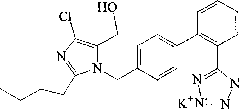
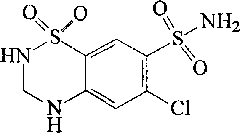
Liposome solid preparation of losartan potassium hydrochlorothiazide pharmaceutical composition
A technology of losartan potassium hydrochlorothiazide and solid preparations, which is applied in the field of liposome solid preparations, and can solve the problems of rapid drug release process, easy peak and trough phenomena, bioavailability, and light and heat instability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The preparation of embodiment 1 losartan potassium hydrochlorothiazide liposome sheet
[0074] Prescription (1000 tablets):
[0076] Hydrochlorothiazide 12.5g
[0077] Hydrogenated egg yolk lecithin 60g
[0078] Cholesterol 30g
[0079] Poloxamer 188 8g
[0080] Starch 30g
[0081] Mannitol 20g
[0082] Low-substituted hydroxypropyl cellulose 10g
[0083] Hypromellose 2g
[0085] Micronized silica gel 3g
[0086] Preparation Process
[0087] (1) 60g of hydrogenated egg yolk lecithin, 30g of cholesterol, and 8g of poloxamer 188 were dissolved in 400ml of dichloromethane and isopropanol mixed solvent with a volume ratio of 1:1, mixed evenly, and reduced on a rotary thin film evaporator. Remove the mixed solvent under pressure to obtain a phospholipid film;
[0088] (2) Add 200ml of citric acid-sodium citrate buffer solution with a pH value of 5.5, shake and stir to fully hydrate the phospholipid membrane,...
Embodiment 2
[0093] The preparation of embodiment 2 losartan potassium hydrochlorothiazide liposome sheet
[0094] Prescription (1000 tablets):
[0096] Hydrochlorothiazide 12.5g
[0097] Hydrogenated egg yolk lecithin 80g
[0098] Cholesterol 50g
[0099] Poloxamer 188 20g
[0100] Starch 65g
[0101] Sorbitol 70g
[0102] Carboxymethyl starch sodium 8g
[0103] Low-substituted hydroxypropyl cellulose 10g
[0104] Sodium Carboxymethyl Cellulose 2g
[0106] Preparation Process
[0107] (1) 80g of hydrogenated egg yolk lecithin, 50g of cholesterol, and 20g of poloxamer 188 were dissolved in 600ml of dichloromethane and isopropanol mixed solvent with a volume ratio of 1:1, mixed evenly, and reduced on a rotary thin film evaporator. Remove the mixed solvent under pressure to obtain a phospholipid film;
[0108] (2) Add 300ml of citric acid-sodium citrate buffer solution with a pH value of 5.5, shake and stir to fully hydrate ...
Embodiment 3
[0114] The preparation of embodiment 3 losartan potassium hydrochlorothiazide liposome dispersible tablet
[0115] Prescription (1000 tablets):
[0117] Hydrochlorothiazide 12.5g
[0118] Hydrogenated egg yolk lecithin 70g
[0119] Cholesterol 40g
[0120] Poloxamer 188 14g
[0121] Starch 70g
[0122] Dextrin 40g
[0123] Crospovidone 10g
[0124] Low-substituted hydroxypropyl cellulose 10g
[0125] Hypromellose 2g
[0127] Micronized silica gel 2g
[0128] The preparation process is the same as in Example 1, and losartan potassium hydrochlorothiazide liposome dispersible tablets are obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com