Application of losartan potassium and dacarbazine combined medicine to preparation of medicine for treating intestinal cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 MTT method detects the sensitivity of different intestinal cancer cells to losartan potassium
[0024] 1. Experimental materials
[0025] (1) Drug: The chemical structural formula of losartan potassium is as follows:
[0026]
[0027] (2) Intestinal cancer cells: human intestinal cancer cells HCT116 and HT29 cells.
[0028] (3) Commercially available MTT kits.
[0029] 2. Experimental grouping
[0030] (1) Control group: blank control, that is, intestinal cancer cells were not treated with any drugs.
[0031] (2) Experimental group: intestinal cancer cells were treated with different concentrations of losartan potassium.
[0032] 3. MTT method to detect the sensitivity of different tumor cells to losartan potassium
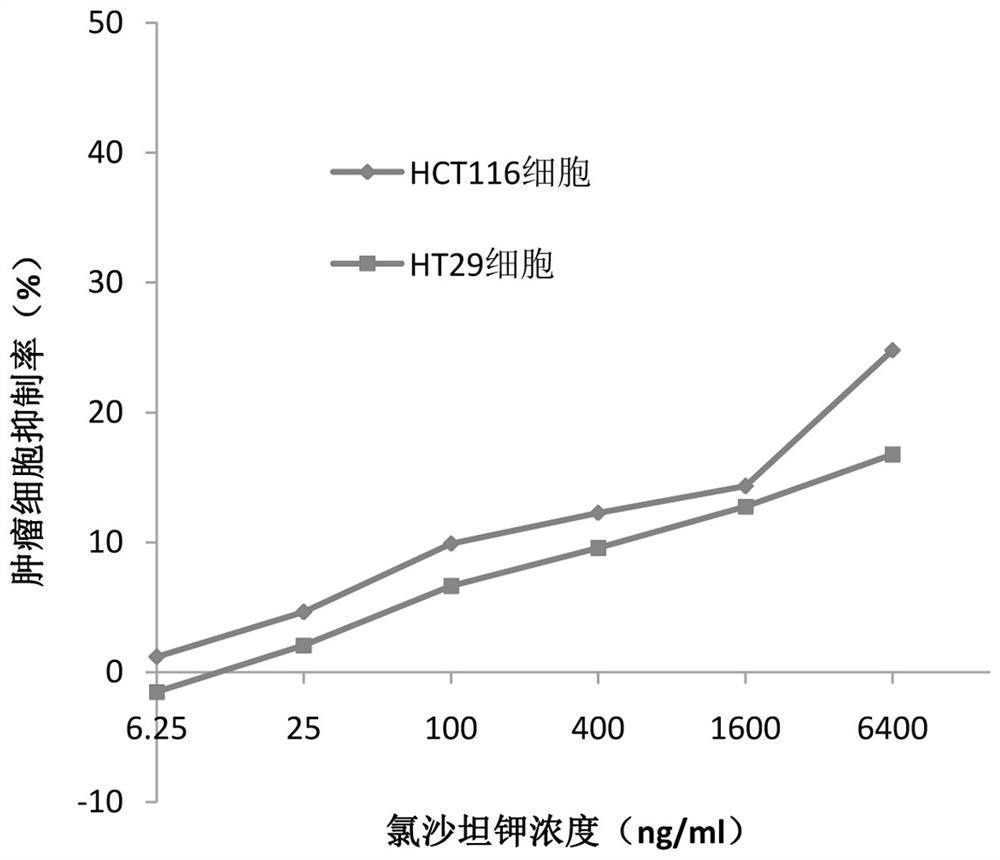
[0033] (1) Inoculate human intestinal cancer cells HCT116 cells and HT29 cells into 96-well plates at the number of 5000-9000 cells per well. After the cells adhere to the wall, add PBS solutions of different concentrations of losartan pot...
Embodiment 2
[0035] Example 2 The effect of dacarbazine alone or the combination of losartan potassium and dacarbazine on the inhibition rate of intestinal cancer cells
[0036] 1. Experimental materials
[0037] (1) Drugs: losartan potassium, dacarbazine.
[0038] (2) Intestinal cancer cells: human intestinal cancer cells HCT116 and HT29 cells.
[0039] (3) Commercially available MTT kits.
[0040] 2. Experimental grouping
[0041] (1) Control group: intestinal cancer cells were only treated with dacarbazine.
[0042] (2) Experimental group: Different concentrations of losartan potassium and dacarbazine were used in combination to treat intestinal cancer cells.
[0043] 3. MTT method to detect the sensitivity of intestinal cancer cells to the combination of losartan potassium and dacarbazine
[0044] (1) Inoculate human intestinal cancer cells HCT116 cells and HT29 cells into 96-well plates at the number of 5000-9000 cells per well. After the cells adhere to the wall, add different r...
Embodiment 3
[0046] Example 3 The effect of dacarbazine alone or combined administration of losartan potassium and dacarbazine on the inhibition rate of human intestinal cancer tissue organoids
[0047] 1. Experimental materials
[0048] (1) Drugs: losartan potassium, dacarbazine.
[0049] (2) Intestinal cancer cells: Human intestinal cancer tissue organoids.
[0050] (3) Commercially available CellTiter-Glo 3D kit.
[0051] 2. Experimental grouping
[0052] (1) Control group: only treated with dacarbazine.
[0053] (2) Experimental group: Different concentrations of losartan potassium and dacarbazine were used in combination to treat human intestinal cancer tissue organoids.
[0054] 3. Sensitivity of human intestinal cancer tissue organoids to the combination of losartan potassium and dacarbazine
[0055] (1) Mix the treated human intestinal cancer cells with Matrigel Matrigel, add 50 μl of mixed Mtrigel Matrigel to each well on a 48-well plate, add 250 μl complete medium to each we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com