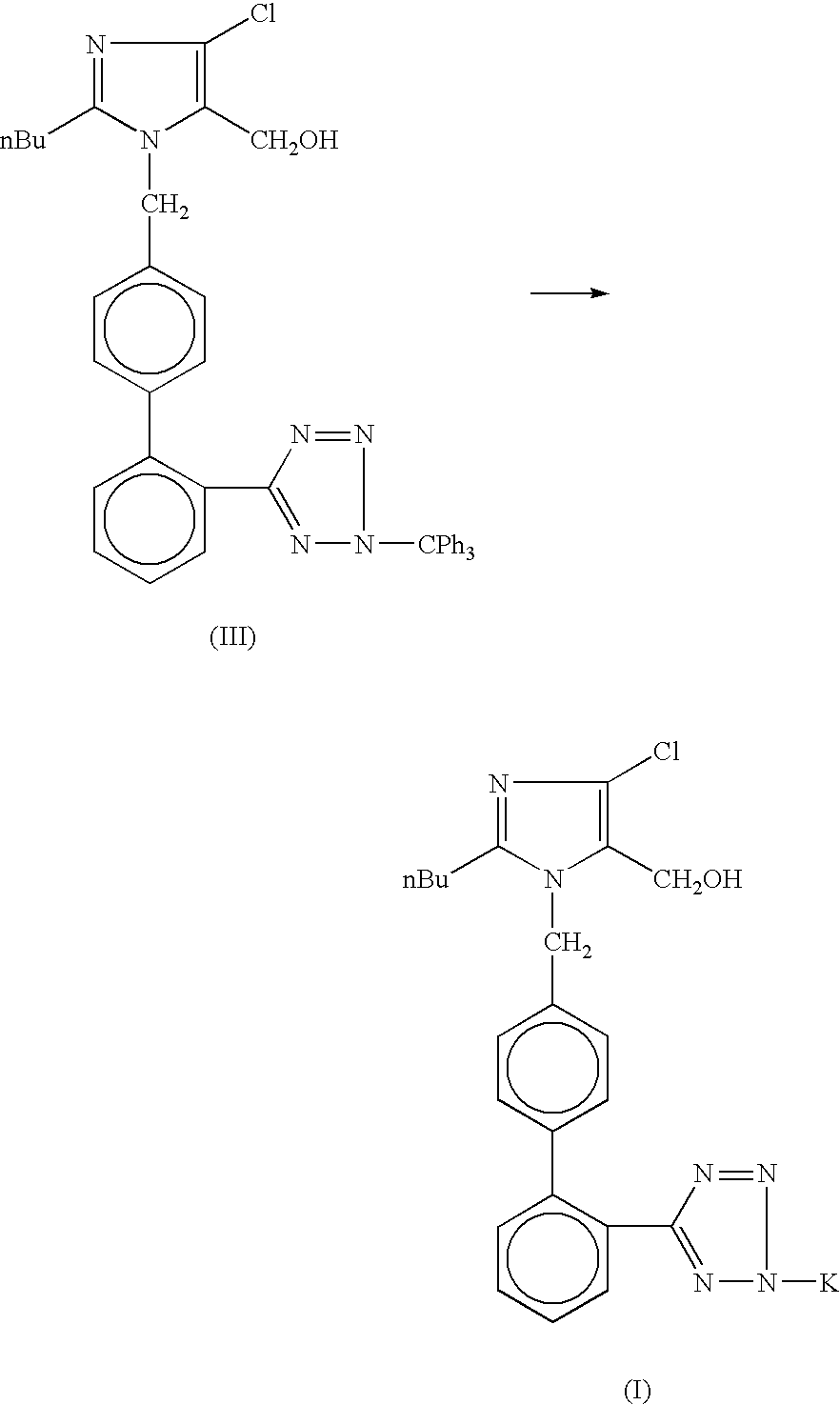
Processes for preparing losartan and losartan potassium
Losartan is prepared by acid catalyzed cleavage of a triarylmethyl group from a triarylmethyl-substituted losartan derivative in a diluent comprising liquid ketone. The reaction mixture is basified and liquid ketone is evaporated from the mixture leaving a residue from which a triarylmethyl alcohol and losartan are each obtained in high yield and high purity. In addition, losartan potassium is prepared by a process that is more convenient that those previously known in the art in which losartan is contacted with potassium ions in substantially pure liquid alcohol and losartan potassium is precipitated from the alcohol.
Owner:TEVA PHARMA IND LTD +1
Preparation method for compound losartan potassium-hydrochlorothiazide pharmaceutical composition
ActiveCN102475707BImprove stabilityAvoid contactOrganic active ingredientsPill deliveryCelluloseCross-link
The invention relates to a preparation method for a losartan potassium-hydrochlorothiazide tablet. The losartan potassium-hydrochlorothiazide tablet is characterized in that: after losartan potassium and starch are mixed, 10% of starch slurry is used to prepare particles with a suitable hardness; hydrochlorothiazide, cross linked sodium carboxymethyl cellulose and lactose are mixed, and particles with a proper hardness are prepared from an obtained mixture by using 5% of a polyvinylpyrrolidone K30 solution; the two kinds of particles and magnesium stearate are uniformly mixed and then are subjected to tabletting. According to the invention, a low dissolution rate caused by interaction among drugs in primary granulation is avoided, and an in vitro dissolution rate can be improved greatly, thereby enhancing bioavailability.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of losartan impurity
InactiveCN113372338AQuality impactShort reaction stepsOrganic chemistryRegioselectivityReaction step
The invention relates to a preparation method of a losartan potassium impurity. In the production process, a novel losartan alkylation impurity is found, and the impurity has obvious influence on the quality of a finished product and is a key impurity in the preparation process. A synthesis method of the impurity is not reported at present. The invention provides a synthesis method of the losartan impurity, and the synthesis method has the advantages of short reaction steps, easily available raw materials, high yield and good regioselectivity.
Owner:润都制药荆门有限公司
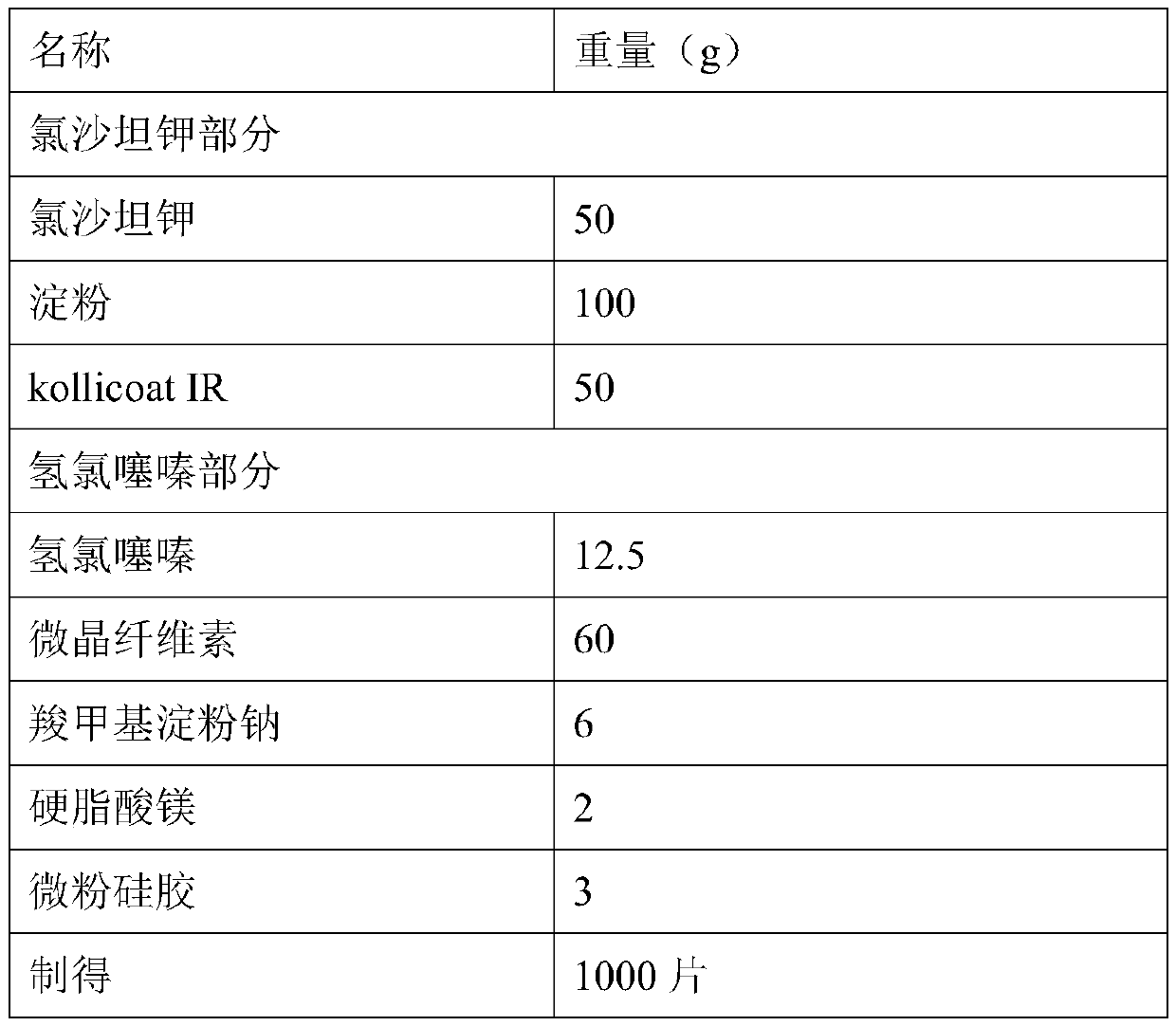
Compound preparation containing losartan potassium and preparation method of compound preparation
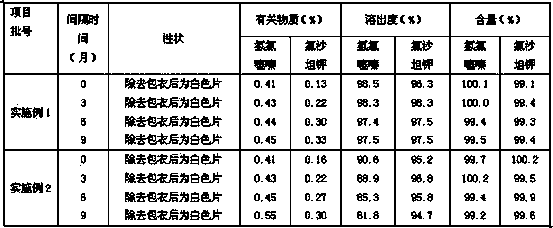
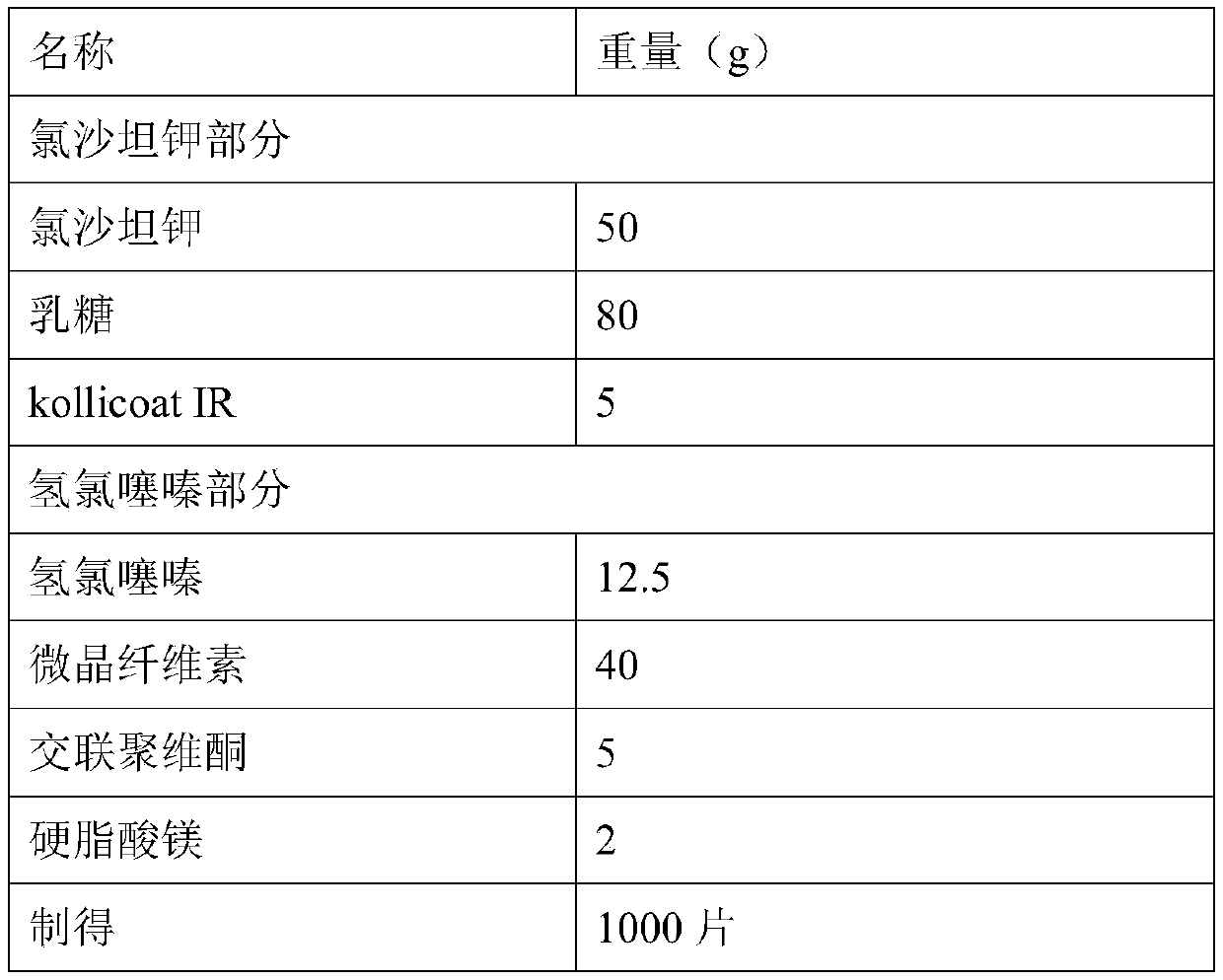
InactiveCN111297812AFully dispersedPromote dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsHydrochlorothiazidePharmaceutical drug
The present invention discloses a compound preparation containing losartan potassium and a preparation method of the compound preparation. The compound preparation is prepared by the following method:dissolving losartan potassium and kollicoat IR in a methanol aqueous solution and conducting granulation on auxiliary materials to obtain losartan potassium granules wrapped by the kollicoat IR; dissolving hydrochlorothiazide in acetone and conducting granulation on the auxiliary materials; mixing the two granules and conducting tableting to obtain a finished product. The prepared compound preparation containing the losartan potassium can fully isolate the losartan potassium and hydrochlorothiazide, and besides, the drug is added in a state of the solution and fully dispersed on the surfacesof the auxiliary materials and dissolves rapidly. The compound preparation containing the losartan potassium prepared by the process steps has a simple and feasible process, stable quality, improved product quality, and guaranteed product stability during long-term storage.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com