Recombinant polymerase isothermal amplification detection method of hepatitis B viruses in ducks
A hepatitis B virus, isothermal amplification technology, applied in the field of molecular biology, to achieve the effect of good specificity, rapid diagnosis and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Design and optimization of primers
[0041] 1. Design of primers:
[0042] In this embodiment, by comparing the C and S gene sequences of 40 strains of duck hepatitis B virus reported in NCBI, a series of RPA primers were designed by selecting the conserved regions of duck hepatitis B virus C and S genes, see for details Table 1.
[0043] Table 1: RPA primers designed for the conserved regions of duck hepatitis B virus C and S genes
[0044]
[0045]
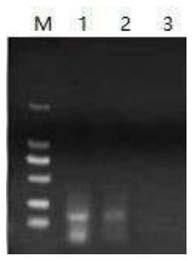
[0046] Utilize the RPA amplification reaction to screen the primers in Table 1, select the standard duck hepatitis B virus serum sample, extract the virus DNA as a template, and carry out RPA detection. The specific steps are as follows:
[0047] Extract the DNA template of the duck hepatitis B virus sample according to the EasyPure@ViralDNA / RNA Kit kit instructions, and perform the RPA reaction system according to the TwistAmp DNA Amplification Kits kit instructions: 1.2 μL each of the upstream and dow...
Embodiment 2
[0050] Embodiment 2: Optimization of RPA reaction conditions
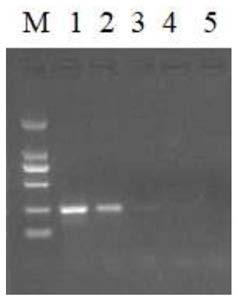
[0051] The usage amount of primers F1 and R1 in primer pair 1 was optimized respectively. The optimization results showed that the concentration of primers F1 and R1 was 20 μmol / μL, and the effect was the best when the usage amount was 1.2 μL. In addition, 6 concentration gradients were set up for the amount of MgAc used. The amount of MgAc added to the RPA system at a concentration of 280 mM was 0.5 μL, 1 μL, 1.5 μL, 2 μL, 2.5 μL and 3 μL. Amplification failed. When 1.5μL, 2μL, 2.5μL and 3μL MgAc were added to the reaction system, amplification products could be obtained, and the amplification effect was the best when the amount of MgAc was 2.5μL.
[0052] The optimized RPA reaction system is: 1.2 μL each of upstream and downstream primers (20 μmol / μL), RehydrationBuffer 29.5 μL, Template 3 μL, ddH 2 O 12.6 μL, 280 mM MgAc 2.5 μL.
[0053] In addition, the RPA reaction time and reaction temperature were optimize...
Embodiment 3
[0055] Embodiment 3: specificity test
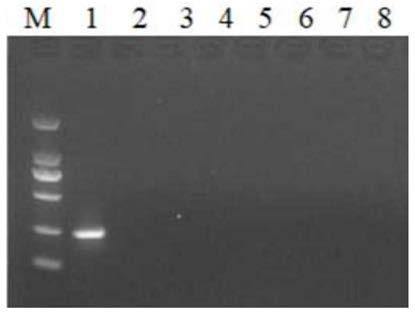
[0056] Duck hepatitis B virus, duck hepatitis A virus type 1 (DHAV-1), duck hepatitis A virus type 3 (DHAV-3), duck plague virus (DPV), duck-derived Escherichia coli (E.coli), duck-derived Xincheng The DNA / cDNA template of epidemic virus (NDV), duck origin H9 subtype avian influenza virus (AIV-H9) and Riemerella anatipestifer (RA) carries out RPA by the optimized reaction condition of embodiment 2, by gel Electrophoretic detection. Experimental results such as figure 1 As shown, only the duck hepatitis B virus is positive, and the others are all negative, indicating that the RPA detection system of the present invention has good specificity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com