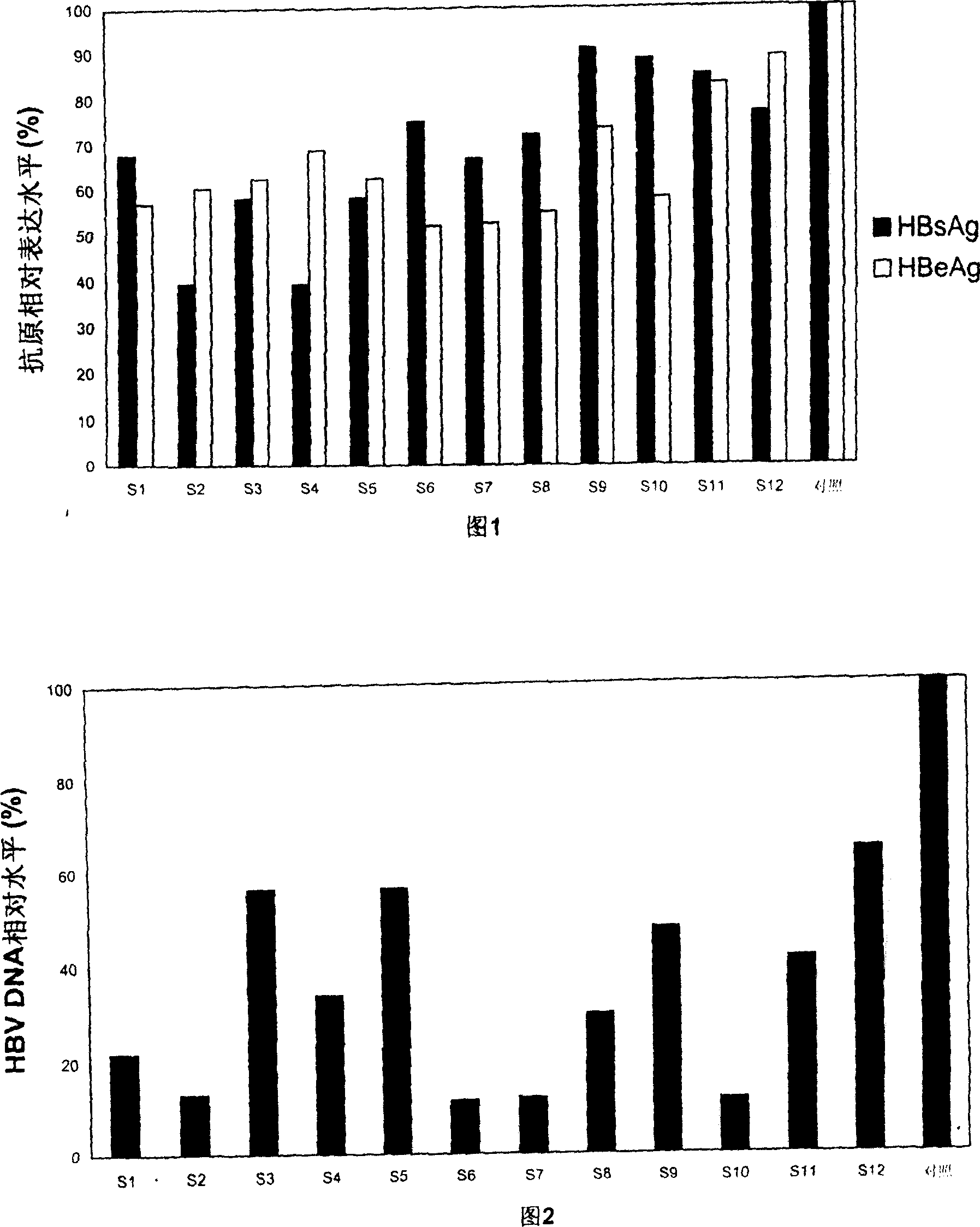
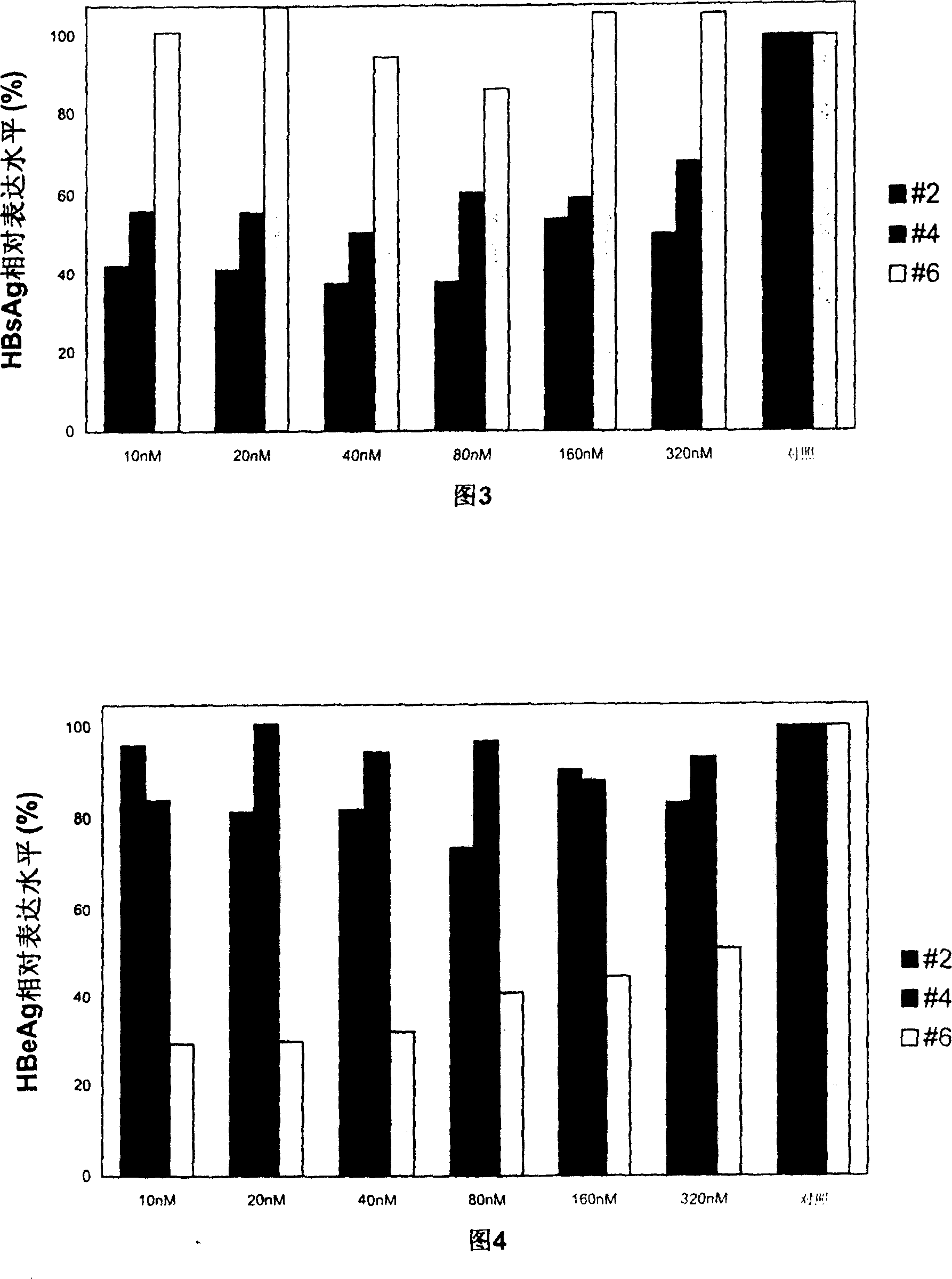
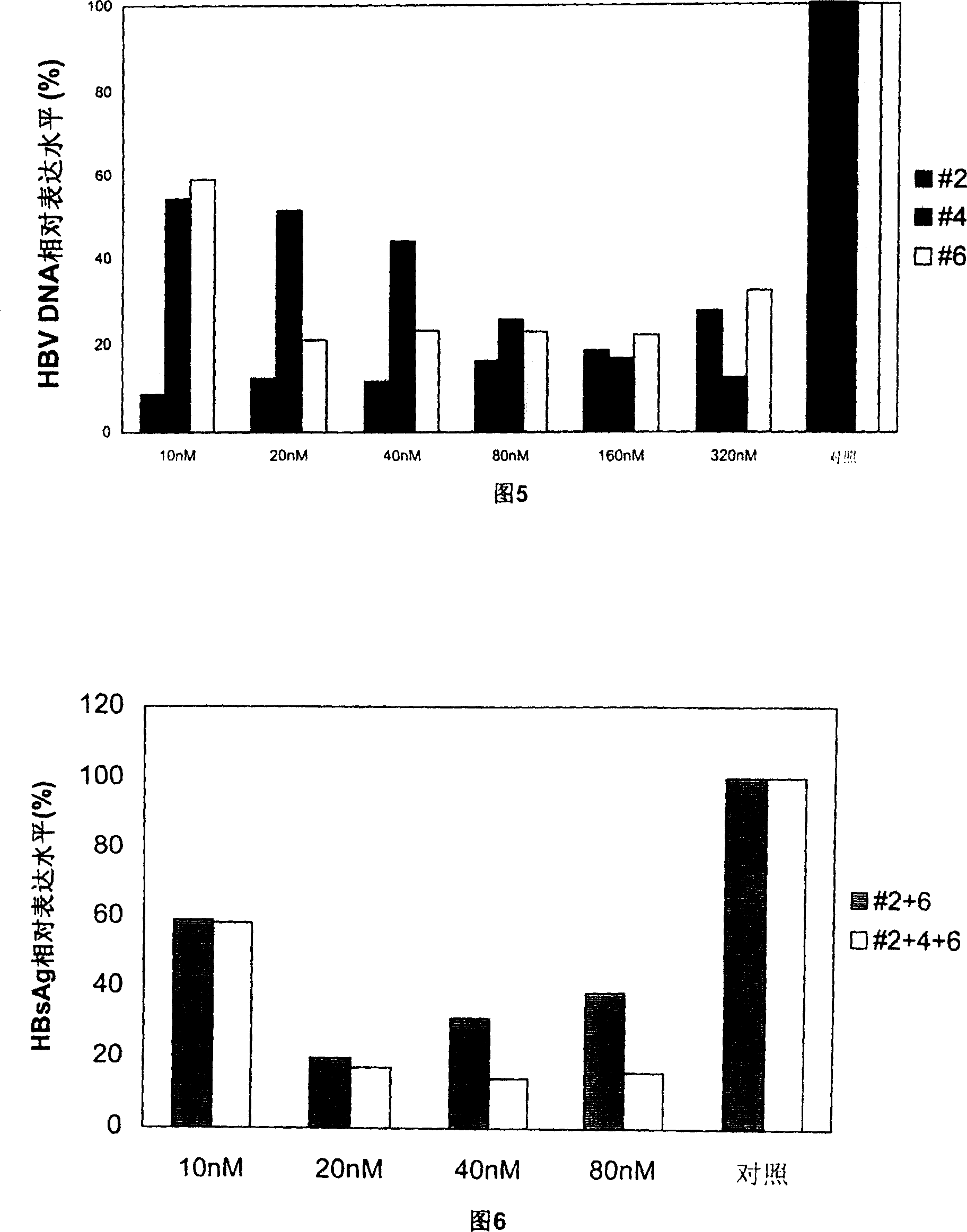
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Duck hepatitis B virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Duck hepatitis B virus, abbreviated DHBV, is part of the genus Avihepadnavirus of the Hepadnaviridae, and is the causal agent of duck hepatitis B. DHBV is a small DNA virus with a diameter of 40–45 nm. The viral envelope is made up from host cell lipid, with viral surface antigens (DHBsAg). The icosahedral nucleocapsid within, is composed of the virus core antigen (DHBcAg) and surrounds the DNA genome and viral polymerase. The viral genome is a circular double stranded DNA molecule about 3000 base pairs long. The genome has three overlapping open reading frames or ORFs...
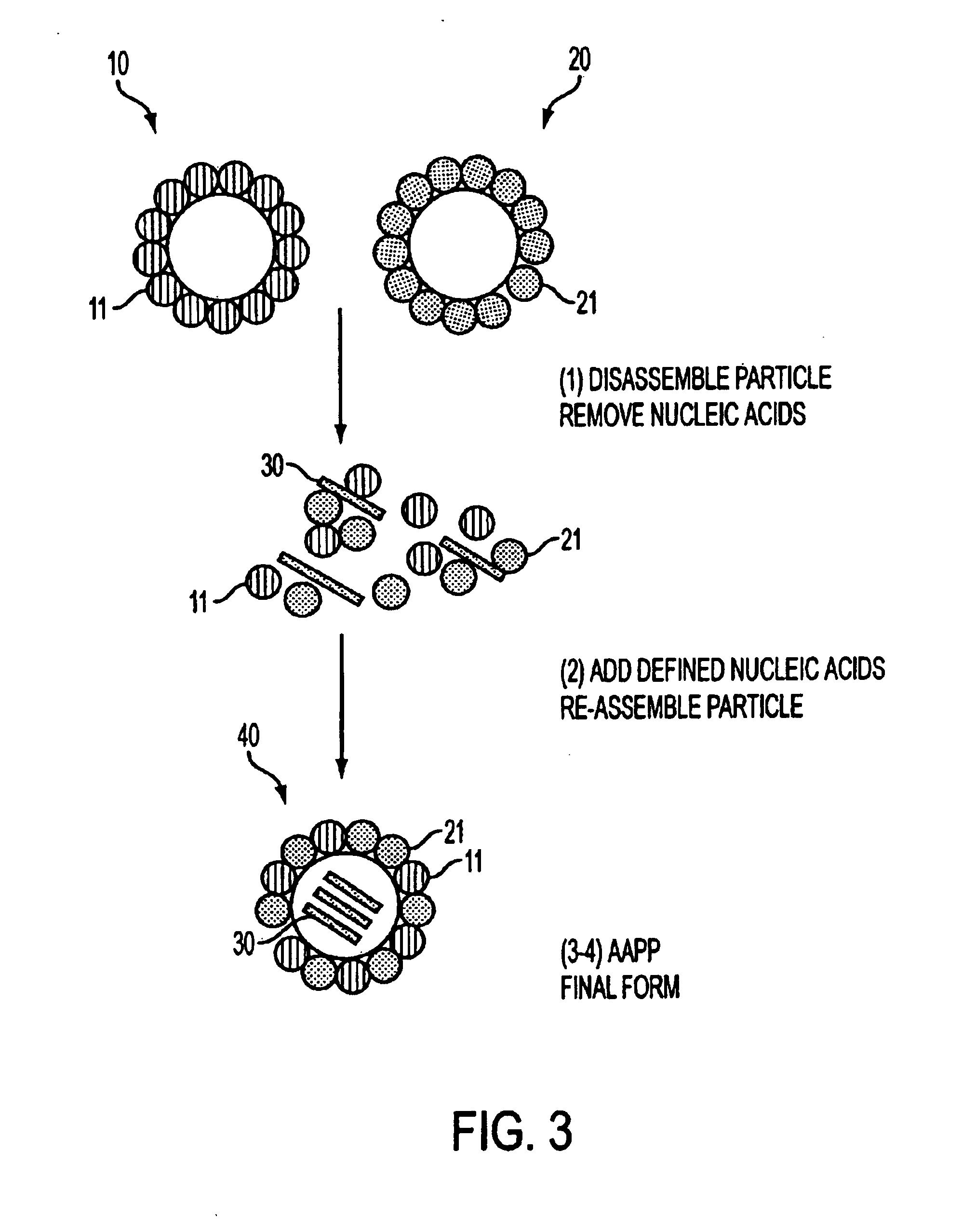
Advanced antigen presentation platform
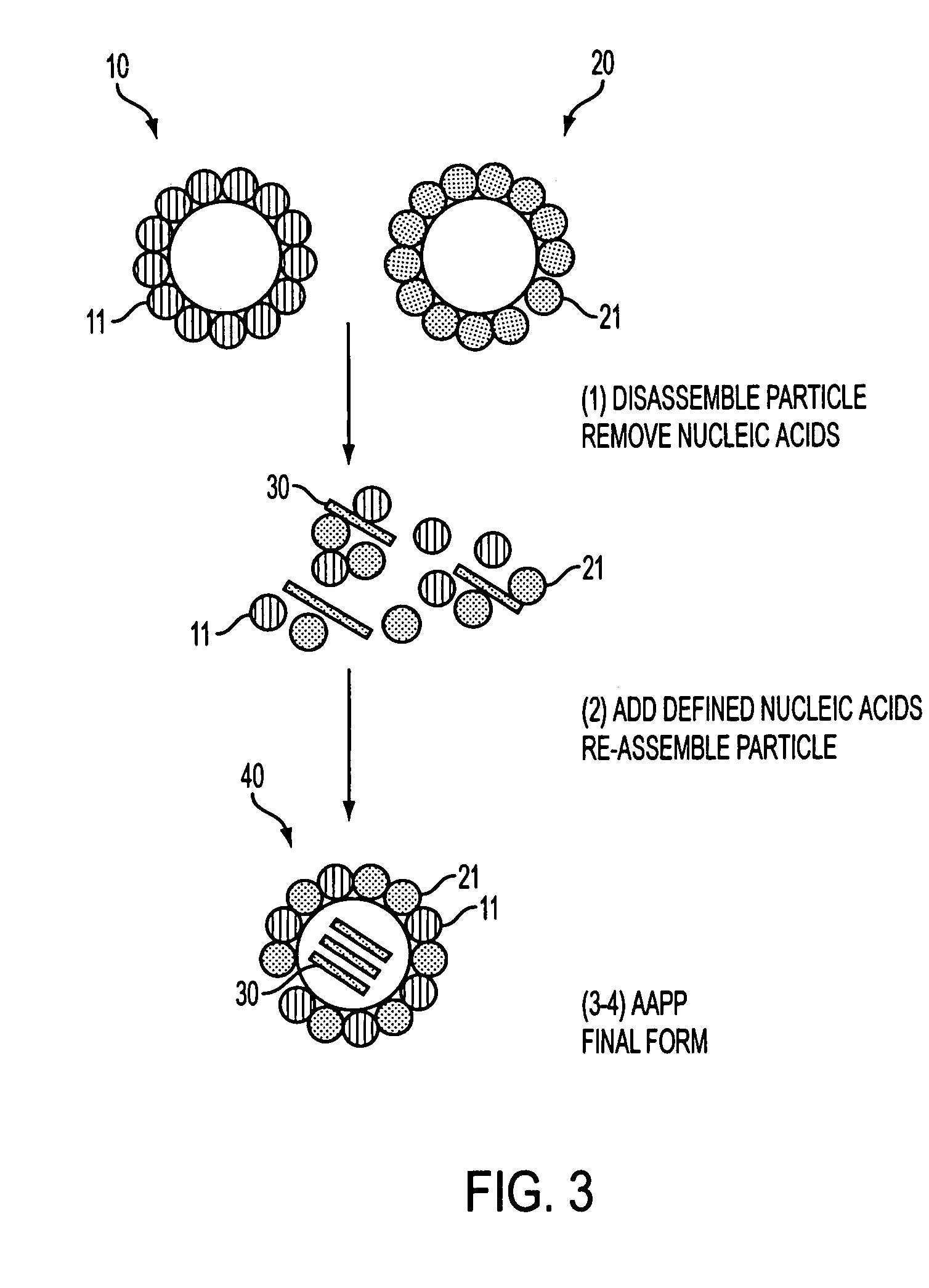
The present invention provides particles for the presentation of haptens for the purpose of eliciting an immune response. The amino acid sequences of the monomers which make up the particles are derived from duck hepatitis B virus core protein. The particles may also deliver nucleic acids. The nucleic acids may be delivered for the purpose of enhancing an immune response, or for other purposes such as gene therapy.
Owner:BIOCACHE PHARMA +1
Small interference RNA molecule SiRNA capable of attacking human hepatitis B virus and application thereof
InactiveCN1566131ABlocking reproductionBlock replicationOrganic active ingredientsSugar derivativesDiseaseNucleotide
The invention provides a small interference RNA molecule SiRNA capable of attacking human hepatitis B virus, which is double chain RNA molecule whose sequence has at least 70% of consanguinity degree with the sequence (I), wherein the antisense chain of the sequence (I) and one nucleic acid mutant has no consanguinity with the known human gene and gene expression segment. The SiRNA can be used for preparing medicament or preparation for prevention or treating hepatitis B and any diseases relating to hepatitis B viral infection.
Owner:丽水瑞德佳生物医药有限公司
Therapeutic hepatitis B vaccine
ActiveCN102462840AInhibition of replicationDigestive systemAntiviralsActive componentHepatitis B Surface Antigens
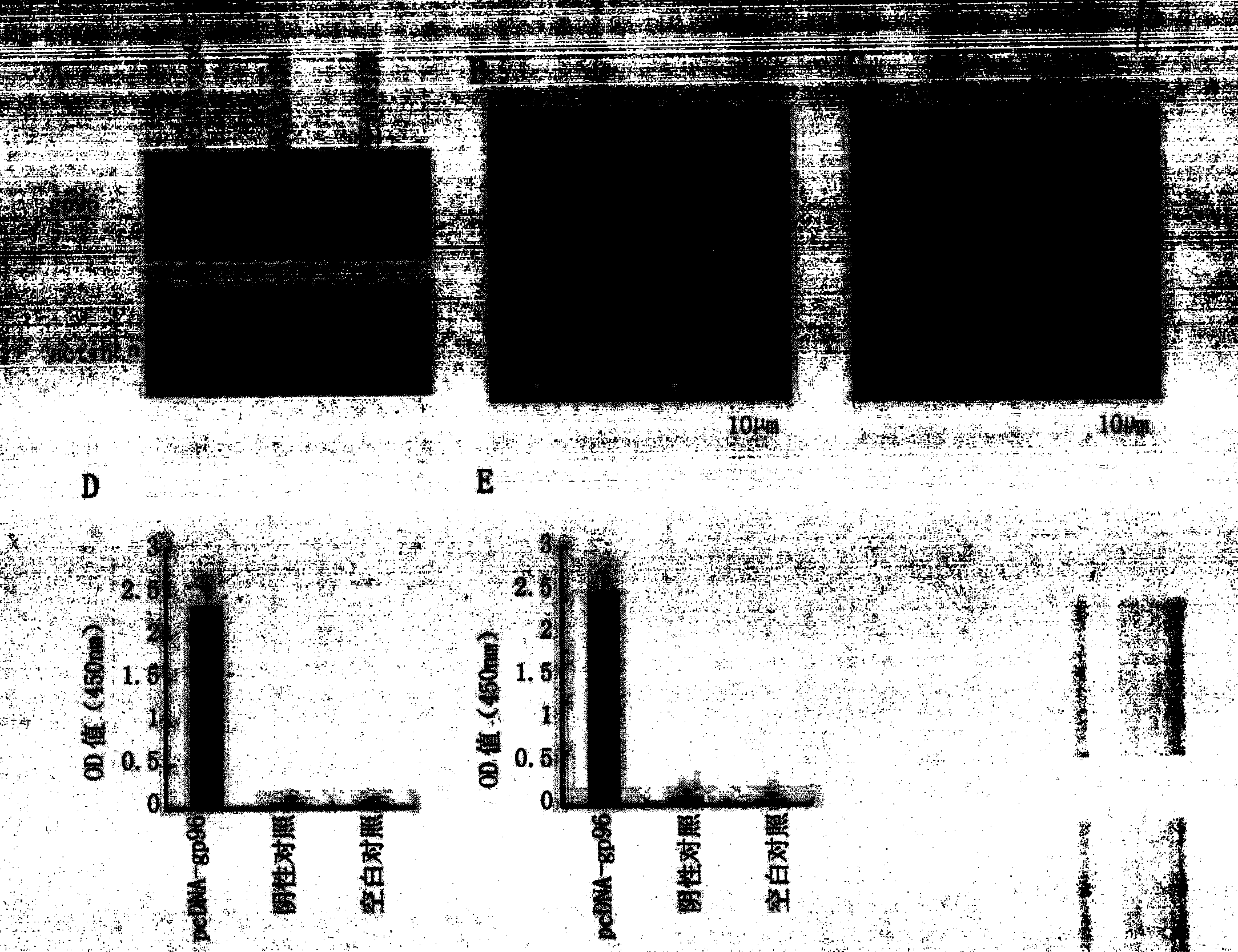
The invention discloses a therapeutic hepatitis B vaccine. Active components of the therapeutic hepatitis B vaccine comprise a protein gp96, a hepatitis B surface antigen and a hepatitis B core protein. The protein gp96 has a sequence shown in the sequence 1 of the sequence table. The hepatitis B surface antigen has a sequence shown in the sequence 5 of the sequence table. The hepatitis B core protein has a sequence shown in the sequence 3 of the sequence table. The active components also comprise a plasmid pcDNA-gp96 containing a coding gene of the protein gp96, a plasmid pcDNA-HB containing a coding gene of the hepatitis B surface antigen and a plasmid pcDNA-HBc containing a coding gene of the hepatitis B core protein. The therapeutic hepatitis B vaccine provided by the invention can effectively inhibit hepatitis B virus (HBV) replication, can eliminate viruses infecting the liver and has a very important value to hepatitis B prevention and treatment.
Owner:北京热休生物技术有限公司
Monoclonal antibody of hepatitis B virus X protein and use thereof
The invention relates to a monoclonal antibody of hepatitis B virus X protein (HBx), which is characterized by having specificity reaction to N-end epitope or C-end epitope of HBx, but having no reaction to keyhole limpet hemocyanin (KLH) of carrier protein and other expressed proteins of hepatitis B virus (HBV). The preparation method of the monoclonal antibody comprises the steps of: fusing N-end and C-end antigen epitope polypeptides of HBx respectively with mice immunized with KLH in crosslinking way, and myeloma cells and screening to obtain the monoclonal antibody. The monoclonal antibody can be used for various immunodetection reagents and directed therapeutic drugs of HBx protein or HBx antibody and applied to diagnosis and treatment of diseases such as hepatitis b virus (HBV) infection, hepatocellular carcinoma (HCC) and the like.
Owner:王虹
Application of gentiopicrin in preparing medication for restraining hepatitis B virus
InactiveCN1660124AEnhanced inhibitory effectOrganic active ingredientsDigestive systemIntramuscular injectionHepatitis B virus
Owner:HARBIN GLORIA PHARMA
Advanced antigen presentation platform
The present invention provides particles for the presentation of haptens for the purpose of eliciting an immune response. The amino acid sequences of the monomers which make up the particles are derived from duck hepatitis B virus core protein. The particles may also deliver nucleic acids. The nucleic acids may be delivered for the purpose of enhancing an immune response, or for other purposes such as gene therapy.
Owner:VIRGINIA COMMONWEALTH UNIV
Fluorescence quantitative PCR (polymerase chain reaction) detection method of duck hepatitis B virus, and reagent
InactiveCN103114153ANot easy to mutateAvoid Vulnerable DefectsMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceHepatitis B virus
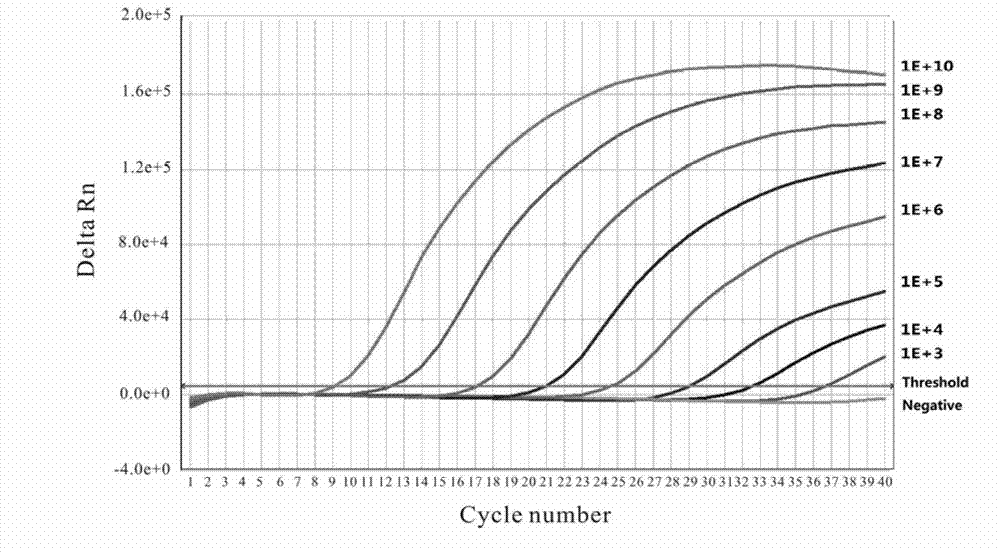
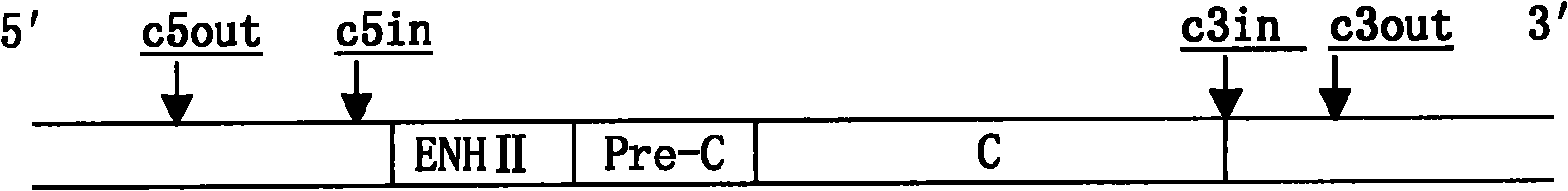
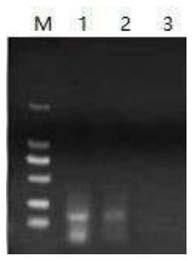
The invention discloses a fluorescence quantitative PCR (polymerase chain reaction) detection method of duck hepatitis B virus (DHBV), and a reagent. Nucleic acid sequences in a conservative region most consistent to a DHBV Core region are used as a template to design and synthesize a primer and a fluorescent probe. A pGEM-T / DHBV Core recombinant plasmid is established to prepare a PCR standard product. A reaction system and amplification conditions are optimized. The fluorescence quantitative PCR detection method provided by the invention has the advantages of strong versatility, high sensitivity, strong specificity, good reproducibility and the like, and is suitable for detection of DHBV DNA (deoxyribonucleic acid) of different duck species in different regions.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Method for preparing silkworm powder, pupa powder or pupa serum powder containing antibacterial peptide and application of same
ActiveCN101530434AAvoid destructionReduce energy consumptionAnthropod material medical ingredientsDigestive systemSerum igeMedicine
Pupa serum is made into dry powder, and the bactericidal activity of antibacterial peptide is kept for 300-3,000 unit / gram. Silkworm powder or pupa powder containing antibacterial peptide after oral taking can inhibit the multiplication of duck hepatitis B virus (DHBV) DNA in a body of duck, and the inhibition ratio reaches more than 80 percent. The silkworm powder, the pupa powder or pupa serum powder containing the antibacterial peptide is taken as a Chinese medicinal raw material and prepared into capsules for treating hepatitis.
Owner:BIOPHARM RES & DEV CENT JINAN +1
Application of phyllanthus urinaria polysaccharide component in preparing drug for resisting hepatitis B virus
InactiveCN103040857AImprove immunityReduce harmOrganic active ingredientsAntiviralsPhyllanthus urinariaIn vivo
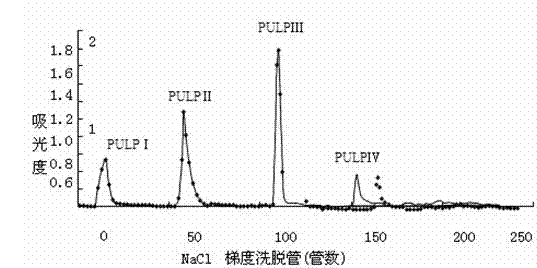
The invention relates to an application of a phyllanthus urinaria polysaccharide component in preparing a drug for resisting a hepatitis B virus, and belongs to the field of medicine. The phyllanthus urinaria polysaccharide component is a phyllanthus urinaria polysaccharide component PUIP II which comprises rhamnose, arabinose, mannose and glucose at a molar ratio of 0.11:0.36:0.08:0.45. The phyllanthus urinaria polysaccharide component is artificially extracted, separated and purified by a medicinal plant, namely phyllanthus urinaria. An in-vitro bioactivity experiment indicates that the phyllanthus urinaria polysaccharide component PUIP II can inhibit secretion of HBsAg and HBeAg and reproduction of HBV-DNA (hepatitis B virus-deoxyribose nucleic acid) in HepG2.2.2.15 cell culture, and has a certain in-vitro HBV activity resistance effect, and an in-vivo activity experiment indicates that the PUIP II has an obvious inhibiting effect on DHBVDNA (duck hepatitis B virus deoxyribose nucleic acid) in a duck, is smaller in bounce, and can be used for preparing the drug for resisting the hepatitis B virus and for preparing health care products for protecting a liver.
Owner:FUJIAN AGRI & FORESTRY UNIV
Application of Tibetan capillaris in preparing a medicine for treating viral hepatitis B
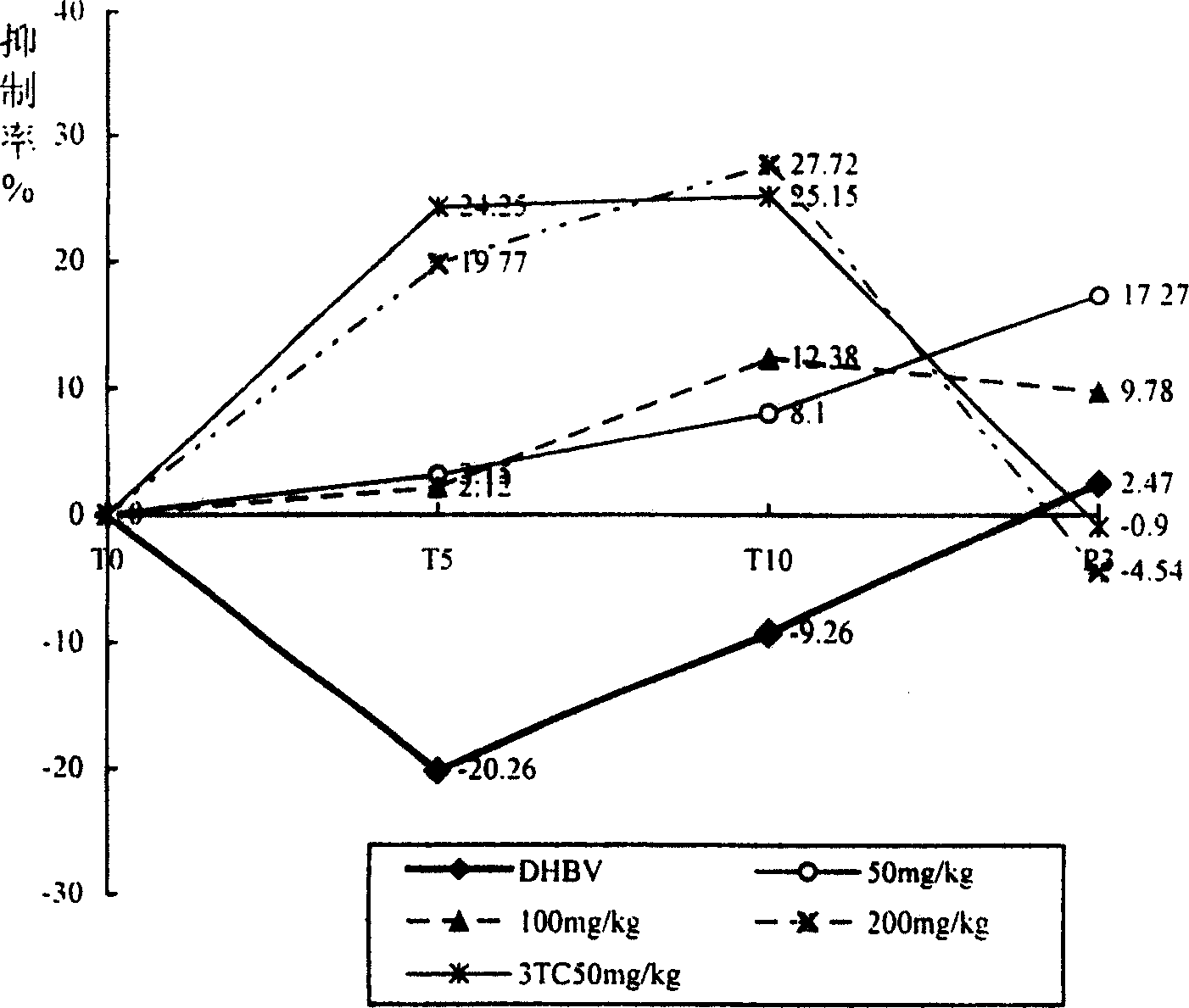
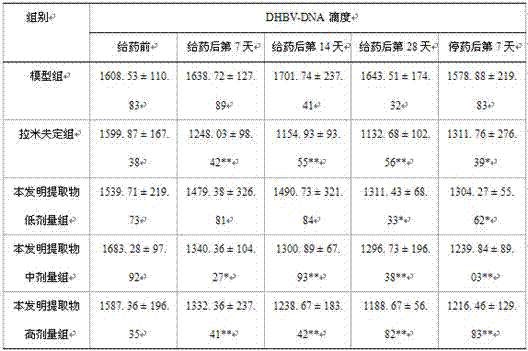
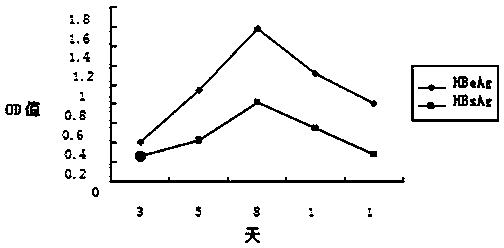
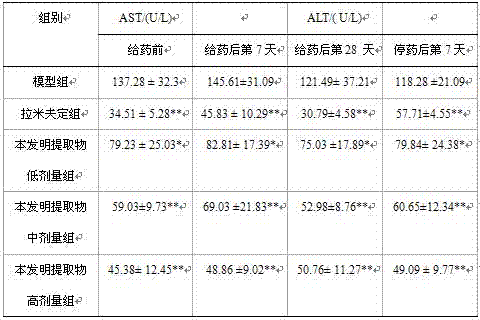
The invention discloses an application of Tibetan capillaris in preparing a medicine for treating viral hepatitis B. The Tibetan capillaris is a Tibetan capillaris extract which is prepared by conducting extracting with water, ethanol or carbon dioxide as a solvent. In accordance with a conventional process, the Tibetan capillaris or the Tibetan capillaris extract is prepared into any one of the following pharmaceutical dosage forms: tablets, capsules, granules, pills and the like. The Tibetan capillaris is applicable to preparation of the medicine for treating the viral hepatitis B; and the medicine, which is suitable for clinical application, is prepared from the Tibetan capillaris. The Tibetan capillaris can obviously reduce a DHBV-DNA titer, an HBsAg level and AST and ALT activities in serum of a hepatitis B model duck; within 7 days of drug withdrawal, a recurrence phenomenon is avoided; and in addition, the Tibetan capillaris can relieve degeneration of hepatic cells and inflammatory cell infiltration. It is indicated that the composition (the medicine) is obvious in effect of resisting hepatitis B virus of the duck; therefore, it is indicated that the composition (the medicine) has a clinical application value. The pharmaceutical preparation of the medicine for treating the viral hepatitis B is scientific and reasonable.
Owner:姚萍
Redback christmashush extract, and preparation method and application thereof in preparation of drugs for treating hepatitis B
ActiveCN104586925AThe extraction process is simple and reasonableEffective part extractDigestive systemAntiviralsSerum igeAlcohol
Owner:SOUTHERN MEDICAL UNIVERSITY
Double expression plasmid of hepatitis B virus, and construction method and application thereof
InactiveCN101824430AEfficient expressionIncrease chance of bindingGenetic material ingredientsMicroorganism based processesAntigenRestriction Enzyme Cut Site
The invention relates to double expression plasmid of hepatitis B virus and a construction method thereof. The method comprises the following steps: firstly and respectively taking former C-C gene / self-replication regulatory element enhancer II and former S-S gene / self-replication regulatory element enhancer I of the hepatitis B virus as target genes, using eukaryotic expression vector VR012, constructing ENH II / former C-C gene vaccine recombinant plasmid VEC and ENH II / former S-S gene vaccine recombinant plasmid VES of the hepatitis B, then adopting a single primer secondary PCR method to mutate the recombinant plasmid VES and inserting restriction enzyme cutting sites into the downstream of the expression regulatory unit; and finally, using the inserted restriction enzyme cutting sites, carrying out amplification on the regulatory and expression unit of recombinant plasmid VEC by PCR, enzyme-cutting and recombining the regulatory and expression unit into mutated VES recombinant plasmid, and obtaining two starters which respectively regulate the double expression plasmid for expressing the former C-C gene and the former S-S gene of the hepatitis B virus as DNA vaccines. The recombinant HBV DNA vaccine can effectively express surface antigens and core antigens of the hepatitis B and can be used for prevention and treatment of the hepatitis B.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Method of preparing extract of Mallotus apelta and uses for fighting hepatitis B virus
InactiveCN101584702ASignificant anti-HBV effectAbundant resourcesOrganic active ingredientsDigestive systemDiseaseBULK ACTIVE INGREDIENT
The invention relates to extract activity of Mallotus apelta and uses in prepartion of medicine for treating related diseases caused by hepatitis B. The extract of Mallotus apelta provided by the invention has basic components of apiolin, apiin-7-O-belta-D-glucoside, and flavonoids compounds of 5,7-dihydroxy-6-isopentene group-4'-methoxyl flavonone, flavonoids compounds Mallotus apelta have obvious inhibiting effect on hepatitis B virus surface antigen and hepatitis B virus e antigen ono surface of HepG 2215, and cabaple of inhibiting duplication of Duck Hepatitis B Virus deoxyribonucleic acid, withdraw rebound is weaker than positive reference medicine lamivudine, and hepatitis B is provided with a subsequent inhibiting effect after withdraw. The medicine according to the invention has strong function for fighting hepatitis B virus, clear active ingredient, which is suitable for industrial production, predictablly used for preparing medicine of preparing diseases infected by hepatitis B virus.
Owner:广西中医学院
Uses of c15-substituted andrographolide derivatives in the preparation of Anti-hepatitis b virus medicament
InactiveUS20140187627A1Improve efficiencyLow toxicityBiocideOrganic chemistryBULK ACTIVE INGREDIENTHepatitis B virus surface Antigen
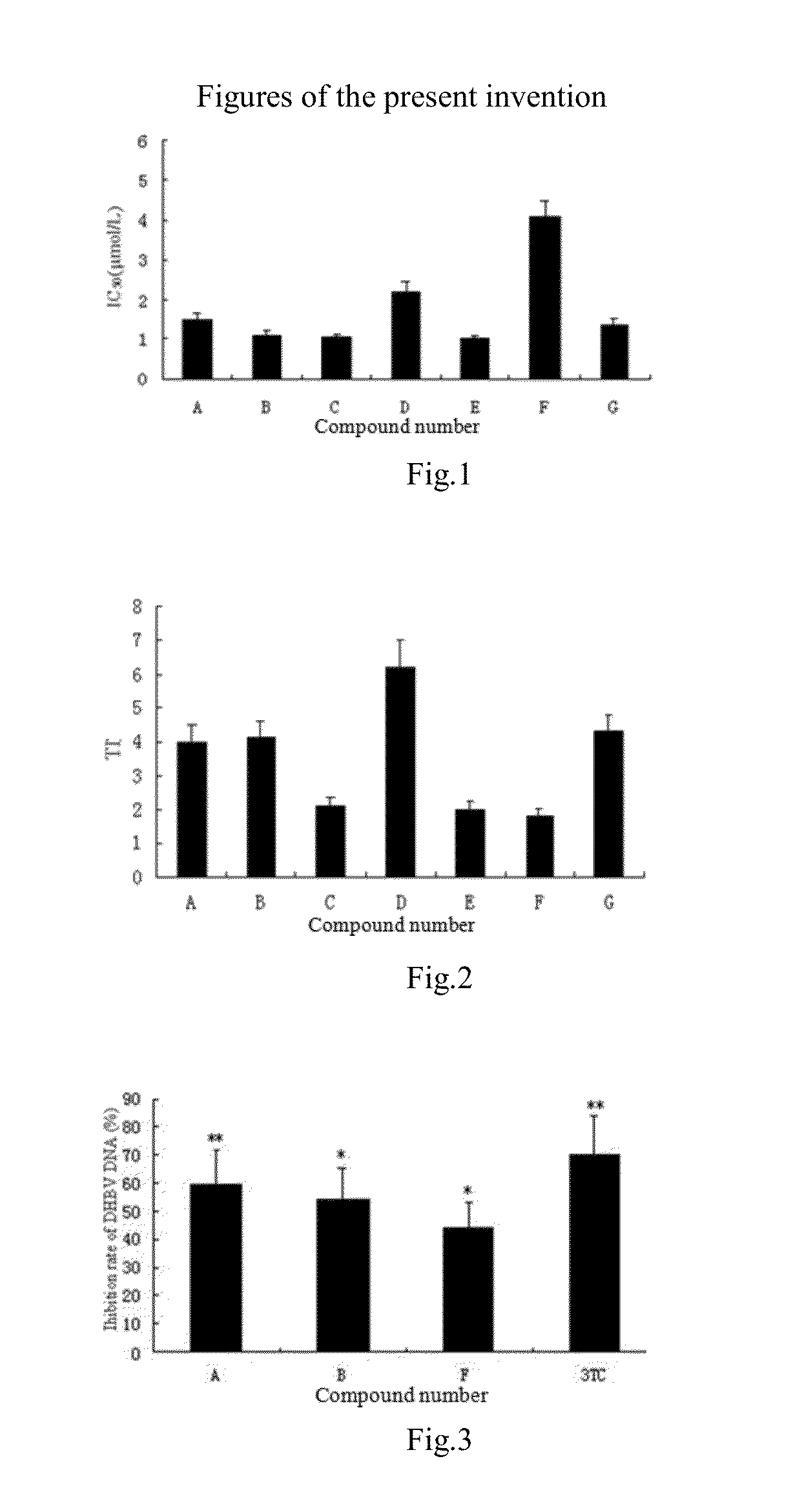
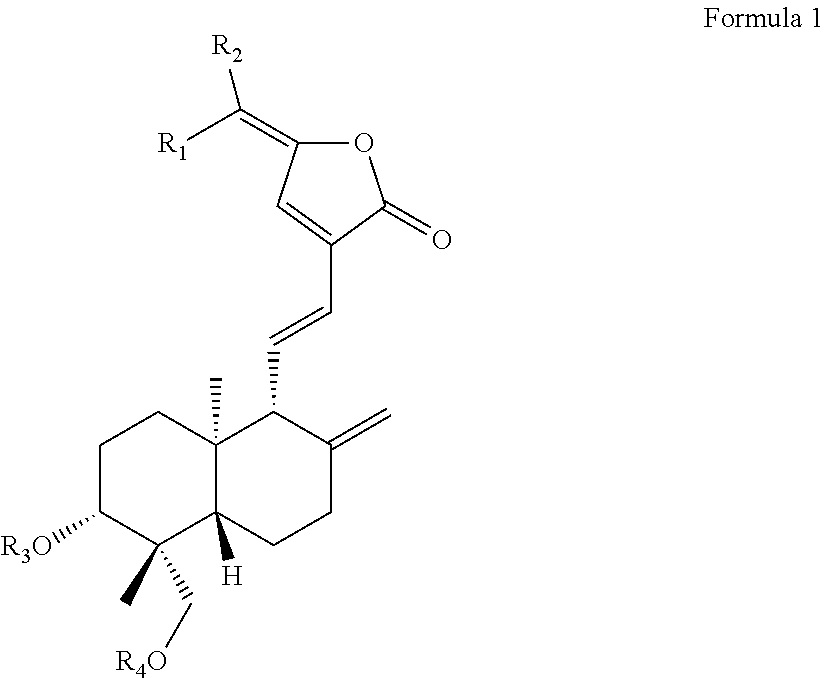
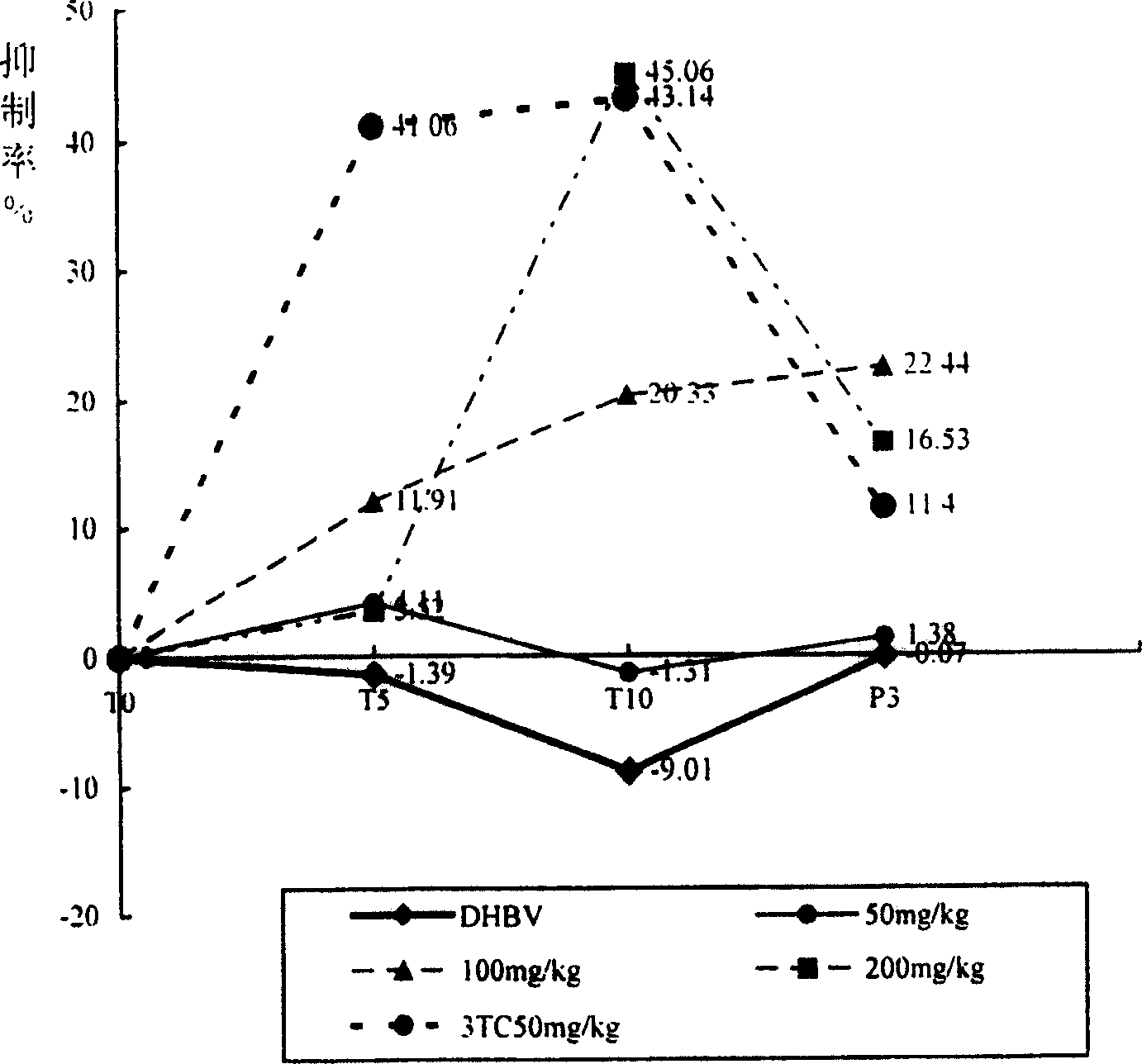
Disclosed is a use of C15-substituted andrographolide derivatives in preparation of anti-hepatitis B virus medicaments. In the present invention, the HepG2.2.15 cells are used to measure the amount of the hepatitis B virus surface antigen (HBsAg) secretion in the supernatant of the culture; the duck hepatitis B virus (DHBV) is used to infect the model and the DHBV-DNA level in serum is measured, and the pathological change in hepatic tissue is observed. A number of andrographolide derivative compounds are screened, compounds having a good anti-HBV effect are preferred, which has a structure represented by general formula 1 set forth herein. Due to high anti-HBV activity and low toxicity, as well as good protection against hepatic injury, the compounds can be used as the active ingredient for preparing anti-HBV medicaments, thereby providing a new pharmaceutical way for treatment of hepatitis, and broadening the range of clinical medicines.
Owner:ZHENGZHOU UNIV
Recombinant polymerase isothermal amplification detection method of hepatitis B viruses in ducks
PendingCN111593138ARapid diagnosisStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationHepatitis B immunizationViral test
The invention discloses a recombinant polymerase isothermal amplification detection method of hepatitis B viruses in ducks, a special RPA primer pair as shown in SEQ ID No. 1 and SEQ ID No. 2 and a kit. Specific primers are designed according to the gene sequence of the duck hepatitis B viruses, and the recombinant polymerase isothermal amplification method for quickly and accurately detecting theduck hepatitis B viruses is further optimized and established. Through the method, the duck hepatitis B viruses can be detected specifically, the minimum detection template amount is 146 pg through the method, and the sensitivity is equivalent to that of traditional PCR. Since only one temperature is needed during the whole gene amplification, no special instruments and equipment are needed, theoperation is simpler and quicker, and the method and kit are suitable for on-site quick detection of the duck hepatitis B virus during production.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Application of gentiopicrin in preparing medication for restraining hepatitis B virus
InactiveCN1259054CEnhanced inhibitory effectOrganic active ingredientsDigestive systemHepatitis B virusBULK ACTIVE INGREDIENT
The application of the active ingredient gentiopicroside in the preparation of drugs for inhibiting hepatitis B virus. The active ingredient gentiopicroside is a compound extracted and separated from the commonly used traditional Chinese medicine Gentiana quinquefolium in my country. The active ingredient gentiopicroside is mixed with pharmaceutically acceptable carriers in preparations such as those suitable for enteral and parenteral administration. Organic or inorganic solid or liquid excipients are mixed, and the pharmaceutical preparation can be in solid form such as tablet, capsule, powder, pill, granule, or in liquid form such as injection, emulsion, etc. Gentiopicroside was prepared into injections, and the drug efficacy test showed that ducks infected with duck hepatitis B virus were treated with intramuscular injection of gentiopicroside on the 7th day after infection. The inhibitory effect of duck serum DHBV-DNA level is remarkable, and the statistical processing results have very significant and significant differences, inhibitory effect (P<0.01-0.05), and the inhibitory effect of three batches of experiments can be repeated.
Owner:HARBIN GLORIA PHARMA CO LTD
Gene clone of hepatitis B virus front S1 region multi-epitope antigen and encoding sequence thereof
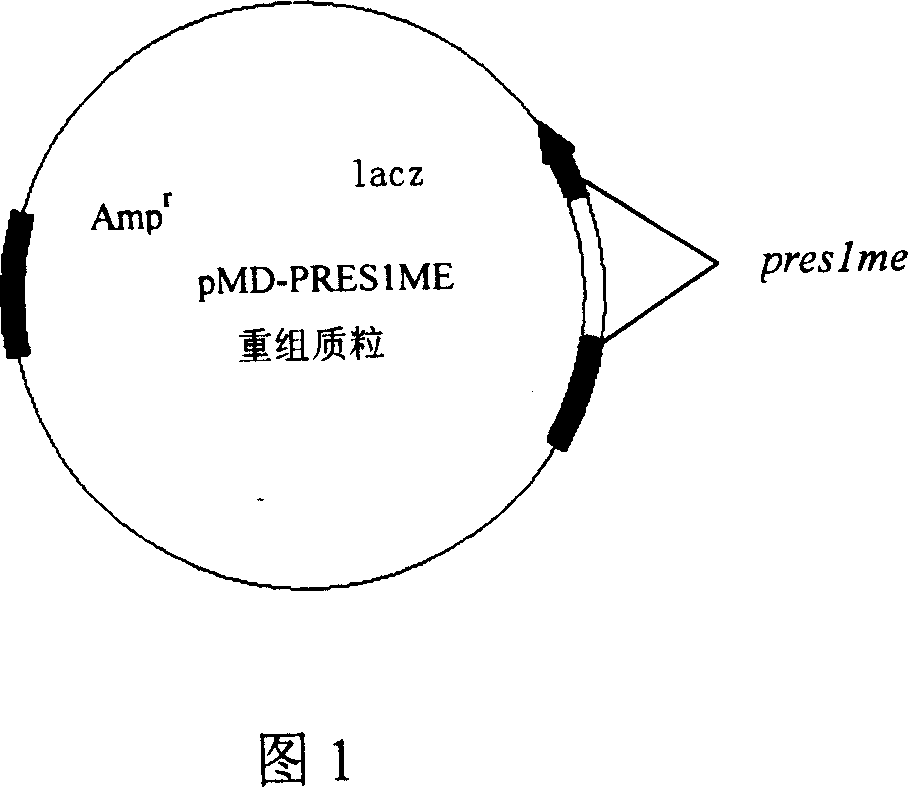
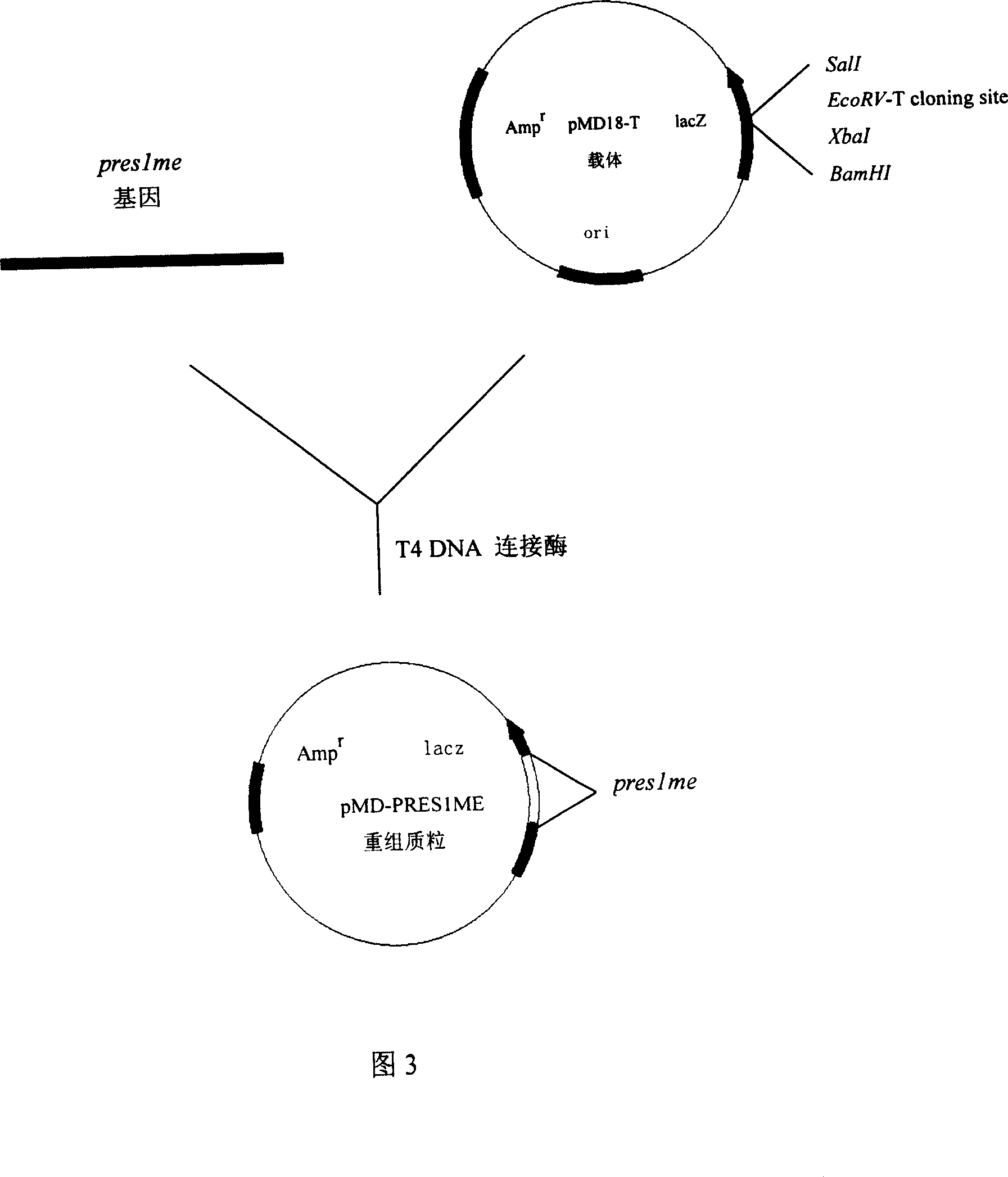
The invention relates to biotechnology field and discloses the gene cloning preslme and its code sequence PRES1ME of multiple-epitope antigen at hepatitis b virus pres region (1) (PreS1). The preslme includes the B cell epitope section 4 at HBV genome pres region (1), and it is indicated that its reaction result with pronuclear fusion expression product GST-PRES1M of gst gene and pre-S1 antigen of hepatitis bvirus ELISA detection kit. The pre-S1 antigen of hepatitis bvirus can be detected in hepatitis b virus serum after peraration of ELISA plate with pres1me, and the serum from GST-PRES1ME immuned mice can identify four Presl epitope synthetic peptide and pre-S1 full- length protein. So pres1me and its expression product PRES1ME can be used to prepare checking agent of HBV pre-S1 antibody or antigen and hepatitis b vaccine.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD
Medicinal composition for treating chronic hepatitis and its preparing method
InactiveCN1781544AInhibition of degenerative lesionsInhibition of fibrous proliferation in liver tissueDigestive systemAntiviralsSide effectChronic hepatitis
The present invention relates to a kind of medicinal composition for treating chronic hepatitis and its preparation process. The medicinal composition is prepared through extracting volatile oil of zedoary; water extracting astragalus root, curcuma root, etc, filtering and concentration to obtain extractum; alcohol extracting fleeceflower root, schisandra, etc, filtering and concentration to obtain extractum; merging the extractum, adding lactose, drying and crushing into fine powder; crushing aweto into fine powder; mixing fine powder, adding lactose, pelletizing, drying and spraying the volatile oil; and packing. It has determined curative effect, treating functions of benefiting vital energy, nourishing yin, removing blood stasis, etc and no toxic side effect. It is suitable for treating chronic viral hepatitis.
Owner:王君
LAMP (Loop-mediated Isothermal Amplification) detection reagent of DHBV (Duck Hepatitis B Virus)
PendingCN108754021AStrong specificityIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesAdditive ingredientLoop-mediated isothermal amplification
The invention belongs to the technical field of virus detection, and specifically relates to a LAMP (Loop-mediated Isothermal Amplification) detection reagent of DHBV (Duck Hepatitis B Virus). A specific LAMP primer is designed and synthesized according to a DHBV gene conserved region, and the specific LAMP primer comprises an upstream inner primer FIP, a downstream inner primer BIP, an upstream outer primer F3 and a downstream outer primer B3. The LAMP detection reagent is prepared from the following ingredients: a 10*Bst Buffer reaction buffer solution, dNTPs (Deoxynucleotide Triphosphates),MgSO4, Bst DNA (Deoxyribonucleic Acid) polymerase, deionized water, a color-producing reagent and the primers. The LAMP detection reagent provided by the invention has the advantages of strong specificity, high sensitivity, simpleness and convenience in operation, low cost and the like during application.
Owner:NANYANG NORMAL UNIV
Small interference RNA molecule SiRNA capable of attacking human hepatitis B virus and application thereof
The invention provides a small interference RNA molecule SiRNA capable of attacking human hepatitis B virus, which is double chain RNA molecule whose sequence has at least 70% of consanguinity degree with the sequence (I), wherein the antisense chain of the sequence (I) and one nucleic acid mutant has no consanguinity with the known human gene and gene expression segment. The SiRNA can be used for preparing medicament or preparation for prevention or treating hepatitis B and any diseases relating to hepatitis B viral infection.
Owner:丽水瑞德佳生物医药有限公司
Method for preparing silkworm powder, pupa powder or pupa serum powder containing antibacterial peptide
ActiveCN101530434BAvoid destructionReduce energy consumptionAnthropod material medical ingredientsDigestive systemSerum igeHepatitis
Owner:BIOPHARM RES & DEV CENT JINAN +1
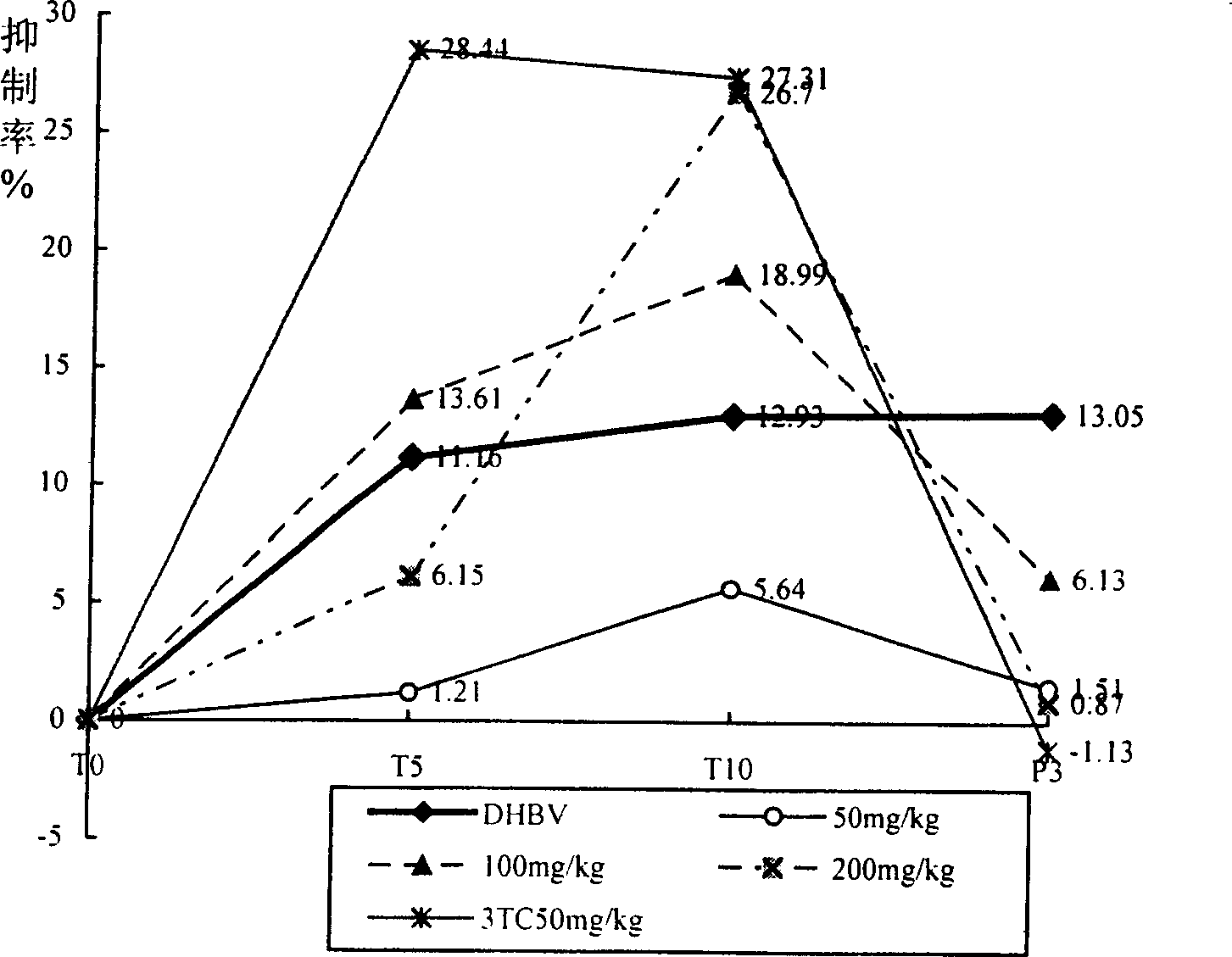
Application of radix gentianae in preparing medicine for treating viral hepatitis B
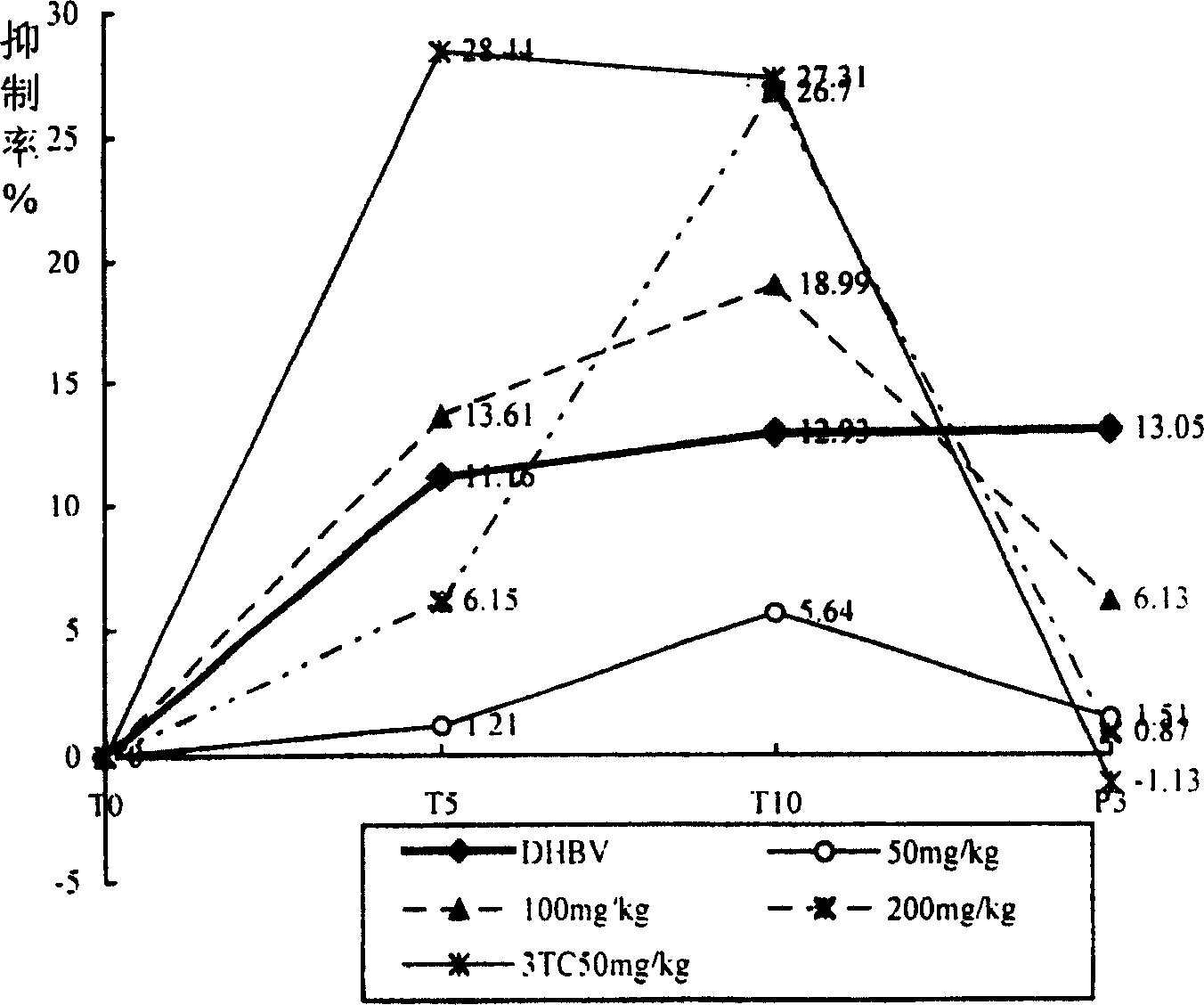
The invention discloses an application of radix gentianae in preparing a medicine for treating viral hepatitis B. The radix gentianae is a radix gentianae extract which is prepared by conducting extracting with water, ethanol or carbon dioxide as a solvent. In accordance with a conventional process, the radix gentianae or the radix gentianae extract is prepared into any one of the following pharmaceutical dosage forms: tablets, capsules, granules, pills and the like. The radix gentianae is applicable to preparation of the medicine for treating the viral hepatitis B; and the medicine, which is suitable for clinical application, is prepared from the radix gentianae. The radix gentianae can obviously reduce a DHBV-DNA titer, an HBsAg level and AST and ALT activities in serum of a hepatitis B model duck; within 7 days of drug withdrawal, a recurrence phenomenon is avoided; and in addition, the radix gentianae can relieve degeneration of hepatic cells and inflammatory cell infiltration. It is indicated that the composition (the medicine) is obvious in effect of resisting hepatitis B virus of the duck; therefore, it is indicated that the composition (the medicine) has a clinical application value. The pharmaceutical preparation of the medicine for treating the viral hepatitis B is scientific and reasonable.
Owner:姚萍
Traditional Chinese medicine prescription for treating viral hepatitis B and preparation method of traditional Chinese medicine prescription
The invention discloses a traditional Chinese medicine prescription for treating viral hepatitis B, and belongs to the field of traditional Chinese medicinal preparations. The traditional Chinese medicinal preparation is a pharmaceutical composition of Tibetan capillaris, radices macrotomiae, chicory herb, radix bupleuri, radix gentianae, Japanese wormwood herb and cornu saigae tataricae, and in accordance with a conventional process, the pharmaceutical composition is prepared into any one of the following pharmaceutical dosage forms: tablets, capsules, granules, pills and the like. The traditional Chinese medicinal preparation for treating the viral hepatitis B provided by the invention is scientific and reasonable in compatibility. The traditional Chinese medicinal composition can obviously reduce a DHBV-DNA titer, an HBsAg level and AST and ALT activities in serum of a hepatitis B model duck; within 7 days of drug withdrawal, a recurrence phenomenon is avoided; and in addition, the composition can relieve degeneration of hepatic cells and inflammatory cell infiltration. It is indicated that the composition is obvious in effect of resisting hepatitis B virus of the duck; therefore, it is indicated that the prescription has a clinical application value.
Owner:姚萍
Application of Phyllostachys polysaccharide component in the preparation of anti-hepatitis B virus medicine
InactiveCN103040857BImprove immunityReduce harmOrganic active ingredientsAntiviralsPhyllanthus urinariaIn vivo
The invention relates to an application of a phyllanthus urinaria polysaccharide component in preparing a drug for resisting a hepatitis B virus, and belongs to the field of medicine. The phyllanthus urinaria polysaccharide component is a phyllanthus urinaria polysaccharide component PUIP II which comprises rhamnose, arabinose, mannose and glucose at a molar ratio of 0.11:0.36:0.08:0.45. The phyllanthus urinaria polysaccharide component is artificially extracted, separated and purified by a medicinal plant, namely phyllanthus urinaria. An in-vitro bioactivity experiment indicates that the phyllanthus urinaria polysaccharide component PUIP II can inhibit secretion of HBsAg and HBeAg and reproduction of HBV-DNA (hepatitis B virus-deoxyribose nucleic acid) in HepG2.2.2.15 cell culture, and has a certain in-vitro HBV activity resistance effect, and an in-vivo activity experiment indicates that the PUIP II has an obvious inhibiting effect on DHBVDNA (duck hepatitis B virus deoxyribose nucleic acid) in a duck, is smaller in bounce, and can be used for preparing the drug for resisting the hepatitis B virus and for preparing health care products for protecting a liver.
Owner:FUJIAN AGRI & FORESTRY UNIV
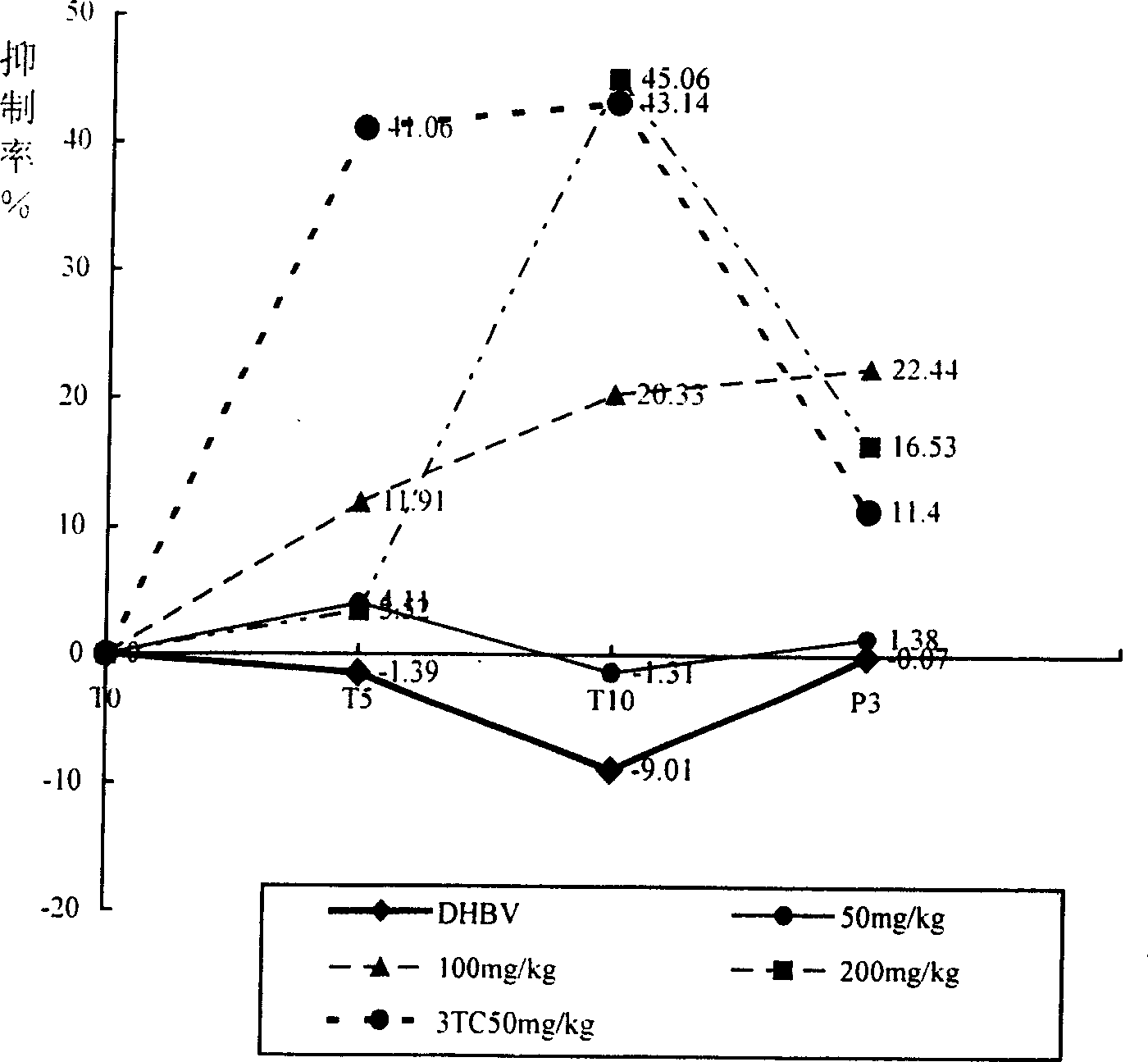
Application of radix bupleuri in preparing medicine for treating viral hepatitis B
The invention discloses an application of radix bupleuri in preparing a medicine for treating viral hepatitis B. The radix bupleuri is a radix bupleuri extract which is prepared by conducting extracting with water, ethanol or carbon dioxide as a solvent. In accordance with a conventional process, the radix bupleuri or the radix bupleuri extract is prepared into any one of the following pharmaceutical dosage forms: tablets, capsules, granules, pills and the like. The radix bupleuri is applicable to preparation of the medicine for treating the viral hepatitis B; and the medicine, which is suitable for clinical application, is prepared from the radix bupleuri. The radix bupleuri can obviously reduce a DHBV-DNA titer, an HBsAg level and AST and ALT activities in serum of a hepatitis B model duck; within 7 days of drug withdrawal, a recurrence phenomenon is avoided; and in addition, the radix bupleuri can relieve degeneration of hepatic cells and inflammatory cell infiltration. It is indicated that the composition (the medicine) is obvious in effect of resisting hepatitis B virus of the duck; therefore, it is indicated that the composition (the medicine) has a clinical application value. The pharmaceutical preparation of the medicine for treating the viral hepatitis B is scientific and reasonable.
Owner:姚萍
Medicinal liquor for treating chronic gastritis
InactiveCN106109634AEffective treatmentCompatibility is simpleDispersion deliveryDigestive systemDuck hepatitis B virusMedicinal herb
The invention discloses a kind of medicinal wine for treating chronic gastritis. Wind 15‑25 grams, white peony root 10‑20 grams, chicken bone grass 10‑20 grams, white back leaf root 5‑15 grams, safflower 5‑15 grams, rice wine 1000 ml, by mixing Atractylodes macrocephala, ghost needle grass, hawthorn , gallinacea, swollen joint wind, white peony root, chicken bone grass, white back leaf root, and safflower are coarsely crushed, put into a cloth bag, placed in a sealed container, soaked in rice wine, and sealed for more than 30 days to obtain. The medicated wine has the effects of invigorating the spleen and stomach, promoting qi and reducing swelling, dispelling stagnation and relieving pain, promoting blood circulation and softening the liver. gastritis; its compatibility is simplified, the source of medicine is wide, the preparation is simple and convenient, and the cost is low.
Owner:黄春荣
Therapeutic hepatitis B vaccine
ActiveCN102462840BInhibition of replicationDigestive systemAntiviralsHepatitis B Surface AntigensHbv replication
The invention discloses a therapeutic hepatitis B vaccine. Active components of the therapeutic hepatitis B vaccine comprise a protein gp96, a hepatitis B surface antigen and a hepatitis B core protein. The protein gp96 has a sequence shown in the sequence 1 of the sequence table. The hepatitis B surface antigen has a sequence shown in the sequence 5 of the sequence table. The hepatitis B core protein has a sequence shown in the sequence 3 of the sequence table. The active components also comprise a plasmid pcDNA-gp96 containing a coding gene of the protein gp96, a plasmid pcDNA-HB containing a coding gene of the hepatitis B surface antigen and a plasmid pcDNA-HBc containing a coding gene of the hepatitis B core protein. The therapeutic hepatitis B vaccine provided by the invention can effectively inhibit hepatitis B virus (HBV) replication, can eliminate viruses infecting the liver and has a very important value to hepatitis B prevention and treatment.
Owner:北京热休生物技术有限公司
Method of preparing extract of Mallotus apelta and uses for fighting hepatitis B virus
InactiveCN101584702BSignificant anti-HBV effectAbundant resourcesOrganic active ingredientsDigestive systemBULK ACTIVE INGREDIENTHepatitis B virus surface Antigen
The invention relates to anti-HBV activity of Mallotus apelta extract and uses in prepartion of medicine for treating related diseases caused by hepatitis B. The extract of Mallotus apelta provided by the invention has basic components of apiolin, apiin-7-O-belta-D-glucoside, and flavonoids compounds of 5,7-dihydroxy-6-isopentene group-4'-methoxyl flavonone, flavonoids compounds Mallotus apelta have obvious inhibiting effect on hepatitis B virus surface antigen and hepatitis B virus e antigen ono surface of HepG 2215, and cabaple of inhibiting duplication of Duck Hepatitis B Virus deoxyribonucleic acid, withdraw rebound is weaker than positive reference medicine lamivudine, and hepatitis B is provided with a subsequent inhibiting effect after withdraw. The medicine according to the invention has strong function for fighting hepatitis B virus, clear active ingredient, which is suitable for industrial production, predictablly used for preparing medicine of preparing diseases infected by hepatitis B virus.
Owner:广西中医学院
Monoclonal antibody capable of simultaneously recognizing duck hepatitis A virus 1 and duck hepatitis A virus 3, and hybridoma cell strain and application thereof
InactiveCN106811444AStable secretionImprove stabilityMicroorganism based processesImmunoglobulins against virusesEscherichia coliAnatis
The invention discloses a monoclonal antibody capable of simultaneously recognizing a duck hepatitis A virus 1 and a duck hepatitis A virus 3, and a hybridoma cell strain and application of the monoclonal antibody. The hybridoma cell strain is high in stability and can still secrete the monoclonal antibody after subculture in vitro and frozen storage and resuscitation of cells, the secreted monoclonal antibody can simultaneously recognize the duck hepatitis A virus 1 and the duck hepatitis A virus 3, the affinity constant with the duck hepatitis A virus 1 is more than 109 according to the order of magnitudes, the monoclonal antibody does not have specific reactions with the duck viral enteritis virus, the duck hepatitis B virus, the duck tembusu virus, the goose plague virus, the riemerella anatipestifer, the escherichia coli from ducks and the salmonella anatis, so that the broad-spectrum high-specificity high-affinity detection antibody is provided for clinics and basic units to avoid leak detection of the duck hepatitis A virus 1 and the duck hepatitis A virus 3, and can be used to prepare reagents for separately detecting the duck hepatitis A virus 1 or the duck hepatitis A virus 3 and simultaneously detecting the duck hepatitis A virus 1 and the duck hepatitis A virus 3.
Owner:SICHUAN AGRI UNIV
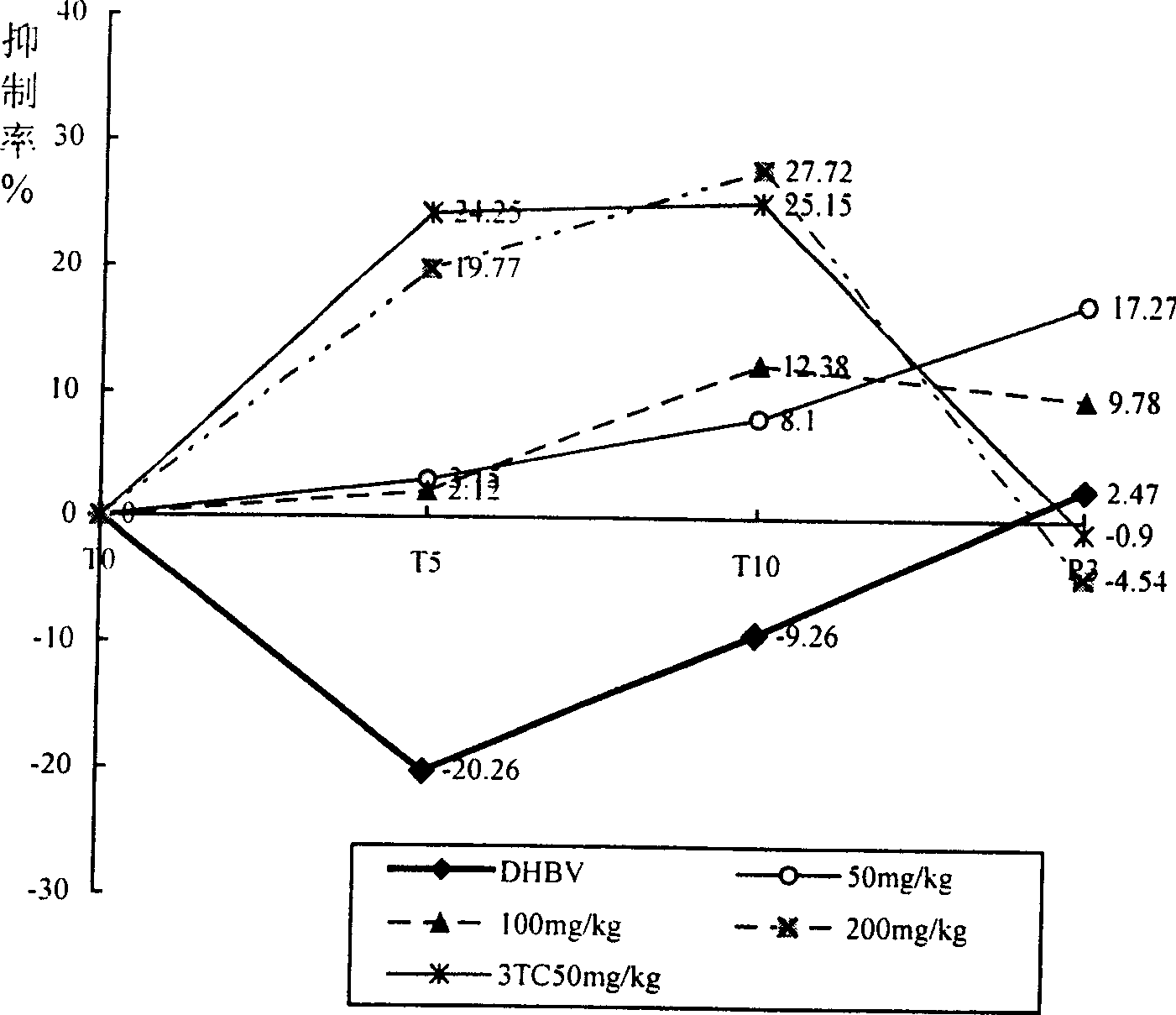
Red back leaf root extract, preparation method and application in preparation of medicine for treating hepatitis B
ActiveCN104586925BThe extraction process is simple and reasonableEffective part extractDigestive systemAntiviralsAlcoholMedicine
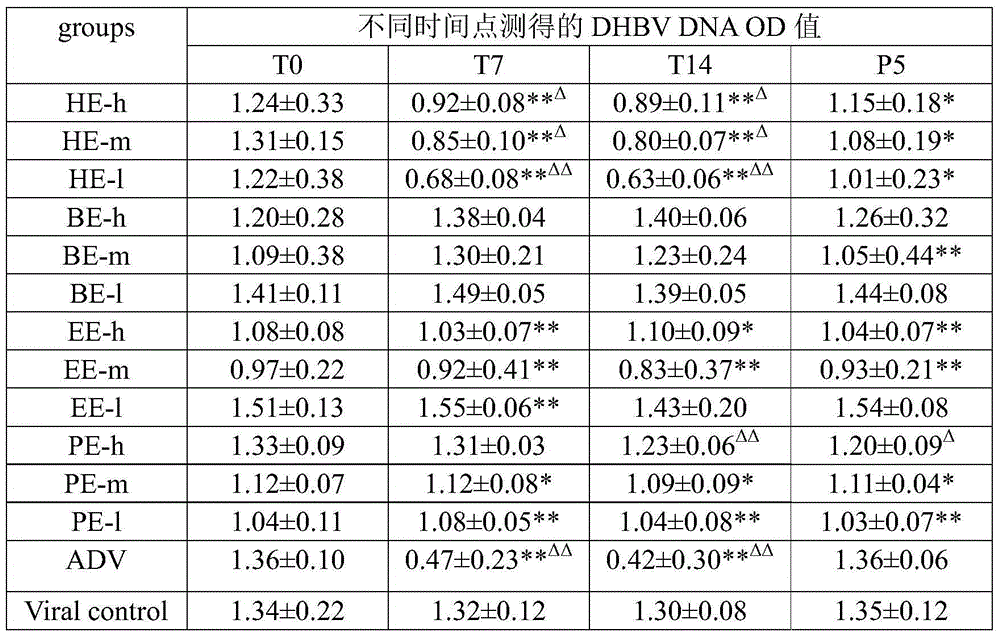
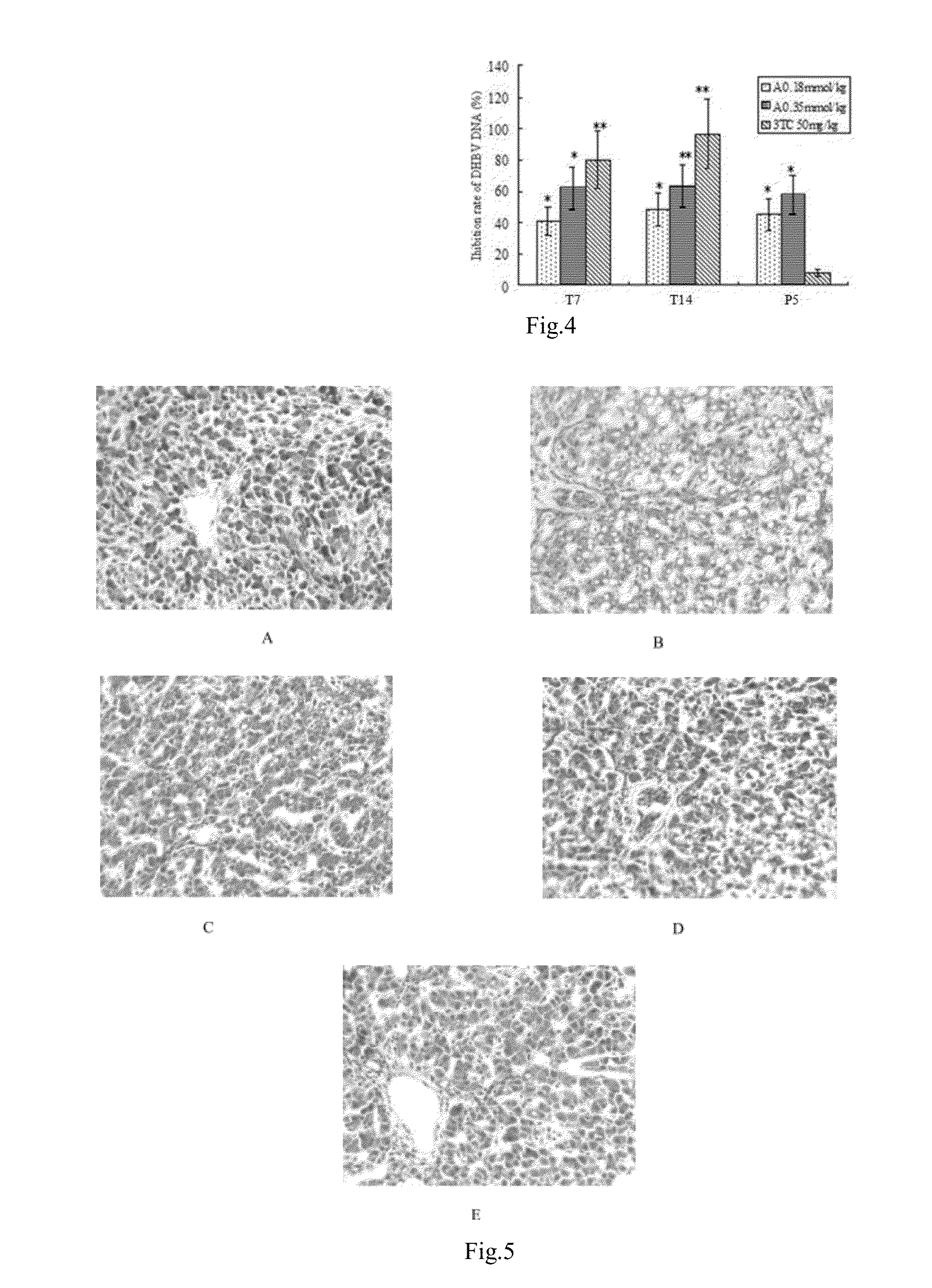
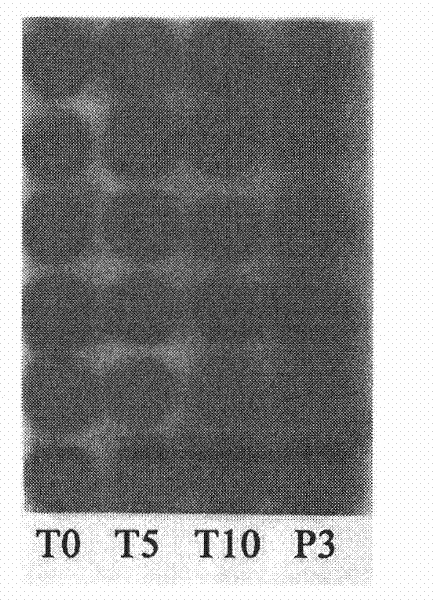
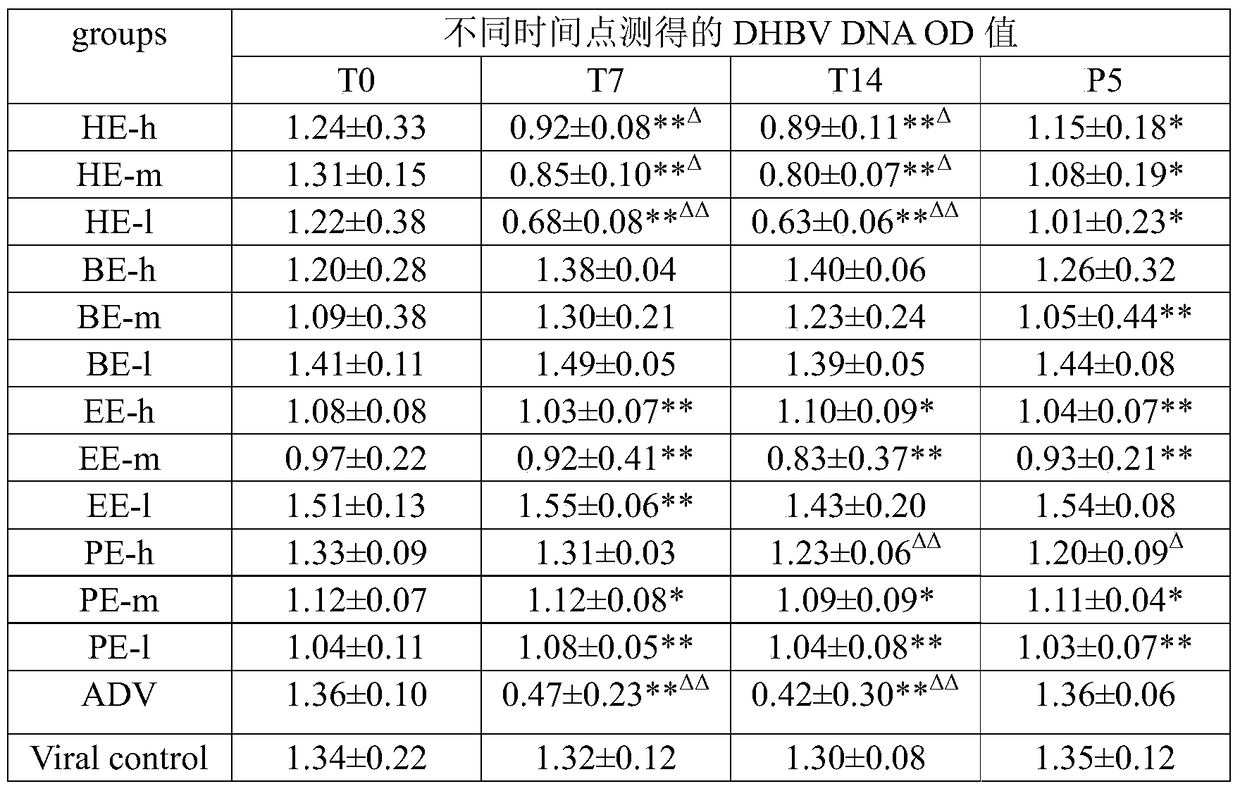
The invention discloses a redback christmashush extract, and a preparation method and application thereof in preparation of drugs for treating hepatitis B. The preparation method comprises the steps of carrying out alcohol extraction, centrifugal concentration, macroporous adsorption resin column treatment, ethanol elution and reduced pressure drying, so as to obtain an effective part extract of redback christmashush. The preparation method has the beneficial effects that an extraction process is simple, convenient and reasonable; by applying the obtained effective part extract of redback christmashush in an anti-duck hepatitis B virus experiment, serum DHBV DNA can be remarkably inhibited on the seventh day and the fourteenth day (T7 and T14) by virtue of three doses of effective part extracts of redback christmashush (P is less than 0.01), and DHBV DNA rises again on fifth day (P5) after the drug delivery is stopped and is still remarkably lower than that before the drug delivery, so that the redback christmashush extract has a relatively ideal anti-hepatitis B virus effect, is a candidate drug with great promise and can be applied to the preparation of anti-hepatitis B drugs.
Owner:SOUTHERN MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com