Preparation method of losartan impurity
A technology for losartan potassium and impurities, applied in the field of drug synthesis, can solve the problems of human toxicity, influence on the raw material drug of losartan potassium, no preparation method reported in literature, etc., and achieves short reaction steps, improved selectivity, and easy availability of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
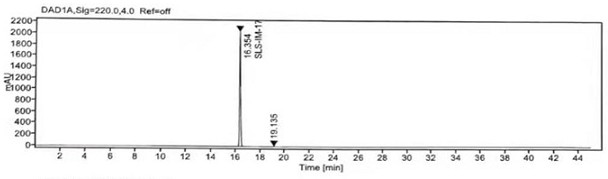
[0021] Add 40g of losartan potassium (0.0868mol, 1eq) and 200ml of acetone into a 500ml four-neck flask, stir and cool down to 10°C, add 7.2g of K 2 CO 3 (0.0511mol, 0.7 equivalent), stir evenly, continue to add 12.9g of iodomethane (0.0911mol, 1.05 equivalent) in 20ml of acetone solution to the reaction solution, return to room temperature after adding, and react at room temperature for 6h. TLC monitors the completion of the reaction of losartan potassium. Concentrate the reaction solution to dryness under reduced pressure, add 100ml of dichloromethane and 100ml of water, stir evenly, add 13g of glacial acetic acid to the reaction solution to adjust pH=4~5, and cool down only 0~5℃ Stir and crystallize for 2 hours, filter, mix the wet product with 60ml of acetone, heat to reflux to dissolve, then cool down naturally, stir and crystallize at room temperature for 4 hours, filter and wash the filter cake with water, continue to filter until dry, and dry the filter cake to obtain ...
Embodiment 2
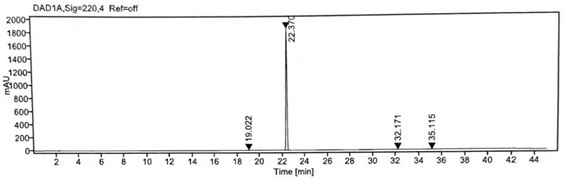
[0023] Add 40g of losartan potassium (0.0868mol, 1eq) and 200ml of acetone into a 500ml four-neck flask, stir and cool down to 10°C, add 7.2g of K 2 CO 3 (0.0511mol, 0.7 equivalent), stir well, continue to add 19.5g of isopropyl p-toluenesulfonate (0.0911mol, 1.05 equivalent) to the reaction solution in 20ml of acetone solution, return to room temperature after adding, and react at room temperature for 8h . TLC monitors that the reaction of losartan potassium is complete, concentrate the reaction solution to dryness under reduced pressure, add 100ml of dichloromethane and 100ml of water, stir well, add glacial acetic acid to the reaction solution to adjust pH=4~5, and cool down to 0~5°C Stir and crystallize for 2 hours, filter, mix the wet product with 60ml of acetone, heat to reflux to dissolve, then cool down naturally, stir and crystallize at room temperature for 4 hours, filter and wash the filter cake with water, continue to filter until dry, and dry the filter cake to o...
Embodiment 3
[0026] Add 40g of losartan potassium (0.0868mol, 1eq) and 200ml of acetone into a 500ml four-neck flask, stir and cool down to 10°C, add 7.2g of K 2 CO 3 (0.5211mol, 0.7 equivalent), stir evenly, continue to add 14.2g iodoethane (0.0911mol, 1.05 equivalent) in 20ml acetone solution to the reaction solution, return to room temperature after adding, and react at room temperature for 8h. TLC monitors the completion of the reaction of losartan potassium. Concentrate the reaction solution to dryness under reduced pressure, add 100ml of dichloromethane and 100ml of water, stir well, add glacial acetic acid to the reaction solution to adjust pH=4~5, and cool down only 0~5℃ Stir and crystallize for 2 hours, filter, mix the wet product with 60ml of acetone, heat to reflux to dissolve, then cool down naturally, stir and crystallize at room temperature for 4 hours, filter and wash the filter cake with water, continue to filter until dry, and dry the filter cake to obtain 32.02g of N Eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com