Preparation method of amlodipine losartan potassium compound composition
A technology for dipine losartan potassium and losartan potassium, applied in the field of medicine, can solve the problems of complex double-layer tablet process, hidden dangers in quality and safety, slow dissolution rate, etc., and achieves improved drug safety, simple manufacturing process, and improved stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
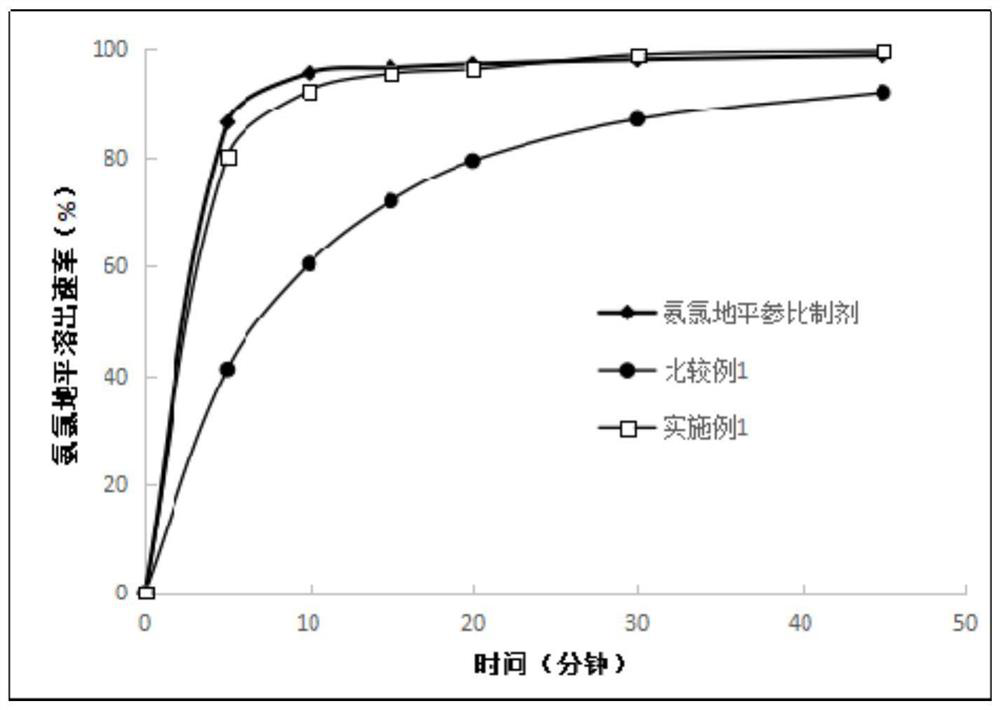
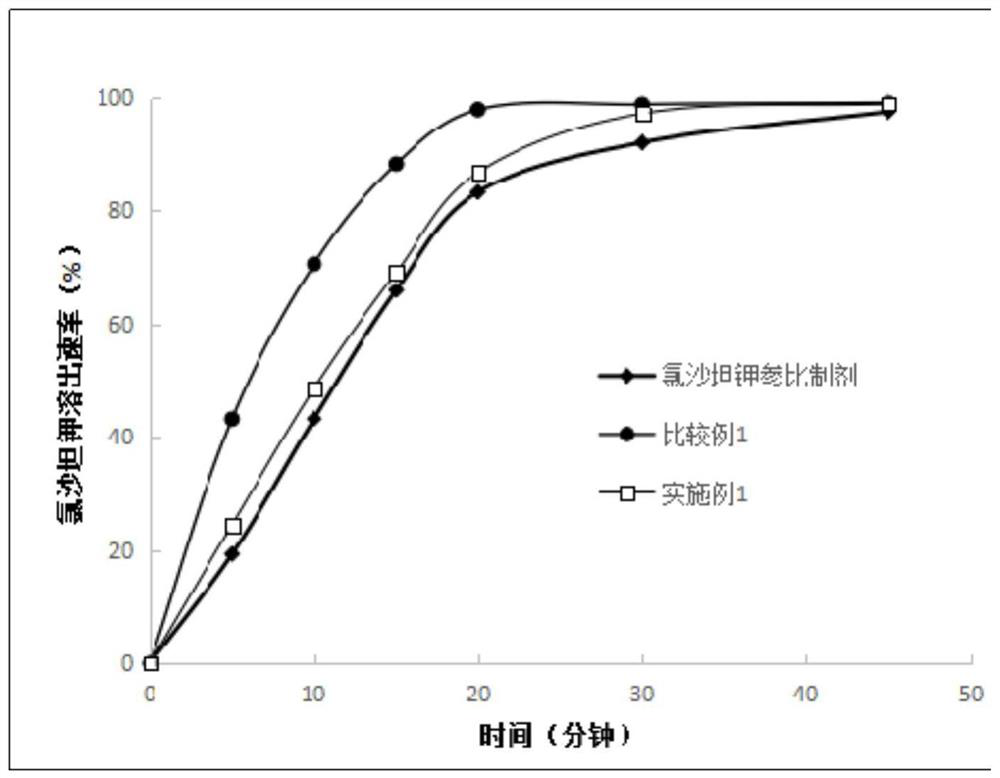
Embodiment 1
[0049] Prescription: (makes 1000 tablets)
[0050] Amlodipine besylate 6.94g (equivalent to 5g amlodipine), losartan potassium 50g, microcrystalline cellulose 100g, pregelatinized starch 100g, hypromellose 3g, sodium carboxymethyl starch 8g, 1 g of silicon oxide, 1.5 g of magnesium stearate, an appropriate amount of 10% ethanol aqueous solution, and an appropriate amount of 80% ethanol aqueous coating solution containing 8% Opadry gastric-soluble film coating premix.
[0051] Preparation:
[0052](1) 50 g of losartan potassium and 3 g of hypromellose are mixed in a mixer, then 50 g of microcrystalline cellulose and 50 g of pregelatinized starch are added and mixed in a mixer to obtain a mixed powder of losartan potassium;
[0053] (2) Add an appropriate amount of 10% ethanol aqueous solution to the mixed powder of losartan potassium, further mix, pass through a 20-mesh sieve and granulate to obtain wet granules of losartan potassium;
[0054] (3) drying the wet granules of l...
Embodiment 2
[0071] Prescription: (makes 1000 tablets)
[0072] Amlodipine besylate 6.94g (equivalent to 5g amlodipine), losartan potassium 50g, microcrystalline cellulose 100g, pregelatinized starch 100g, hypromellose 5g, sodium carboxymethyl starch 16g, 3g of silicon oxide, 0.5g of magnesium stearate, an appropriate amount of 50% ethanol aqueous solution, and an appropriate amount of 80% ethanol aqueous coating solution containing 8% Opadry gastric-soluble film coating premix.
[0073] Preparation:
[0074] (1) 50 g of losartan potassium and 5 g of hypromellose are mixed in a mixer, then 25 g of microcrystalline cellulose and 75 g of pregelatinized starch are added and mixed in a mixer to obtain a mixed powder of losartan potassium;
[0075] (2) Add an appropriate amount of 50% ethanol aqueous solution to the mixed powder of losartan potassium, further mix, pass through a 20-mesh sieve and granulate to obtain wet granules of losartan potassium;
[0076] (3) drying the wet granules of l...
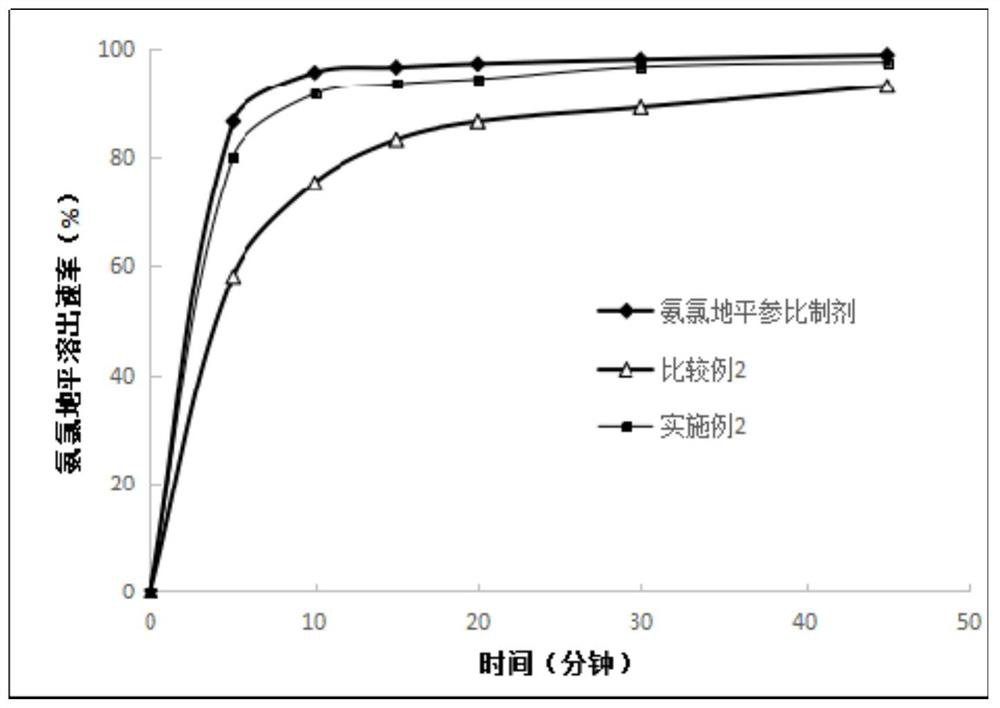
Embodiment 3
[0093] Prescription: (makes 1000 tablets)
[0094] Amlodipine besylate 6.94g (equivalent to 5g amlodipine), losartan potassium 50g, microcrystalline cellulose 100g, pregelatinized starch 100g, hypromellose 4g, sodium carboxymethyl starch 12g, 1.5 g of silicon oxide, 1 g of magnesium stearate, an appropriate amount of 30% ethanol water solution, and an appropriate amount of 80% ethanol water coating solution containing 8% Opadry gastric-soluble film coating premix.
[0095] Preparation:
[0096] (1) 50 g of losartan potassium and 4 g of hypromellose are mixed in a mixer, then 40 g of microcrystalline cellulose and 60 g of pregelatinized starch are added and mixed in a mixer to obtain a mixed powder of losartan potassium;
[0097] (2) Add an appropriate amount of 30% ethanol aqueous solution to the mixed powder of losartan potassium, further mix, pass through a 20-mesh sieve and granulate to obtain wet granules of losartan potassium;
[0098] (3) drying the wet granules of los...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com