Fluorescence quantitative PCR (polymerase chain reaction) detection method of duck hepatitis B virus, and reagent
A hepatitis B virus, fluorescence quantitative technology, applied in the direction of microbial determination/inspection, fluorescence/phosphorescence, biochemical equipment and methods, etc., can solve the problems of lack, lack of versatility, complex operation, etc., to achieve strong immunogenicity , the effect of strong versatility and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the design of duck hepatitis B virus fluorescent quantitative PCR diagnostic method primer, probe
[0070] A total of 44 duck hepatitis B virus genome sequences were retrieved in GenBank, from China (including Beijing, Hubei, Shanghai, Chongqing, Sichuan, Guilin, Henan, Guangdong, etc.) and foreign countries (the United States, Germany, India, Canada, South Africa, Australia, etc.), through the comparison of DNASIS software, there are many differences in the DHBV gene sequences in different regions, but the core region sequence bp241-414 reported in different regions is basically the same, with a minimum of 98% and a maximum of 100%.
[0071] Therefore, use this region as a template to design primers, and match multiple pairs of primers with all DHBV Core region nucleic acid sequences to obtain the most matched primers and probes. The designed primer sequences and fluorescent
[0072] The photoprobe sequence is as follows:
[0073] Upstream primer F: DHB...
Embodiment 2
[0084] Embodiment 2. Preparation of PCR standards:
[0085] (1). Construction of the pGEM-T / DHBV Core recombinant plasmid: Based on the gene sequence of the DHBVCore region provided by GenBank, the forward and negative primers were designed using the principle of negative strand extension, and BamHI and Xhol enzymes were added to both ends of the positive and negative primers respectively Cutting site and protective base G, the sequence is as follows (the underline is the introduced enzyme cutting site):
[0086] F1:G GGATCC ATATCAATGCTTCTAG;
[0087] R1:G CTCGAG TTATTTTCCTAGGCGAG;
[0088] PCR amplifies the DHBV Core gene of the pBR322 / 2DHBV plasmid, and the 100 μl reaction system is as follows:
[0089] 10×buffer 10μl, 10mM dNTP2μl, 1U / μl HotStart Taq Polymerase1μl, 25mM MgCl 2 6 μl, 50 mM F primer 1 μl, 50 mM R primer 1 μl, DEPC-H 2 O 77μl, DHBV plasmid (10-fold dilution) template 1μl.
[0090] PCR amplification conditions: first, pre-denaturation at 94°C for 30...
Embodiment 3
[0099] Embodiment 3: Optimization of FQ-PCR reaction system:
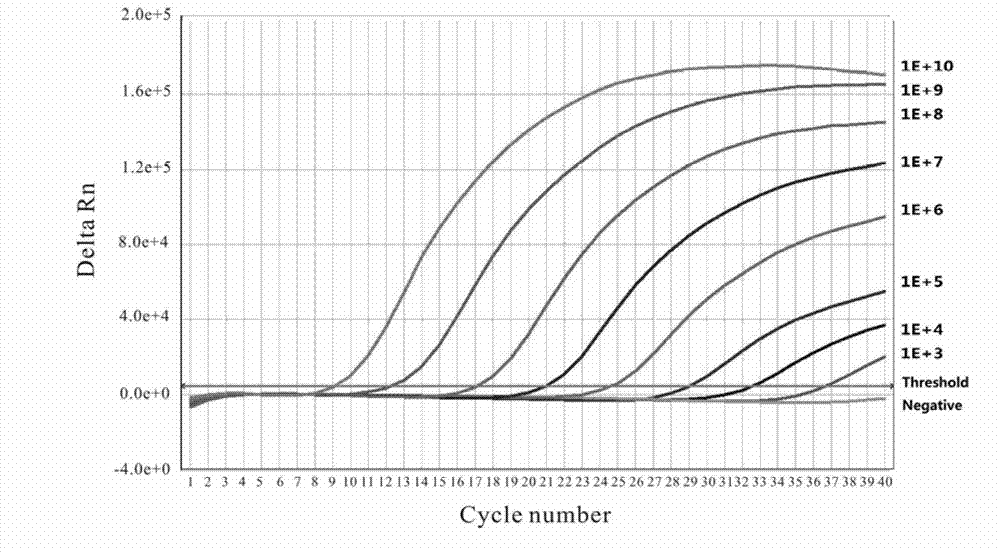
[0100]The matrix method was used to screen for the best optimization of primer and probe concentrations to obtain the lowest Ct value and higher fluorescence intensity. Optimize PCR amplification conditions, choose FAM (490nm) for fluorescence signal collection, and set data collection at the end of extension. Positive control, negative control, blank control and standards of different concentrations are set on the same machine.
[0101] The optimized FQ-PCR 25μl reaction system is:
[0102] 2×HotStart Taq PCR MasterMix12.5μl (including 0.1HotStart Taq Polymerase / μl, 500μM dNTP each, 20mM Tris-HCl (pH8.3), 100mM KCl, 3mM MgCl2), F primer 0.5μl (50mM), R primer 0.5μl (50mM), DHBV-Probe 0.5μl (10mM), DEPC-H2O 9μl, DHBV template 2μl.
[0103] Optimized PCR amplification conditions: firstly, pre-denaturation at 94°C for 2 minutes, followed by 40 cycles at 94°C for 15s, 49°C for 30s, and 72°C for 30s.
[0104] fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com