Pharmaceutic preparation of losartan potassium
A technology for losartan potassium and pharmaceutical preparations, which is applied in directions such as pharmaceutical combinations, pharmaceutical formulations, and non-active ingredients medical preparations, can solve the problems of long mixing process time, high viscosity of materials, influence on drug efficacy, etc., and achieves excellent flow performance, improve stability, and improve water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
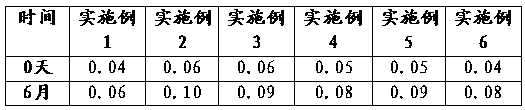
Examples
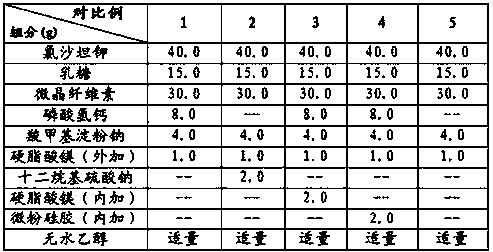
Embodiment 1
[0024]
[0025] The preparation process comprises the following steps:
[0026] a. Fully mix losartan potassium, sodium lauryl sulfate, lactose, microcrystalline cellulose, calcium hydrogen phosphate, and sodium carboxymethyl starch (internal addition) to obtain a mixed powder;
[0027] b. Add absolute ethanol to the above mixed powder, sieve, dry, and granulate to obtain drug granules;
[0028] c. Mix magnesium stearate, sodium carboxymethyl starch (additional) and the drug granules in step b evenly, and fill the aforementioned mixture into capsules.
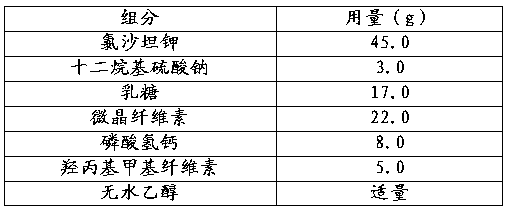
Embodiment 2
[0030]
[0031] The preparation process comprises the following steps:
[0032] a. Fully mix losartan potassium, sodium lauryl sulfate, lactose, microcrystalline cellulose, and calcium hydrogen phosphate to obtain mixed powder;
[0033] b. Add absolute ethanol to the above mixed powder, sieve, dry, and granulate to obtain drug granules;
[0034] c. Filling capsules with drug particles.
Embodiment 3
[0036]
[0037] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com