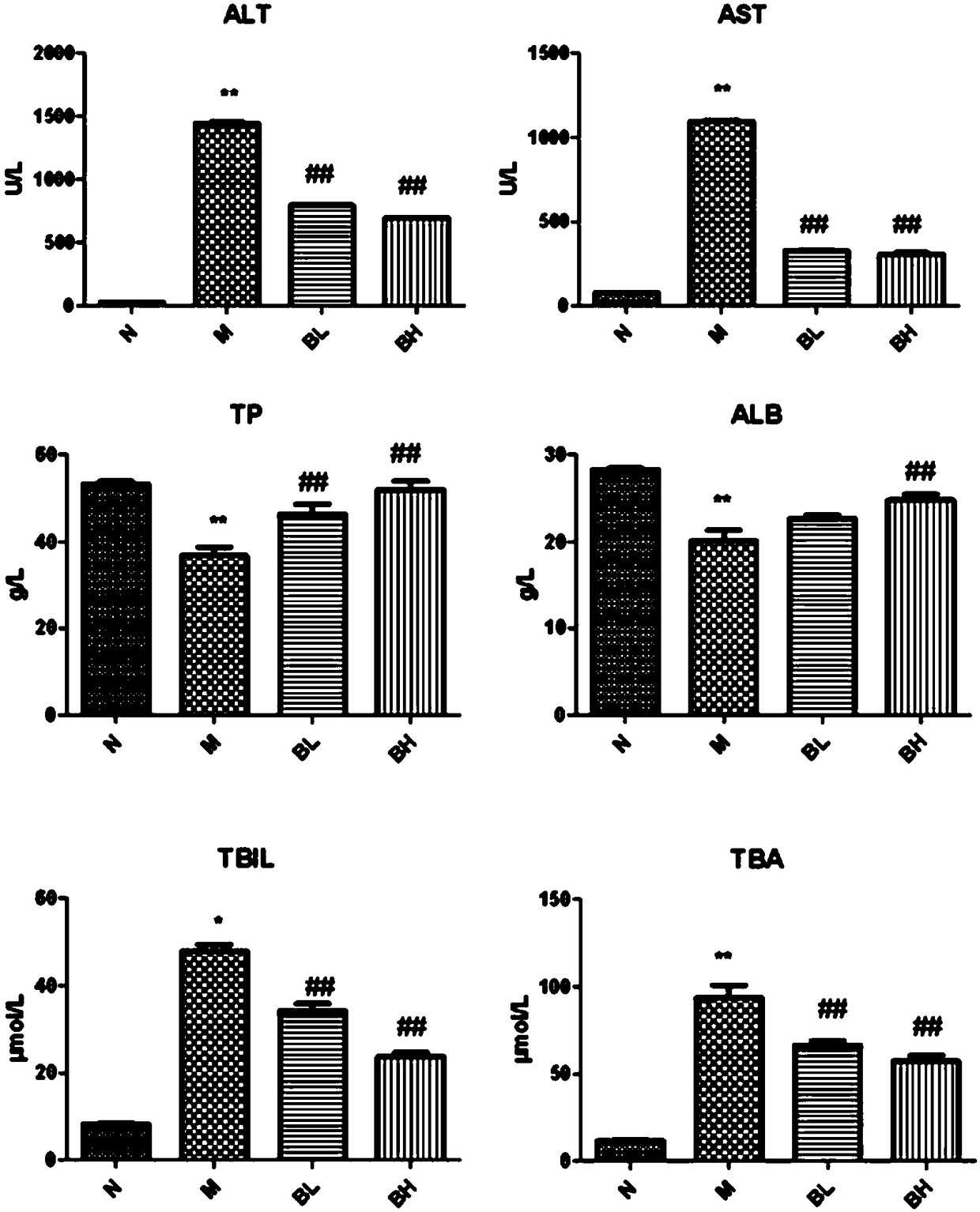
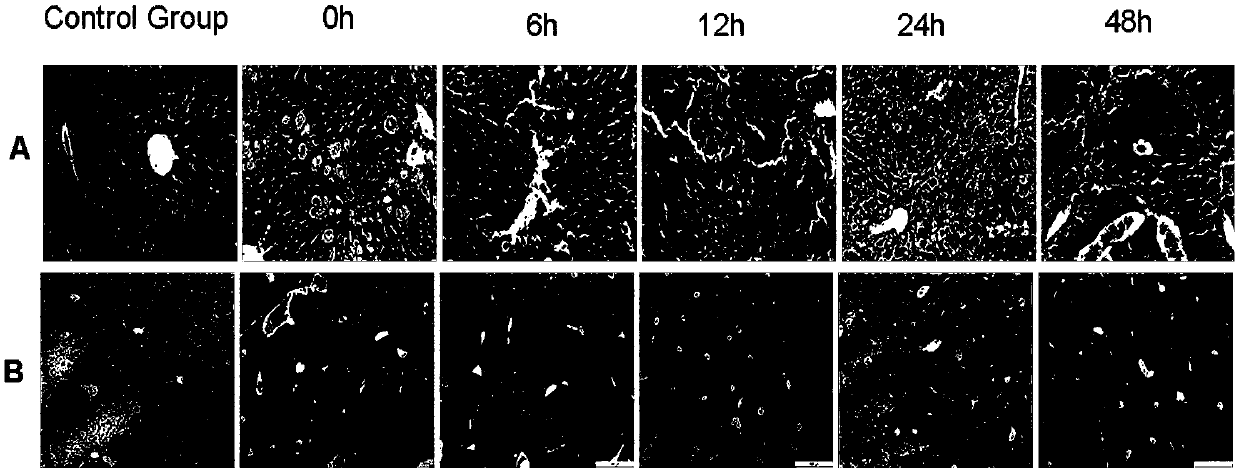
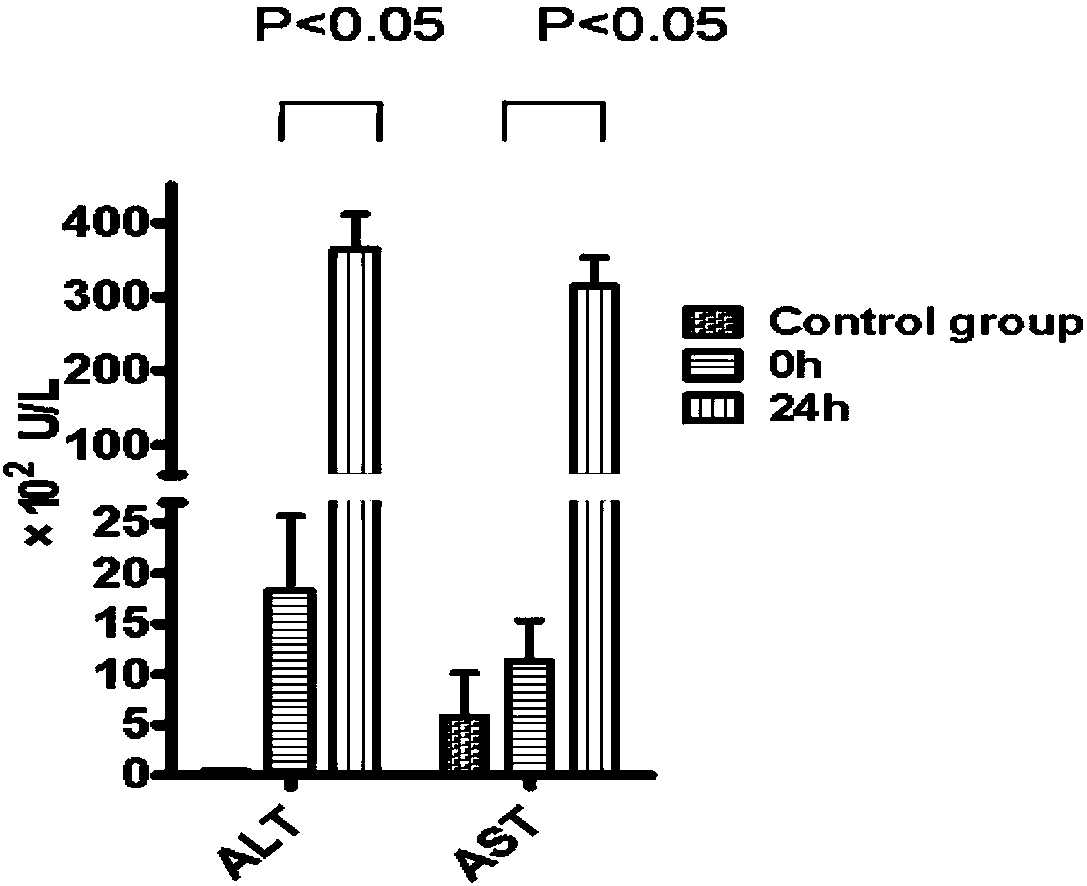
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Hepatocellular necrosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The liver has the central role in the synthesis of almost all coagulation factors and some inhibitors of coagulation and fibrinolysis. Hepatocellular necrosis leads to impaired synthesis of many coagulation factors and their inhibitors.
Acetylcysteine composition and uses therefor
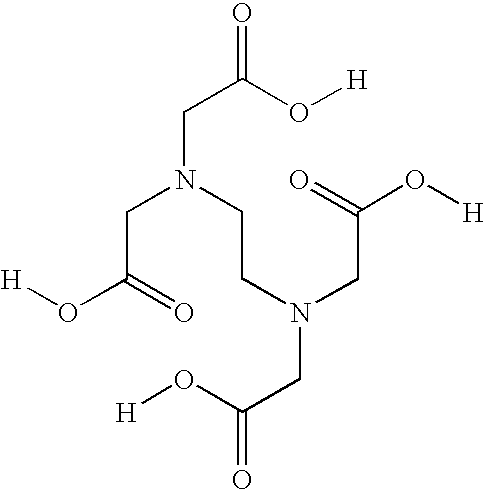
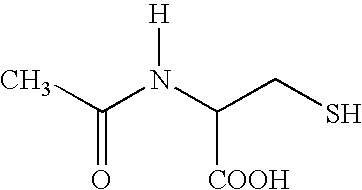
This invention relates to novel acetylcysteine compositions in solution, comprising acetylcysteine and which are substantially free of metal chelating agents, such as EDTA. Further, this invention relates to methods of making and using the acetylcysteine compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time maintaining the stability of the pharmaceutical formulation. The compositions and methods of this invention are useful in the treatment of acetaminophen overdose, acute liver failure, various cancers, methacrylonitrile poisoning, reperfusion injury during cardio bypass surgery, and radiocontrast-induced nephropathy, and can also be used as a mucolytic agent.
Owner:CUMBERLAND PHARM INC
Acetylcysteine composition and uses therefor
This invention relates to novel acetylcysteine compositions in solution, comprising acetylcysteine and which are substantially free of metal chelating agents, such as EDTA. Further, this invention relates to methods of making and using the acetylcysteine compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time maintaining the stability of the pharmaceutical formulation. The compositions and methods of this invention are useful in the treatment of acetaminophen overdose, acute liver failure, various cancers, methacrylonitrile poisoning, reperfusion injury during cardio bypass surgery, and radiocontrast-induced nephropathy, and can also be used as a mucolytic agent.
Owner:CUMBERLAND PHARM INC
Implantable bioartificial liver
InactiveCN101850136AEasy to attachSpecific function preservationPharmaceutical containersMedical packagingCell-Extracellular MatrixBioartificial liver device
The invention provides a combining reagent for preparing a bioartificial liver stent. The reagent comprises perfusate 1, perfusate 2, perfusate 3, perfusate 4, eluent 1 and eluent 2, wherein the perfusate is alkalescent hypotonic solution or pure water; the perfusate 2 is a group of ionic detergent aqueous solutions with gradient concentration; the perfusate 3 is aqueous electrolyte solution; theperfusate 4 is anticoagulant factor aqueous solution; the eluent 1 is distilled water; and the eluent 2 is non-ionic detergent aqueous solution. The invention also provides a method for preparing an implantable bioartificial liver by using the combining reagent, and the implantable bioartificial liver prepared by using the method. The bioartificial liver stent prepared by using the combining reagent is decellularized completely; and the structure of an extracellular matrix is kept complete. The bioartificial liver can accommodate a large number of cells and provide adequate blood supply, so that the survival time of a rate with an acute hepatic failure is prolonged obviously; and a liver function is improved obviously. Therefore, the bioartificial liver has an extremely good clinical application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
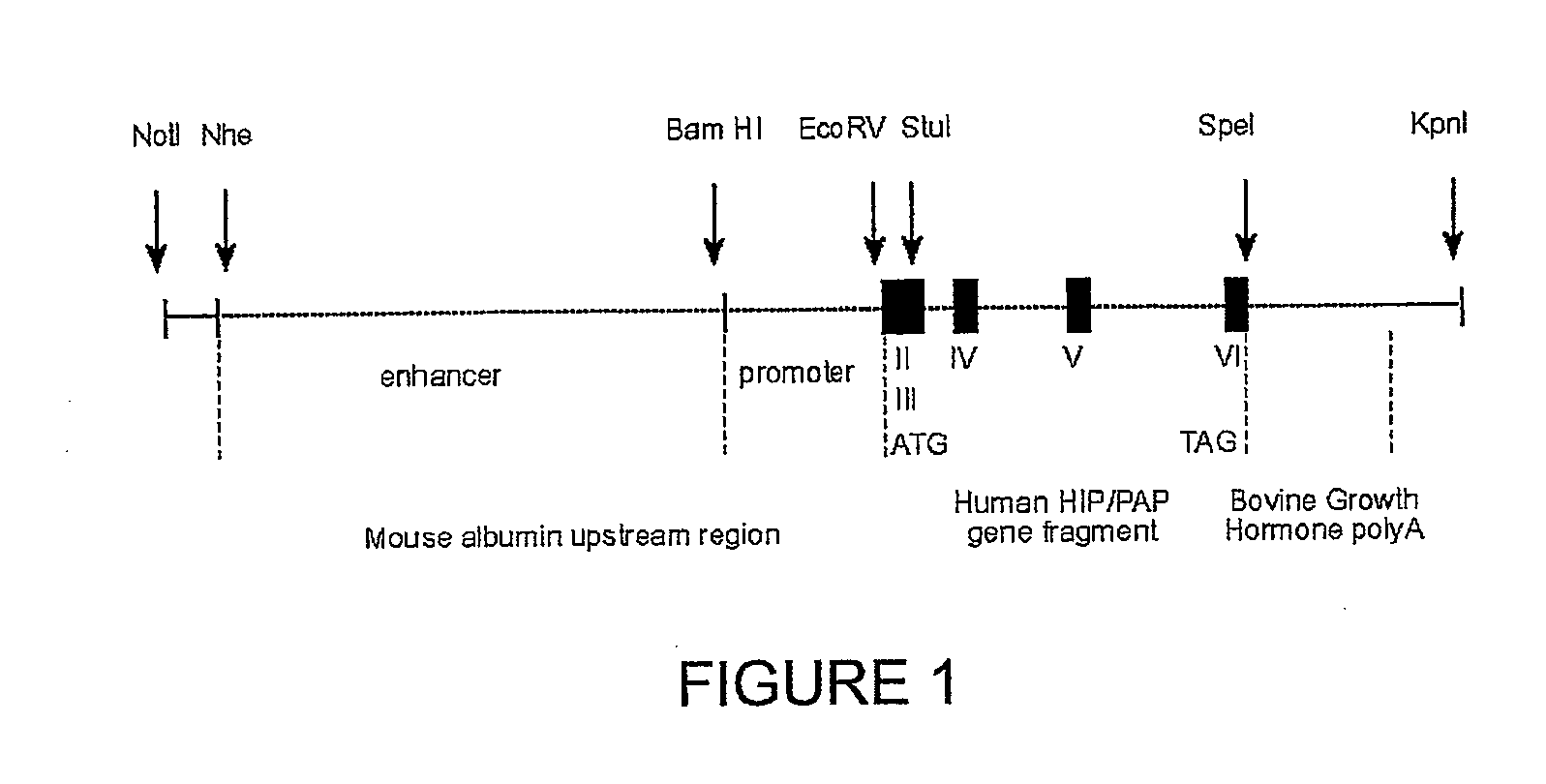
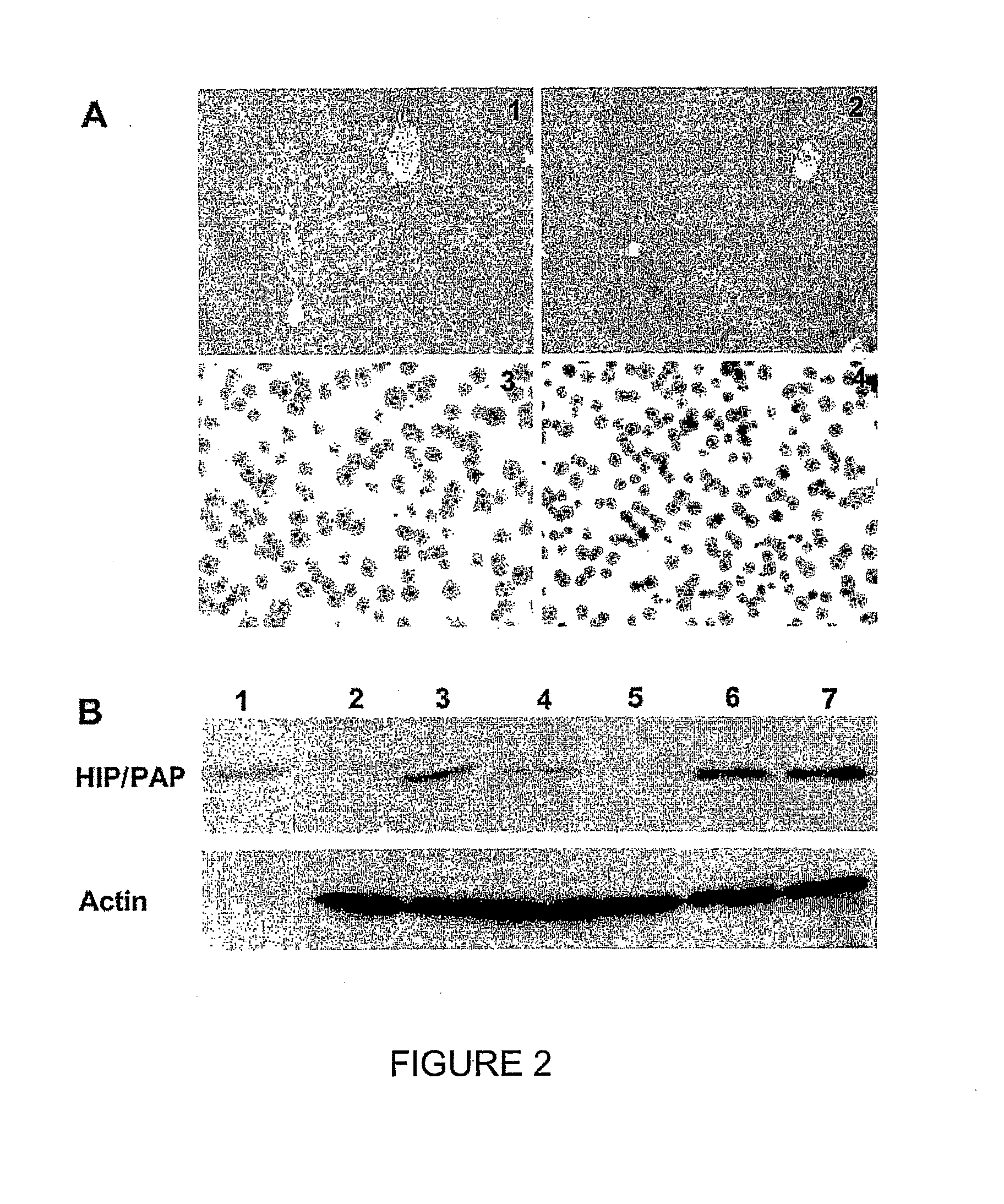
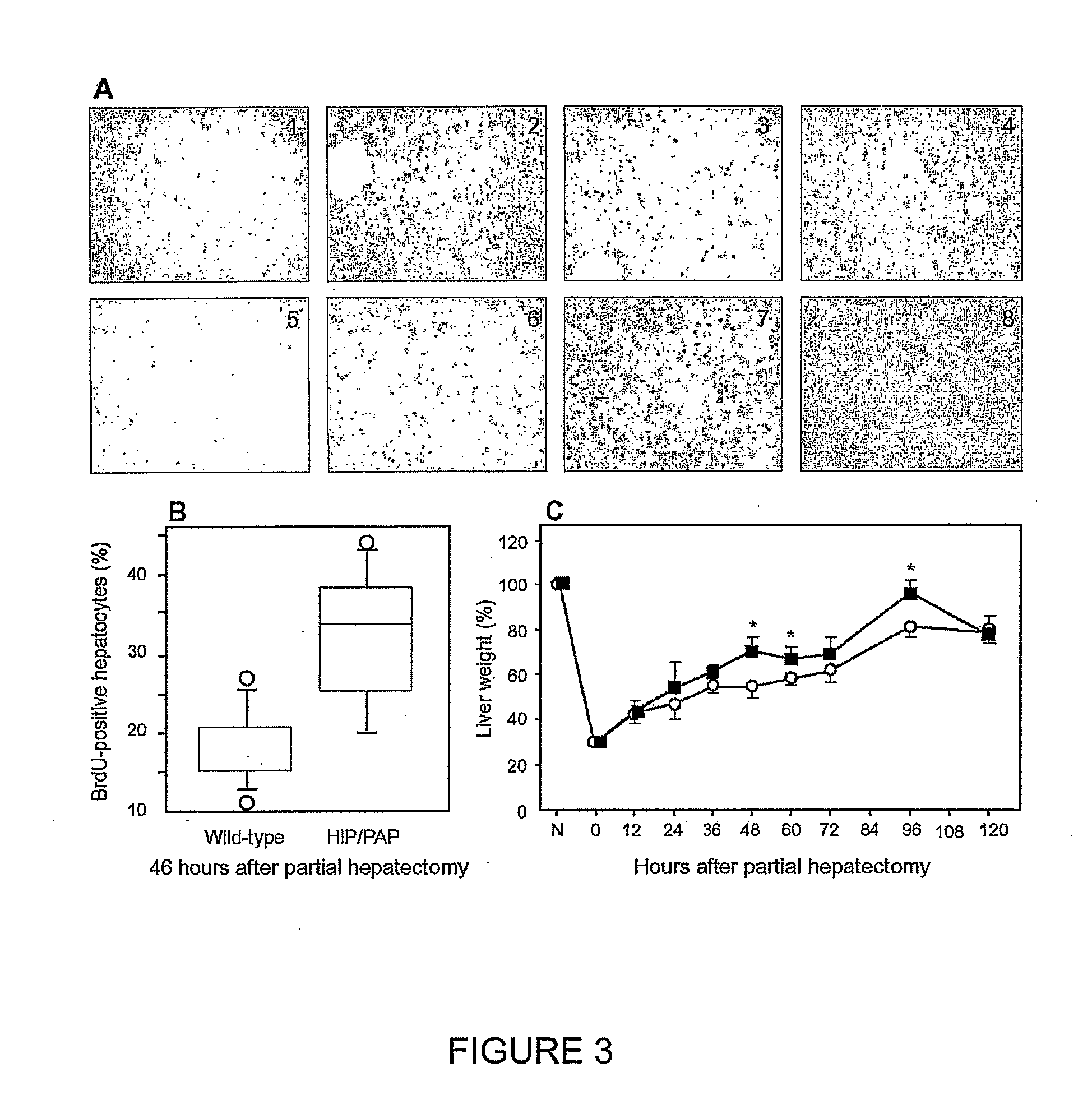
HIP/PAP Polypeptide Composition for Use in Liver Regeneration and for the Prevention of Liver Failure
Owner:VIVO BIOSCI CO LTD
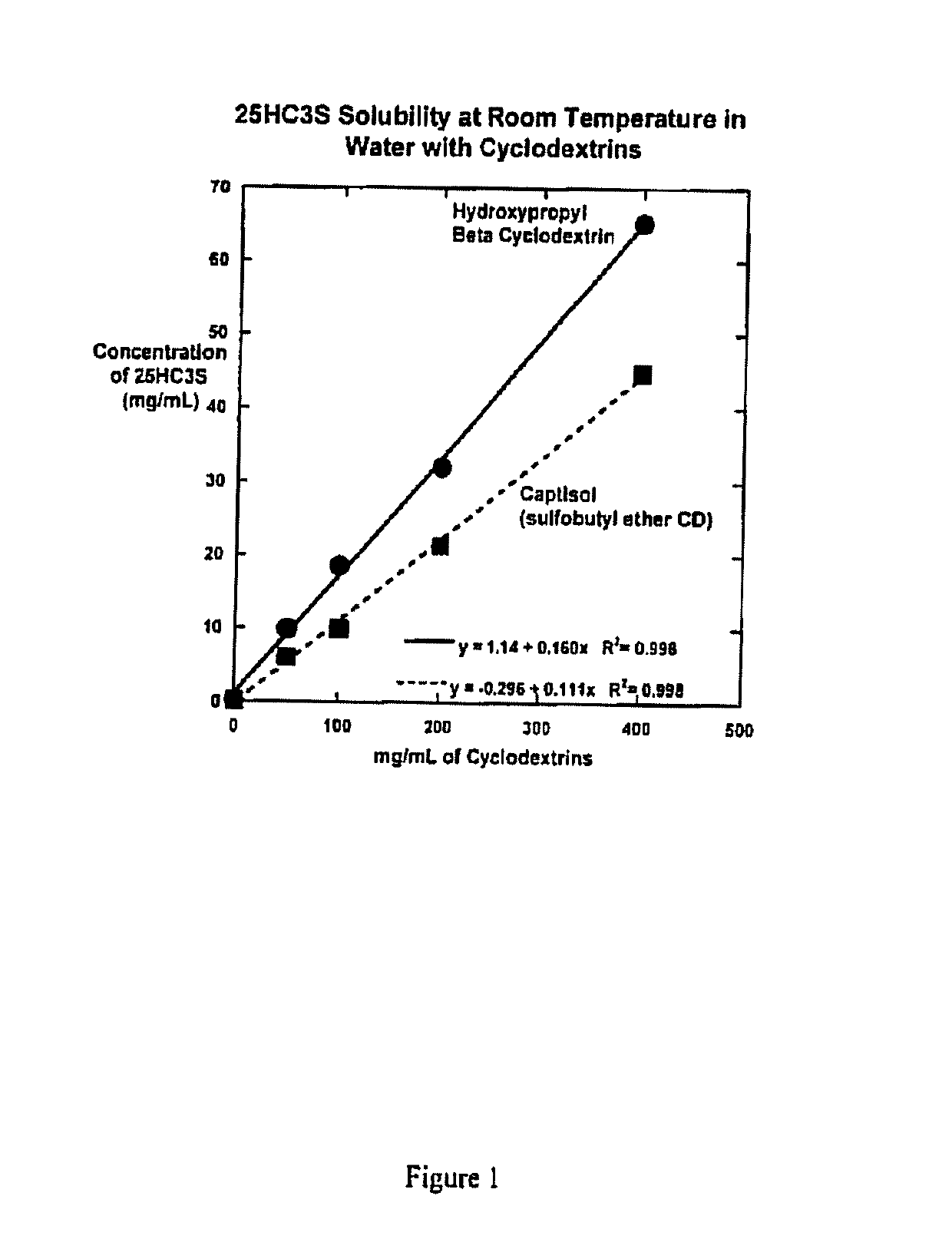
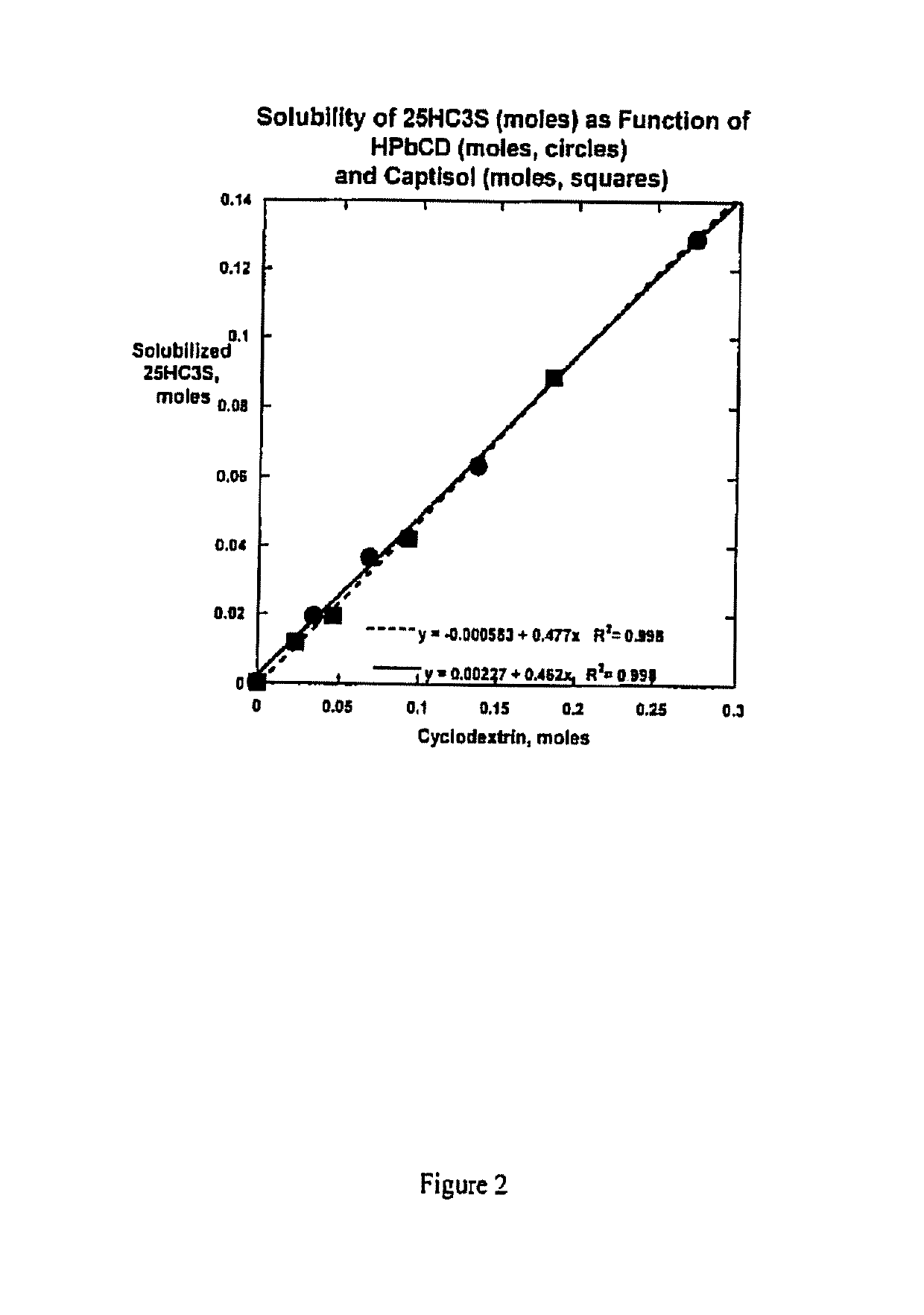
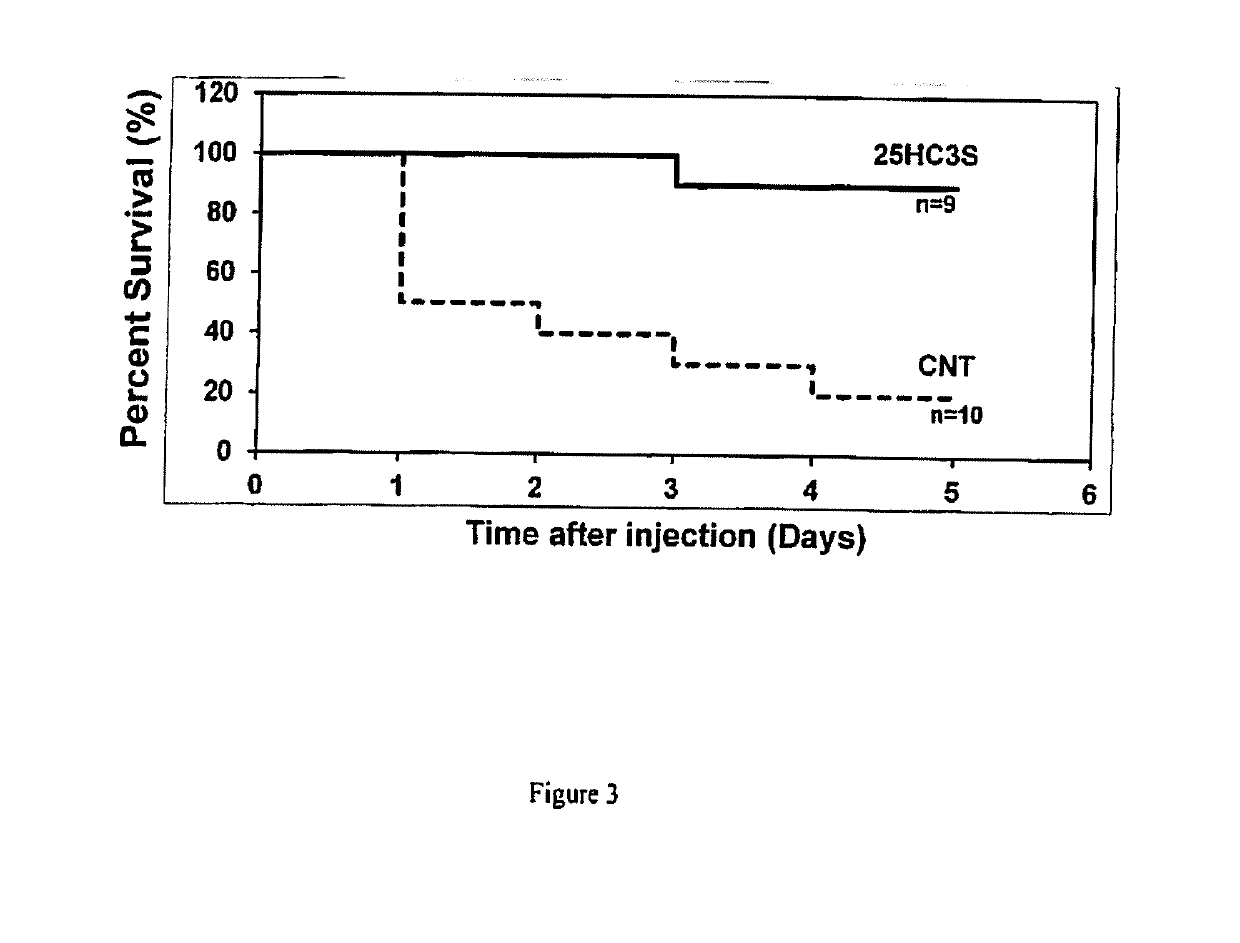
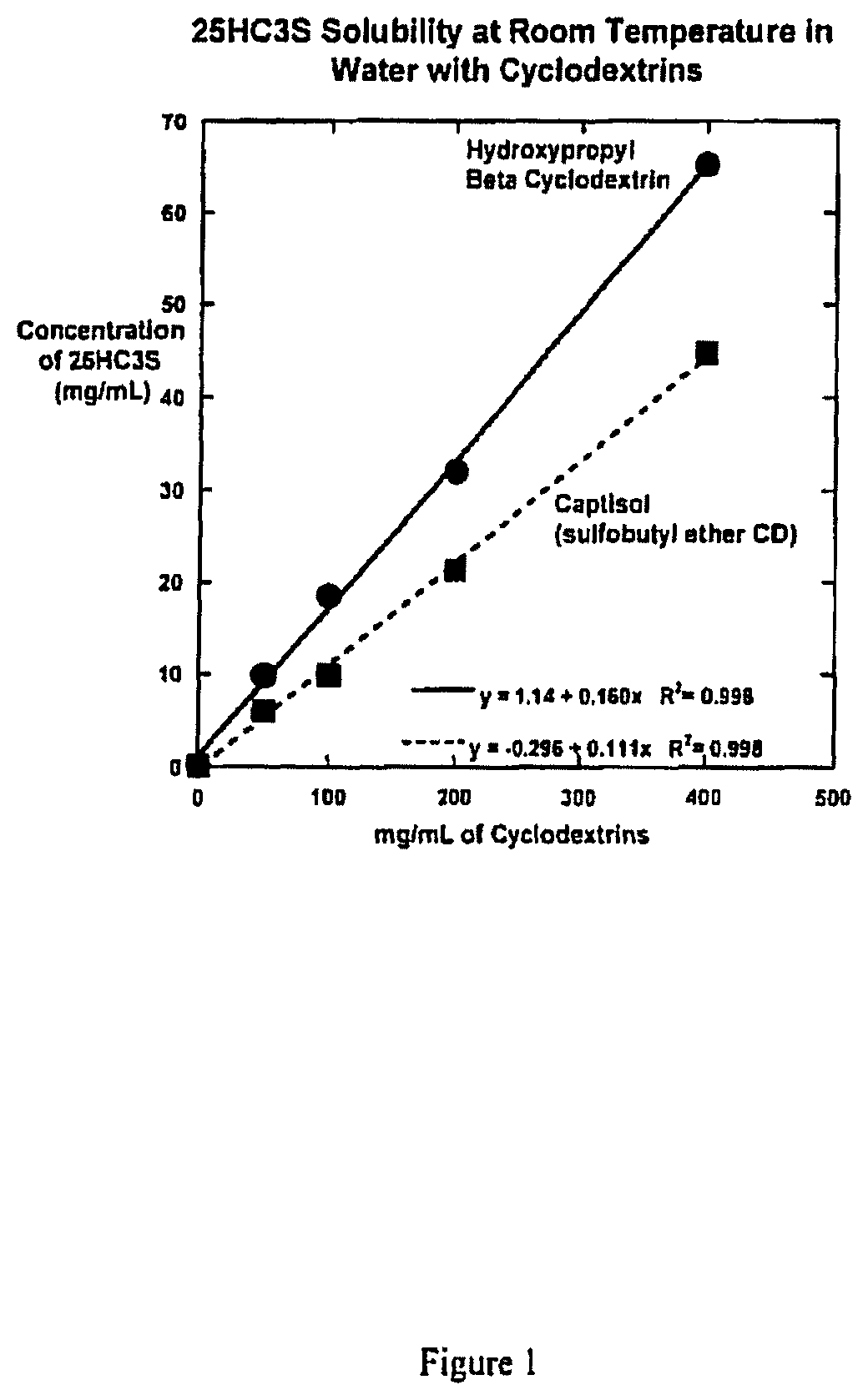
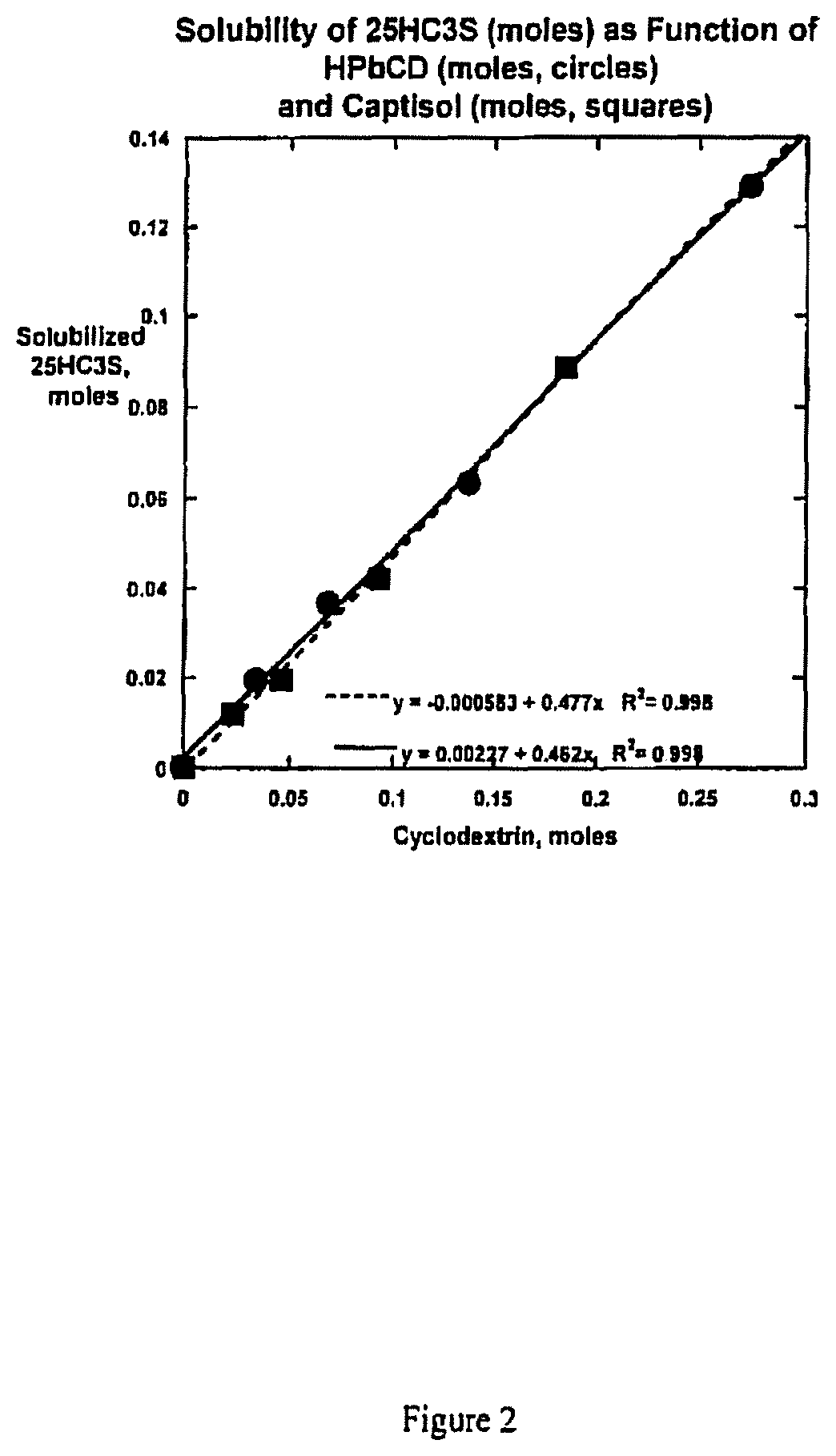
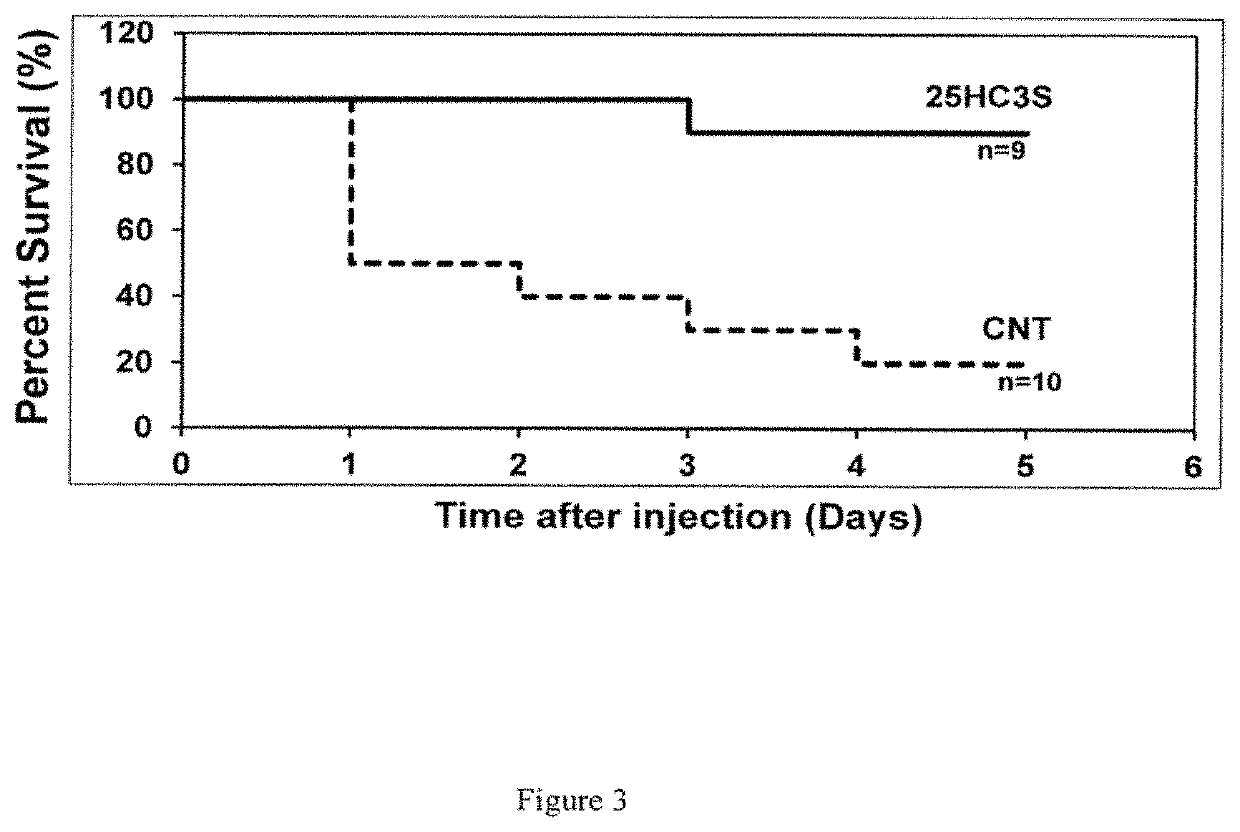
Compositions comprising 5-cholesten-3, 25-diol, 3-sulfate (25hc3s) or pharmaceutically acceptable salt thereof and at least one cyclic oligosaccharide
Compositions comprising 5-cholesten-3, 25-diol, 3-sulfate (25HC3S) or pharmaceutically acceptable salt thereof and at least one cyclic oligosaccharide, e.g., a cyclodextrin (CD), are provided. The compositions may be used to prevent and / or treat a variety of diseases and conditions, including organ failure (e.g. acute liver failure), high cholesterol / high lipids, and various inflammatory diseases and conditions.
Owner:VIRGINIA COMMONWEALTH UNIV +2
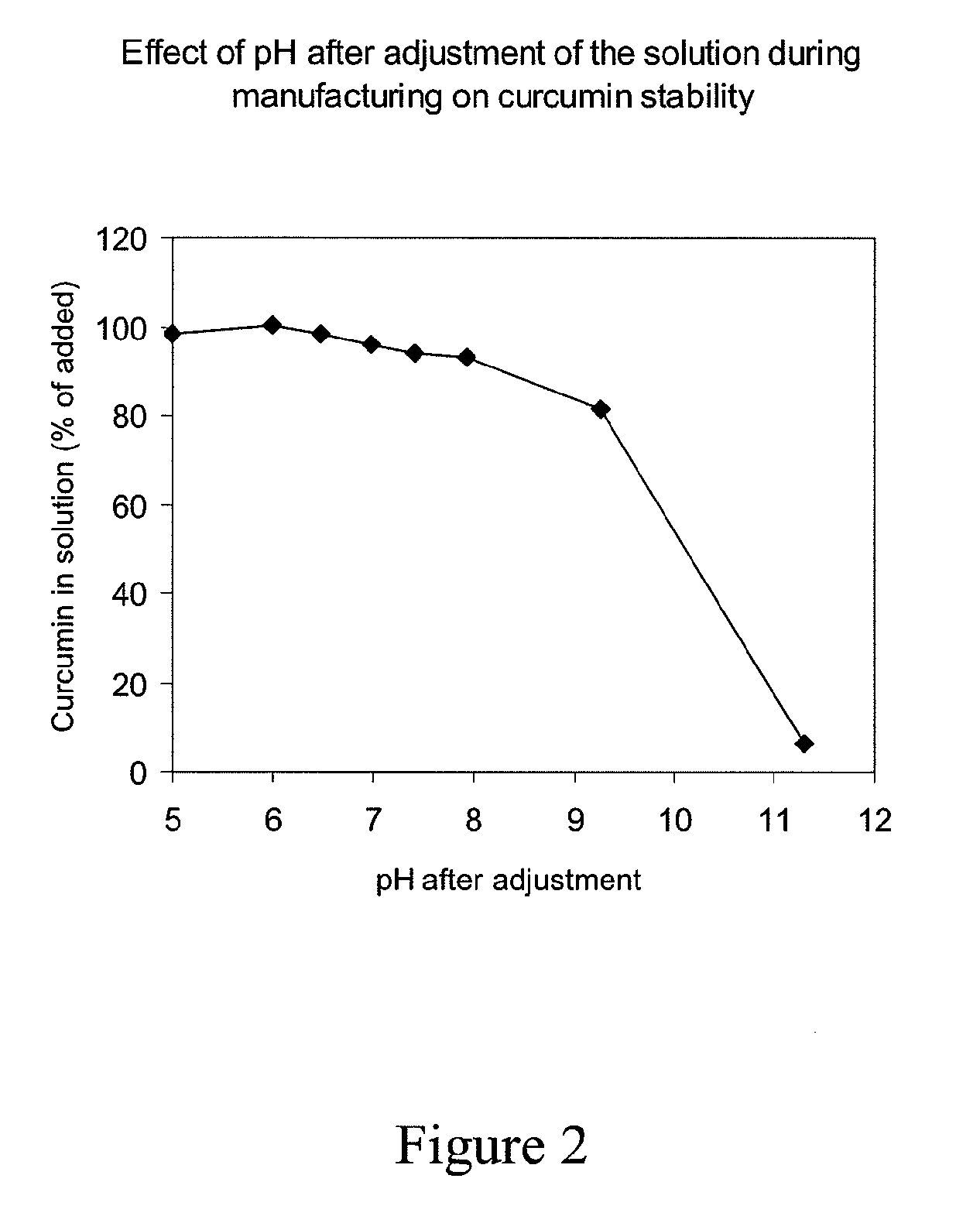
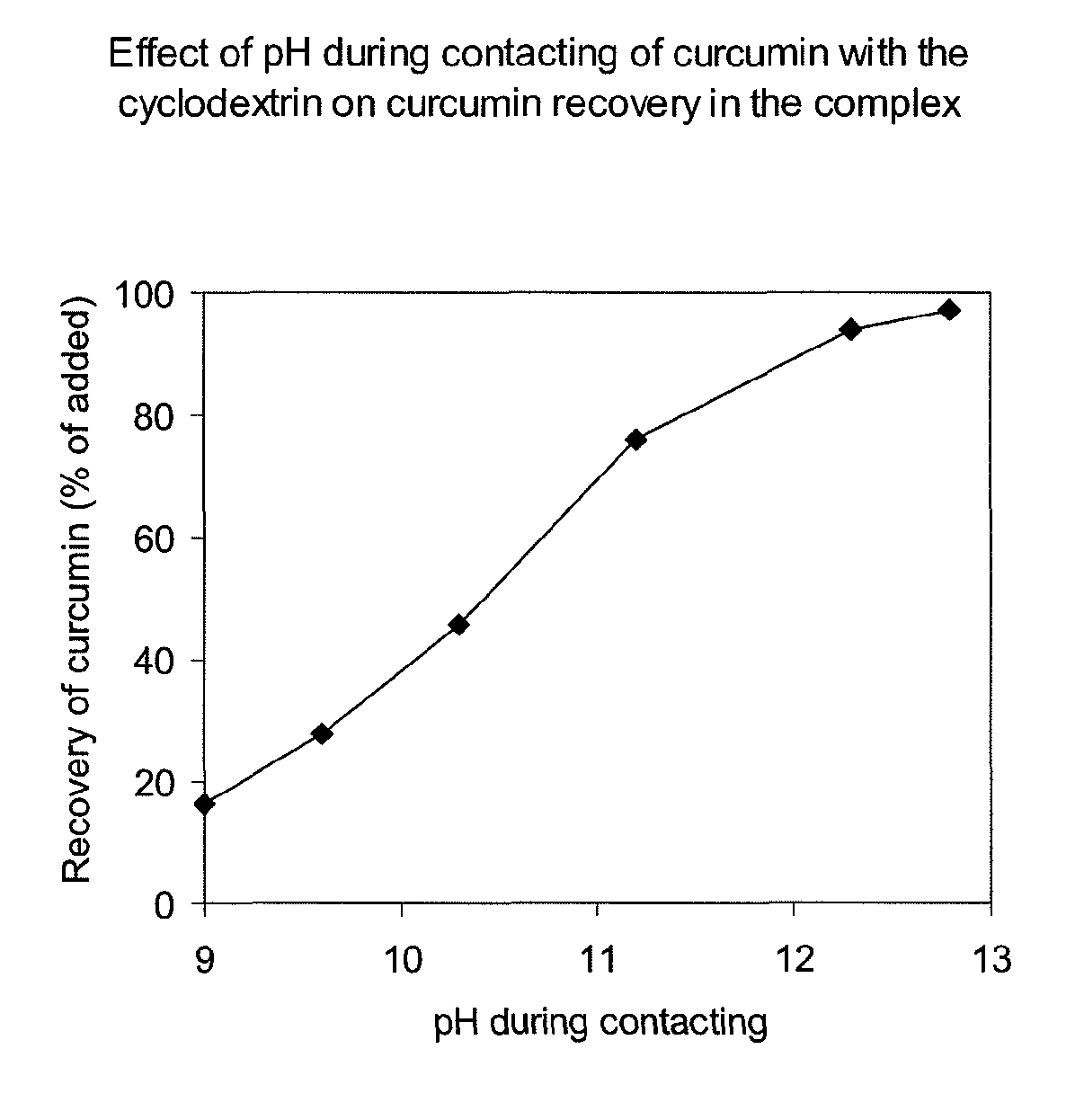
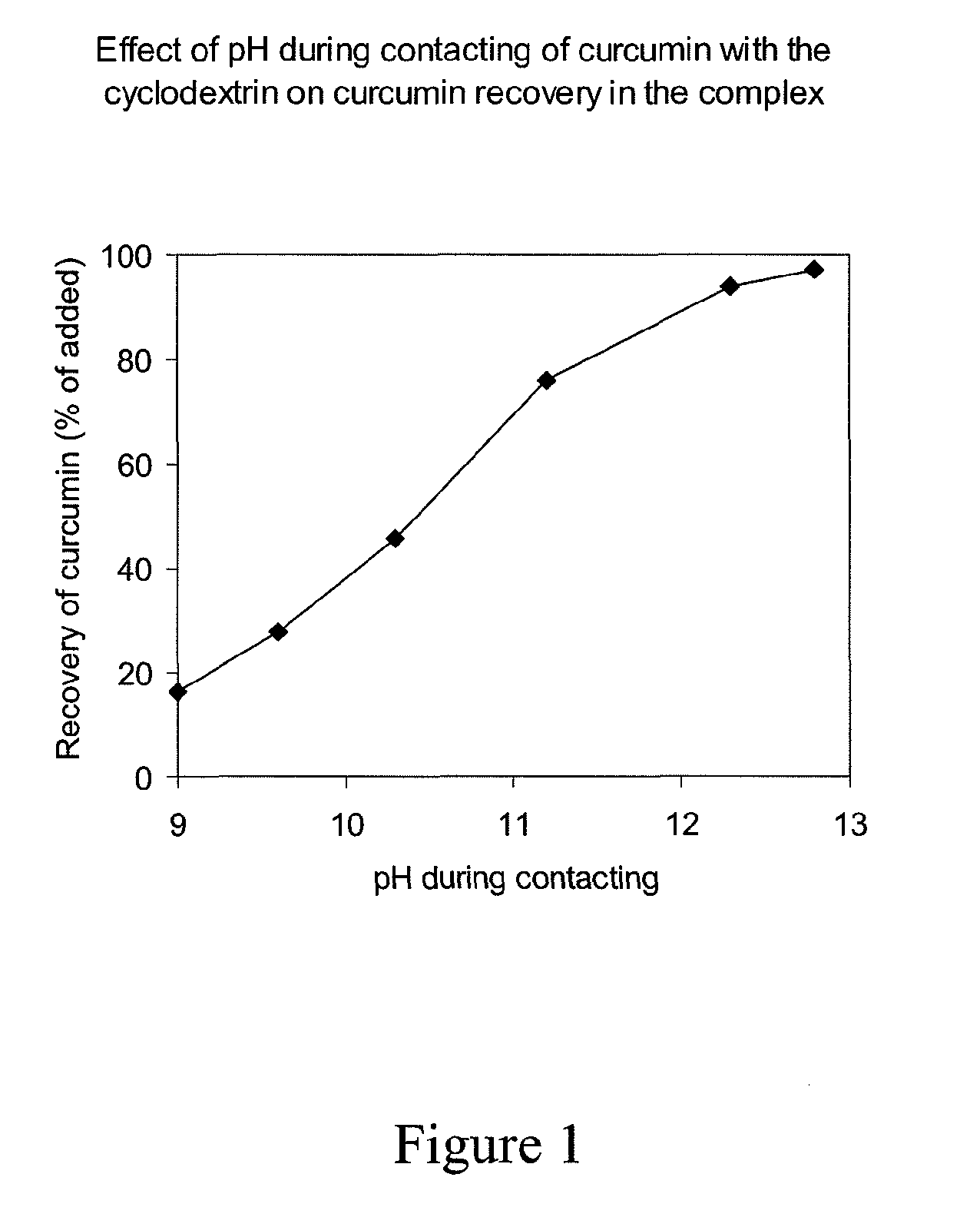
Soluble complexes of curcumin
InactiveUS20110034564A1Improve stabilityIncrease concentrationAntibacterial agentsBiocideALI - Acute lung injuryRisk stroke
A composition comprising a water-soluble and stable complex formed by an alkyl ether derivative of gamma-cyclodextrin and curcumin and optionally comprising non-complexed cyclodextrin, the molar ratio of curcumin to cyclodextrin being between 1:1 and 1:6, and a method of manufacturing such a composition. The water-soluble and stable complex of curcumin is useful in therapy, e.g. for treatment of cancer, leukemia, myocardial infarction, stroke, sepsis, acute lung injury, acute liver failure, acute tubular necrosis, acute pancreatitis, radiation injury and other life-threatening conditions in a human or animal subject, as well as for preserving human or animal organs, tissues or cells at a hypothermic temperature.
Owner:NOVOBION OY
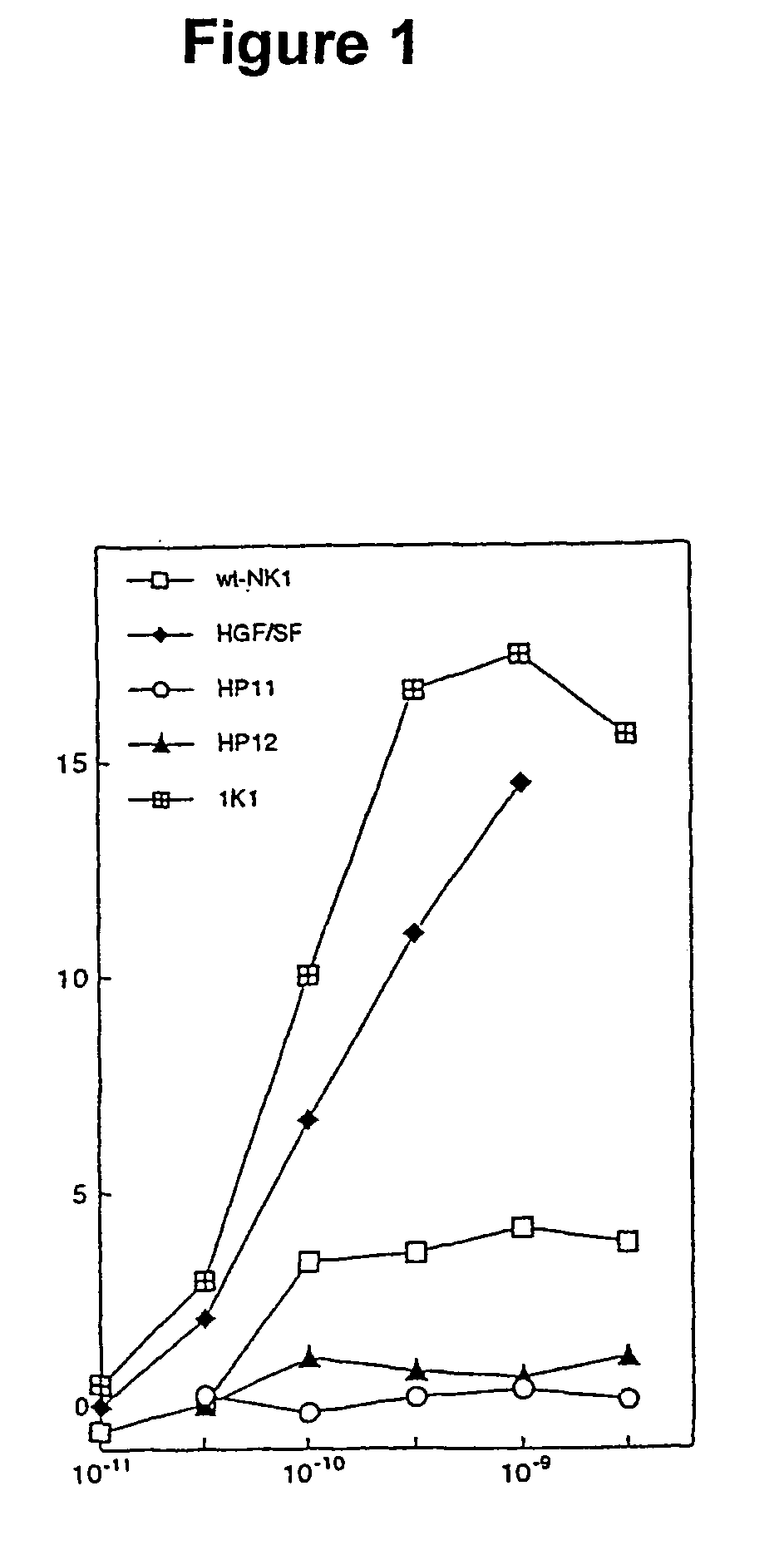
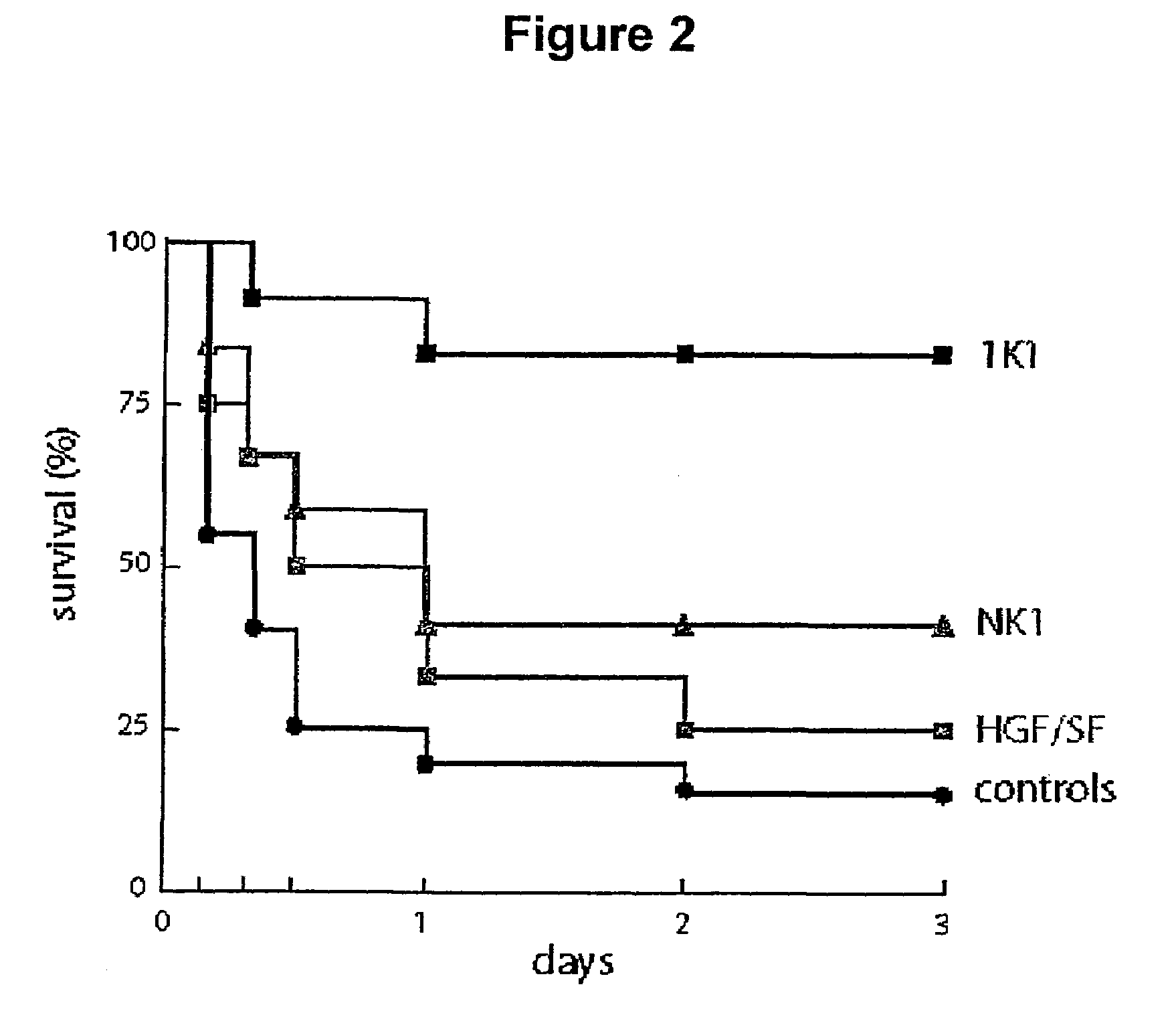
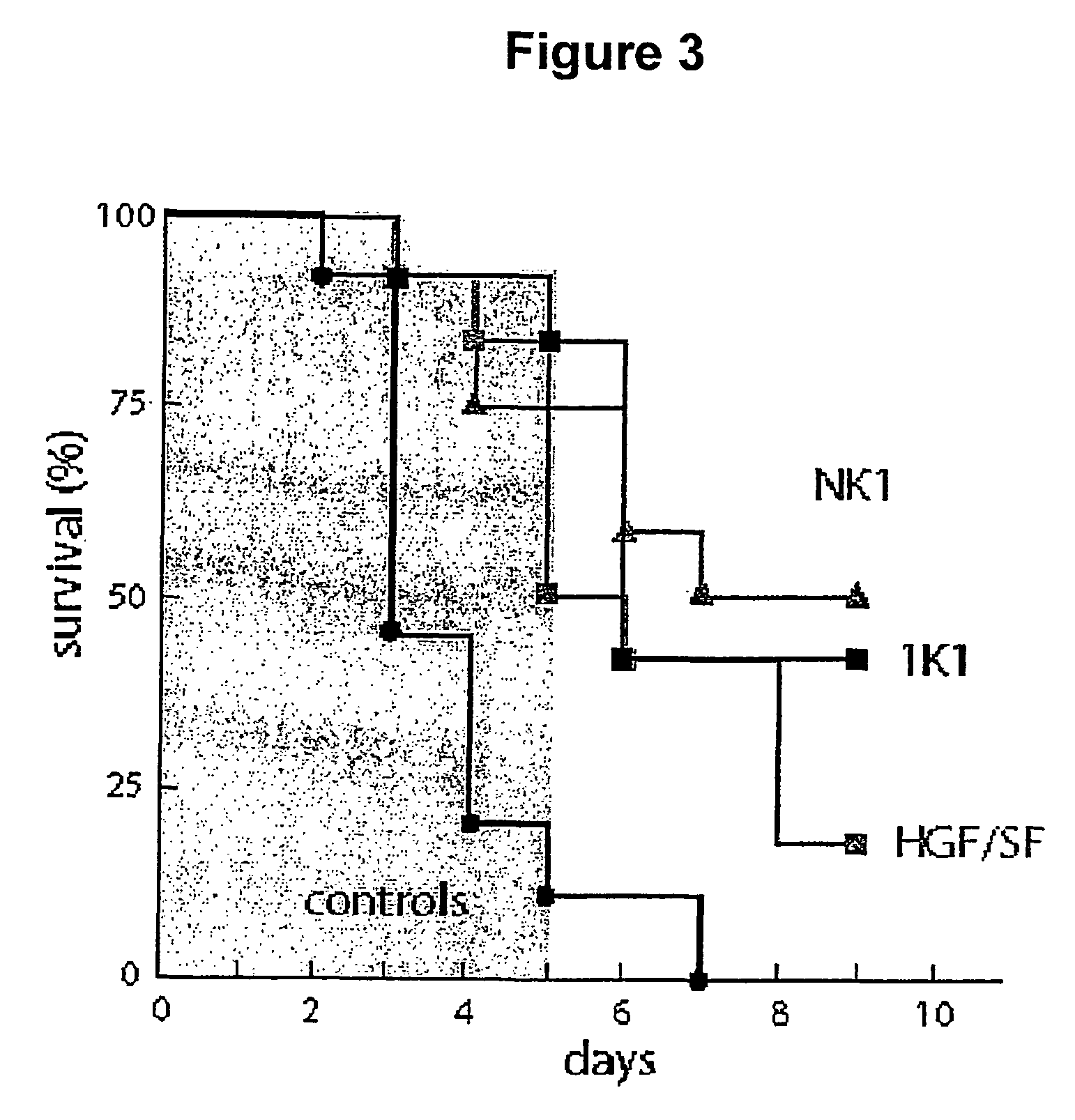
Variants of the NK1 fragment of hepatocyte growth factor/scatter factor (HGF/SF) and their use
Variants of the NK1 fragment of the polypeptide growth factor HGF / SF which act as agonists of the MET receptor and their use are disclosed. The agonists comprise at least one substitution at positions equivalent to 132, 134, 170 and 181 of full length HGF / SF (SEQ ID NO:2) and these substitutions provide variants which show scatter factor activity and induce DNA synthesis. In vivo, the variants provide protection from liver damage in a model of acute liver failure.
Owner:CAMBRIDGE UNIV TECH SERVICES LTD +1
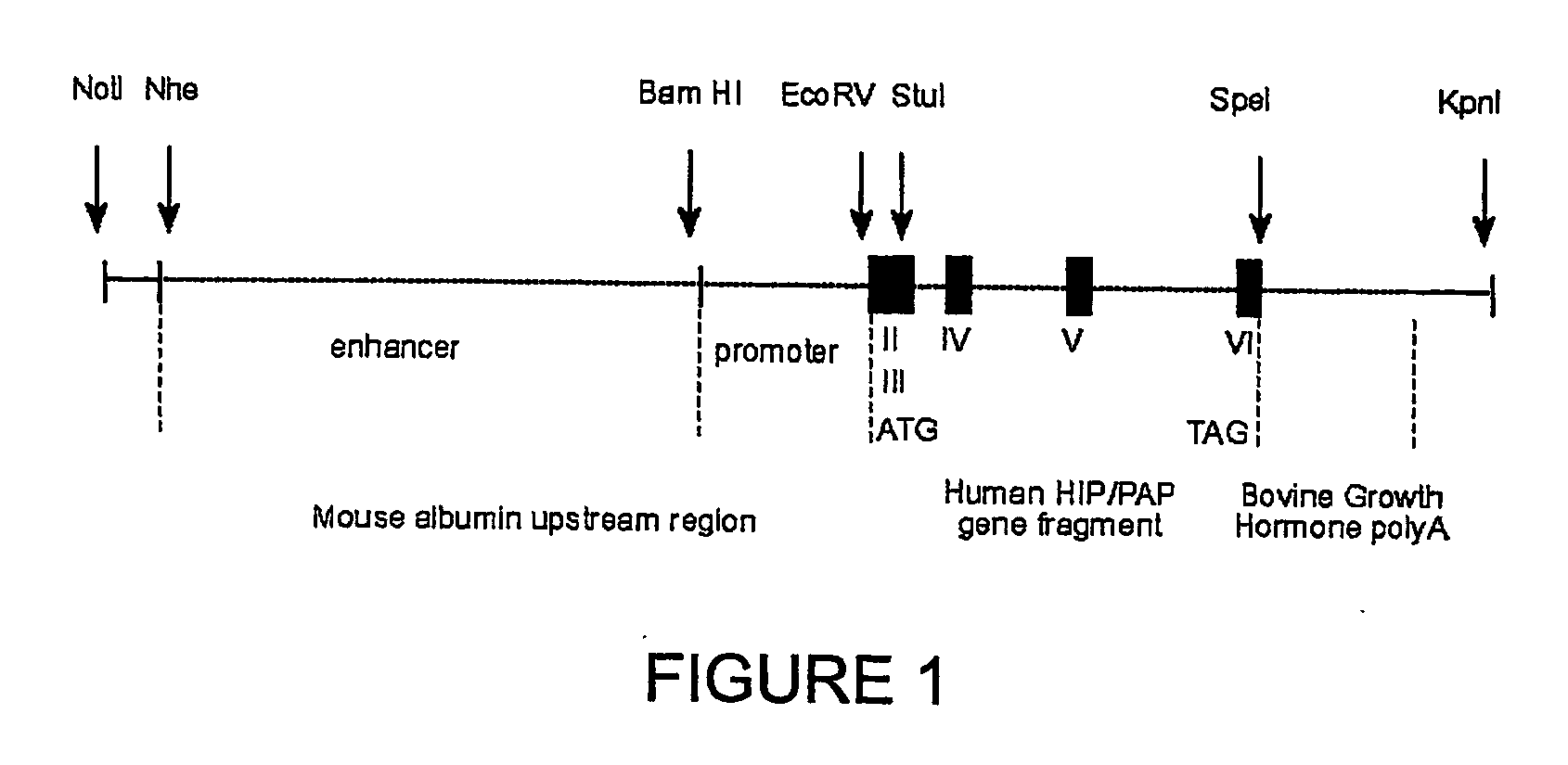
Hip/pap polypeptide compositions for use in liver regeneration and for the prevention of liver failure
This invention is based on the experimental finding that HIP / PAP has mitogenic and antiapoptotic effects in vitro on hepatocytes in primary culture. Moreover, HIP / PAP is a mitogenic and anti-apoptotic molecule for hepatocytes, in vivo, during liver failure and liver regeneration. The present invention is also based on the experimental finding that HIP / PAP administration has no adverse effects in mammals. This invention concerns a pharmaceutical composition for stimulating liver regeneration in vivo including after chronic / acute liver failure, comprising an effective amount of a polypeptide comprising an amino acid sequence having 90% amino acid identity with the polypeptide consisting of the amino acid sequence starting at the amino acid residue (36) and ending at the amino acid residue (175) of sequence SEQ ID No. 1, in combination with at least one physiologically acceptable excipient.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
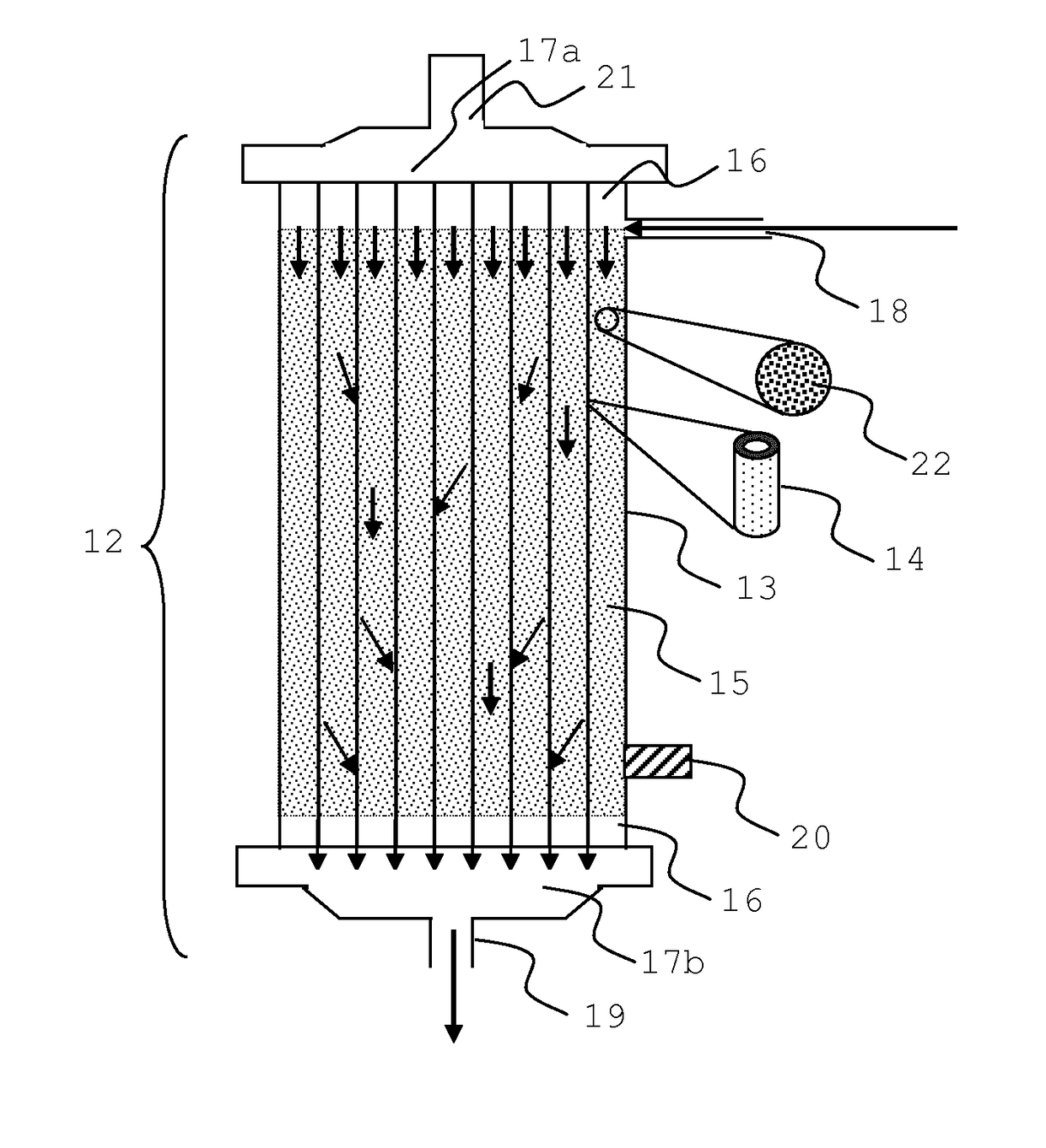
Integrated device for liver support system
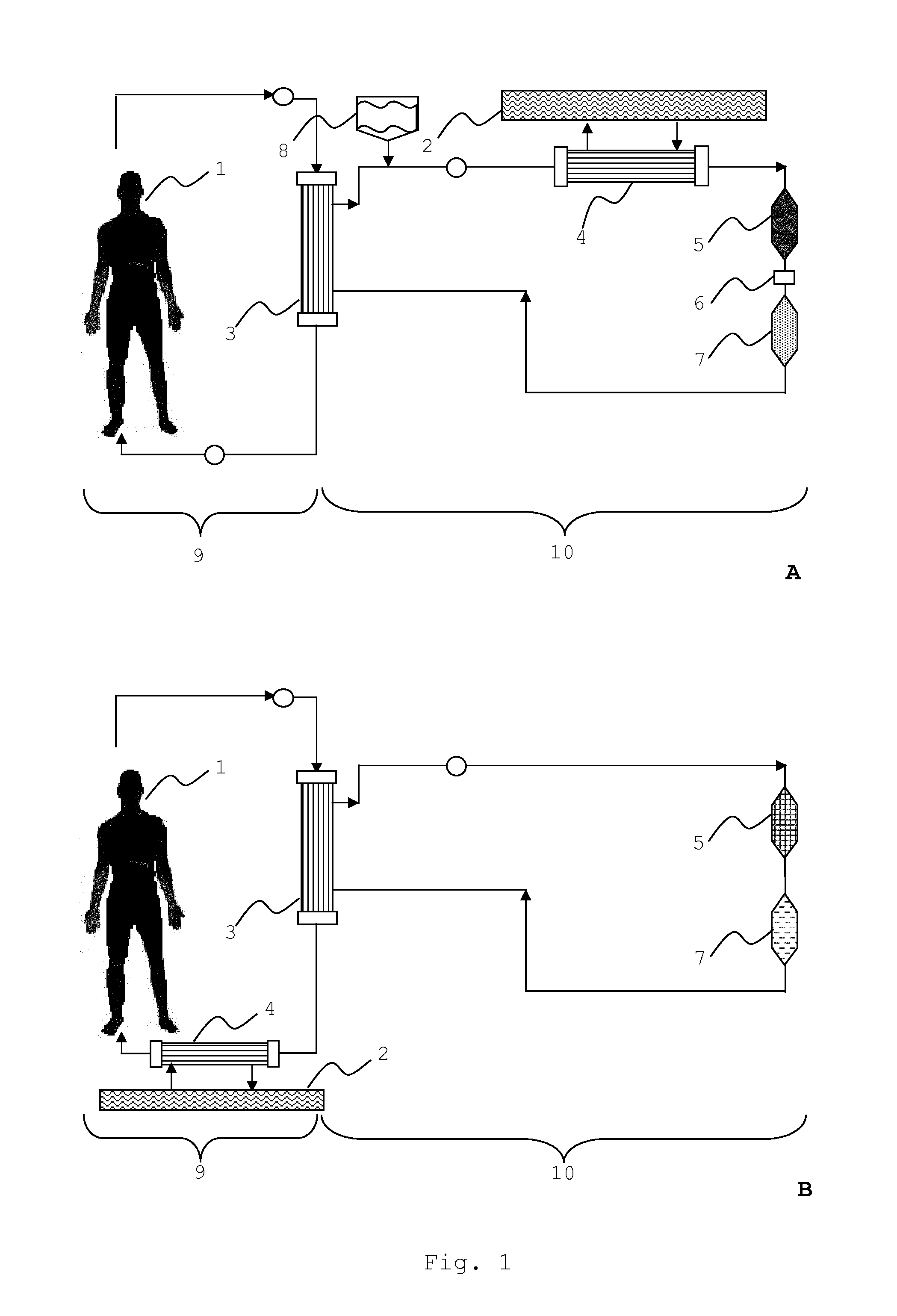
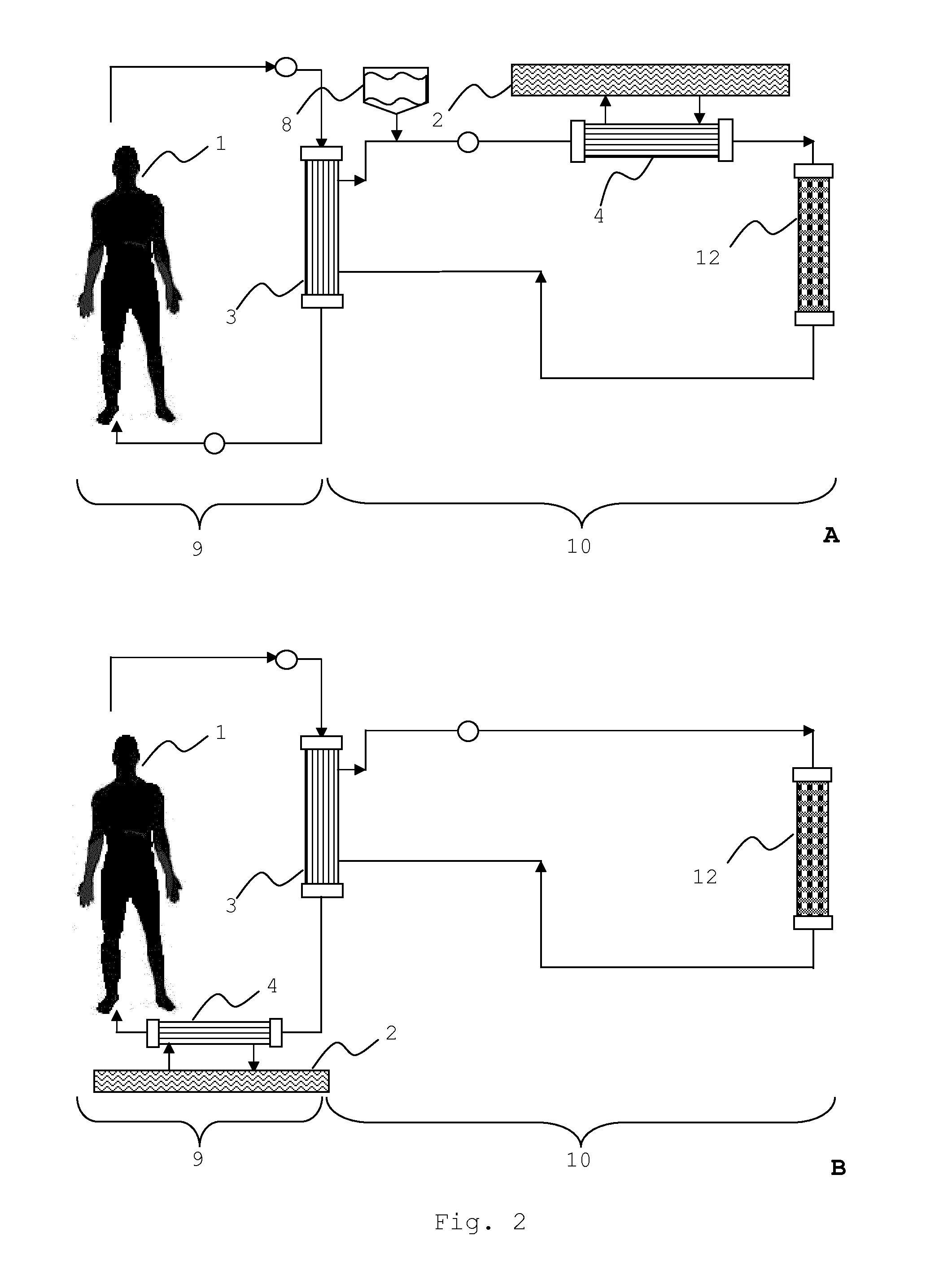
An extracorporeal system for liver dialysis comprises a filter device having hollow fibers with integrated ion-exchange particles and hydrophobic adsorbent particles in the filtrate space. The system can be used for the treatment of acute liver failure and acute-on-chronic liver failure.
Owner:GAMBRO LUNDIA AB
Drug for treating end-stage liver diseases caused by chronic hepatitis B and preparation method of drug
InactiveCN104383055AEasy to prepareAbundant raw materialsDigestive systemPlant ingredientsAngelica Sinensis RootRadix Astragali seu Hedysari
The invention discloses a drug for treating end-stage liver diseases caused by chronic hepatitis B. The drug is prepared from the following raw materials in parts by weight: 30-60 parts of astragalus, 20-30 parts of radix pseudostellariae, 20-30 parts of fried rhizoma atractylodis macrocephalae, 10-15 parts of tangerine peel, 10-20 parts of angelica sinensis, 10-15 parts of tuckahoe, 3-6 parts of honey-fried licorice roots, 10-20 parts of scutellaria baicalensis and 10-20 parts of ligusticum wallichii. The traditional Chinese medicine composition is prepared by using a conventional traditional Chinese medicine preparation method and comprises the dosage forms such as a decoction, a capsule or powder. The drug for effectively treating end-stage liver diseases including chronic and acute liver failure, chronic liver failure and decompensated cirrhosis caused by chronic hepatitis B is created on the basis of multi-year clinical experience and is remarkable in curative effect.
Owner:THE SECOND HOSPITAL OF NANJING
Acute hepatic insufficiency depressant and method for evaluating drug efficacy thereof
ActiveUS20140234341A1Constant effectPoor prognosisPeptide/protein ingredientsHepatocyte-growth/scatter/tumor-cytotoxic factorHepatic comaDepressant
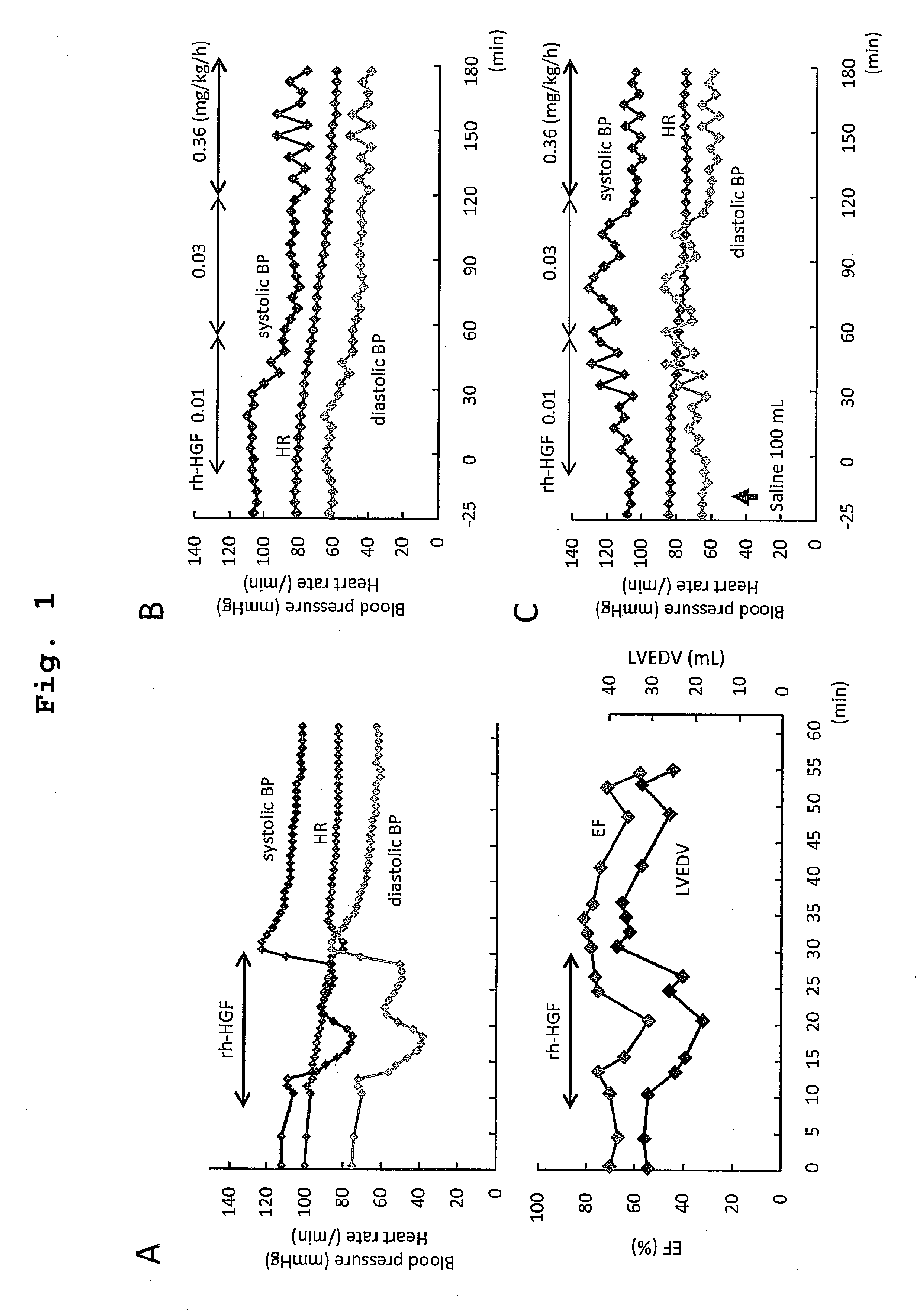
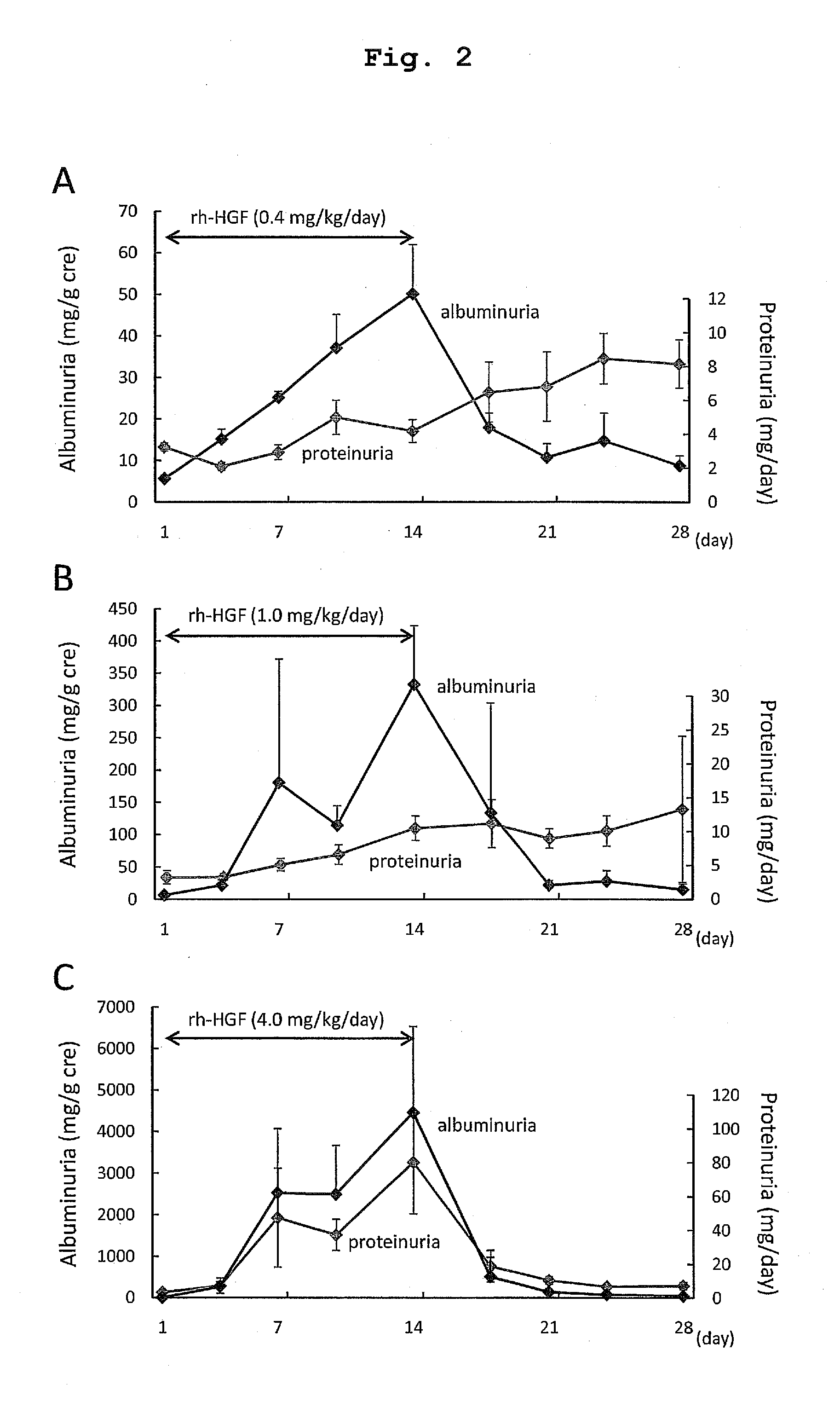
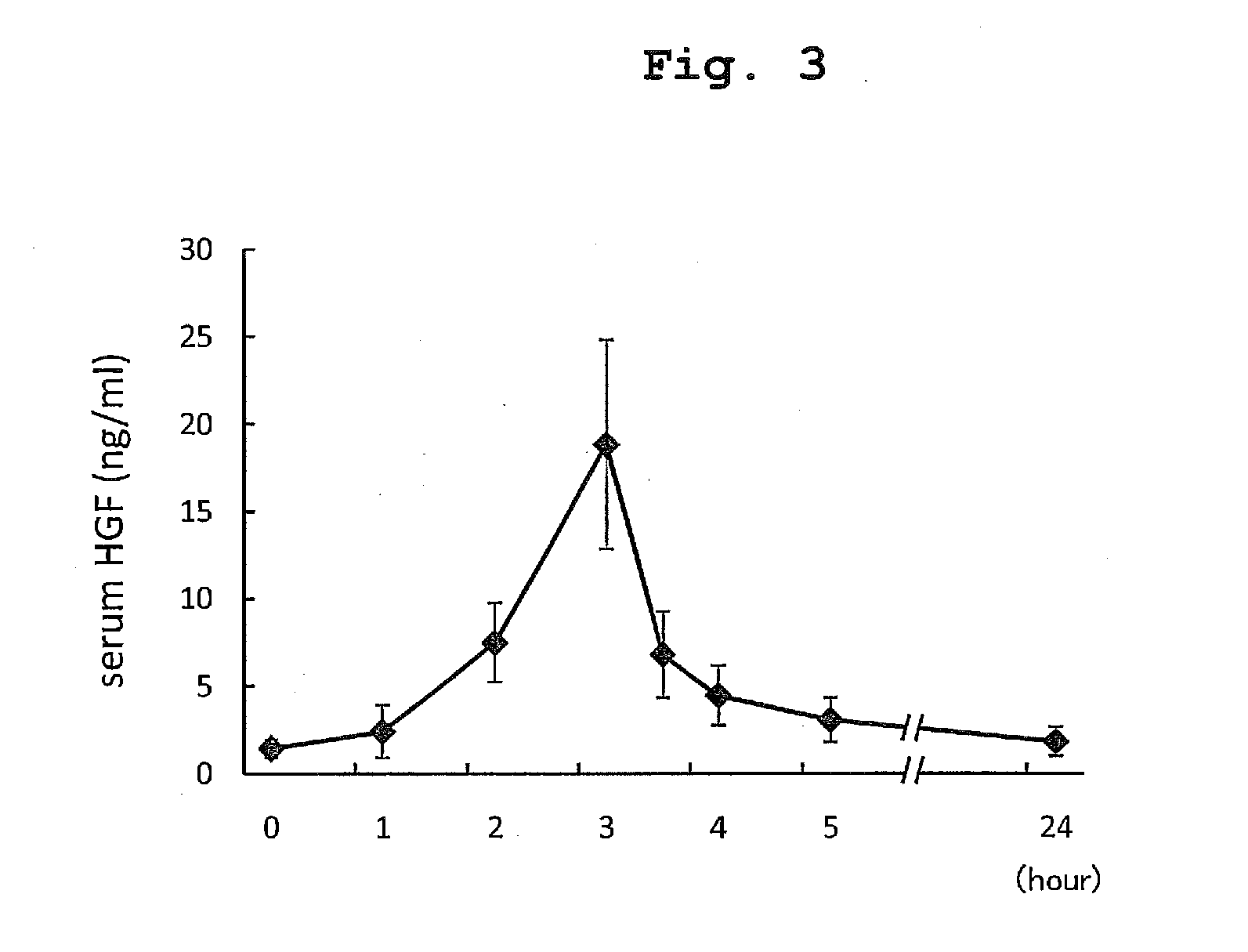
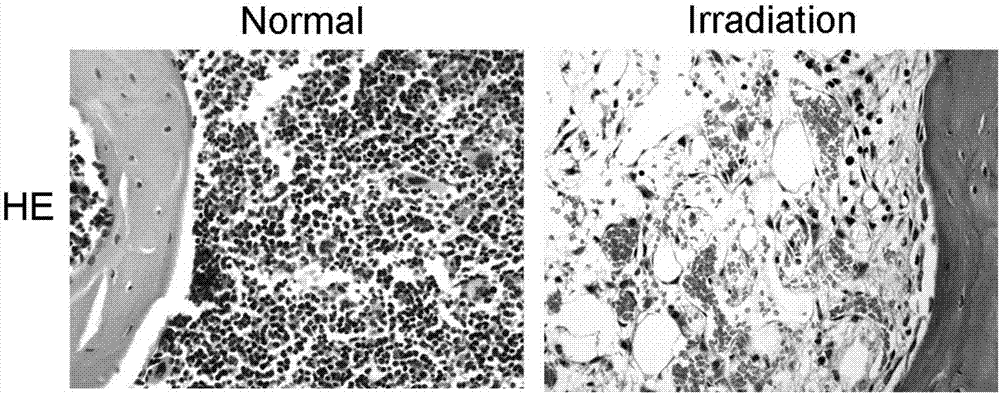
The present invention provides a therapeutic agent for acute liver failure containing a hepatocyte growth factor (HGF), particularly an agent for treating fulminant hepatitis or late onset hepatic failure or suppression of progression of acute liver failure without hepatic coma to fulminant hepatitis or late onset hepatic failure. The present invention also provides a method for evaluating the efficacy of HGF including measuring the amount of α-fetoprotein (liver regeneration biomarker) and / or soluble Fas (anti-apoptotic biomarker) in a sample obtained from a liver injury patient administered with HGF.
Owner:KYOTO UNIV
Application of peripheral blood mononuclear cell activation inhibitors to preparing medicines for treating acute hepatic failure
ActiveCN106963780APrevention of Acute Liver InjuryDigestive systemMammal material medical ingredientsAcute hepatic failureBiological activation
The invention discloses application of peripheral blood mononuclear cell activation inhibitors to preparing a medicine for treating and / or preventing acute hepatic failure, and further provides the medicine for treating and / or preventing the acute hepatic failure. The medicine is a preparation, the peripheral blood mononuclear cell activation inhibitors are used as active substances for the preparation, and pharmacologically acceptable excipients or auxiliary components are added into the peripheral blood mononuclear cell activation inhibitors to prepare the preparation. The application and the medicine have the advantages that the fact that activation of mononuclear cells can be effectively inhibited is proved, accordingly, acute hepatic injury can be effectively treated and prevented, a novel option can be provided for clinically treating the acute hepatic injury, and the medicine has an excellent application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Medicine composition with acetylcysteine and medical application of medicine composition
InactiveCN105777856AGood treatment effectHighlight substantive featuresOrganic active ingredientsDigestive systemPharmacologic actionAcetylcysteine
The invention discloses a medicine composition with acetylcysteine and medical application of the medicine composition. The medicine composition with acetylcysteine contains clenbuterol hydrochloride and a natural product compound (I) which adopts a novel structure; when acetylcysteine and the compound (I) work independently, the effects of reducing serum transaminase, bilirubin and urea nitrogen contents of liver damaged animals can be achieved, jaundice and renal dysfunction are relieved, and the toxin content of plasma can be reduced; when acetylcysteine and the compound (I) are combined, the pharmacologic action is more obvious, and the medicine composition can be prepared into a medicine for treating acute liver failure; compared with the prior art, the medicine composition disclosed by the invention has outstanding substantive features and a remarkable progress.
Owner:陈昊
Compositions comprising 5-cholesten-3, 25-diol, 3-sulfate (25hc3s) or pharmaceutically acceptable salt thereof and at least one cyclic oligosaccharide
Compositions comprising 5-cholesten-3, 25-diol, 3-sulfate (25HC3S) or pharmaceutically acceptable salt thereof and at least one cyclic oligosaccharide, e.g., a cyclodextrin (CD), are provided. The compositions may be used to prevent and / or treat a variety of diseases and conditions, including organ failure (e.g. acute liver failure), high cholesterol / high lipids, and various inflammatory diseases and conditions.
Owner:DURECT CORP +2
Soluble complexes of curcumin
InactiveUS8568815B2Improve stabilityIncrease concentrationAntibacterial agentsBiocideDiseaseRisk stroke
Owner:NOVOBION OY
Medicinal composition for treating acute renal failure
The invention relates to the use of a group of Chinese medicinal compositions in treating acute kidney failure and / or enhancing immunity, wherein the medicinal composition comprises the following raw materials (by weight portion): rhizome of Sichuan lovage 15-200 weight parts, astragalus root 40-450 weight parts, Chinese angelica root 20-220 weight parts, epimedium 15-185 weight parts, dried rehmannia root 15-205 weight parts, alisol 15-185 weight parts and poria cocos wolf 15-185 weight parts.
Owner:LIVZON PHARM GRP INC
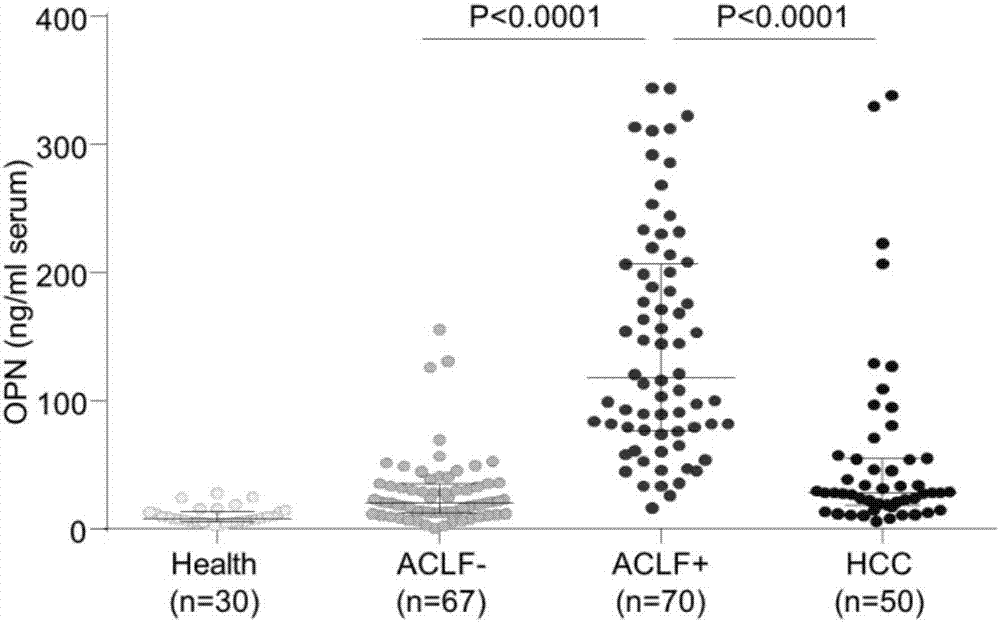
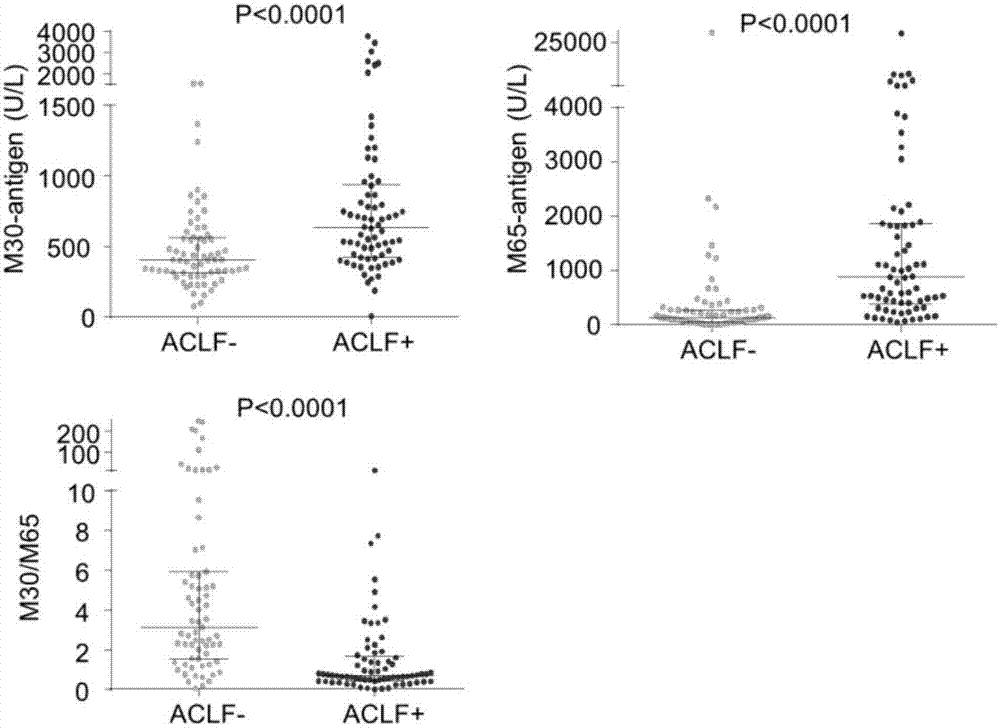
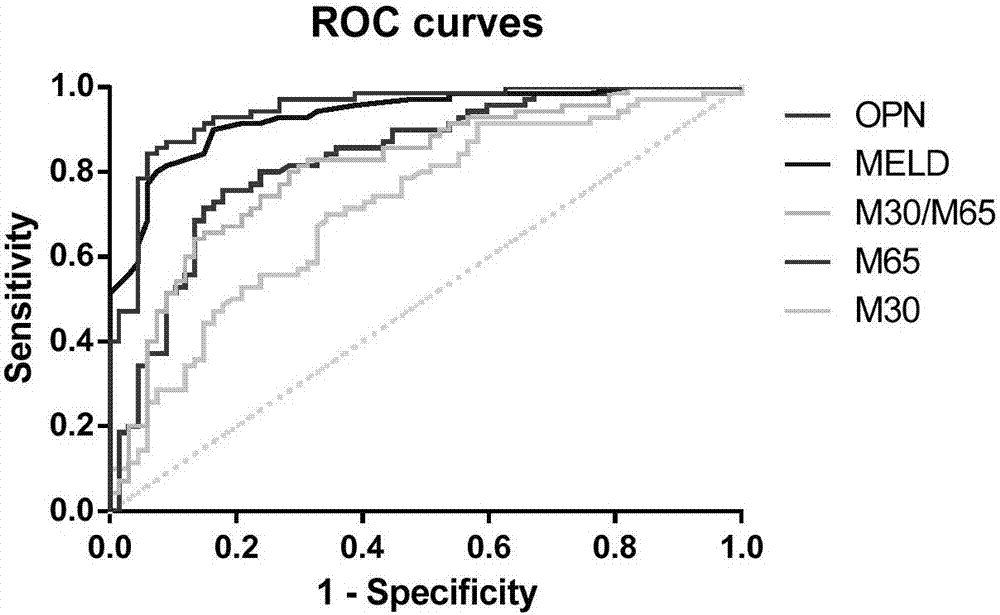
Application of osteopontin in preparation or screening of diagnostic reagents for acute-on-chronic liver failure
The invention belongs to the field of biomedicine and disease diagnosis and particularly relates to application of osteopontin in the preparation or screening of diagnostic reagents for acute-on-chronic liver failure. It is disclosed herein for the first time that osteopontin can act as a biomarker for distinguishing acute-on-chronic liver failure (with diagnostic effect up to 0.9454), significantly higher than M30 (0.7167), M65 (0.8319) and M30 / M65 (0.8100), and almost flush with clinically common index MELD (model for end-stage liver disease) (0.9317).
Owner:孔晓妮 +1
New application of sodium copper chlorophyllin to preparation of medicines for treating liver disease
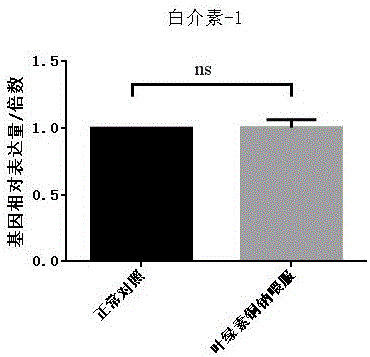
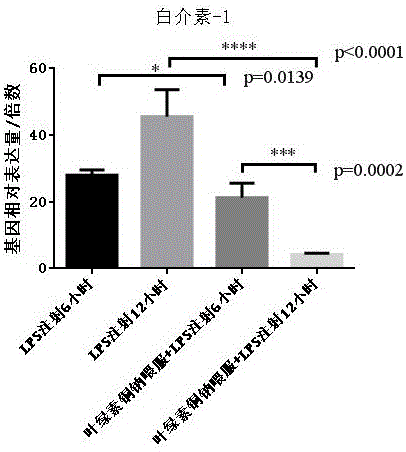
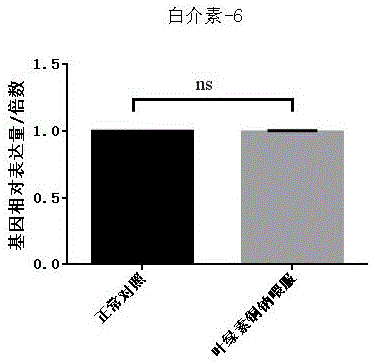
The invention discloses new application of sodium copper chlorophyllin to preparation of medicines for treating liver disease. Acute liver failure is caused by severe liver damage due to various factors, and can lead to acute necrosis and apoptosis of liver cells to show a group of clinical syndromes mainly represented by disturbances of blood coagulation, jaundice, hepatic encephalopathy, ascites and the like, and moreover, inflammatory response, particularly proinflammatory factors in a liver, plays a key role in incidence, deterioration and cure of the disease, so that slowing the inflammatory response can be one of approaches for adjuvant therapy of acute liver failure. Sodium copper chlorophyllin provided by the invention can inhibit interleukin-1 and interleukin-6 in liver tissues, as well as tumor necrosis factor alpha in the liver tissues. Experiments show that sodium copper chlorophyllin can be used to preparation of the medicines for treating liver disease.
Owner:CHENGDU TONGDE PHARMA
Application of Babaodan in preparing medicine for preventing and treating acute liver failure
The invention relates to the technical field of new indication of a medicine, and specifically relates to an application of Babaodan in preparing a medicine for preventing and treating acute liver failure. The application of Babaodan in preparing a medicine for acute liver failure designed in the invention is revealed for the first time; the traditional Chinese medicine Babaodan provided by the invention is verified repeatedly by thioacetamide (TAA) induced acute liver failure rat model experiments, and the result proves that the Babaodan can remarkably improve the liver function and relieve the degrees of liver necrosis and liver injury. Therefore, the Babaodan can be used for preparing a medicine for preventing and treating acute liver failure, and has a good prospect in development andapplication.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M +2
Method for inducing chronic and acute liver failure mouse model by using carbon tetrachloride
InactiveCN110664788ASimple groupingSimple methodCompounds screening/testingHalogenated hydrocarbon active ingredientsBALB/cLaboratory mouse
The invention relates to a method for inducing a chronic and acute liver failure mouse model, in particular to a method for inducing the chronic and acute liver failure mouse model by using carbon tetrachloride, which comprises the following steps of: a grouping method is characterized by randomly and averagely dividing Balb / c inbred line mice into a model group and a control group; an administration method is characterized by diluting carbon tetrachloride to concentrations of 10% and 50% by using olive oil, continuously injecting 10% carbon tetrachloride into the abdominal cavity of a mouse in the model group according to 5-8ml / kg body weight, administrating twice a week, and continuously administrating for 8 weeks; after 8 weeks, injecting the 50% carbon tetrachloride into the abdominalcavity once according to 5-8 ml / kg body weight; and a detection method is characterized by performing biochemical index detection and liver pathological examination on the experimental mouse. The method has the beneficial effects that Balb / c inbred line mice are adopted as model animals, most inbred lines can be adopted, the repeatability is better, the cost is lower compared with rats, and the operation is simpler and more convenient. The model better conforms to the disease development process of clinical hepatitis B virus (HBV) related chronic and acute liver failure.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Integrated device for liver support system
An extracorporeal system for liver dialysis comprises a filter device having hollow fibers with integrated ion-exchange particles and hydrophobic adsorbent particles in the filtrate space. The system can be used for the treatment of acute liver failure and acute-on-chronic liver failure.
Owner:GAMBRO LUNDIA AB
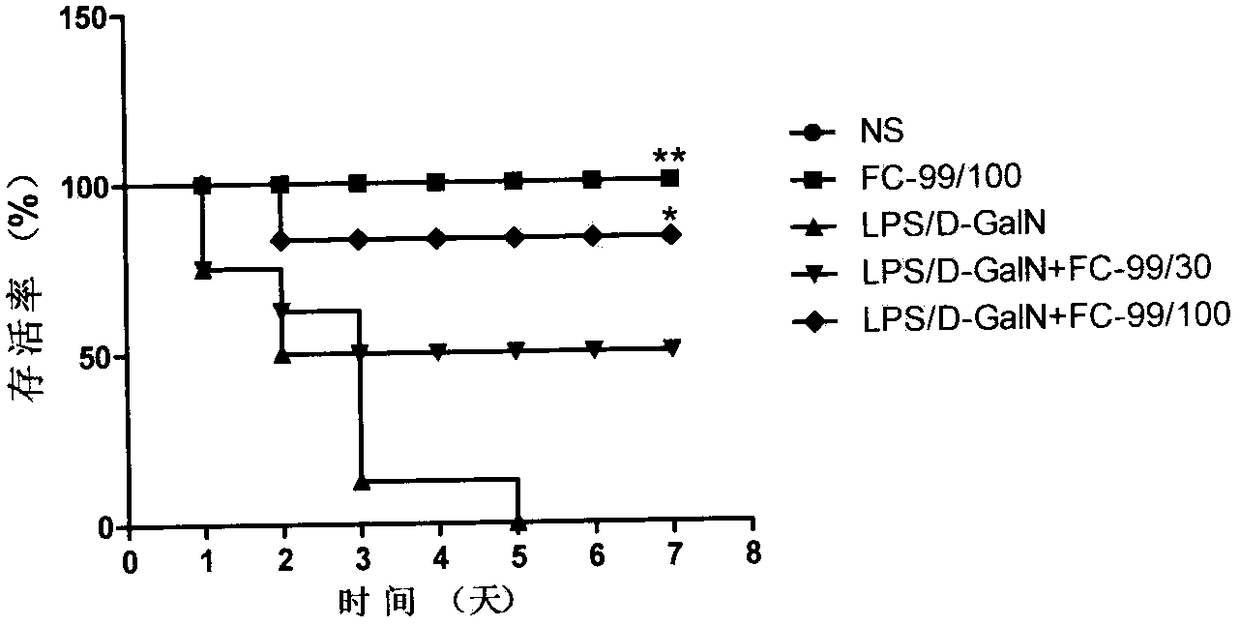
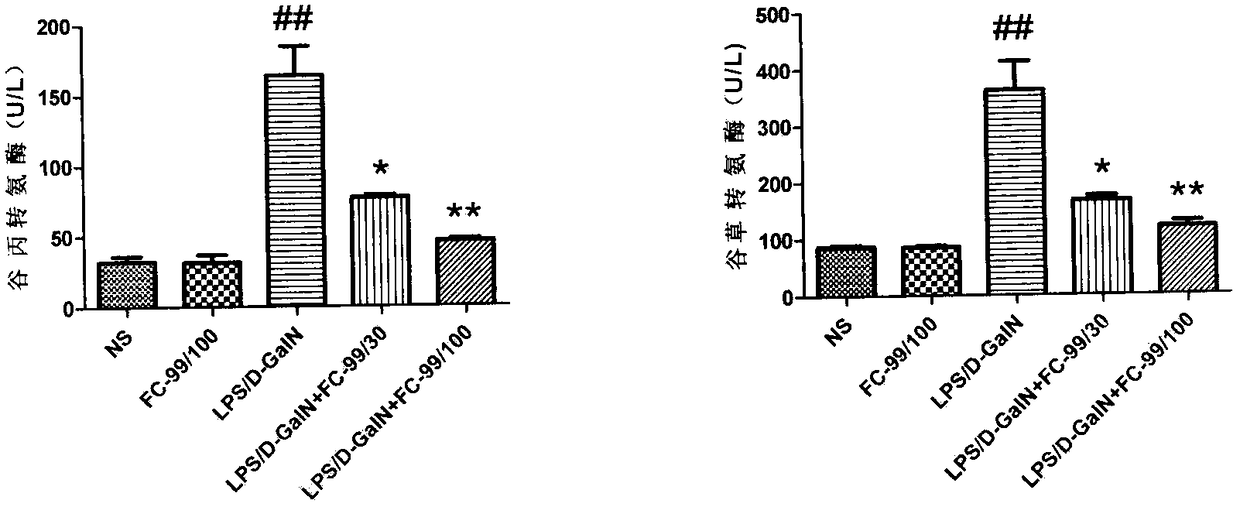
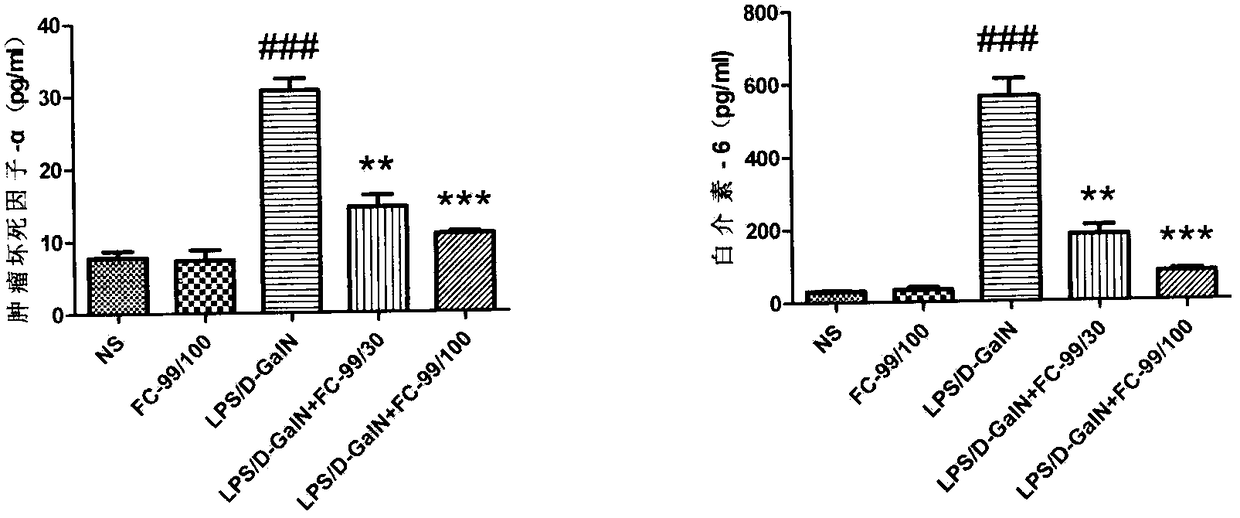
Application of compound FC-99 in preparing drug for preventing or treating acute hepatic failure
The invention discloses application of FC-99 shown as a formula (I) in preparing drug for preventing or treating acute hepatic failure, particularly relates to application of FC-99 in inducing expression of Let-7a (microRNA Let-7A) to inhibit occurrence and development of hepatic failure so as to prevent or treat the hepatic failure and belongs to the field of biomedicine. By building an acute hepatic failure mouse animal model induced by D-aminogalactose combined with lipopolysaccharide and intervening fulminant hepatic failure through FC-99, a result shows that FC-99 can obviously lower transaminase, inhibit inflammation factor release, reduce hepatic cell apoptosis and maintain liver microscopic structure stable and can remarkably lower death rate of mice with acute hepatic failure; bioinformatic analysis and molecular biological detection find that FC-99 is realized by inducing high expression of Let-7a and inhibiting gene expression of IL-6, IRF4, CD86 and SOCS1 in a targeted manner. Therefore, research results show that the compound FC-99 can be applied in preparing drug for preventing or treating acute hepatic failure. In the above application, dosage range for injection andoral administration is 0.01-1000mg per day, and the range can be deviated according to illness degree or dosage form difference.
Owner:NANJING UNIV
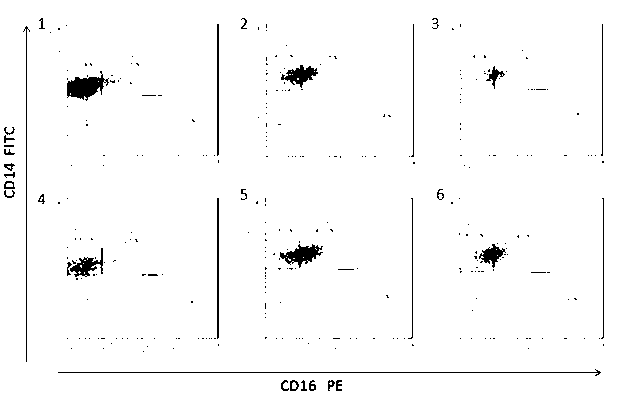
Detection kit used for severe septicopyemia and acute liver failure
InactiveCN103033620AGood outcomePoor outcomeMaterial analysisAntiendomysial antibodiesChronic liver failure
The invention belongs to the technical field of the immunology, and relates to a detection kit for the severe septicopyemia and the acute liver failure. The detection kit comprises a sodium citrate contained anticoagulant tube, an erythrocyte lysate, a PBS solution, a CD45 human monoclonal antibody, a CD14 human monoclonal antibody, a CD16 human monoclonal antibody, and a 1% paraformaldehyde fixing solution. The detection kit can detect the peripheral bloods of septicopyemia and acute / acute-on-chronic liver failure patients through detecting the monocyte differentiation capability in order to determine the prognosis of the above diseases. Detection results show that the obstacles of the monocyte differentiation capability of the diseases still exist; and the peripheral blood monocyte differentiation repression is closely related with the disease prognosis because the mortality of monocyte differentiation repression patients obviously increases and the monocyte differentiation repression patients can survive after the disappearance of the differentiation repression. The detection kit provides positive meaningful reference data for further treatment schemes.
Owner:李海
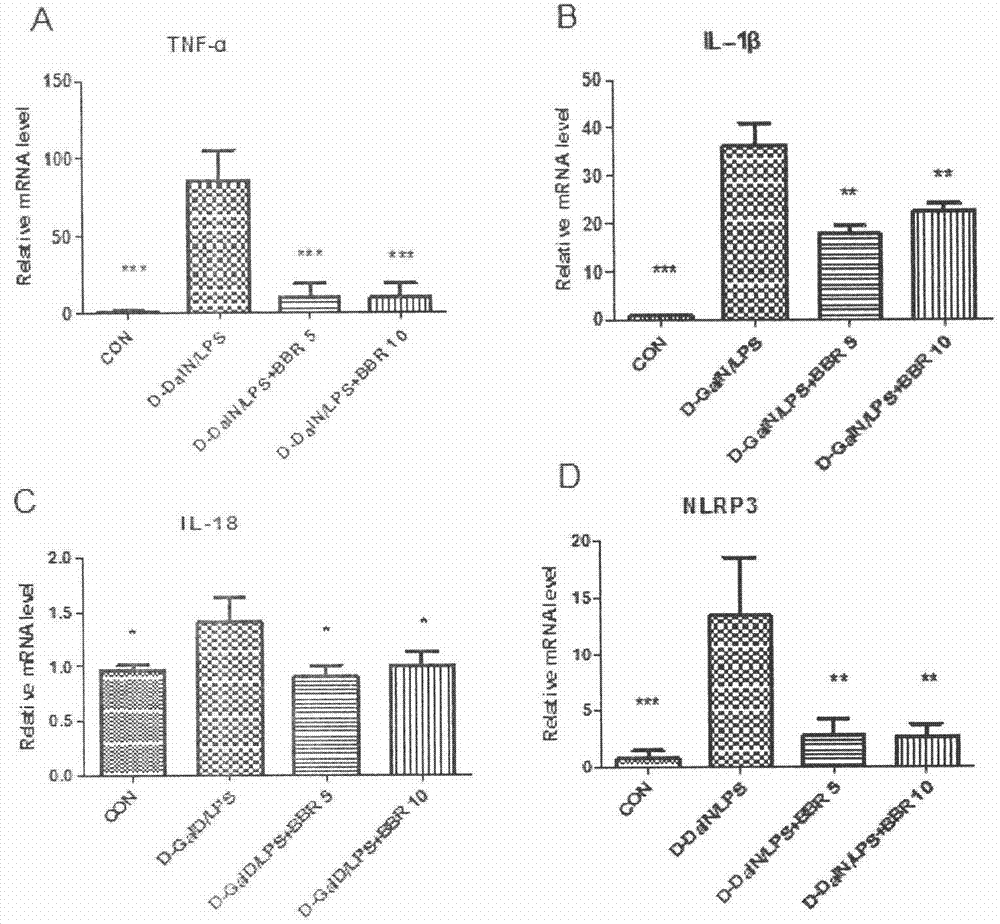
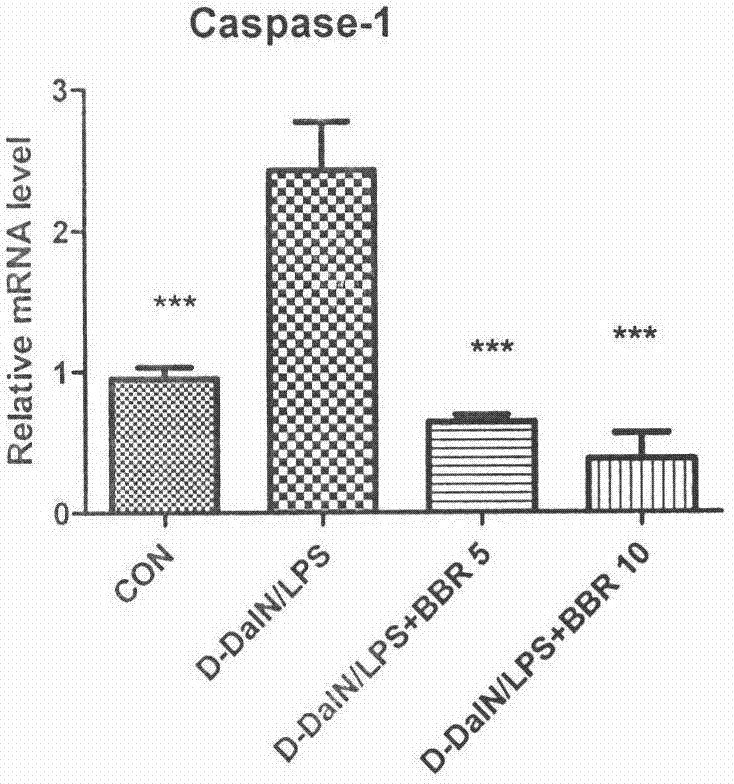
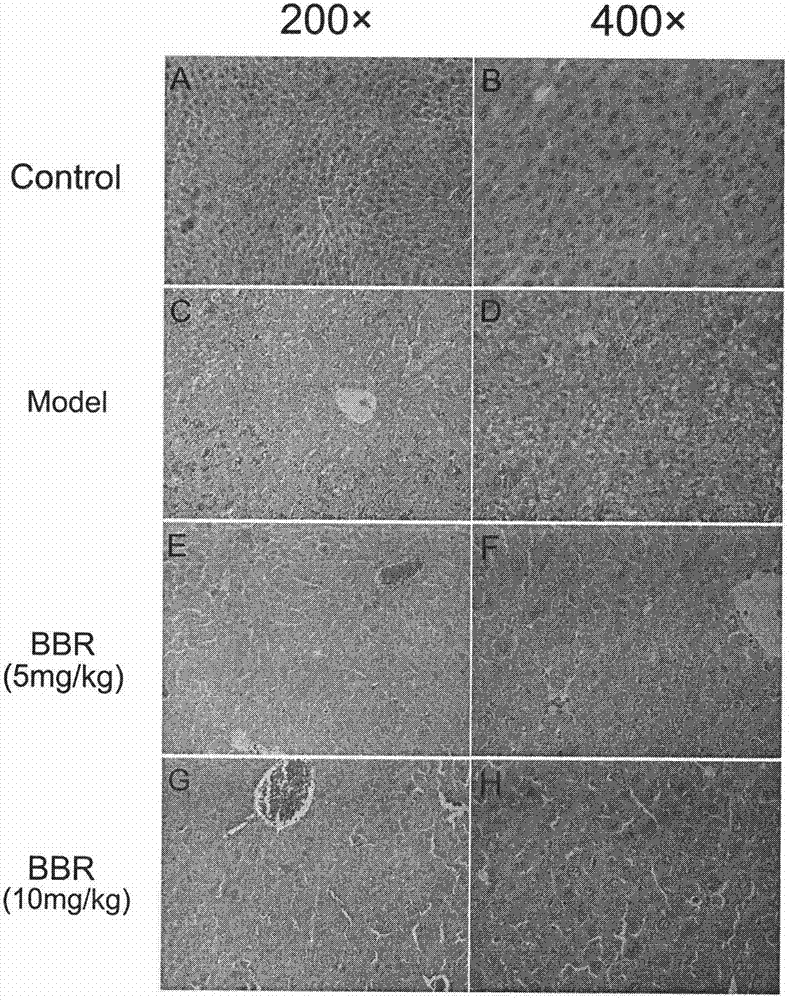
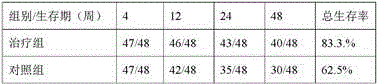
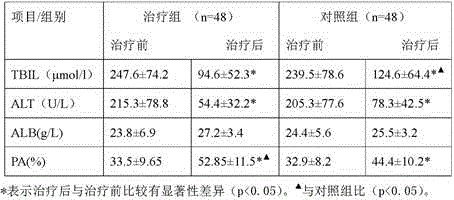
Application of berberine hydrochloride in preparation of medicine used for preventing and/or treating acute hepatic failure
The invention discloses application of berberine hydrochloride shown as a formula (I) in preparation of a medicine used for preventing and / or treating acute hepatic failure and belongs to the field of biological medicine. According to the invention, the prevention and treatment effects of berberine hydrochloride to acute hepatic failure are inspected by building a D-galactosamine and LPS (lipopolysaccharide)-induced acute hepatic failure mouse animal model; results of experimental analysis in biology, pharmacology and molecular biology show that the effects of obviously reducing transaminase, inhibiting oxidative stress injury and inhibiting release of inflammatory factors can be realized by virtue of administration of berberine hydrochloride, and the death rate of acute hepatic failure mice can be obviously reduced, so that research results of the invention show that berberine hydrochloride can be applied to preparation of the medicine used for preventing and / or treating the acute hepatic failure. The formula (I) is described in the specification.
Owner:CHINA PHARM UNIV
Traditional Chinese medicine composition for treating chronic and acute liver failure enterogenic endotoxemia and enema
InactiveCN105456448AEasy dischargeAdjustment disorderAntibacterial agentsDigestive systemOfficinalisGut flora
The invention discloses a traditional Chinese medicine composition for treating chronic and acute liver failure enterogenic endotoxemia. The composition is prepared from, by weight, 20-23 parts of rhubarb, 10-20 parts of cortex magnoliae officinalis, 10-20 parts of fructus aurantii immaturus, 20-40 parts of red peony roots, 10-20 parts of fructus mume, 20-40 parts of dandelions and 10-15 parts of coptidis rhizomes. The traditional Chinese medicine composition can be prepared into an enema through the method including the steps that all the raw materials are evenly mixed, water is added and gets over the medicine surface, the materials are soaked for 20-40 min and decocted for two to five times, decoction solutions obtained each time are combined, and the enema is obtained. The traditional Chinese medicine enema can be used for treating enterogenic endotoxemia, protecting intestinal mucosa, improving intestinal flora and facilitating disease recovery, the length of stay can be shortened, and medical cost is reduced.
Owner:THE FIRST AFFILIATED HOSPITAL OF HENAN UNIV OF TCM
Treatment of necroptosis
ActiveUS20210072242A1Reduce inflammationReduce expressionOrganic active ingredientsDigestive systemDiseaseChronic liver failure
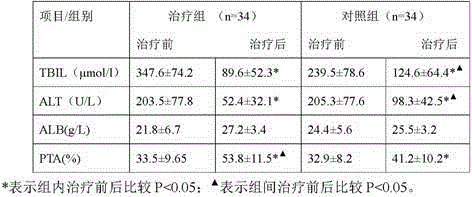
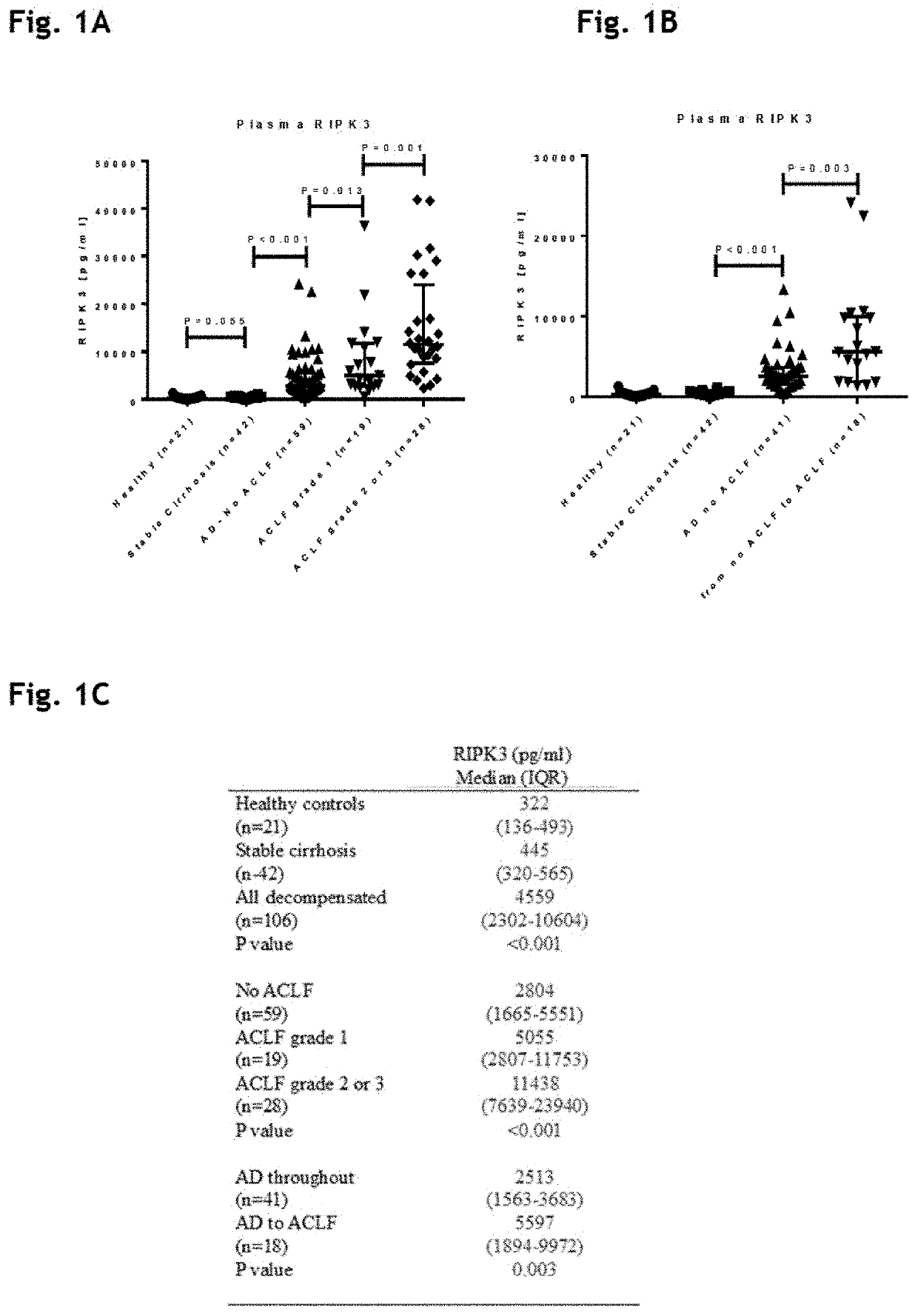
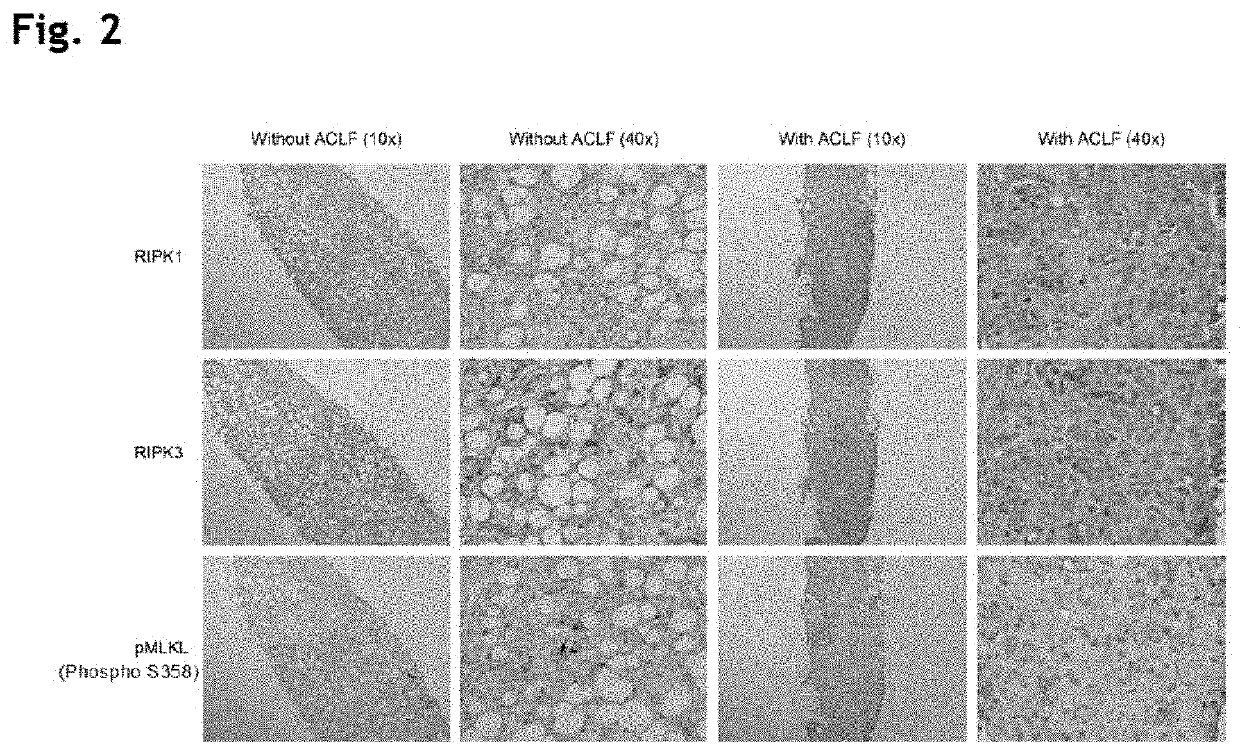
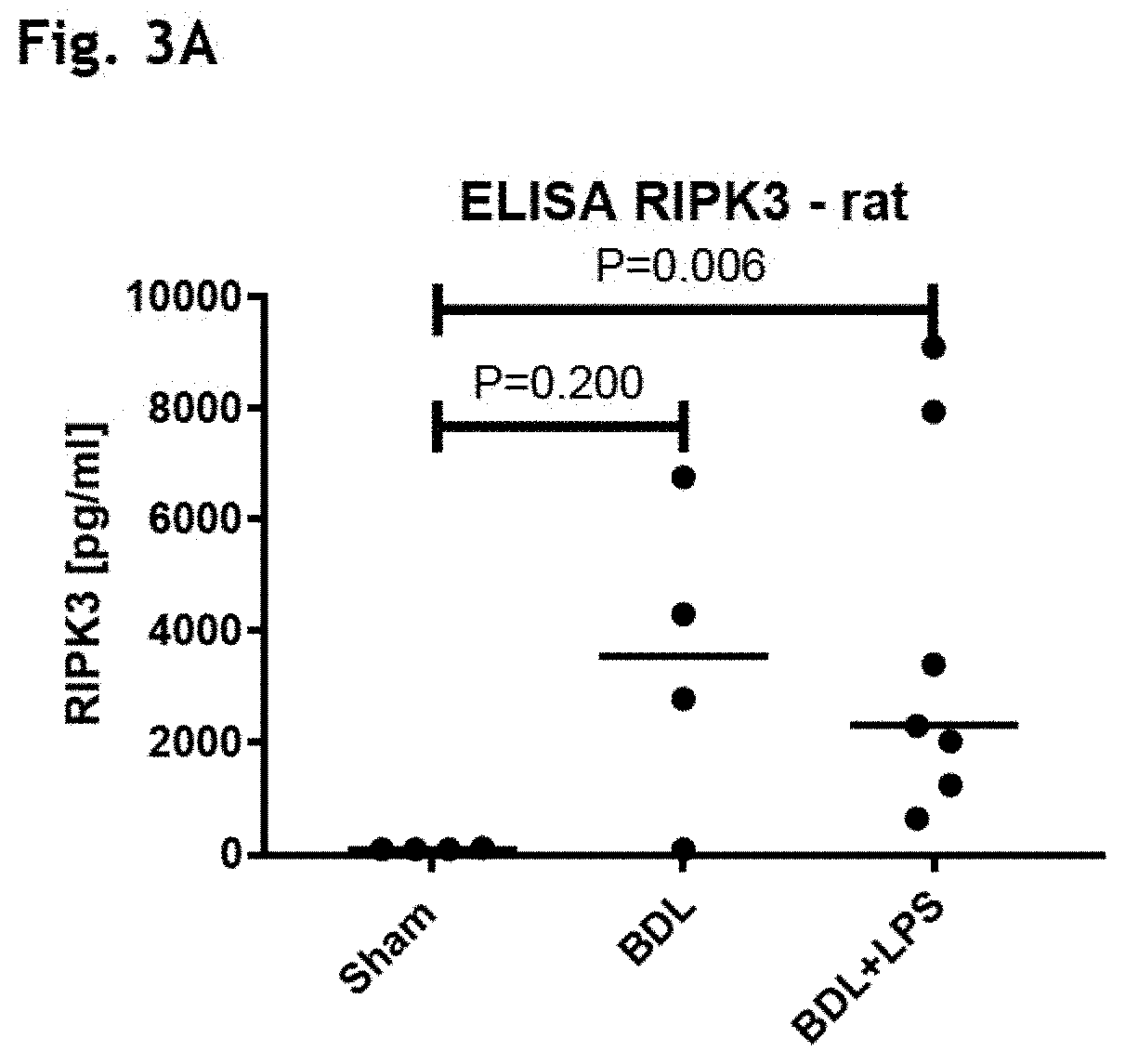
The present invention derives from the unexpected finding that necroptosis is a novel biomarker and target for therapy in patients with liver failure such as acute liver failure (ALF) and acute-on-chronic liver failure (ACLF). RIPK1, MLKL or RIPK3 can be detected and quantified in serum or plasma, and used as a biomarker for outcome in ACLF and other diseases involving aberrant necroptosis. By antagonising RIPK1, MLKL or RIPK3 many of the unwanted consequences or symptoms of acute-on-chronic liver failure (ACLF) may be reduced. The present invention utilises these findings to identify and provide antagonists of RIPK1, MLKL and RIPK3 that may be used in the treatment or prevention of ACLF. The present invention utilises these findings to identify and provide antagonists of RIPK1, MLKL or RIPK3 that may be used in the treatment or prevention of aberrant necroptosis in the kidney, brain, liver or other organ of the body.
Owner:JALAN RAJIV +3
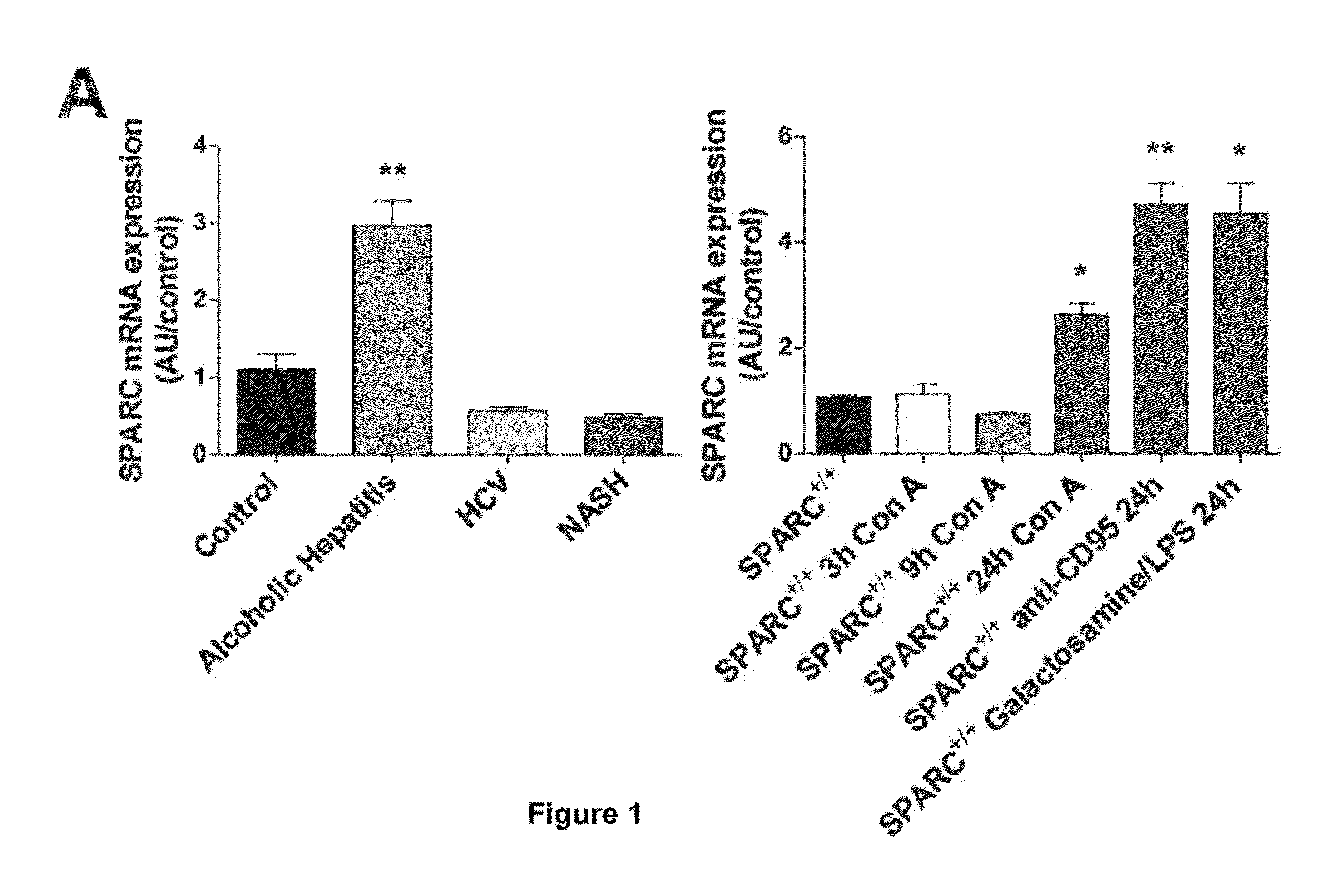
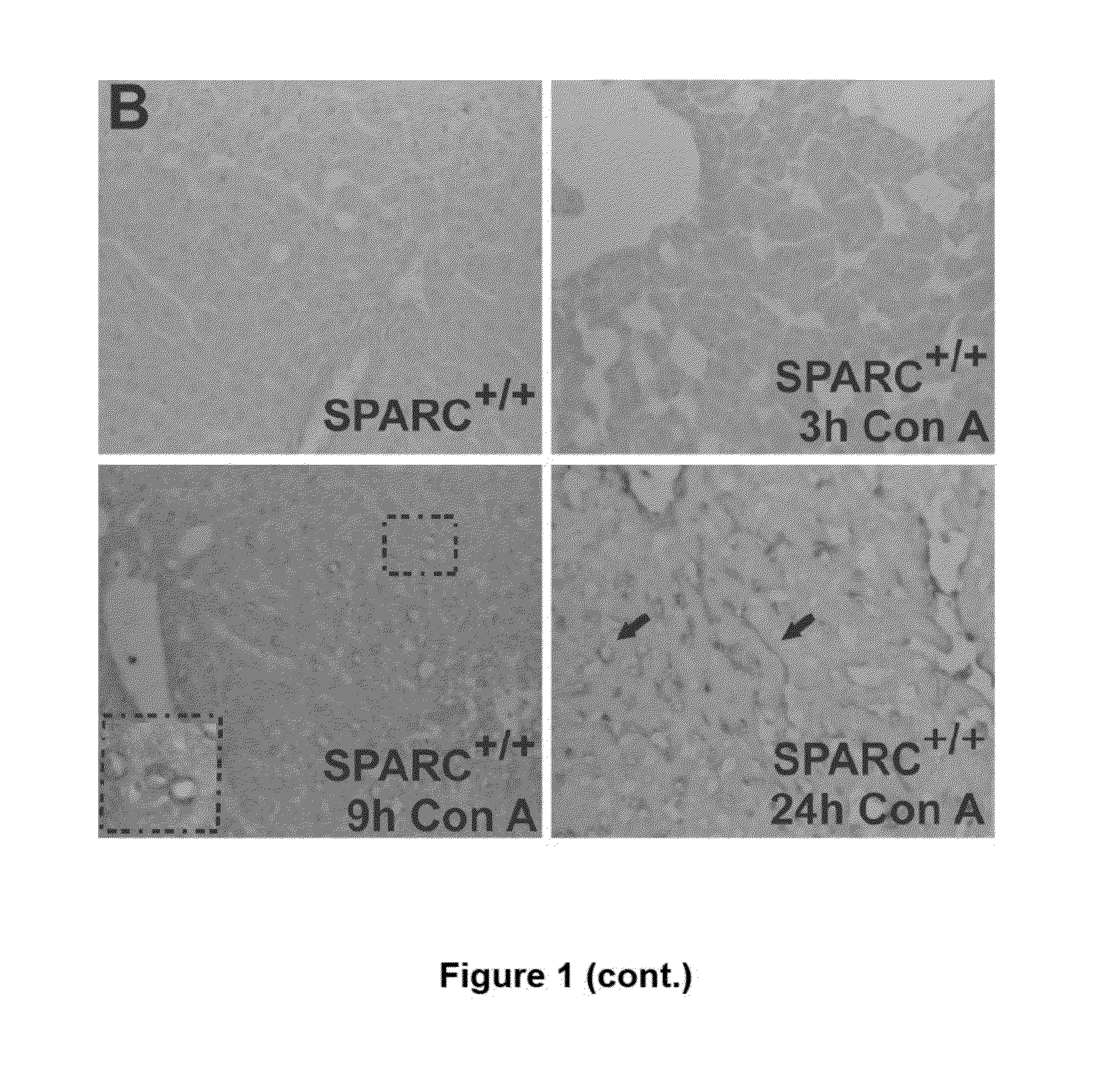
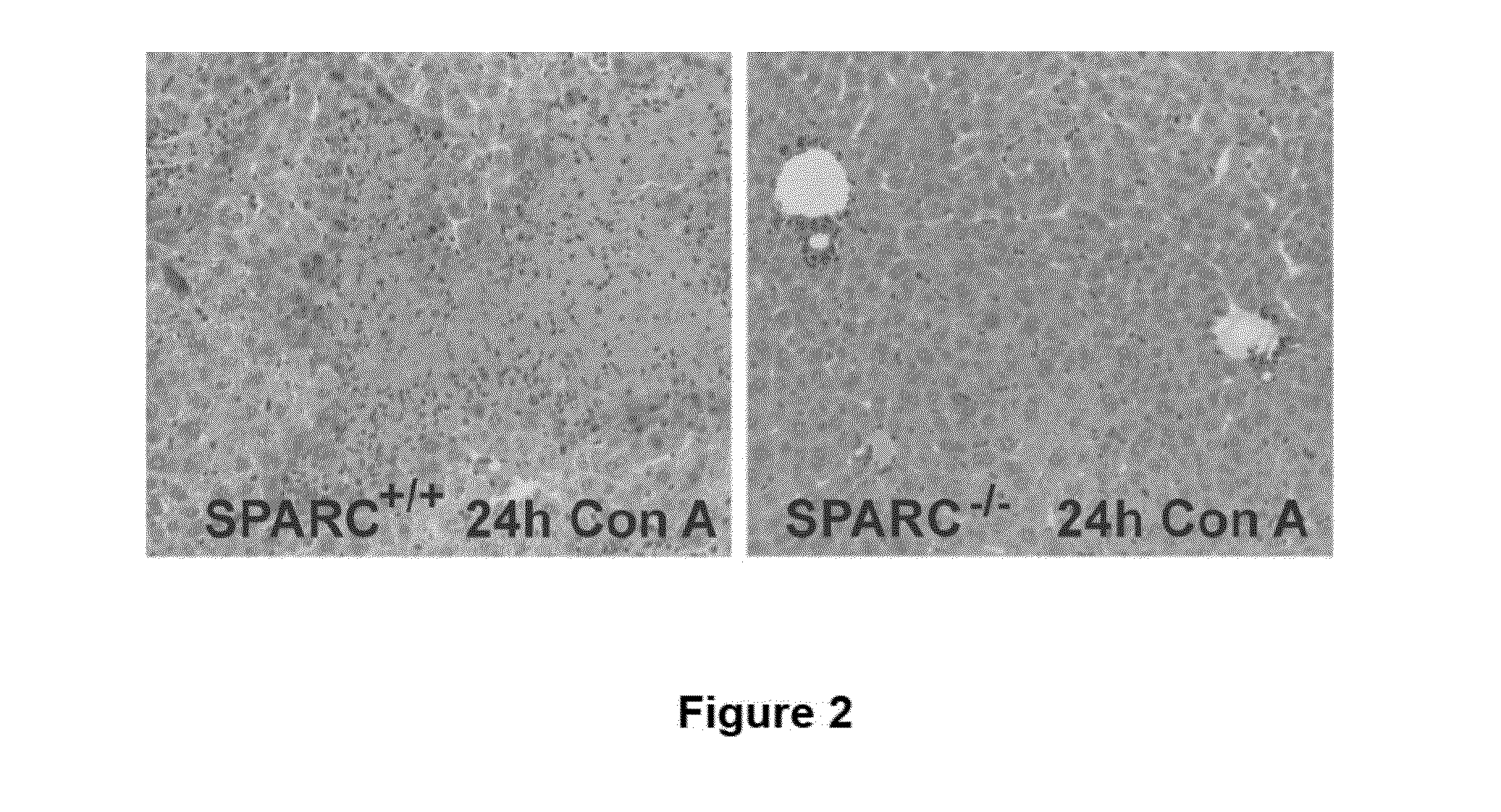
SPARC (Secreted Protein, Acidic and Rich in Cysteine), A New Target for the Treatment and Prevention of Acute Liver Failure
The invention relates to the identification of Secreted Protein, Acidic and Rich in Cysteine (SPARC) as a new therapeutic target in patients with fulminant hepatitis and allows the development of a strategy destined to protect the liver form damage. The invention relates to the treatment of acute liver failure or fulminant hepatitis by administering to a subject in need thereof an agent that inhibits at least partially the expression of SPARC and / or interferes with its biological function.
Owner:INIS BIOTEC LLC +3
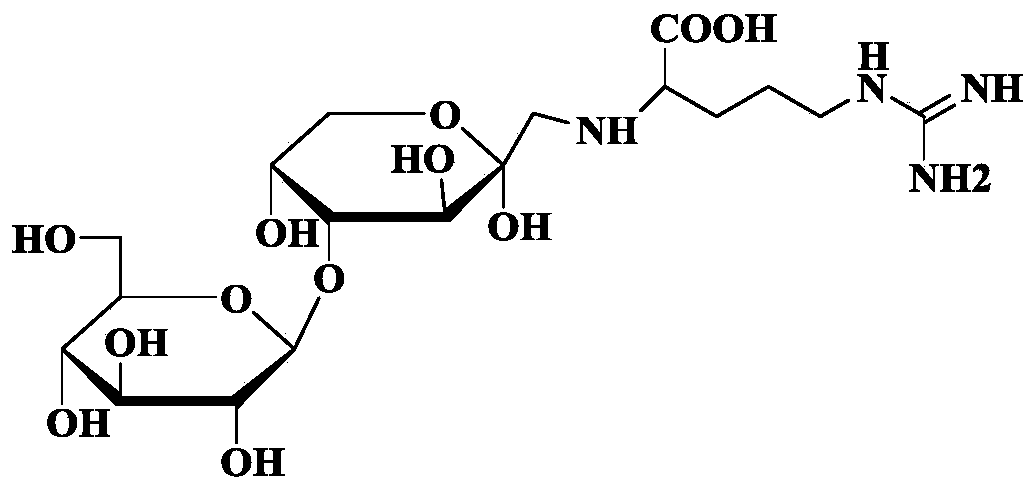
Pharmaceutical use of argininyl fructosy glucose
ActiveCN110123821AReduce apoptosisReduce oxidative stressOrganic active ingredientsDigestive systemDiseaseAcute hepatic failure
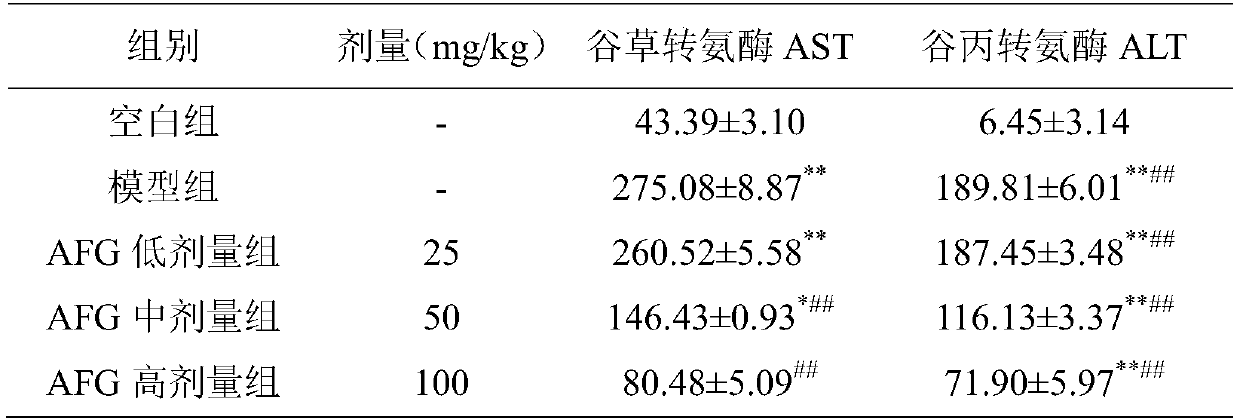
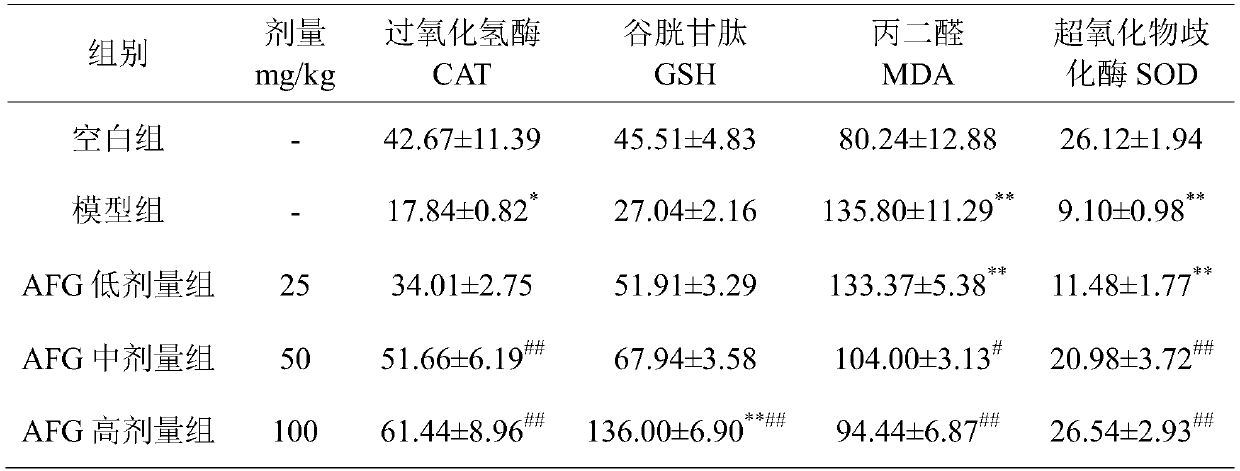
The invention discloses a pharmaceutical use of argininyl fructosy glucose, and belongs to the field of research and development of pharmaceutical products. The pharmaceutical use refers to application of the argininyl fructosy glucose, as an active ingredient, in preparation of drugs for treating or preventing acute hepatic failure. The acute hepatic failure is oxidative stress injury caused by the combination of lipopolysaccharide and D-galactosamine, liver cells are protected from injuries, and apoptosis caused by LPS / D-GalN is reduced. The purity of the argininyl fructosy glucose is 80-99.99%. The argininyl fructosy glucose is applied to prevention or treatment of the acute hepatic failure for the first time, so that a new raw material is provided for the preparation of the drugs for the acute hepatic failure, and a new method is also provided for the prevention and treatment of the acute liver failure.
Owner:DALIAN NATIONALITIES UNIVERSITY
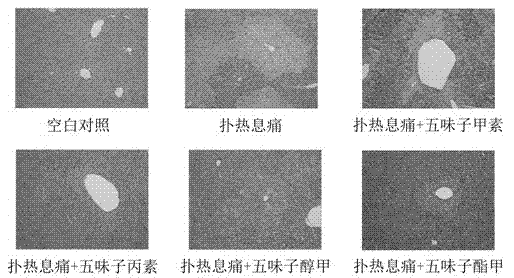
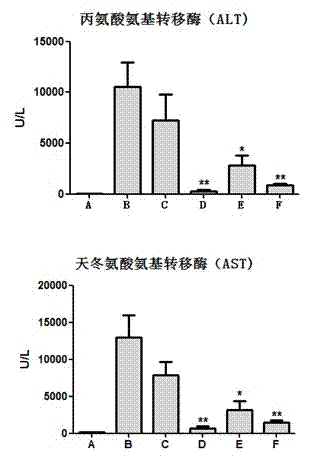
Application of schisandra chinensis monomer compound in preparation of drugs for prevention and treatment of hepatotoxicity caused by acetaminophen
ActiveCN103751174ACurb consumptionImprove necrosisDigestive systemEther/acetal active ingredientsLiver necrosisAlanine aminotransferase
The invention discloses an application of a schisandra chinensis monomer compound in preparation of drugs for prevention and treatment of hepatotoxicity caused by acetaminophen (APAP), wherein the schisandra chinensis monomer compound is deoxyschizandrin, schisandrin C, schisandrin or schisantherin a. Experiment results show that: with the schisandra chinensis monomer compound, the ALT content and the AST content in the APAP-induced liver injury animal model can be significantly reduced, consumption of glutathione in liver cells can be inhibited, the liver cell necrosis degree can be effectively improved, and the schisandra chinensis monomer compound provides significant treatment and protection effects for hepatotoxicity symptoms caused by APAP.
Owner:SUN YAT SEN UNIV
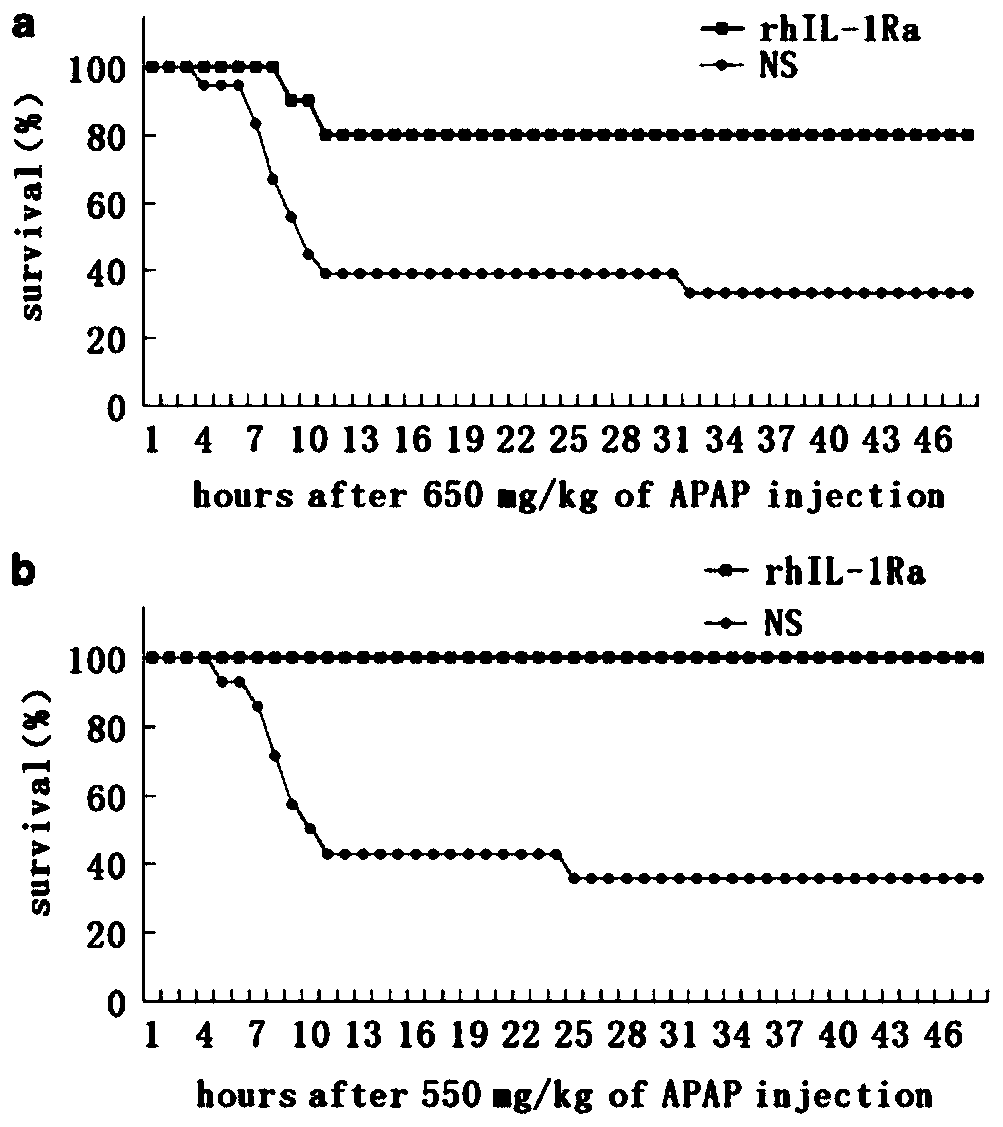
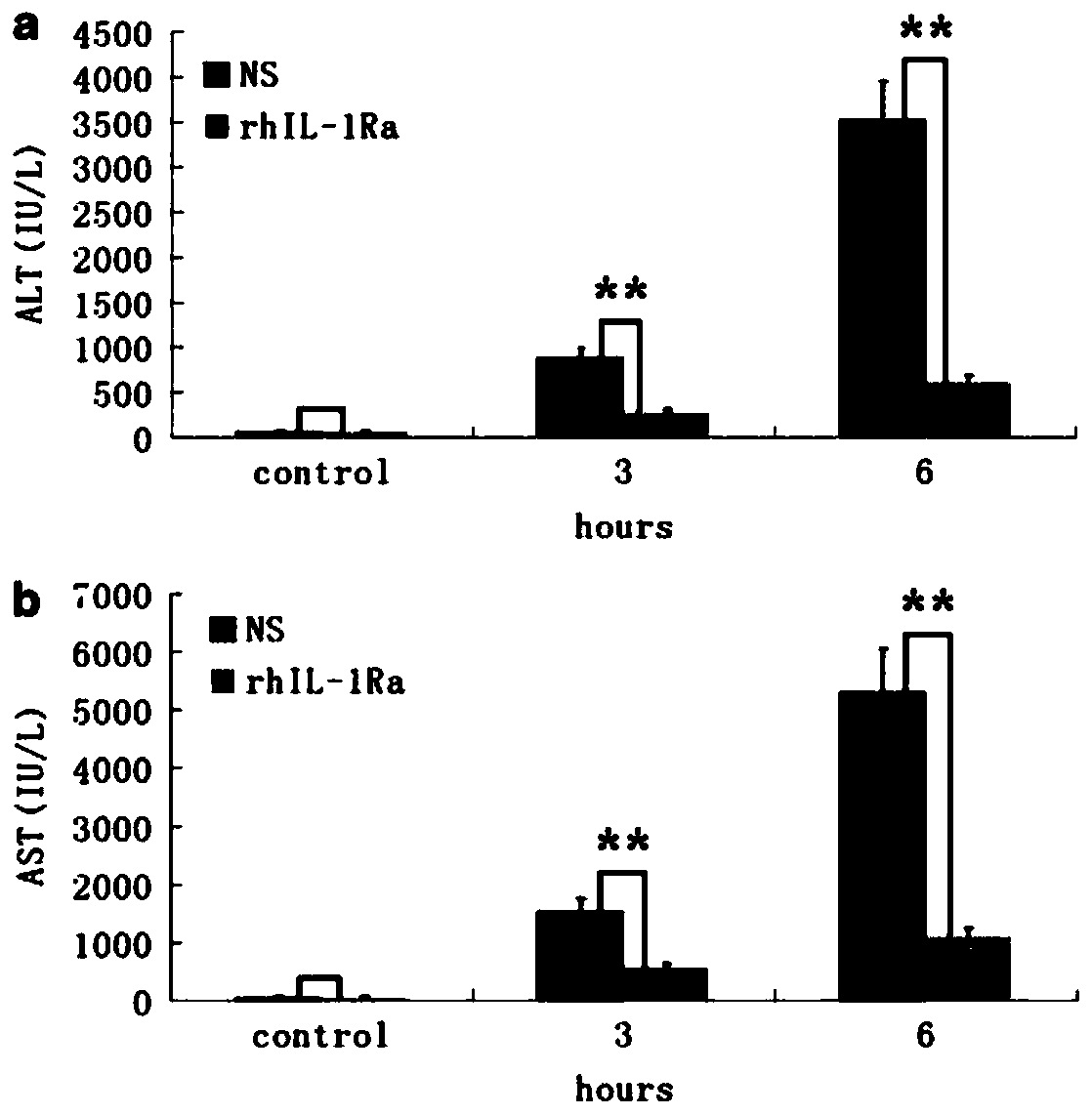
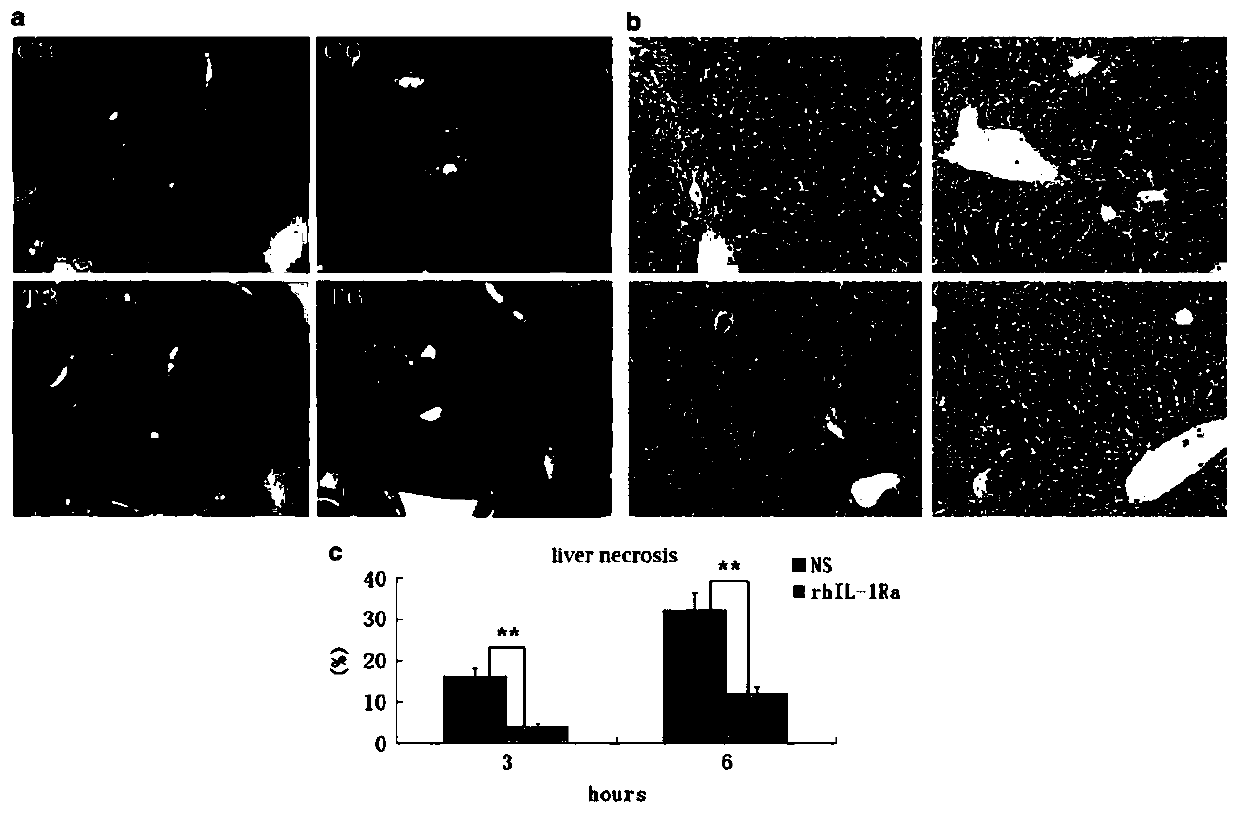
Application of rhIL-1Ra in preparing medicaments for treating acute liver failure
InactiveCN110772637APromote proliferationInhibit apoptosisDigestive systemPharmaceutical active ingredientsHepatocyte apoptosisAlanine aminotransferase
The invention provides application of rhIL-1Ra in preparing medicaments for treating acute liver failure. Compared with a control group, the rhIL-1Ra can obviously inhibit the activity of alanine aminotransferase and aspartate aminotransferase in serum, the death of liver cells is reduced, and the proliferation of the liver cells is promoted, so that the rhIL-1Ra has obvious therapeutic effects ofprotecting liver injury and promoting liver regeneration. In addition, the rhIL-1Ra inhibits apoptosis mediated by caspase-3, caspase-8 and caspase-9 activation in liver tissue by decreasing mitochondrial cytochrome c release. In summary, the rhIL-1Ra provides a strategy for the preparation of candidate drugs capable of reducing hepatocyte apoptosis for the treatment of APAP-induced acute liver failure and liver injury.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com