Preparation method of azilsartan tablets
A technology of azilsartan tablets and lactose, which is applied in the field of medicine, can solve the problems of large dust production, complex process, and slight toxicity, and achieve the effects of improving dissolution rate, ensuring drug safety, and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
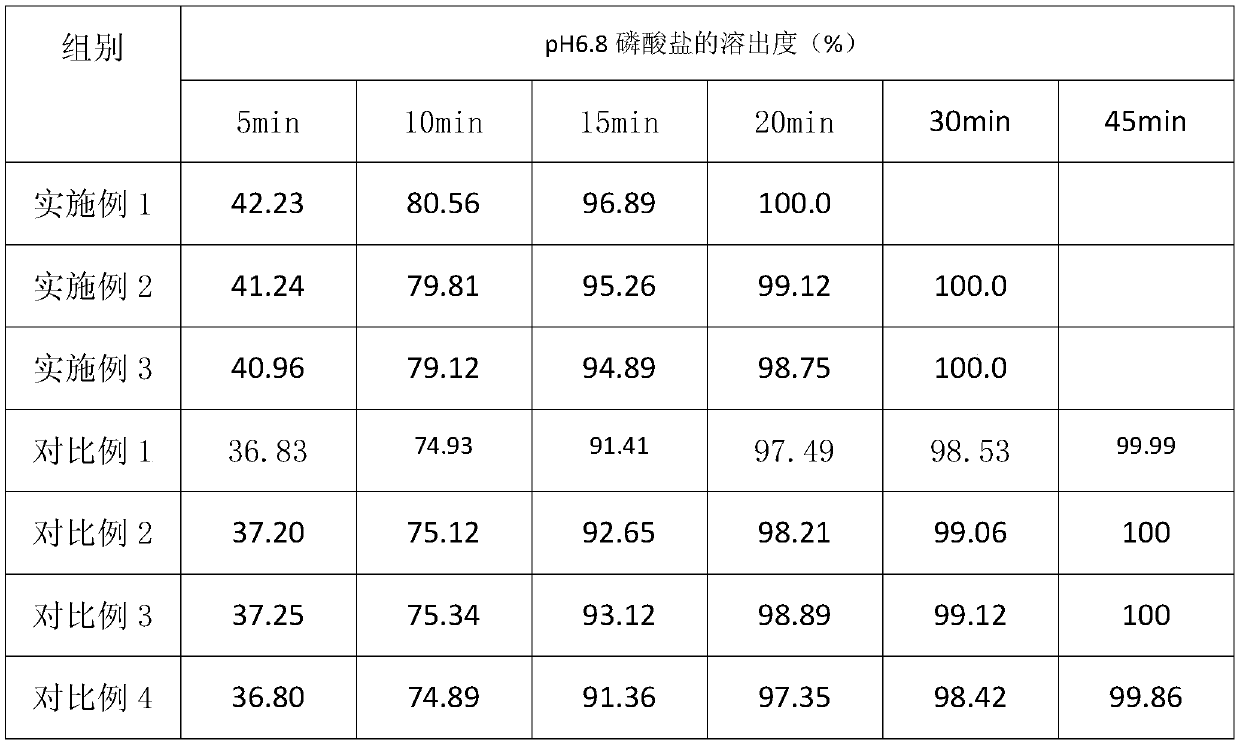
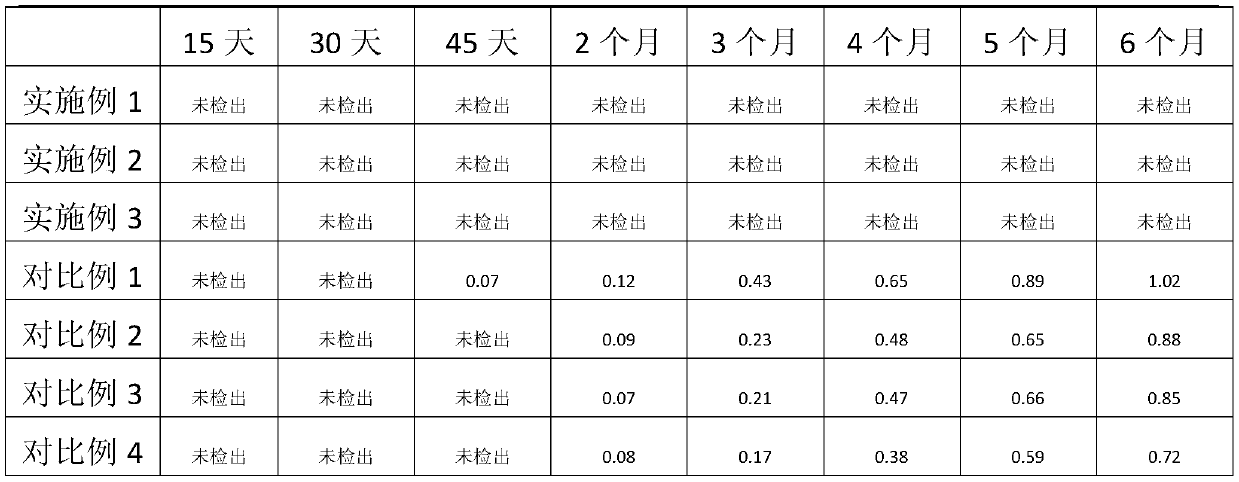
Embodiment 1
[0026] Embodiment 1, prepare 5000 pieces of azilsartan tablets of 20mg
[0027] Azilsartan 100g, lactose 135g, corn starch 215g, hydroxypropyl cellulose 8g, copovidone S630 2g, hypromellose acetate succinate HF 2g, polyethylene glycol 6000 23g, low-substituted hypromellose Vegetable 155g, microcrystalline cellulose PH101 5g, magnesium stearate 5g, film coating premix (stomach-soluble type) 65g.
[0028] Prepared by a method comprising the steps of:
[0029] 1) Azilsartan is crushed through a 60-mesh sieve, lactose, corn starch, low-substituted hydroxypropyl cellulose, and microcrystalline cellulose PH101 are passed through a 60-mesh sieve, magnesium stearate is passed through a 80-mesh sieve, and set aside;
[0030] 2) Dissolving hydroxypropyl cellulose and copovidone S630 in ethanol to obtain a solution 1 with a concentration of 5% (W / V);
[0031] 3) Put corn starch, polyethylene glycol 6000, microcrystalline cellulose PH101, azilsartan, lactose and 40% low-substituted hydr...
Embodiment 2
[0035] Embodiment 2, prepare 5000 pieces of azilsartan tablets of 20mg
[0036] Azilsartan 100g, lactose 120g, corn starch 220 parts, hydroxypropyl cellulose 10 parts, copovidone S6301 parts, hypromellose acetate succinate HF 1, polyethylene glycol 6000 25 parts, low substitution 160 parts of hydroxypropyl cellulose, 7 parts of microcrystalline cellulose PH101, 6 parts of magnesium stearate, 65 parts of film coating premix (gastric soluble type).
[0037] The preparation process is the same as in Example 1.
Embodiment 3
[0038] Embodiment 3, prepare 5000 pieces of azilsartan tablets of 20mg
[0039] Azilsartan 100g, lactose 140g, corn starch 210 parts, hydroxypropyl cellulose 6 parts, copovidone S6304 parts, hypromellose acetate succinate HF 3, polyethylene glycol 6000 24 parts, low substitution 150 parts of hydroxypropyl cellulose, 7 parts of microcrystalline cellulose PH101, 6 parts of magnesium stearate, 65 parts of film coating premix (gastric soluble type).
[0040] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com