Enteric coating composition, solid preparation and method for producing solid preparation
A technology of enteric coating and composition, which is applied in the field of aqueous dispersion, and can solve problems such as difficulty in exerting the effect of drug administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
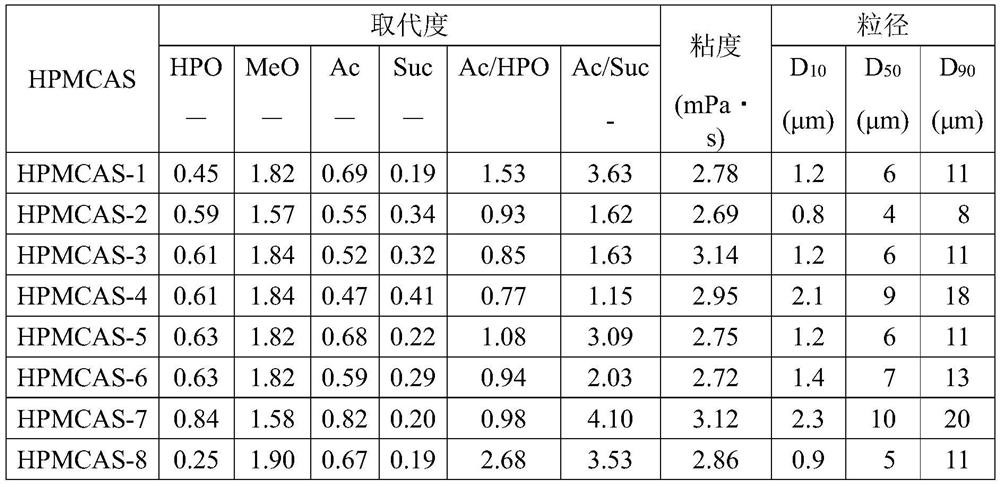
[0019] From the viewpoint of the production rate of the HPMCAS, the molar substitution (MS) of hydroxypropoxy in the HPMCAS is preferably 1.00 or less, more preferably 0.90 or less, further preferably 0.85 or less, particularly preferably 0.80 or less.
[0020] More specifically, the molar substitution (MS) of hydroxypropoxy in the HPMCAS is preferably 0.40 to 1.00, more preferably 0.42 to 0.90, even more preferably 0.50 to 0.85, even more preferably 0.55 to 0.85, particularly preferably 0.60 ~0.80.
[0021] The degree of substitution (DS) of the methoxy group in this HPMCAS is not particularly limited. From the viewpoint of the production rate of the HPMCAS and the film strength, it is preferably 0.70 to 2.50, more preferably 1.00 to 2.20, and still more preferably 1.40 to 1.95.
[0022] The degree of substitution (DS) of the acetyl group in this HPMCAS is not particularly limited. From the viewpoint of acid resistance and enteric properties, it is preferably 0.10 to 2.50, ...
Embodiment 1~5 and comparative example 1
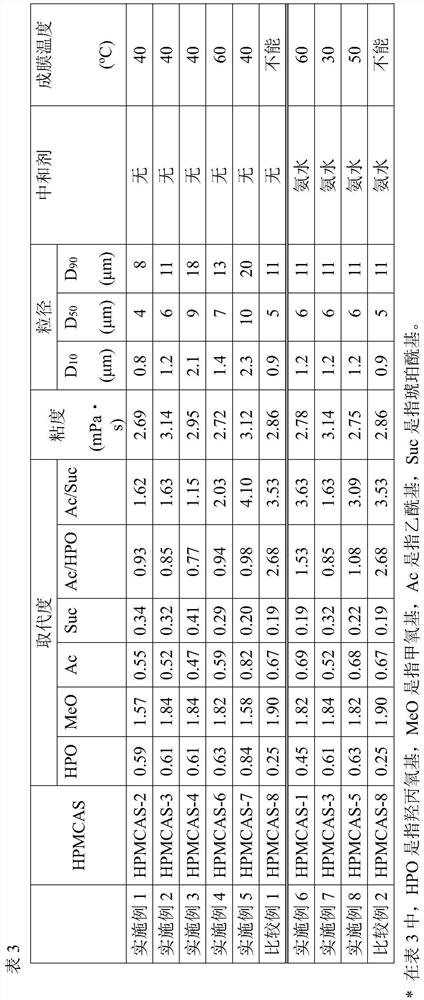
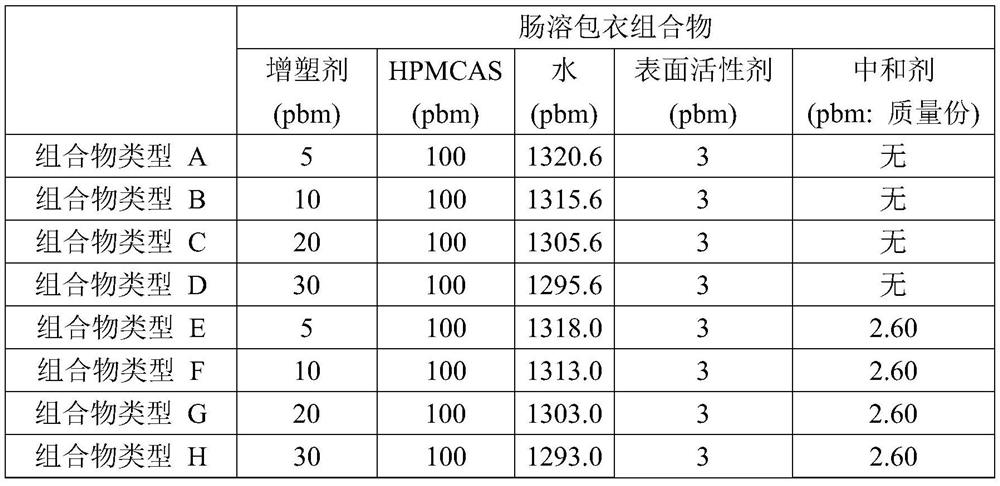
[0138] Pure water (1325.6 parts by mass) was added to a 200-ml beaker and stirred at 200 rpm using a propeller stirrer (NZ-1100, a product of Tokyo Rikakikai Co., Ltd.) while stirring on ice Cool in the bath to about 10°C. Next, while cooling to about 10° C., sodium lauryl sulfate (SLS) (3 parts by mass) as a surfactant was added thereto and mixed, and then HPMCAS-2 to 4 and 6 to 8 were added. HPMCAS (100 parts by mass), followed by stirring at 200 rpm for 30 minutes while cooling to about 10° C., to prepare an enteric coating composition, which is an aqueous dispersion of HPMCAS.
[0139] In order to evaluate the coating performance (film-forming performance) of each prepared enteric coating composition, the film-forming property on a glass plate was tested. A 100 μl aliquot of the enteric coating composition was poured onto five glass plates and dried in an oven for 2 hours at each temperature (25, 30, 40, 50 or 60° C.) to visually inspect the film Formation. The lowest d...
Embodiment 6~8 and comparative example 2
[0141] Pure water (1323.0 parts by mass) was added to a 200 mL beaker at room temperature, and sodium lauryl sulfate (SLS) (3 parts by mass) was added thereto as a surfactant, and while using a propeller stirrer (NZ-1100, Tokyo Rikaki Co., Ltd.) was mixed while stirring at 200 rpm. Next, HPMCAS-1, 3, 5, or 8 (100 parts by mass) was added thereto, and stirred at 200 rpm at room temperature to prepare an aqueous dispersion. Then, 10% by mass of ammonia solution (2.60 parts by mass in terms of ammonia) was added thereto, and stirred at room temperature at 200 rpm for 30 minutes to prepare an enteric coating composition, which was an aqueous dispersion of partially neutralized HPMCAS .
[0142] In order to evaluate the coating performance (film-forming property) of each prepared enteric coating composition, film formation on a glass plate was tested in the same manner as in Examples 1 to 5. The results are shown in Table 3.
[0143]
[0144] The results of Examples 1 to 5 sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com