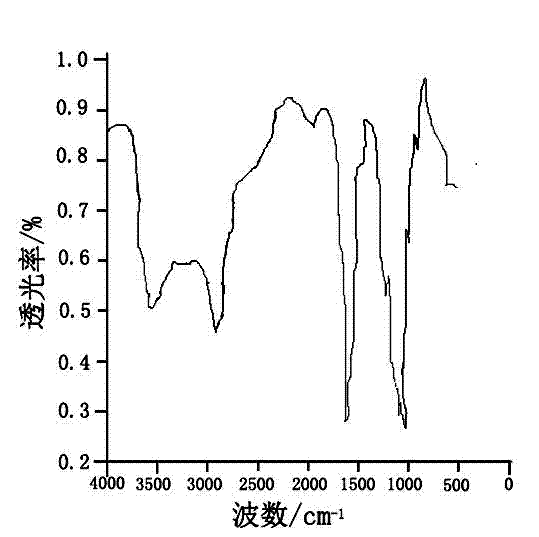
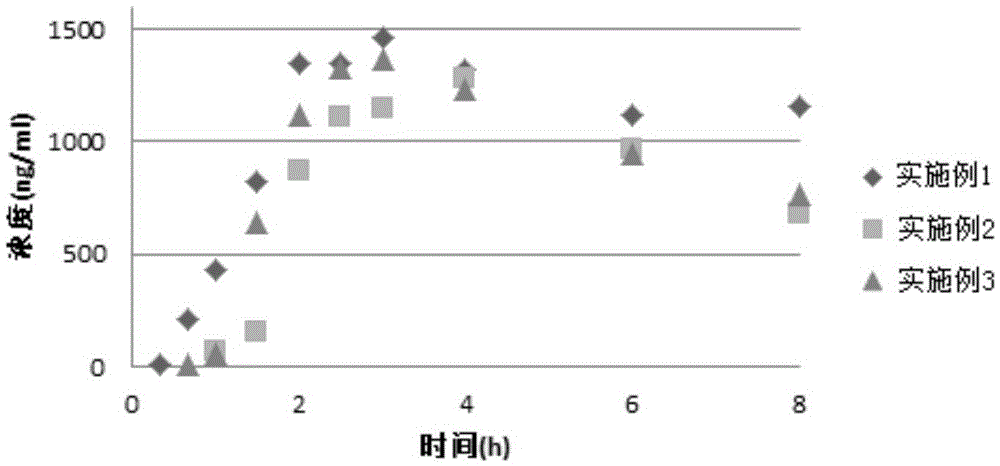
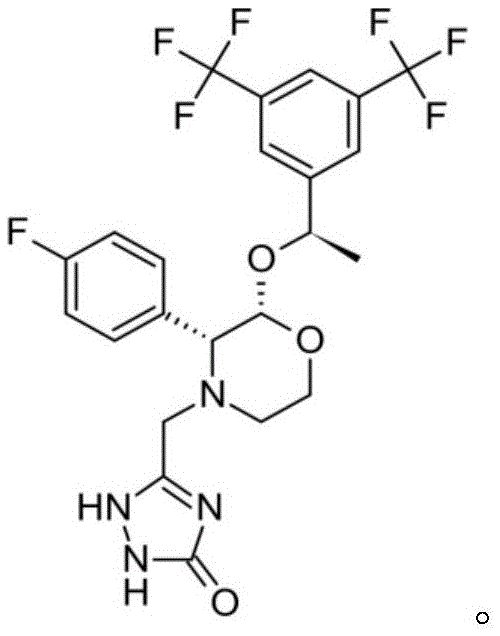
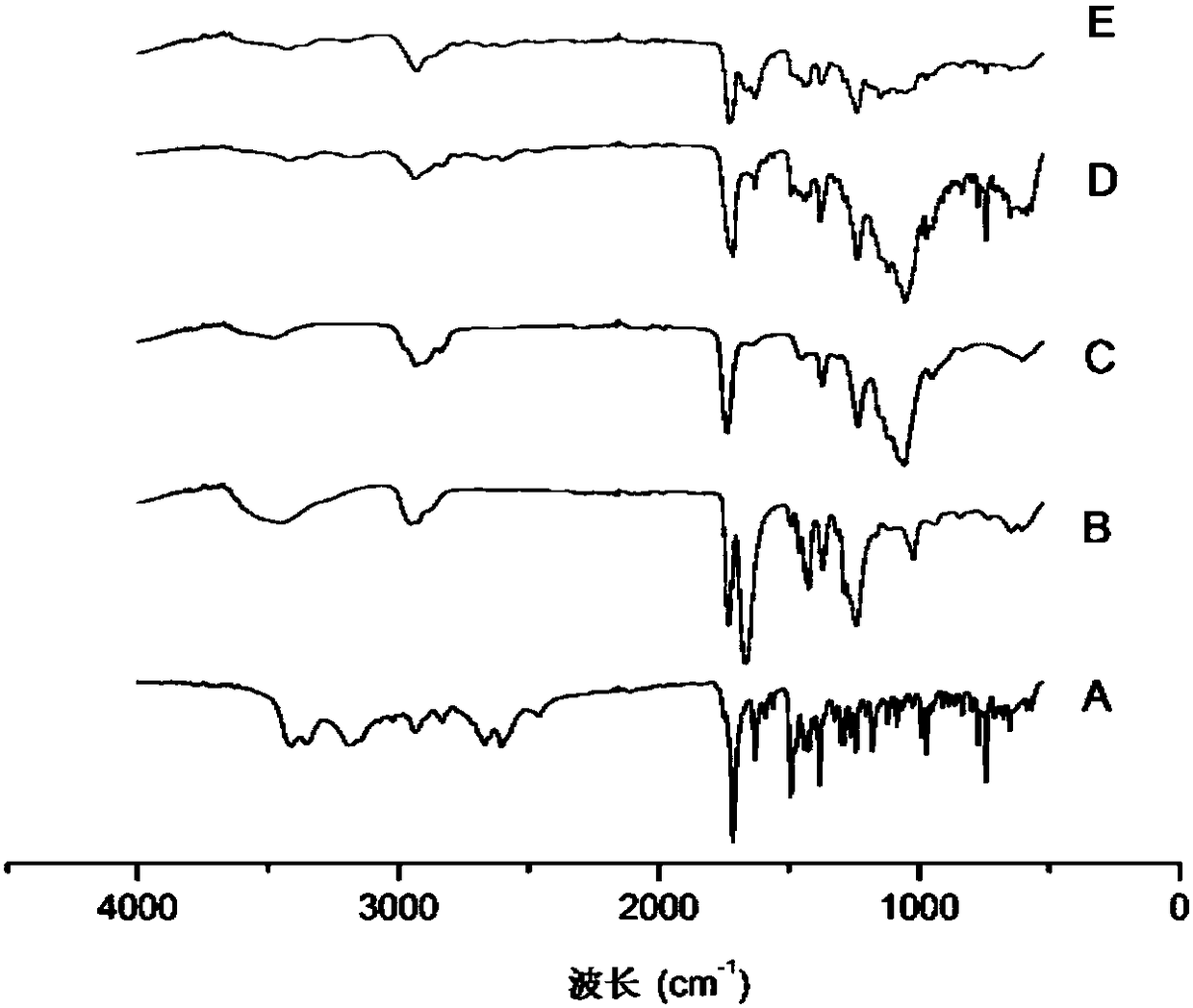
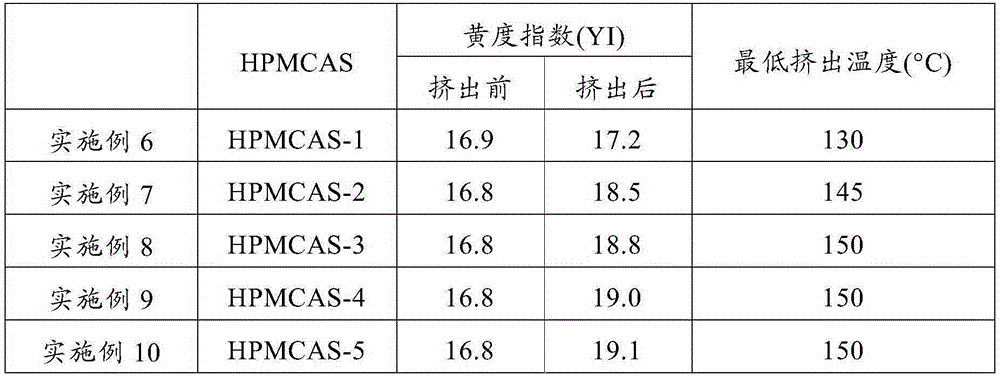
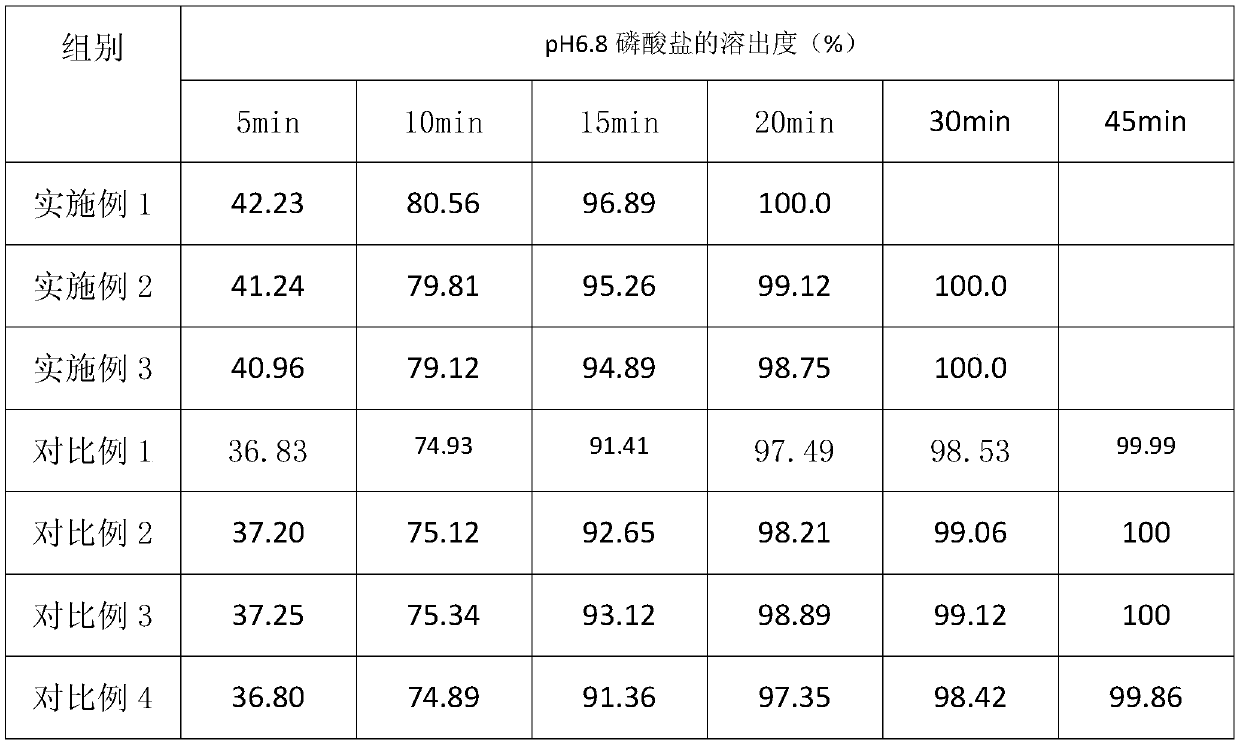
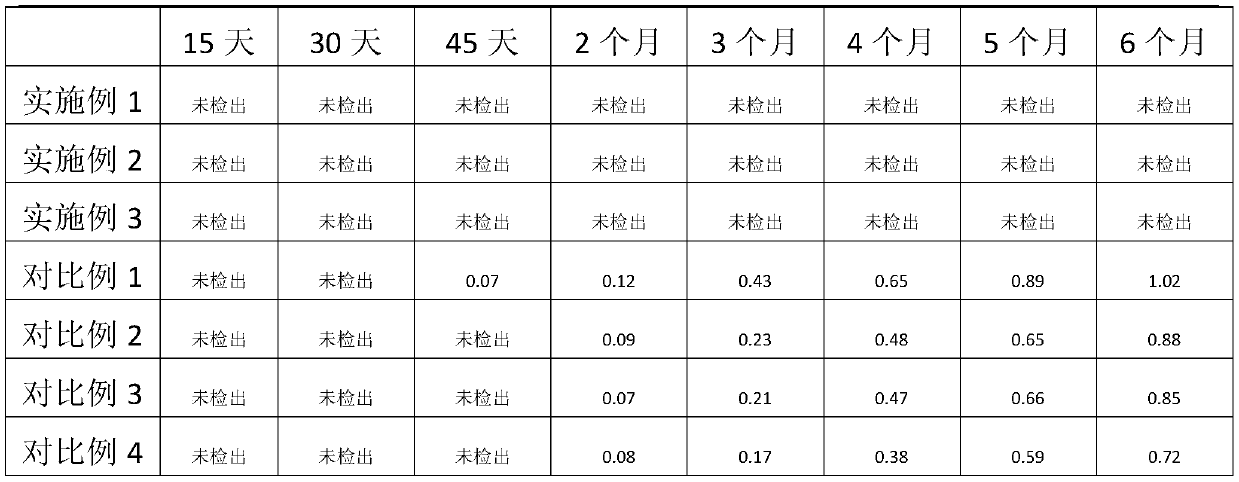
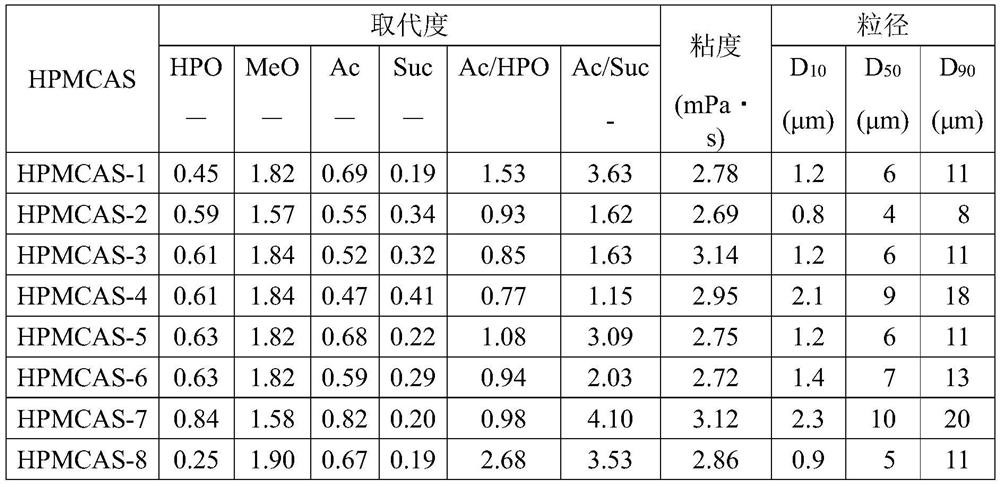
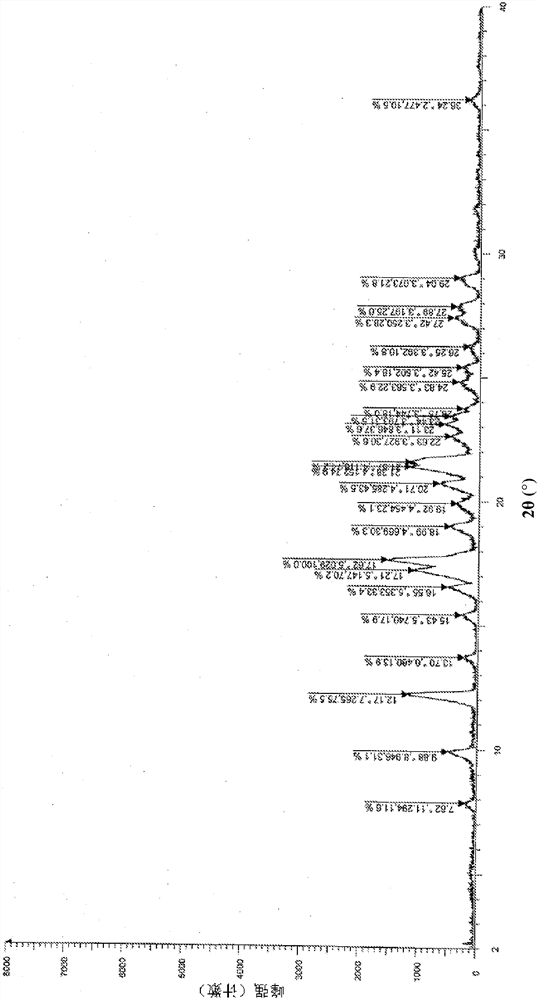
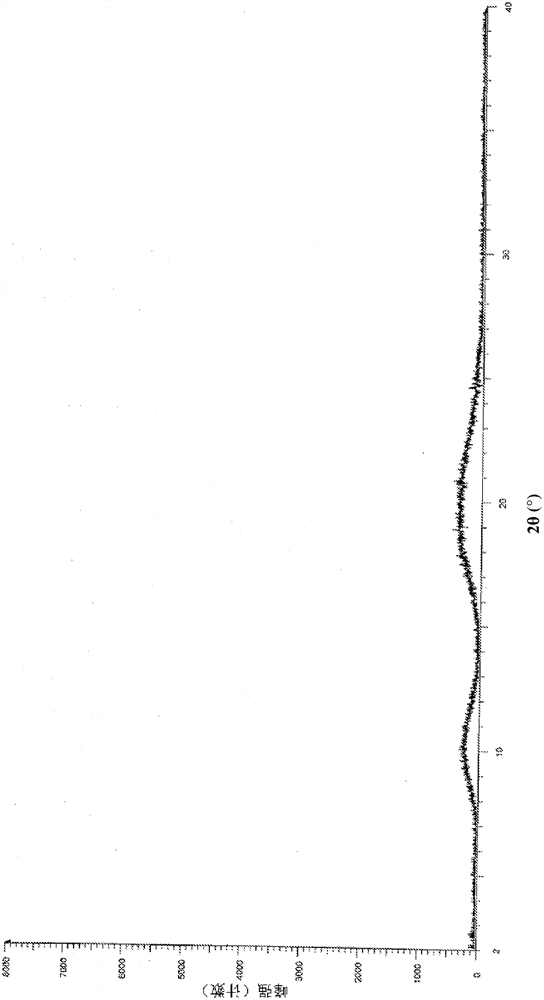
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
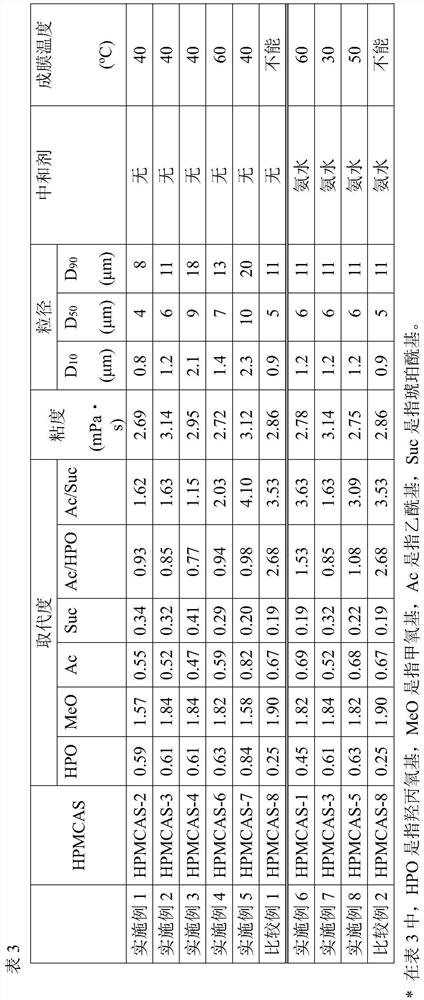
43 results about "Hypromellose acetate succinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
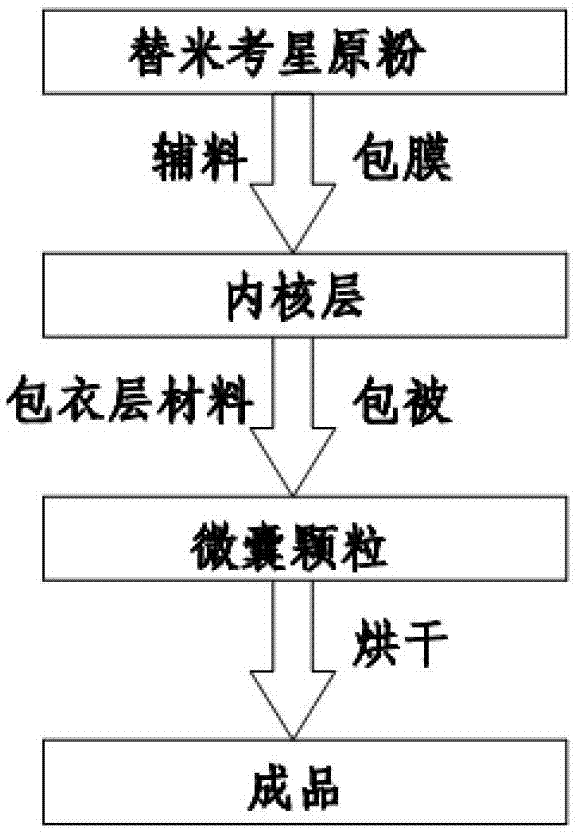
Enteric-coated tilmicosin slow-release micro-capsule preparation and preparation method thereof
InactiveCN103083281AProlong the action timeTo achieve the purpose of sustained releaseAntibacterial agentsOrganic active ingredientsMonoglycerideAcrylic resin
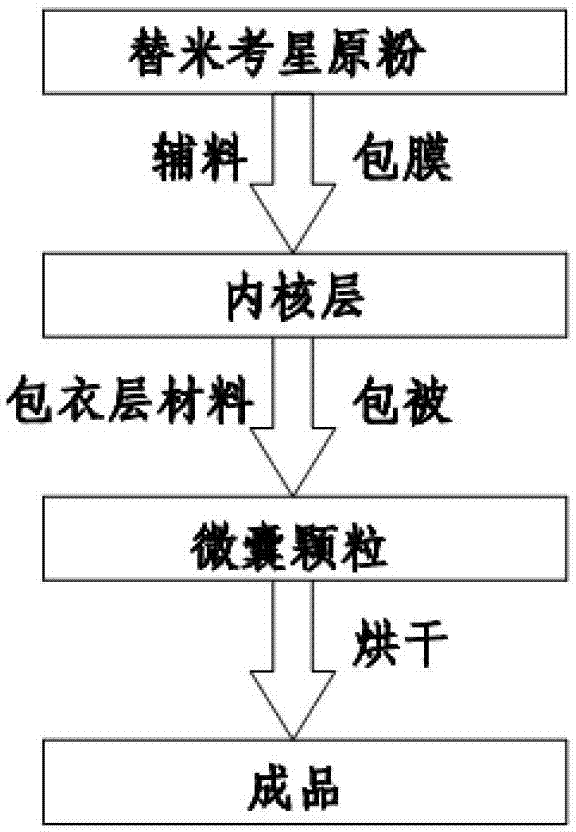
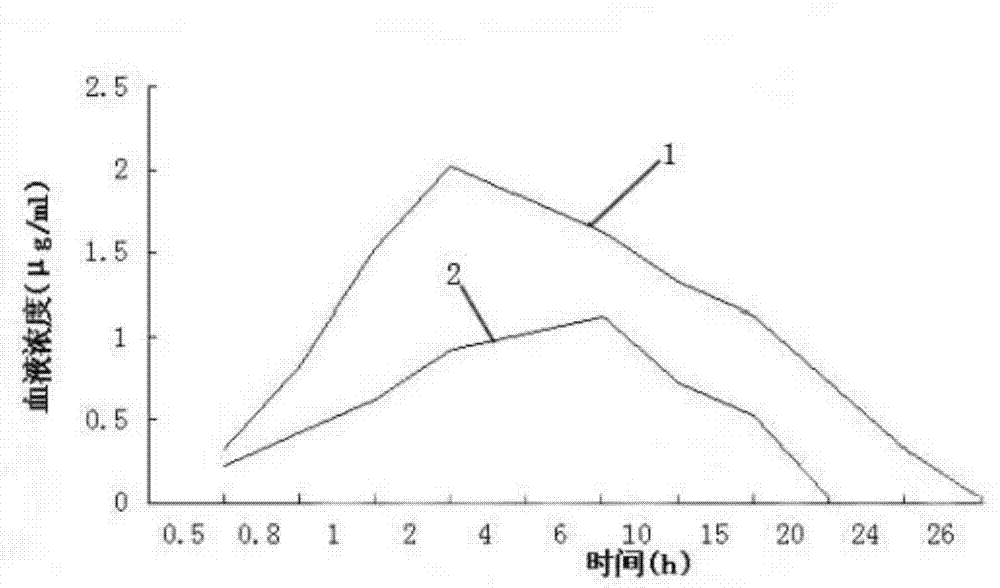
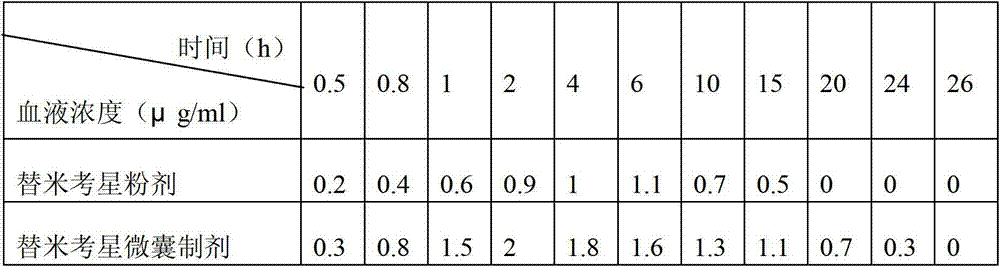
The invention relates to an enteric-coated tilmicosin slow-release micro-capsule preparation and a preparation method thereof and belongs to the field of tilmicosin preparations. The enteric-coated tilmicosin slow-release micro-capsule preparation provided by the invention comprises an inner core layer and a coating layer, wherein the inner core layer comprises tilmicosin raw powder and an auxiliary material; the auxiliary material comprises one or more than one of stearic acid, glycerin monostearate, stearyl alcohol, saturated triglyceride, monoglyceride and paraffin; and the coating layer is made from one or more than one of cellulose acetate phthalate, hydroxypropyl methyl cellulose phthalate, acrylic resin, polyvinyl acetate phthalate and acetic hydroxypropyl methylcellulose succinate. The preparation method comprises the following steps of: carrying out primary coating on the tilmicosin raw powder and the auxiliary material, carrying out secondary coating by using the materials of the coating layer, and drying to obtain the finished product. According to the invention, the tilmicosin is coated by using high polymer materials and the coated tilmicosin micro-capsule is undissolved in acid environment and slowly dissolved in alkaline environment of enteric canal, so that the purpose of slow release is achieved and the action time of the tilmicosin is prolonged.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
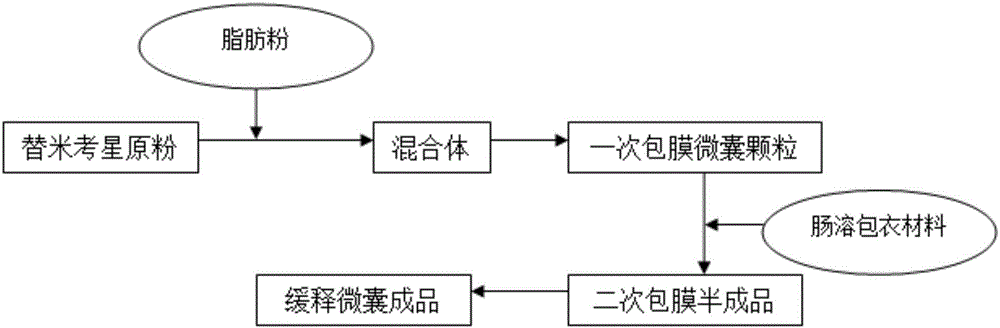
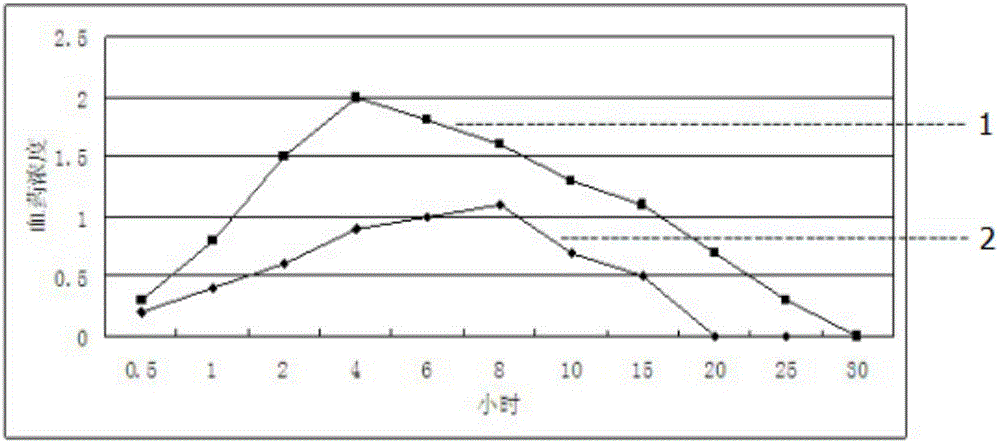
Enteric-coated tilmicosin sustained release microcapsule and preparation method thereof
InactiveCN106176680AProlong the action timeTo achieve the purpose of sustained releaseAntibacterial agentsOrganic active ingredientsDispersityAcrylic resin
The invention discloses an enteric-coated tilmicosin sustained release microcapsule and a preparation method thereof, and belongs to the field of tilmicosin preparations. The enteric-coated tilmicosin sustained release microcapsule is prepared from 10wt%-50wt% of raw tilmicosin powder, 40wt%-88wt% of an auxiliary fatty powder material and 2wt%-10wt% of an enteric coating material, wherein the enteric coating material is prepared from one or more of cellulose acetate phthalate, hydroxypropyl methylcellulose phthalate, L-type acrylic resin, S-type acrylic resin, polyvinyl acetate phthalate and hydroxypropyl methylcellulose acetate succinate; the diameter of the prepared microcapsule is 50-200 mu m. The preparation method comprises the steps as follows: the raw tilmicosin powder and the auxiliary material are subjected to primary coating, are subjected to secondary coating with the enteric coating material and then are dried, and a finished product is obtained. According to the enteric-coated tilmicosin sustained release microcapsule and the preparation method thereof, the sustained release purpose is achieved, the acting time of tilmicosin is prolonged, the fluidity and the dispersity of a drug are improved, the pharmacodynamical function is remarkably improved, and the dosage of the drug is reduced.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
Method for preparing hydroxypropyl methylcellulose acetate succinate
The invention discloses a method for preparing hydroxypropyl methylcellulose acetate succinate. The method comprises the following two steps: an esterification step: adding hydroxypropyl methylcellulose, acetic acid, anhydrous sodium acetate, acetic anhydride, succinic anhydride and a catalyst in proportion to a reaction vessel, and heating and stirring to undergo an esterification reaction; a purification step: after the completion of the reaction, slowly pouring a product into water while stirring, washing the product, crushing and drying to obtain the hydroxypropyl methylcellulose acetate succinate, wherein a three-step temperature raising method is adopted by heating. The method provided by the invention has the advantages of simplicity in industrial production operation, less by-products, mild reaction conditions, and adjustability of viscosity within 100-10000mpa.s, and is suitable for different application requirements.
Owner:ANHUI SUNHERE PHARMA EXCIPIENTS
Preparation method of taste-masked suspension granules of Gegenqinlian decoction
ActiveCN102309562AGood taste masking effectSimple processAntibacterial agentsAntipyreticAdhesiveHypromellose phthalate
The invention relates to a preparation method of taste-masked suspension granules of Gegenqinlian decoction. The preparation method comprises the following steps of 1, taking appropriate amounts of dispensing granules of three traditional Chinese medicines comprising radix puerariae, coptis chinensis and scutellaria baicalensis and respectively carrying out coating processes in a fluidized bed through adopting one or more polymers as coating materials to obtain coated granules for next use, 2, taking an appropriate amount of at least one suspending agent, mixing uniformly the at least one suspending agent and radix glycyrrhizae preparata dispensing granules, then adding an appropriate amount of an adhesive into the mixture to prepare into granules by a wet method, drying the prepared granules in an oven, and then spraying an appropriate amount of an ethanol solution as an aromatic to obtain suspending agent-containing radix glycyrrhizae preparata dispensing granules after ethanol is volatilized, and 3, weighing appropriate amounts of the suspending agent-containing radix glycyrrhizae preparata dispensing granules and the coated granules containing radix puerariae, coptis chinensisand scutellaria baicalensis, mixing well, and carrying out sub-packaging to obtain the taste-masked suspension granules of Gegenqinlian decoction, wherein the one or more polymers as coating materials are selected from enteric-coated polyacrylic resin, hypromellose acetate succinate and hydroxypropyl methylcellulose phthalate. The invention provides the preparation method of the taste-masked suspension granules of Gegenqinlian decoction. The preparation method is also suitable for taste masking of a traditional Chinese medicine with a bitter taste or a fishy smell.
Owner:安徽天祥药业有限公司
Method for preparing hydroxypropyl methylcellulose acetate succinate
The invention relates to a method for preparing hydroxypropyl methylcellulose acetate succinate, and belongs to the field of chemical synthesis. The method comprises the following steps of: (1) preparing 100 weight parts of hydroxypropyl methylcellulose, 10 to 50 weight parts of anhydrous sodium acetate and 20 to 60 weight parts of glacial acetic acid, mixing, and adding 25 to 75 weight parts of succinic anhydride and 30 to 50 weight parts of acetic anhydride; (2) performing microwave radiation on the mixture obtained in the step (1) for 4 to 7 minutes under the power of 400 to 750 kw, and cooling naturally to the temperature of between 50 and 70 DEG C to obtain a reaction product; (3) pouring the reaction product into water, and after precipitating the reaction product completely, washing and performing suction filtration until precipitated liquid is neutral; and (4) drying filter cakes obtained by the suction filtration under vacuum to obtain the hydroxypropyl methylcellulose acetate succinate. The method is high in speed of acylation reaction, short in reaction time and few in byproducts.
Owner:美信佳中维药业股份有限公司
Aprepitant oral pharmaceutical preparation
InactiveCN105534987ADissolution rate is fastImprove bioavailabilityOrganic active ingredientsDigestive systemLow-substituted hydroxypropylcelluloseHypromellose phthalate
The invention discloses an aprepitant oral pharmaceutical preparation. The aprepitant oral pharmaceutical preparation comprises 15wt%-25wt% of aprepitant, 45wt%-75wt% of hydroxypropyl methylcellulose phthalate / hydroxypropyl methylcellulose acetate succinate, 10wt%-25wt% of microcrystalline cellulose, lactose or mannitol, 2wt%-8wt% of low-substituted hydroxypropyl cellulose as well as croscarmellose sodium and / or crospovidone, 0-2wt% of silicon dioxide and / or talc and 0-2wt% of magnesium stearate. The pharmaceutical preparation can be prepared in a form of tablets or capsules andhas high stability and good bioavailability.
Owner:北京颐诺赛医药科技有限公司
Ziprasidone hydrochloride solid dispersible tablets and hot melt extrusion method thereof
ActiveCN108078934AObvious retentionExtended release timeOrganic active ingredientsNervous disorderTherapeutic effectGlass transition
The invention discloses ziprasidone hydrochloride solid dispersible tablets and a hot melt extrusion method thereof. Based on ziprasidone hydrochloride as a main component, one or two of mixed carriers of copolyvidone S630 or HPMCAS-HF (hydroxypropyl methylcellulose acetate succinate) as a base, a coating material is added, and the glass transition temperature of an extruded mixture can also be reduced; then, a surfactant is added. The ziprasidone hydrochloride solid dispersible tablets are prepared through steps as follows: the raw and auxiliary materials are evenly mixed by a three-dimensional mixing machine, extruded by a hot melt extruder at a high temperature and ground into powder with proper size; the powder is then mixed with a fixed quantity of filler, disintegrant and lubricant sufficiently and uniformly, and direct tableting is preformed, and the ziprasidone hydrochloride solid dispersible tablets are prepared. The prepared ziprasidone hydrochloride solid dispersible tabletshave the advantages that the problem of poor in-vitro dissolution of ziprasidone hydrochloride is solved, bioavailability of ziprasidone hydrochloride under the condition of empty stomach is improved, and the treatment effect of the tablets is improved finally.
Owner:江苏省药物研究所有限公司
Posaconazole solid dispersion composition capable of inhibiting separation by crystallization as well as preparation method thereof
ActiveCN108125921AStrong ability to inhibit crystallizationPromote absorption in the bodyAntibacterial agentsOrganic active ingredientsAcrylic resinActive component
The invention discloses a posaconazole solid dispersion composition capable of inhibiting separation by crystallization as well as a preparation method thereof, and relates to the field of posaconazole preparations. The posaconazole solid dispersion composition contains acrylic resin and posaconazole serving as an active component. The research of the invention discovers that compared with hydroxypropyl methylcellulose acetate succinate (HPMCAS) serving as a carrier material, the posaconazole solid dispersion composition taking acrylic resin as a carrier material has the following advantages:after a posaconazole crystal seed is induced in the intestinal environment simulated pH 6.8 dissolution test, the crystallization inhibiting capability is higher and the medicine can be absorbed in vivo better.
Owner:GUANGZHOU BOSITAO CONTROLLED RELEASE PHARMA CO LTD
Composition for heat melt extrusion and method for producing heat melt extruded product using same
ActiveCN105283203APromote absorptionEnhanced bioabsorbabilityPowder deliveryOrganic active ingredientsPolymer scienceHot melt
Provided is a composition for heat melt extrusion, which contains at least a medicament and a hypromellose acetate succinate (HPMCAS) that has a degree of molar substitution of hydroxypropoxy groups of 0.40 or more and a ratio (molar ratio) of acetyl groups to succinyl groups of less than 1.6. Also provided is a method for producing a heat melt extruded product, which comprises at least a step wherein a composition for heat melt extrusion containing at least a medicament and a hypromellose acetate succinate that has a degree of molar substitution of hydroxypropoxy groups of 0.40 or more and a ratio (molar ratio) of acetyl groups to succinyl groups of less than 1.6 is heated, melted and extruded at a heat melt temperature that is not less than the melting temperature of the hypromellose acetate succinate or not less than the temperature at which both the hypromellose acetate succinate and the medicament are melted.
Owner:SHIN ETSU CHEM IND CO LTD
Preparation method of azilsartan tablets
ActiveCN111096955AHigh dissolution rateGuaranteed stabilityOrganic active ingredientsPharmaceutical non-active ingredientsEthylic acidMagnesium stearate
The invention relates to a preparation method of azilsartan tablets. The preparation method comprises the steps of dissolving hydroxypropyl cellulose and copovidone S630 with ethanol to obtain a solution 1 of which the concentration is 3%-8%; putting corn starch, Macrogol 6000, microcrystalline cellulose PH101, azilsartan, lactose and 40% of low substituted hydroxypropy cellulose in a wet granulation machine; performing premixing, adding the solution 1, performing stirring, and performing granulation; placing prepared granules in a multi-functional fluidized bed, performing drying, wherein thetemperature of inlet wind is 60-65 DEG C, performing sampling for moisture detection, and if moisture is smaller than 4 %, stopping heating; taking out the dried granules, adding 50% of hydroxypropylmethylcellulose acetate succinate, uniformly mixing the remaining low substituted hydroxypropy cellulose and magnesium stearate, and performing tabletting; and compounding a film coating premix agentinto a coating solution of which the mass concentration is 13%, with purified water, adding the remaining hydroxypropyl methylcellulose acetate succinate to the obtained coating solution, and performing coating on the prepared tablets.
Owner:北京阳光诺和药物研究股份有限公司
Enteric coating composition, solid preparation and method for producing solid preparation
PendingCN111789960ASolve operational problemsPharmaceutical non-active ingredientsCoatingsMedicineEthylic acid
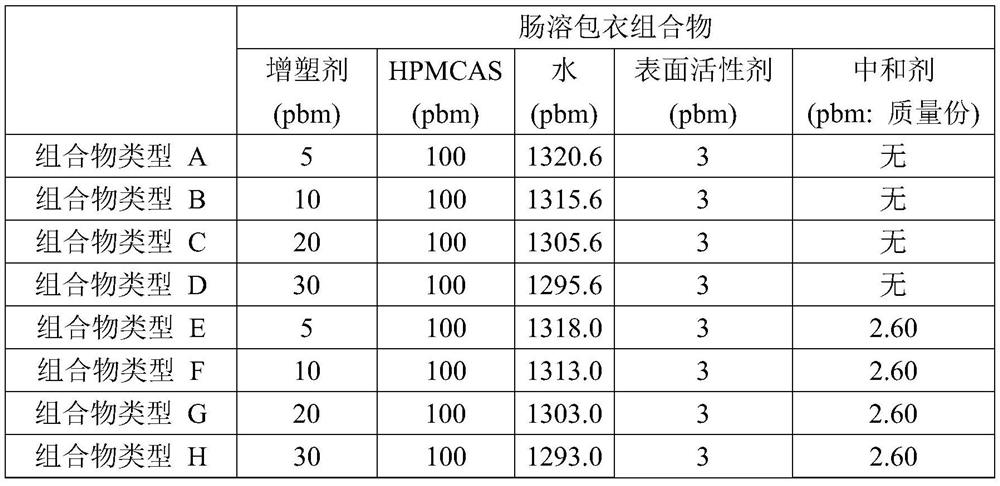
There are provided an enteric coating composition having an excellent film-forming property, being capable of forming a film at a lower temperature than conventional compositions, and / or being capableof avoiding decomposition of a drug due to a high temperature and operational troubles due to or nozzle clogging; and others. More specifically, there are provided an enteric coating composition containing hypromellose acetate succinate having a molar substitution of hydroxypropoxy groups of 0.40 or more, and water; a method for producing a solid preparation including steps of coating a drug-containing core with the enteric coating composition to obtain a coating layer, and drying the coating layer; and a solid preparation containing a drug-containing core, and a coating layer directly or indirectly on the core, the coating layer containing a hypromellose acetate succinate having a molar substitution of hydroxypropoxy groups of 0.40 or more.
Owner:SHIN ETSU CHEM CO LTD
Solution for spray drying comprising hypromellose acetate succinate and method for producing solid dispersion
ActiveUS20160136283A1High light transmittanceReduce generationPowder deliveryOrganic active ingredientsHypromelloseSolvent
There are provided a solution for spray drying having a high transmittance and markedly reduced generation of undissolved matter; and a method for producing a solid dispersion by using the solution for spray drying so that clogging with the undissolved matter is reduced and dissolution is improved. More specifically, there is provided a solution for spray drying comprising hypromellose acetate succinate (HPMCAS) having a hydroxypropoxy molar substitution of 0.40 or more, a solvent, and a drug. There is also provided a method for producing a solid dispersion comprising the step of removing the solvent from the spray drying solution.
Owner:SHIN ETSU CHEM IND CO LTD
Hypromellose acetate succinate for use as hot-melt extrusion carrier, hot-melt extrusion composition, and method for producing hot-melt extrudate
Provided are hypromellose acetate succinates (HPMCAS) for use as a hot-melt extrusion carrier having a volume average particle size (D50) of from 70 to 300 as measured by dry laser diffraction and a loose bulk density of from 0.25 to 0.40 g / cm3; and a hot-melt extrusion composition comprising the HPMCAS and a drug. Also provided is a method for producing a hot-melt extrudate including the steps of: hot-melting the hot-melt extrusion composition at a hot-melt temperature equal to or higher than a melting temperature of the HPMCAS, or at a hot-melt temperature equal to or higher than a temperature at which both of the HPMCAS and the drug become melt; and extruding the hot-melted composition.
Owner:SHIN ETSU CHEM IND CO LTD
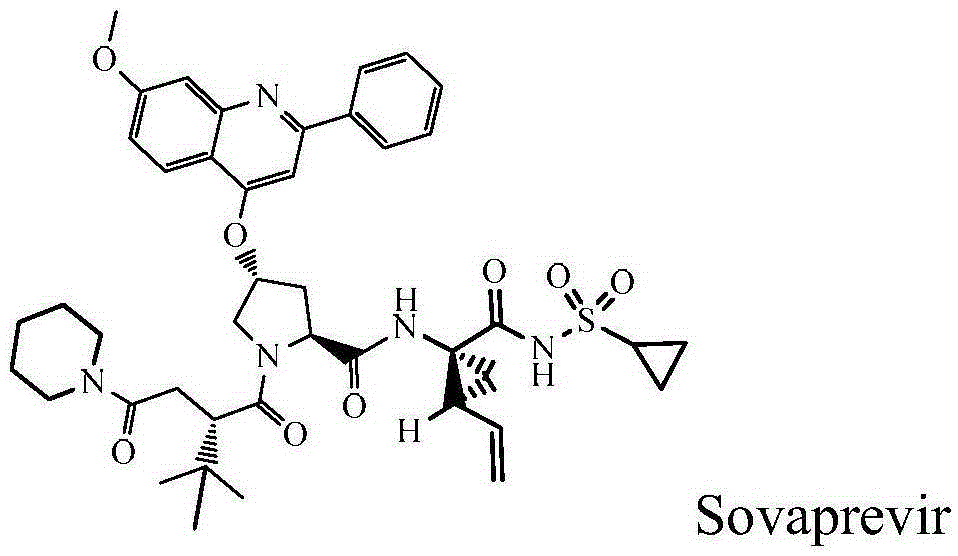
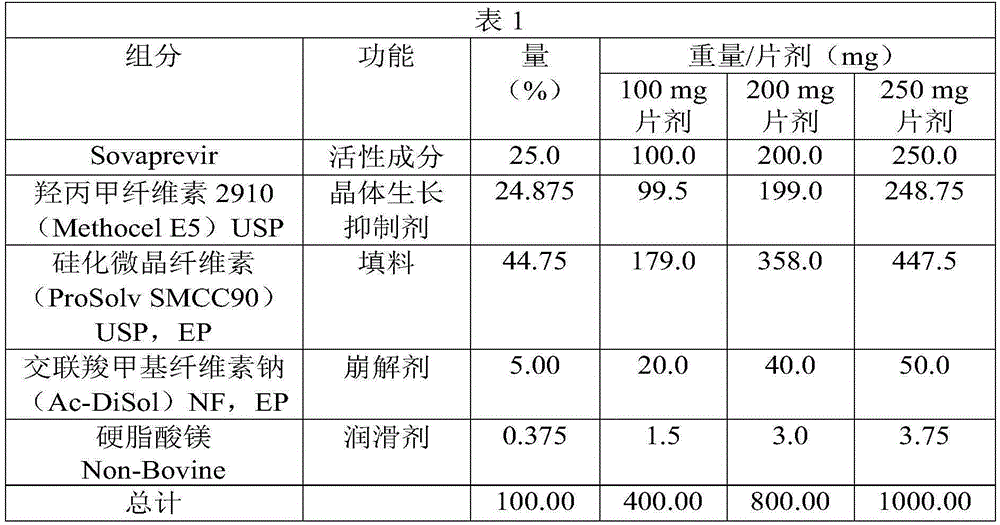
Sovaprevir tablets
The disclosure includes tablet cores comprising Sovaprevir and a crystal growth inhibitor selected from hydroxypropyl methyl cellulose (HPMC), HPC (hydroxypropyl cellulose), hypromellose acetate succinate (HPMCAS), polyvinyl pyrrolidone (PVP), copovidone (PVP-VA), a copolymer of methacrylic acid and ethyl acrylate, or any combination of the foregoing, wherein ratio of Sovaprevir to crystal growth inhibitor is from about 40:60 (w / w) to about 60:40 (w / w). The disclosure further includes film coated tables. The disclosure also includes methods of treating HCV with the tablets described herein.
Owner:ACHILLION PHARMA INC
Antifungal drug posaconazole solid dispersion, preparation method and application
InactiveCN106265526AIncrease dissolution rateImprove bioavailabilityPowder deliveryOrganic active ingredientsAntifungalAlcohol
The invention discloses an antifungal drug posaconazole solid dispersion, a preparation method and an application. The solid dispersion comprises posaconazole and an enteric-coating material, wherein the enteric-coating material is hydroxypropyl methylcellulose acetate succinate. A formulated amount of the posaconazole and the hydroxypropyl methylcellulose acetate succinate is weighed, mixed and added into mixed solution with one or two of acetone or ethyl alcohol solution, and spin flash drying is performed after dissolution to obtain the posaconazole solid dispersion. The dissolution rate of the posaconazole can be increased by the hydroxypropyl methylcellulose acetate succinate, absorption of the posaconazole is promoted, and bioavailability of the posaconazole is improved.
Owner:SHANDONG UNIV
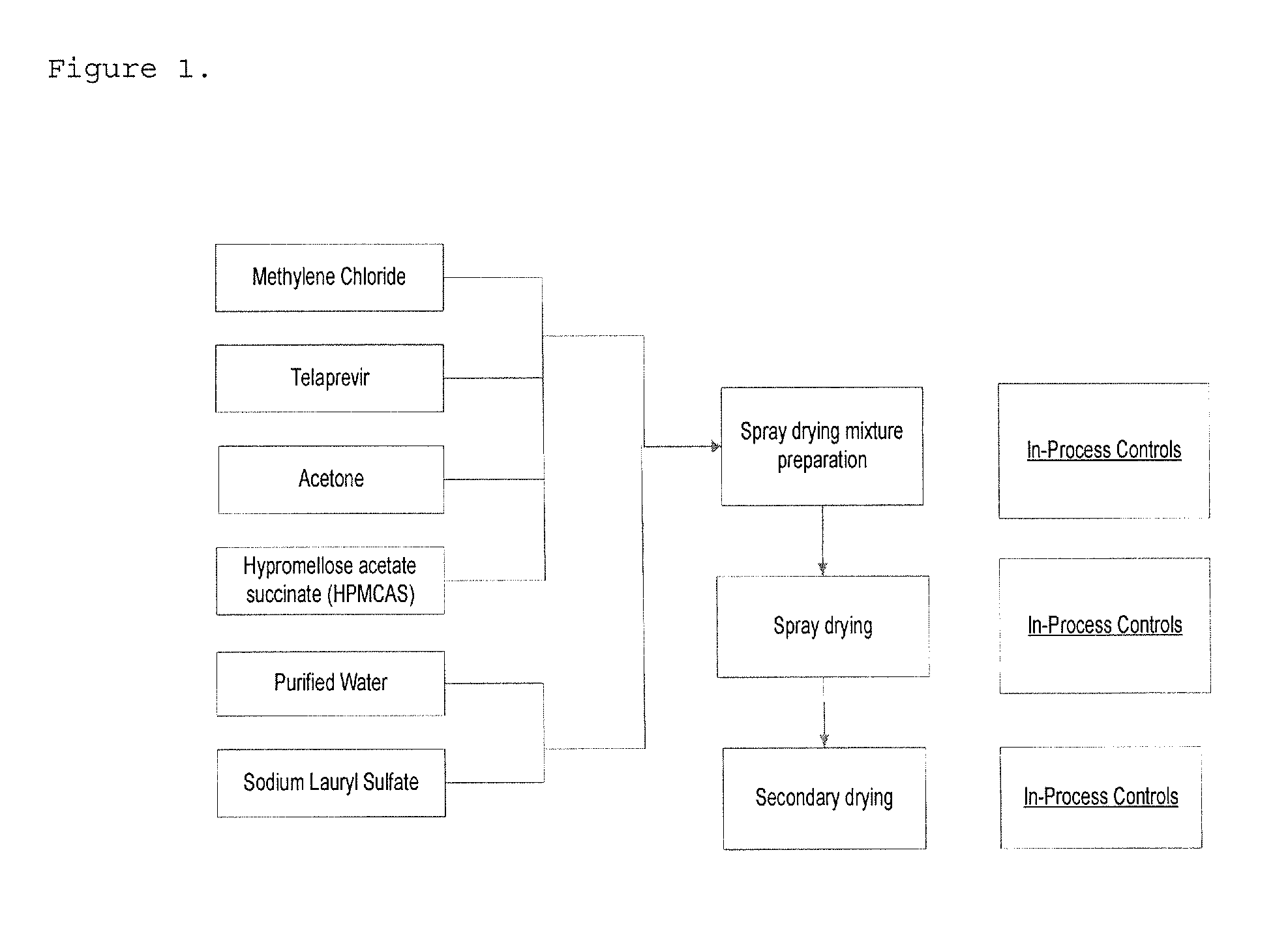
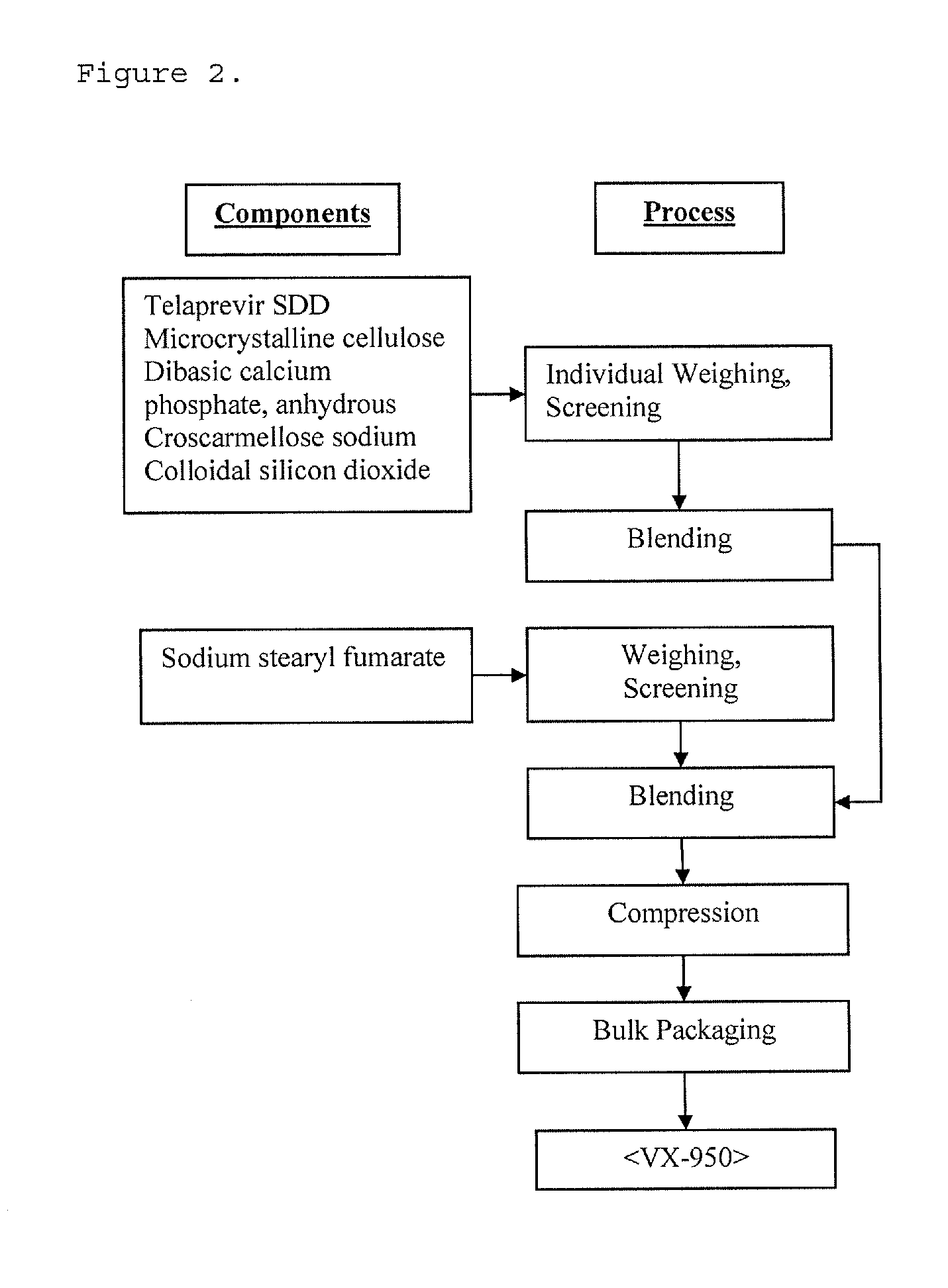
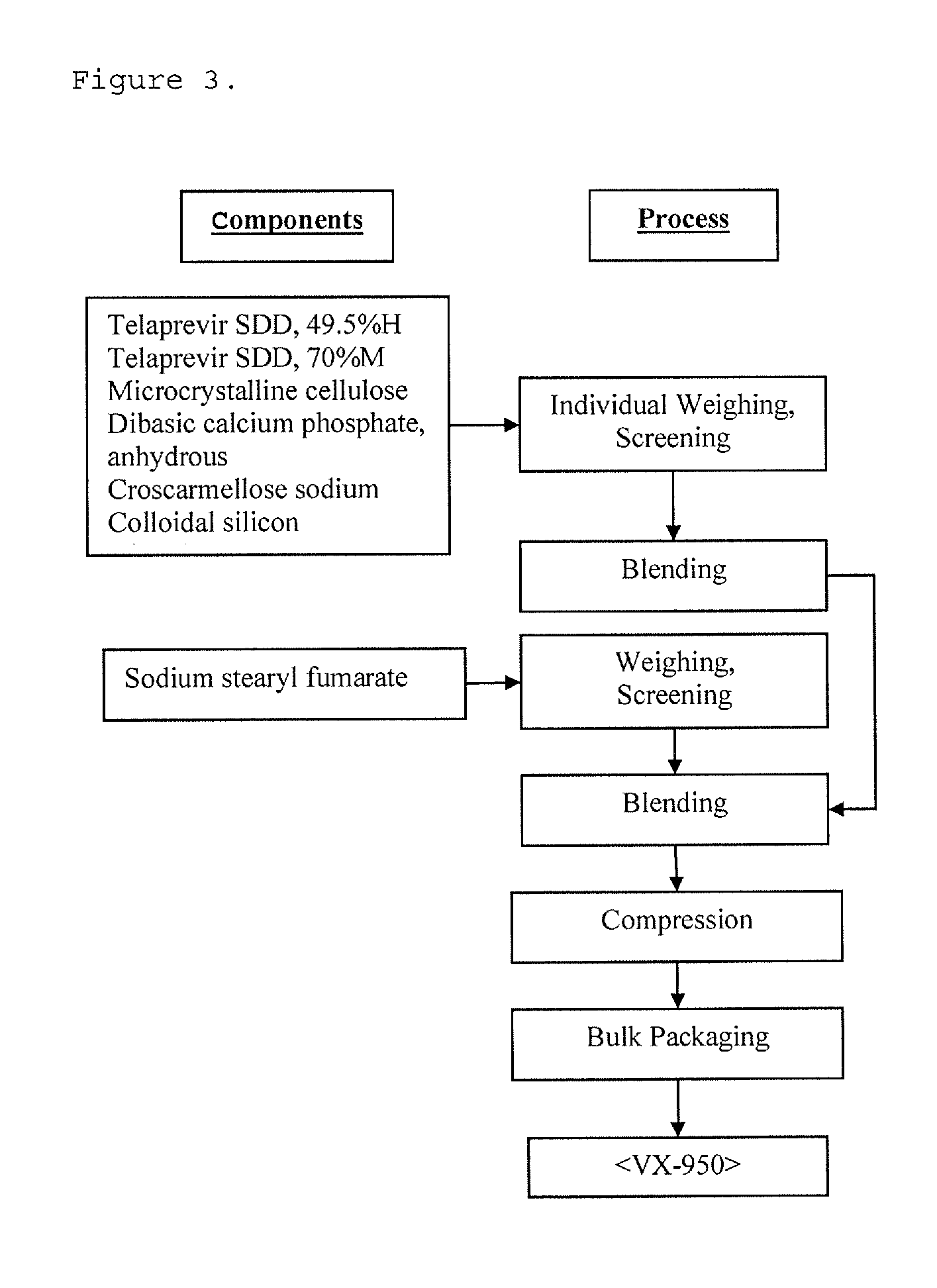
High potency formulations of vx-950
InactiveUS20130195797A1Decreases amount of crystallization and rate of crystallizationOrganic active ingredientsBiocideSulfateNuclear chemistry
High potency pharmaceutical compositions comprising VX-950, sodium lauryl sulfate and a polymer selected from the group consisting of hypromellose acetate succinate-M, hypromellose acetate succinate-L and hypromellose acetate succinate-H.
Owner:VERTEX PHARMA INC
Composition for enteric hard capsule and method for producing enteric hard capsule
ActiveUS20180015045A1Improve acid resistanceHigh mechanical strengthPharmaceutical non-active ingredientsCapsule deliveryHard CapsuleHypromellose
A composition for an enteric hard capsule by taking advantage of conventionally unknown thermal gelation characteristics of a neutralized aqueous solution of an enteric polymer, so as to obtain an enteric capsule having sufficient water and acid resistances. More specifically, the composition has hypromellose acetate succinate having a molar substitution with an acetyl group per anhydroglucose unit of 0.6 to 0.8 and a ratio of the molar substitution with an acetyl group to a molar substitution with a succinyl group per anhydroglucose unit of 2.0 to 4.0, a neutralizer, and water method produces an enteric hard capsule having the steps of: immersing a core pin heated at 50 to 80° C. in the composition, taking the immersed core pin out of the composition, and drying a gel layer of the hypromellose acetate succinate formed on the taken-out core pin.
Owner:SHIN ETSU CHEM IND CO LTD
Composition for enteric hard capsule and method for producing enteric hard capsule
ActiveUS11141381B2Solve the lack of resistanceIncrease resistancePharmaceutical non-active ingredientsCapsule deliveryHard CapsuleEthylic acid
A composition for an enteric hard capsule by taking advantage of conventionally unknown thermal gelation characteristics of a neutralized aqueous solution of an enteric polymer, so as to obtain an enteric capsule having sufficient water and acid resistances. More specifically, the composition has hypromellose acetate succinate having a molar substitution with an acetyl group per anhydroglucose unit of 0.6 to 0.8 and a ratio of the molar substitution with an acetyl group to a molar substitution with a succinyl group per anhydroglucose unit of 2.0 to 4.0, a neutralizer, and water method produces an enteric hard capsule having the steps of: immersing a core pin heated at 50 to 80° C. in the composition, taking the immersed core pin out of the composition, and drying a gel layer of the hypromellose acetate succinate formed on the taken-out core pin.
Owner:SHIN ETSU CHEM IND CO LTD
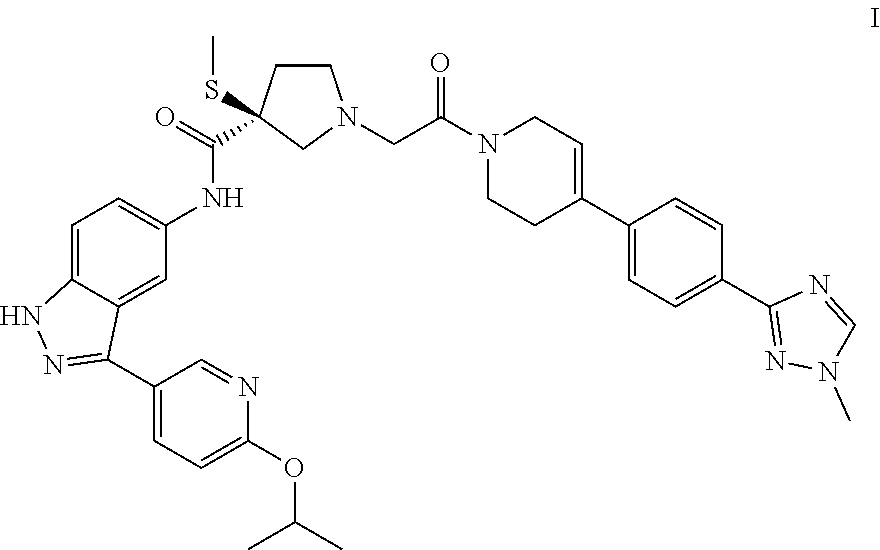
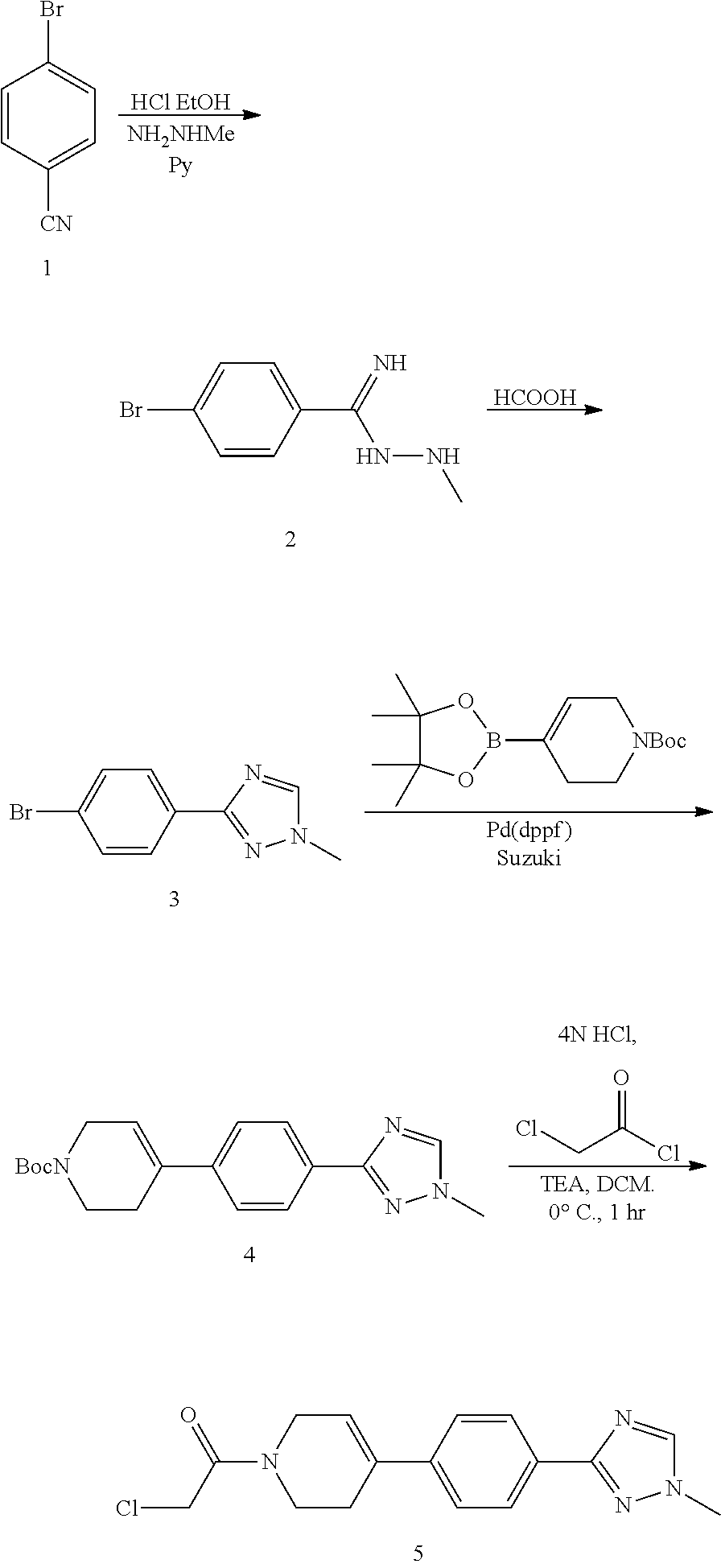
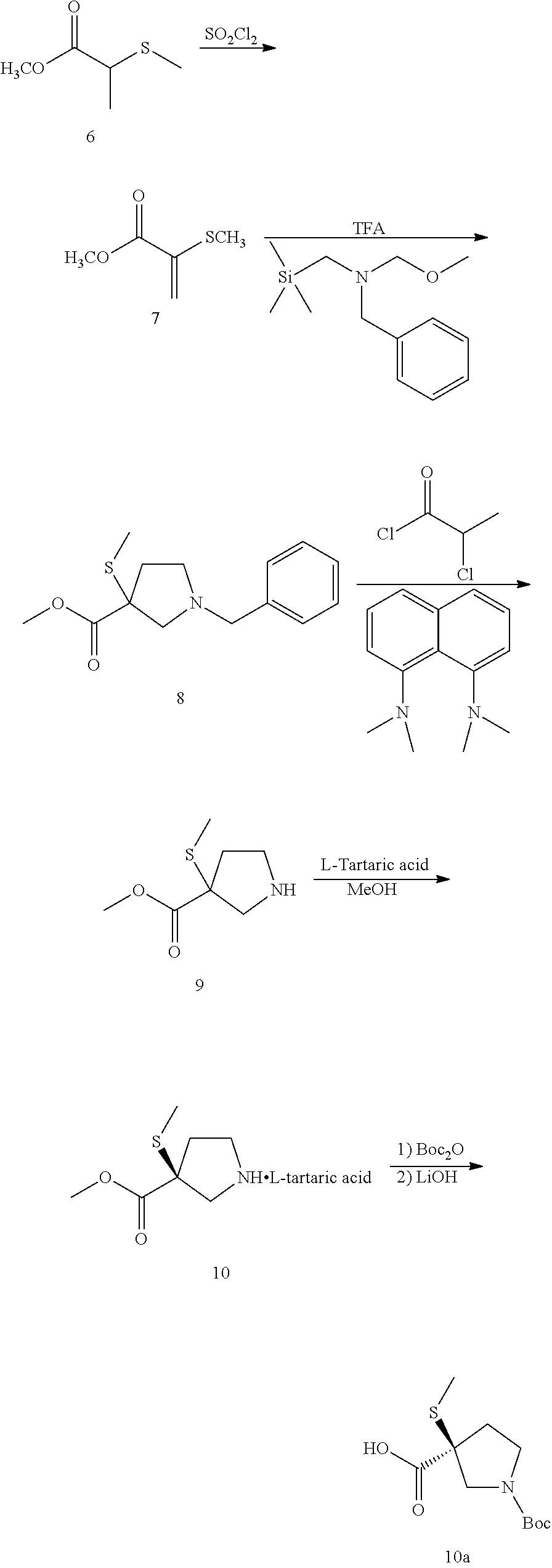
Process for preparing spray dried solid dispersions of (s)-n-(3-(6-isopropoxypyridin-3-yl)-1h-indazol-5-yl)-1-(2-(4-(4-(1-methyl-1h-1,2,4-triazol-3-yl)phenyl)-3,6-dihydropyridin-1(2H)-yl)-2-oxoethyl)-3-(methylthio)pyrrolidine-3-carboxamide for pharmaceutical preparations
A process for preparing spray dried solid dispersions of (S)—N-(3-(6-isopropoxypyridin-3-yl)-1H4ndazol-5-yl)-1-(2-(4-(4-(1-methyl-1H-1,2,4-triazol-3-yl)phenyl)-3,6-dihydropyridin-1(2H)-yl)-2-oxoethyl)-3-(methylthio)pyrrolidine-3-carboxamide amorphous free base and hypromellose acetate succinate for pharmaceutical preparations including pharmaceutical capsule preparations.
Owner:MERCK SHARP & DOHME CORP
Enteric-coated tilmicosin slow-release micro-capsule preparation and preparation method thereof
InactiveCN103083281BProlong the action timeTo achieve the purpose of sustained releaseAntibacterial agentsOrganic active ingredientsMonoglycerideAcrylic resin
The invention relates to an enteric-coated tilmicosin slow-release micro-capsule preparation and a preparation method thereof and belongs to the field of tilmicosin preparations. The enteric-coated tilmicosin slow-release micro-capsule preparation provided by the invention comprises an inner core layer and a coating layer, wherein the inner core layer comprises tilmicosin raw powder and an auxiliary material; the auxiliary material comprises one or more than one of stearic acid, glycerin monostearate, stearyl alcohol, saturated triglyceride, monoglyceride and paraffin; and the coating layer is made from one or more than one of cellulose acetate phthalate, hydroxypropyl methyl cellulose phthalate, acrylic resin, polyvinyl acetate phthalate and acetic hydroxypropyl methylcellulose succinate. The preparation method comprises the following steps of: carrying out primary coating on the tilmicosin raw powder and the auxiliary material, carrying out secondary coating by using the materials of the coating layer, and drying to obtain the finished product. According to the invention, the tilmicosin is coated by using high polymer materials and the coated tilmicosin micro-capsule is undissolved in acid environment and slowly dissolved in alkaline environment of enteric canal, so that the purpose of slow release is achieved and the action time of the tilmicosin is prolonged.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
Double-layer film agent for treating oral ulcer and preparation method thereof
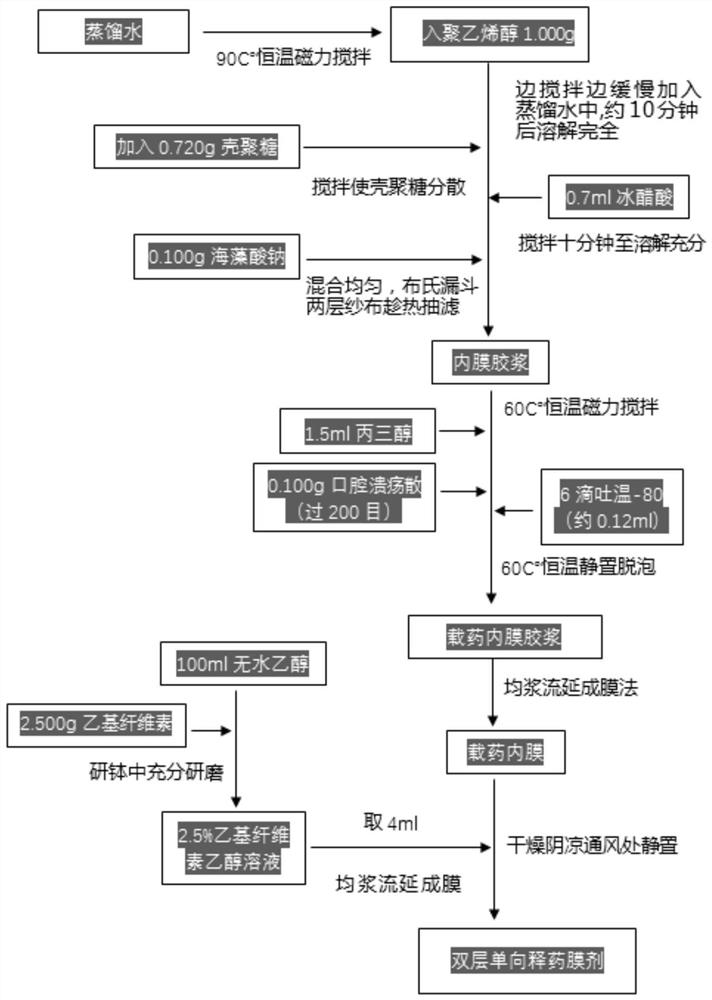
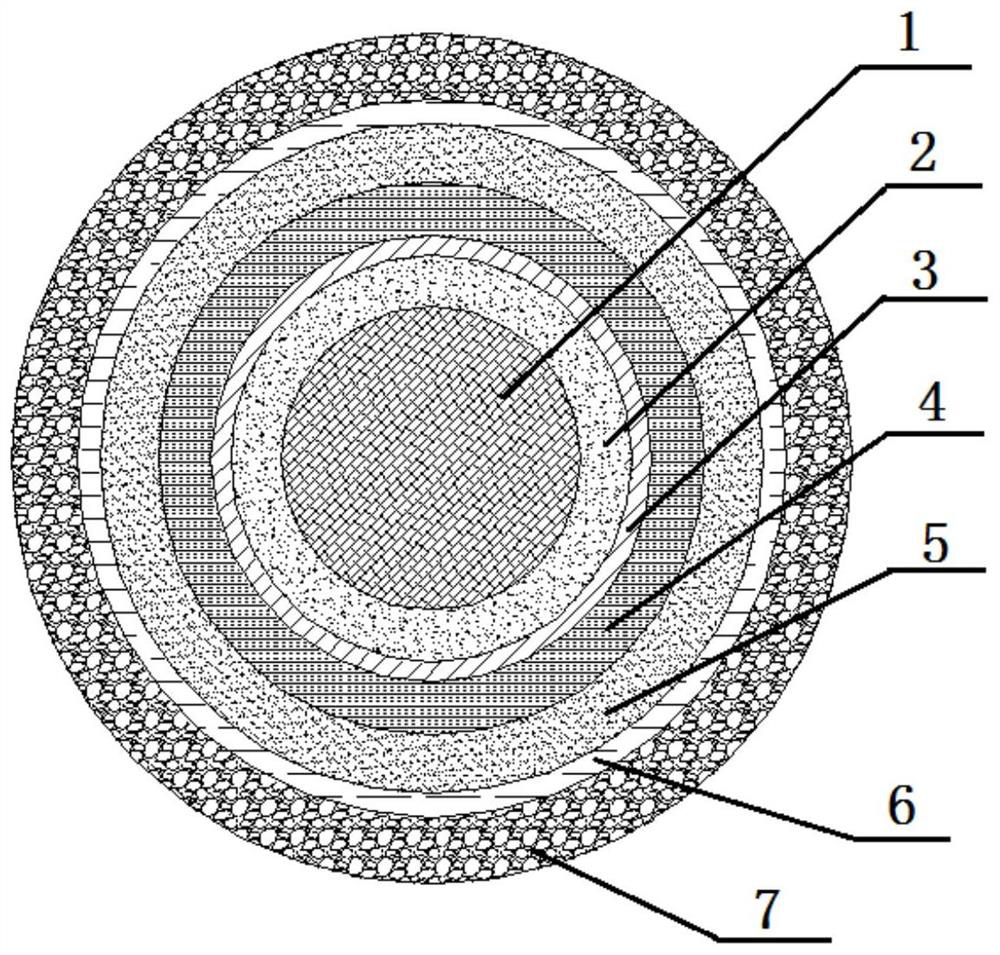
PendingCN113616627AFacilitate dissolution and precipitationReduce stimulationHydroxy compound active ingredientsDigestive systemCelluloseEthylic acid
The invention discloses a double-layer film agent for treating oral ulcer and a preparation method thereof. The double-layer film agent for treating oral ulcer is a double-layer one-way drug release film agent, and comprises a porous drug-loaded inner film and a waterproof outer film; the drug-loaded inner film comprises 1-60 parts of chitosan, 1-37 parts of sodium alginate and 1-7 parts of polyvinyl alcohol; the waterproof outer film is prepared by dissolving any one or a combination of more of ethyl cellulose, hydroxyethyl methylacrylate, a methyl methacrylate copolymer and hydroxypropyl methylcellulose acetate succinate in absolute ethyl alcohol, and the concentration is 1%-5%. The double-layer film agent solves the problems that OTC (Over The Count) Chinese patent medicine oral ulcer powder is inconvenient to use, drugs are easy to lose, and the action time of one-time medication is short. In addition, the invention further provides a preparation method of the double-layer film agent for treating oral ulcer.
Owner:GUANGDONG MEDICAL UNIV
Hypromellose acetate succinate and method for producing the same
ActiveUS10888525B2Avoid stickingOrganic active ingredientsPharmaceutical non-active ingredientsSodium acetateCellulose
There are provided HPMCAS (hypromellose acetate succinate) having such a property that a solution of the HPMCAS in a solvent has a controlled viscosity; and a method for producing the HPMCAS. More specifically, provided are HPMCAS having such property that a solution of 10 parts by weight of the HPMCAS in 100 parts by weight of a mixed solvent having a weight ratio of methylene chloride to methanol of 1:1 has a viscosity at 20° C. of 135 mPa·s or less; and a method for producing the HPMCAS including an esterification step of adding acetic anhydride and succinic anhydride to a solution of hypromellose in glacial acetic acid in the presence of sodium acetate to obtain a reaction product mixture, wherein the succinic anhydride is added intermittently, and a precipitation step of mixing the reaction product mixture with water to precipitate the HPMCAS.
Owner:SHIN ETSU CHEM IND CO LTD
Injection molding composition containing hypromellose acetate succinate and method for producing same
InactiveUS20180282527A1High strengthEasy injection moldingPharmaceutical non-active ingredientsCapsule deliveryHypromelloseMaterials science
An injection molding composition is capable of being injection-molded easily at a temperature lower than that of a conventional composition, and an injection molded product has improved strength. More specifically, an injection molding composition containing HPMCAS has a hydroxypropoxy molar substitution of 0.40 or more. In addition, a method produces an injection molded product including a step of injecting into a mold the injection molding composition at from 50 to 250° C.
Owner:SHIN ETSU CHEM IND CO LTD
Hypromellose acetate succinate powder excellent in dissolved state and production method thereof, and production methods for composition for solid dispersion, coating composition, drug-containing particle, and solid preparation
ActiveUS10604588B2Hinder advantageOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseAcetic acid
Provided are HPMCAS powder having high solubility when dissolved in a solvent and being capable of suppressing generation of undissolved materials; and a method for producing the powder. More specifically, provided is hypromellose acetate succinate powder having an average ratio of L to D of from 2.0 to 3.0, wherein L and D mean maximum and minimum diameters of each particle, respectively. Also provided is a method for producing a hypromellose acetate succinate, comprising the steps of: dissolving hypromellose powder in a solvent, esterifying the dissolved hypromellose with succinic anhydride and acetic anhydride in the presence of a catalyst to obtain a reaction mixture, and mixing the reaction mixture with water to precipitate hypromellose acetate succinate, wherein the reaction mixture just before being mixed with the water has a viscosity of from 100 to 200 Pa·s.
Owner:SHIN ETSU CHEM IND CO LTD
Hypromellose acetate succinate powder excellent in dissolved state and production method thereof, and production methods for composition for solid dispersion, coating composition, drug-containing particle, and solid preparation
ActiveUS20200181291A1Hinder advantageOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseAcetic acid
Provided are HPMCAS powder having high solubility when dissolved in a solvent and being capable of suppressing generation of undissolved materials; and a method for producing the powder. More specifically, provided is hypromellose acetate succinate powder having an average ratio of L to D of from 2.0 to 3.0, wherein L and D mean maximum and minimum diameters of each particle, respectively. Also provided is a method for producing a hypromellose acetate succinate, comprising the steps of: dissolving hypromellose powder in a solvent, esterifying the dissolved hypromellose with succinic anhydride and acetic anhydride in the presence of a catalyst to obtain a reaction mixture, and mixing the reaction mixture with water to precipitate hypromellose acetate succinate, wherein the reaction mixture just before being mixed with the water has a viscosity of from 100 to 200 Pa·s.
Owner:SHIN ETSU CHEM CO LTD
Preparation method of taste-masked suspension granules of Gegenqinlian decoction
ActiveCN102309562BDoes not affect bioavailabilityGood taste masking effectAntibacterial agentsAntipyreticAdhesiveHypromellose phthalate
The invention relates to a preparation method of taste-masked suspension granules of Gegenqinlian decoction. The preparation method comprises the following steps of 1, taking appropriate amounts of dispensing granules of three traditional Chinese medicines comprising radix puerariae, coptis chinensis and scutellaria baicalensis and respectively carrying out coating processes in a fluidized bed through adopting one or more polymers as coating materials to obtain coated granules for next use, 2, taking an appropriate amount of at least one suspending agent, mixing uniformly the at least one suspending agent and radix glycyrrhizae preparata dispensing granules, then adding an appropriate amount of an adhesive into the mixture to prepare into granules by a wet method, drying the prepared granules in an oven, and then spraying an appropriate amount of an ethanol solution as an aromatic to obtain suspending agent-containing radix glycyrrhizae preparata dispensing granules after ethanol is volatilized, and 3, weighing appropriate amounts of the suspending agent-containing radix glycyrrhizae preparata dispensing granules and the coated granules containing radix puerariae, coptis chinensisand scutellaria baicalensis, mixing well, and carrying out sub-packaging to obtain the taste-masked suspension granules of Gegenqinlian decoction, wherein the one or more polymers as coating materials are selected from enteric-coated polyacrylic resin, hypromellose acetate succinate and hydroxypropyl methylcellulose phthalate. The invention provides the preparation method of the taste-masked suspension granules of Gegenqinlian decoction. The preparation method is also suitable for taste masking of a traditional Chinese medicine with a bitter taste or a fishy smell.
Owner:安徽天祥药业有限公司
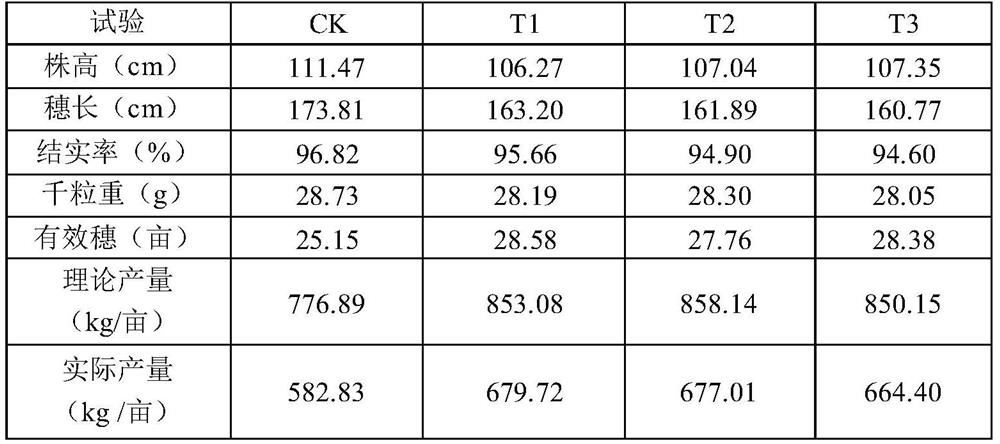
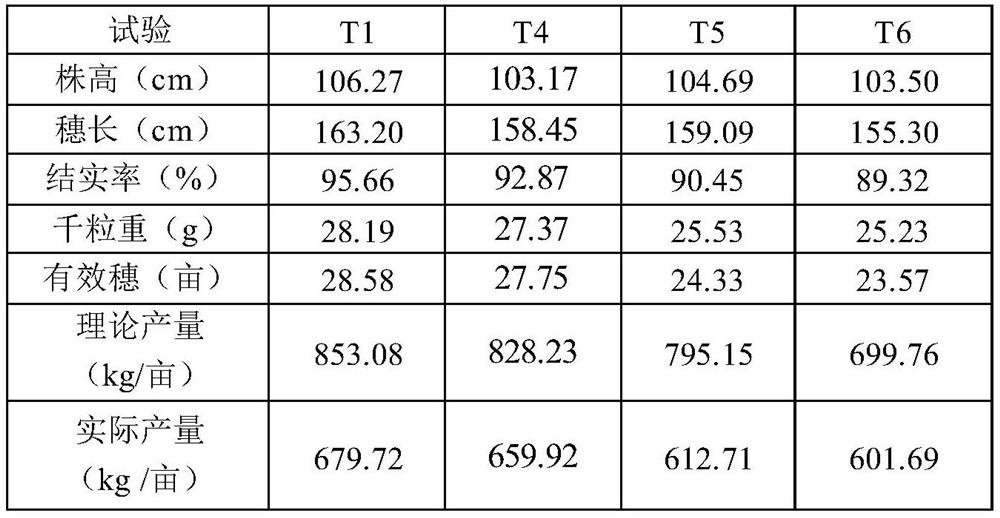
Special multi-stage controlled-release compound microbial fertilizer for rice and preparation method thereof
ActiveCN114315467AProvide accuratelyBalanced offerSolid/semi-solid fertilisersLayered/coated fertilisersCelluloseMicrobial agent
The invention relates to a special multi-stage controlled-release compound microbial fertilizer for rice and a preparation method thereof. The special multi-stage controlled-release compound microbial fertilizer structurally comprises a rice booting fertilizer layer, a dry farmland nutrient microbial agent layer, a water-soluble sensitive layer with the pH value larger than or equal to 6, a rice seedling fertilizer layer with the pH value smaller than 6, a paddy field biocontrol microbial agent layer, a hydrolysis coating layer and a decomposed microorganism / organic matter fertilizer layer from inside to outside in sequence. Wherein the water-soluble sensitive layer with the pH value greater than or equal to 6 is made of one or more of cellulose acetate phthalate, hydroxypropyl methylcellulose acetate succinate, a methacrylic acid copolymer L100, a methacrylic acid copolymer S100 and a methacrylic acid copolymer L100; wherein the hydrolysis coating layer is made of one or more of Arabic gum, gelatin and peach gum. The compound microbial fertilizer disclosed by the invention can release required nutrients according to soil environment control in different growth periods of rice, meets nutrient requirements of the whole growth period of the rice, and improves the utilization rate of the fertilizer.
Owner:SHANGHAI NUOTONG INFORMATION TECH CO LTD
Hypromellose acetate succinate powder excellent in dissolved state and production method thereof, and production methods for composition for solid dispersion, coating composition, drug-containing particle, and solid preparation
ActiveUS20170283514A1Limited amountHinder advantageOrganic active ingredientsPharmaceutical non-active ingredientsAcetic anhydrideSolid mass
Provided are HPMCAS powder having high solubility when dissolved in a solvent and being capable of suppressing generation of undissolved materials; and a method for producing the powder. More specifically, provided is hypromellose acetate succinate powder having an average ratio of L to D of from 2.0 to 3.0, wherein L and D mean maximum and minimum diameters of each particle, respectively. Also provided is a method for producing a hypromellose acetate succinate, comprising the steps of: dissolving hypromellose powder in a solvent, esterifying the dissolved hypromellose with succinic anhydride and acetic anhydride in the presence of a catalyst to obtain a reaction mixture, and mixing the reaction mixture with water to precipitate hypromellose acetate succinate, wherein the reaction mixture just before being mixed with the water has a viscosity of from 100 to 200 Pa·s.
Owner:SHIN ETSU CHEM IND CO LTD
Solid dispersion and pharmaceutical composition
PendingCN111904960AImprove solubilityPromote absorptionOrganic active ingredientsPharmaceutical non-active ingredientsFormic Acid EstersPharmaceutical drug
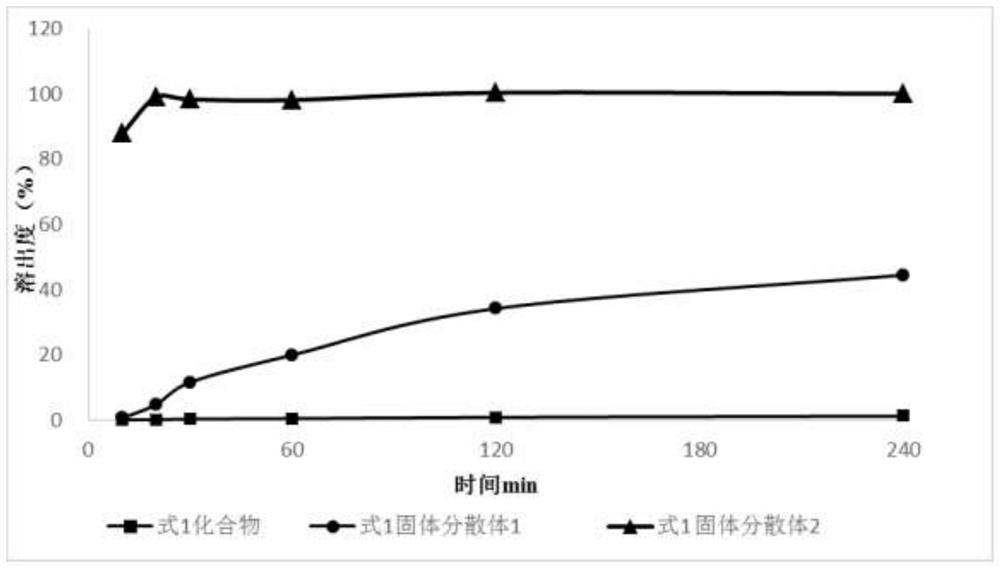
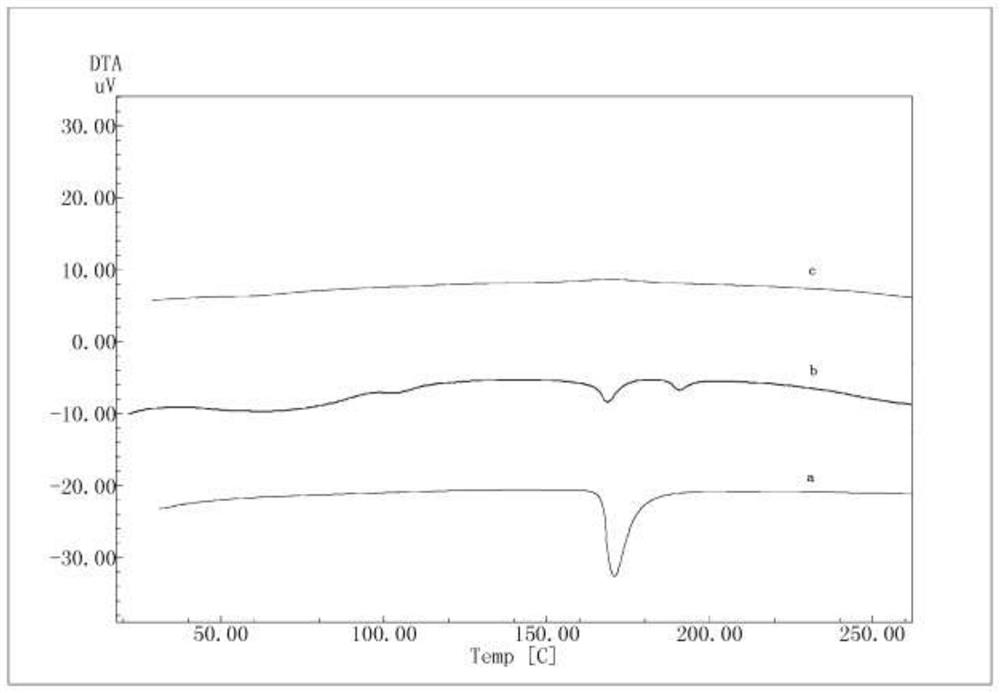
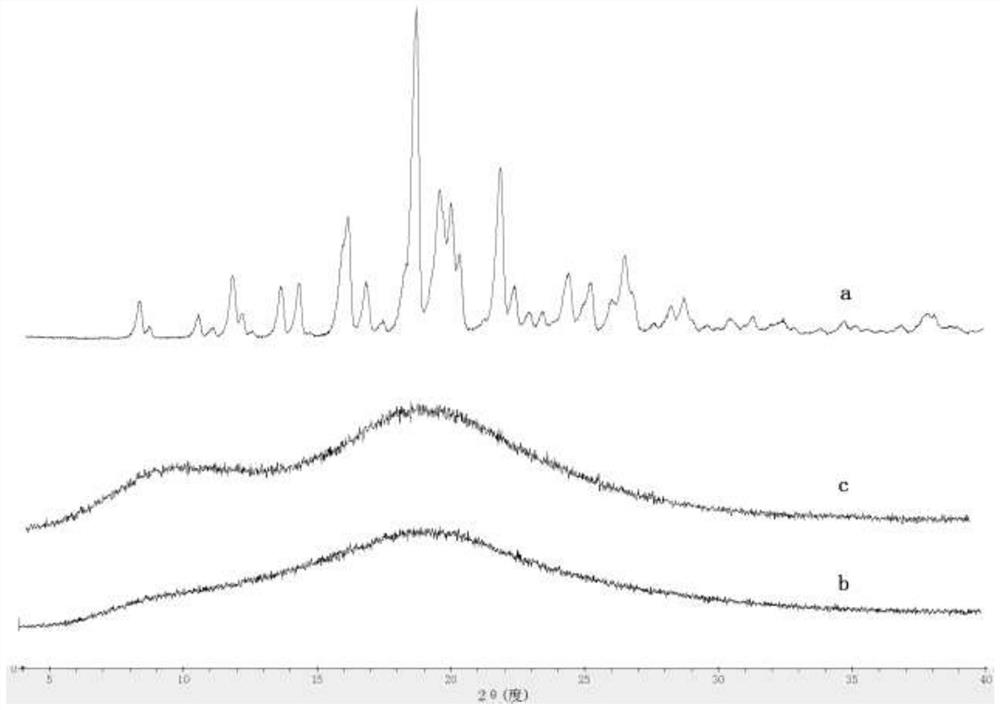
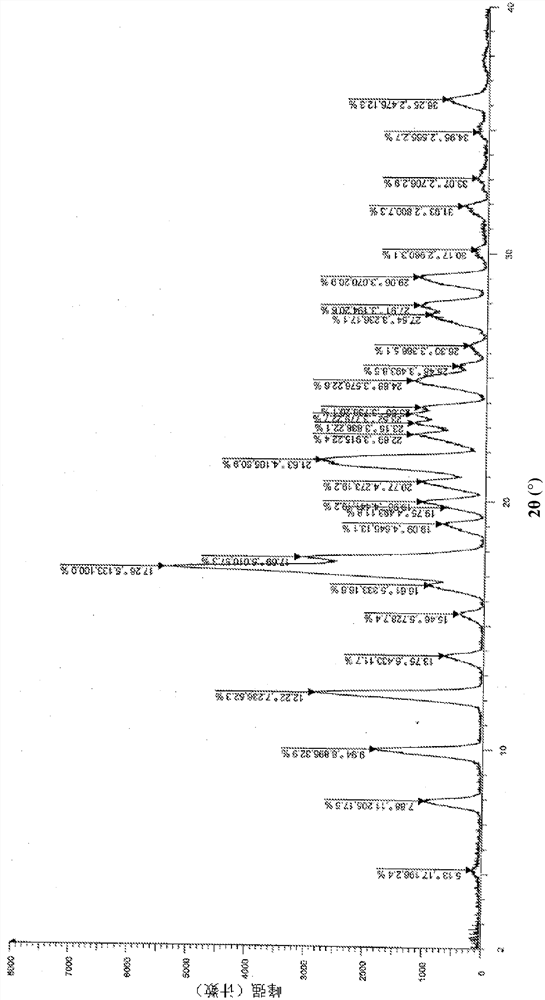
The invention discloses a solid dispersion and a pharmaceutical composition, and relates to the technical field of medicines. The solid dispersion contains an active drug and a carrier material, wherein the active drug is a compound in a formula 1 or formula 2; and the carrier material is any one of hydroxypropyl methylcellulose acetate succinate and hydroxypropyl methylcellulose phthalate. The solid dispersion can improve the solubility and absorption properties of the compound in the formula 1 or formula 2, thereby reducing the clinical dosage. Characterization shows that the compound in theformula 1 or formula 2 is dispersed in the carrier material in an amorphous form and still exists in an amorphous state after being placed for 3 months under an acceleration condition (40 DEG C / 75% RH), and the performance is stable. A preparation prepared from the solid dispersion can greatly reduce the influence of environmental temperature, humidity and the like on product dissolution and bioavailability in the processes of production, transportation, storage and the like of medicines.
Owner:HEFEI COSOURCE PHARMA CO LTD
A kind of pharmaceutical composition and preparation method thereof
A solid dispersion, a preparation method thereof, and a solid formulation comprising the solid dispersion. The solid dispersion contains (R)-4-amino-1-(1-(but-2-ynoyl)pyrrolidin-3-yl)-3-(4-(2,6-difluorophenoxy )phenyl)-1,6-dihydro-7H-pyrrolo[2,3-d]pyridazin-7-one or a pharmaceutically acceptable salt thereof and a carrier material selected from hypromellose acetate Sucuccinate, Hypromellose Phthalate.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com