A kind of pharmaceutical composition and preparation method thereof
A technology for medicinal salts and solid preparations, applied in the field of pharmaceutical preparations, can solve the problems of poor prognosis and low survival rate of B-cell lymphoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
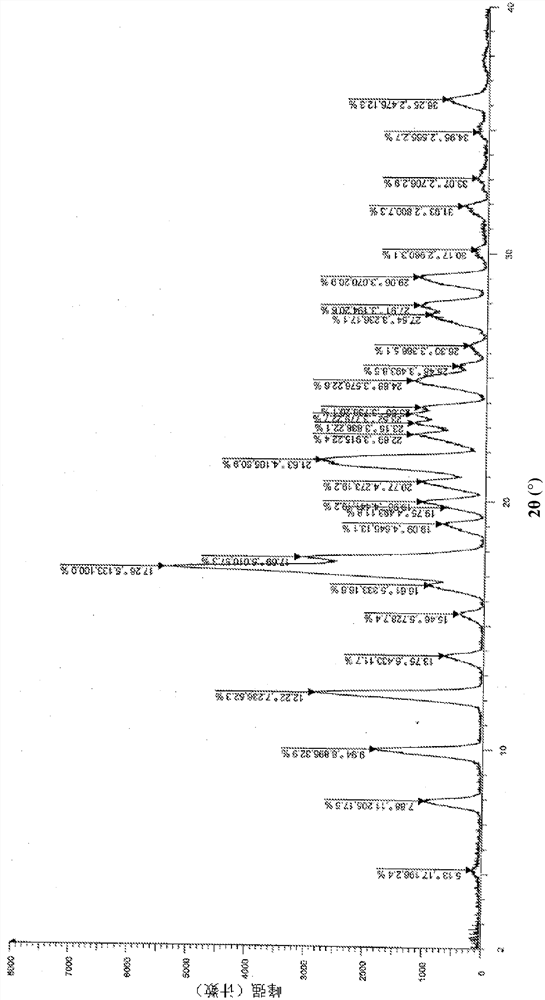
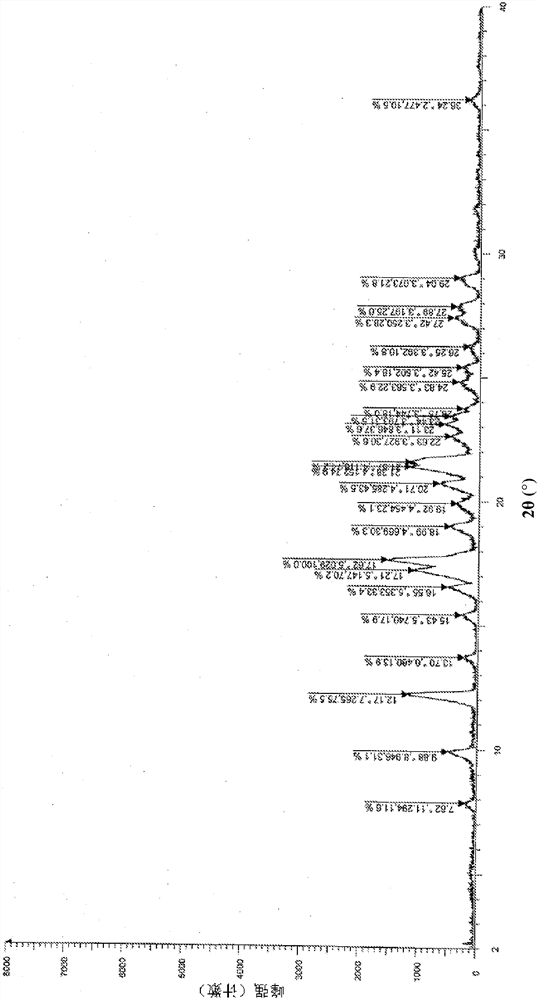
Image
Examples
Embodiment 1
[0057] Example 1: Solid dispersion
[0058]The (R) -4- amino-1- (1- (but-2-ynyl group) pyrrolidin-3-yl) -3- (4- (2,6-difluorophenoxy) phenyl) - 1,6-dihydro -7H- pyrrolo [2,3-d] pyridazin-7-one (referred to as compound a) and different types of support materials for preparing solid dispersions, particular formulation as shown in table 1:
[0059] Table 1
[0060]
[0061] Preparation method (a coprecipitation method):
[0062] According to the formula of compound A and the amount of said carrier material, and completely dissolved in N, N- dimethylacetamide (DMF) in accordance with N, N- dimethylacetamide with purified water 1:15 (g / ratio g), the weighed prescribed amount of purified water, the solvent containing the compound a and the support material was added dropwise to the water flow rate of 30g / min, the precipitated white flocculent precipitate was filtered, washed, and dried to obtain.
[0063] Dissolution experiments
[0064] Determination Method II (paddle) according ...
Embodiment 2
[0073] Compound A and hypromellose acetate succinate weight ratio of 1 to prepare a solid dispersion, and the resulting solid dispersion was ground to achieve a particle size suitable for requirements, in accordance with the formulation design weighed amount of a formulation solid dispersion, microcrystalline cellulose and lactose, were added and crosslinked sodium carboxymethylcellulose, crosslinked povidone, sodium carboxymethyl starch, low-substituted hydroxypropylcellulose as a disintegrator, with hydroxypropyl granulated cellulose as a binder, granulated poured into the tank, after mixing, granulating binder is added; wet granulated material on marshy and dried, then the dry granules (moisture less than 3%) were dry sieved, added prescribed amount of magnesium stearate, mixed uniformly. The resulting total mixed particles were pressed into a tablet. DETAILED proportion prescription of Table 4.
[0074] Table 4
[0075]
[0076] Dissolution experiments
[0077] Determination...
Embodiment 3
[0082] Compound A and hypromellose acetate succinate mass ratio of 1 by the preparation of solid dispersions by coprecipitation, the resulting solid dispersion was ground in accordance with the formulation design weighed amount of a solid dispersion formulation , lactose and microcrystalline cellulose, crosslinked sodium carboxymethyl cellulose, hydroxypropyl methyl cellulose, respectively, polyvinylpyrrolidone, pregelatinised starch, hydroxypropyl cellulose as binder granulated, it is poured into the granulation tank, after mixing, granulating binder is added; wet granulated material on marshy and dried, then the dry particles (less than 3% water content) were dry sieved, added prescribed amount of stearyl magnesium mixed. The resulting total mixed particles were pressed into a tablet. DETAILED proportion prescription in Table 6.
[0083] Table 6
[0084]
[0085] Dissolution experiments
[0086] Determination Method II (paddle) according to China Pharmacopoeia 2015 Edition Gen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com