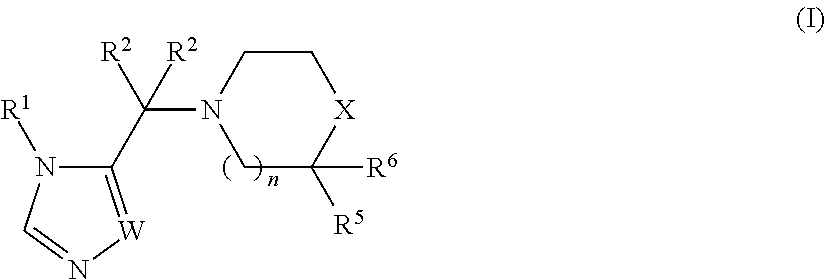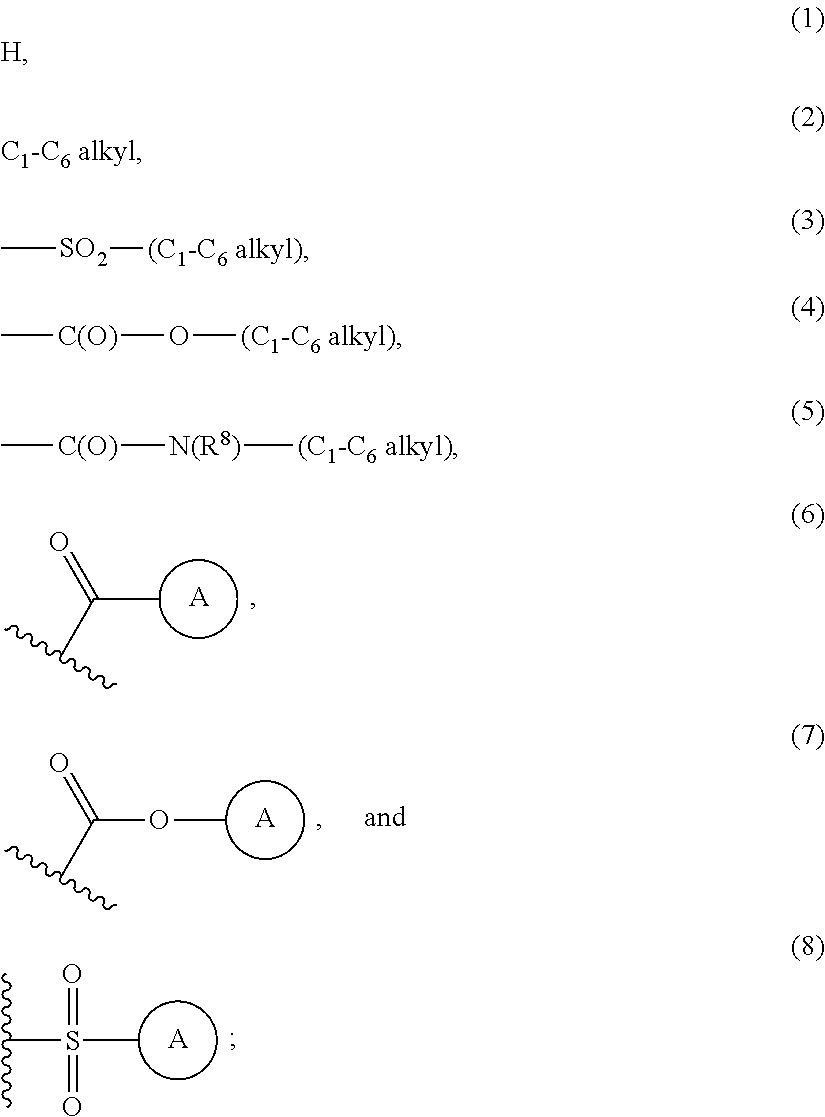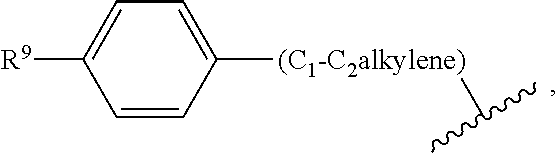Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
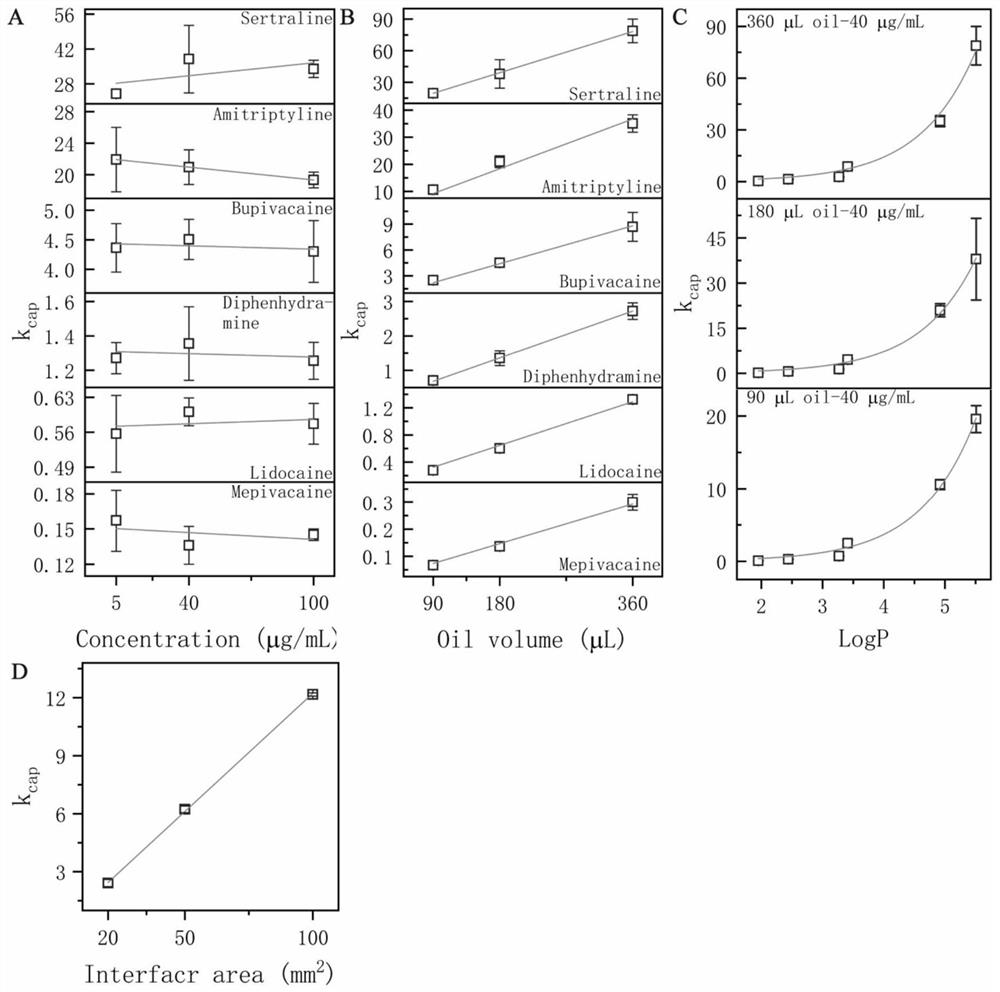
39 results about "Drug pharmacokinetics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmacokinetics is a branch of medicinal pharmacology that deals with the effects of drugs administered externally. It is derived from the Greek word 'pharmacon' (drug) and kineticos " (motion / momentum).
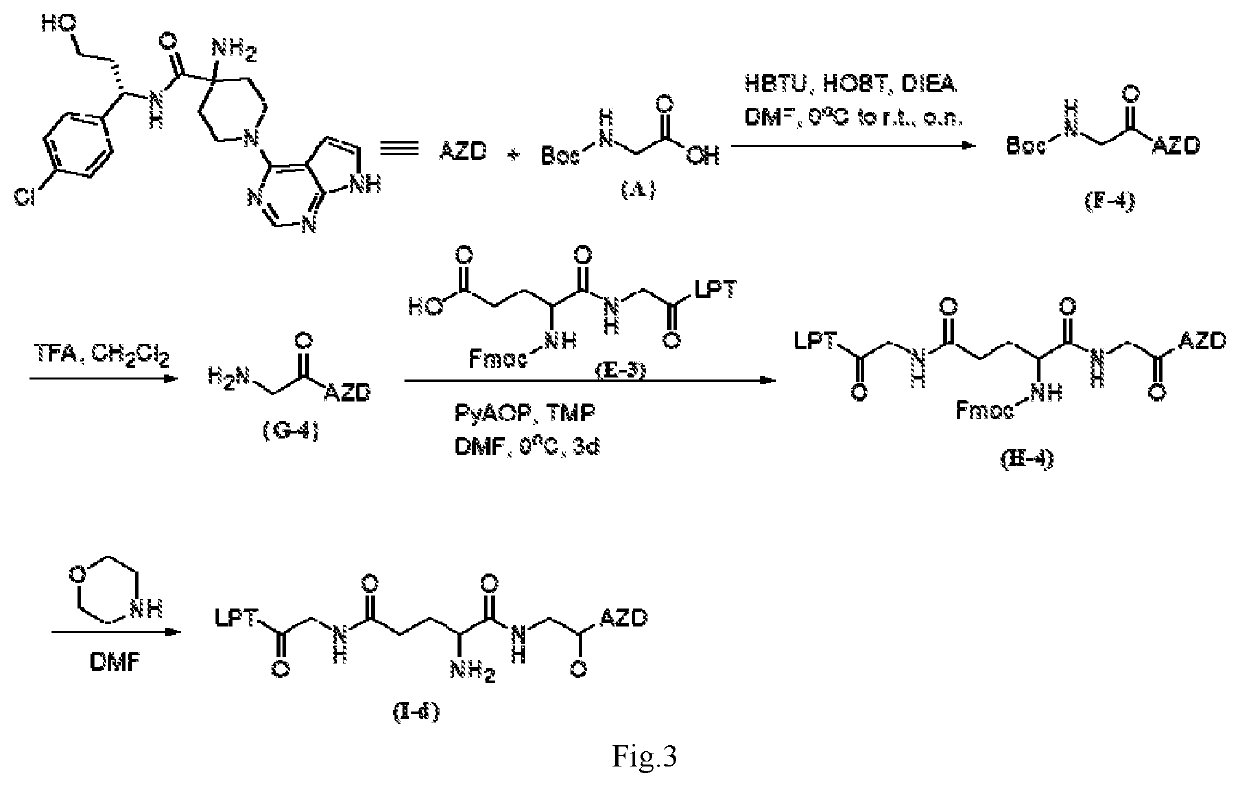
Method for altering drug pharmacokinetics based on medical delivery platform
InactiveUS20020095134A1Increase uptakeRapid uptake rateOrganic active ingredientsAmpoule syringesMedicineSurgery
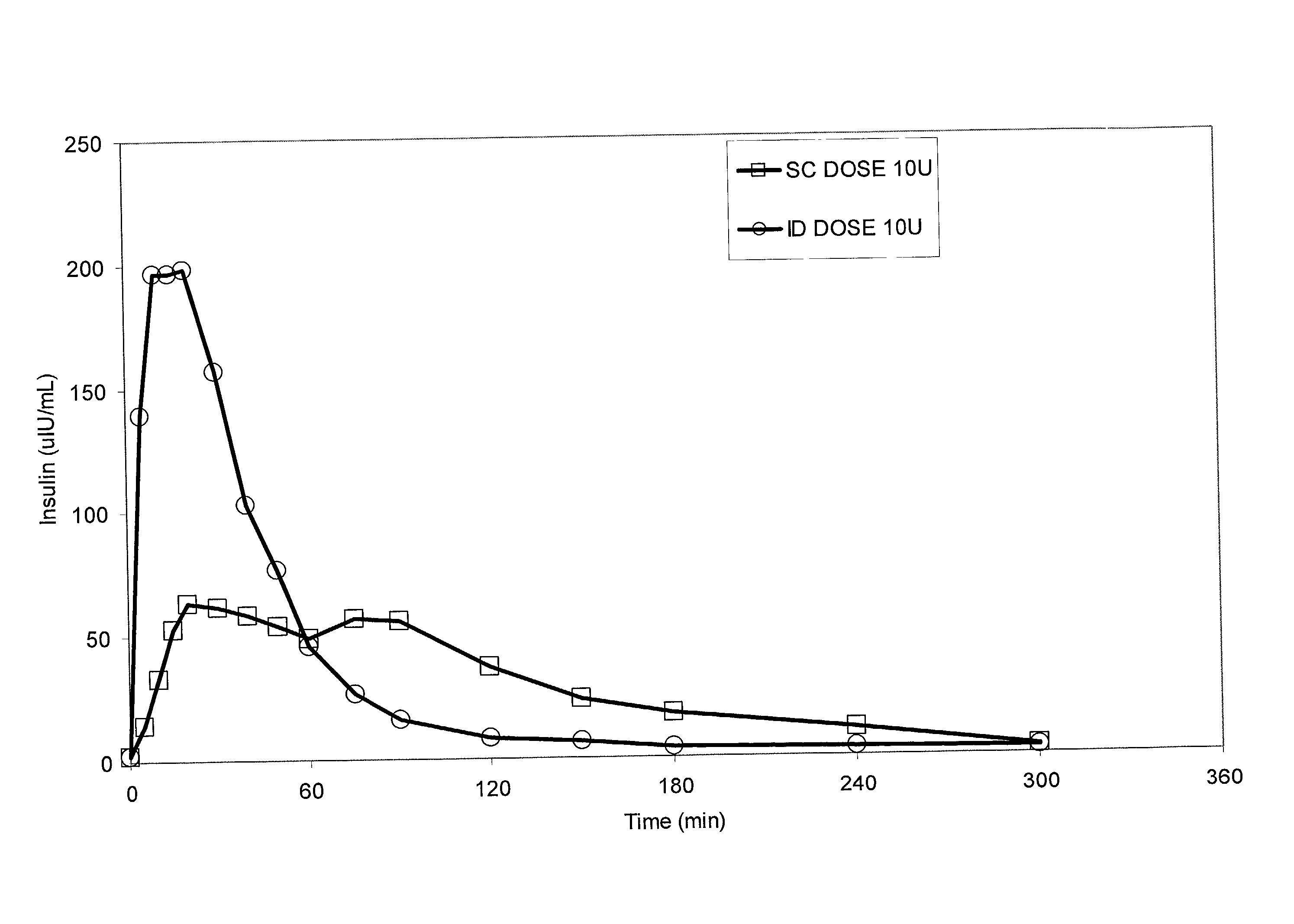
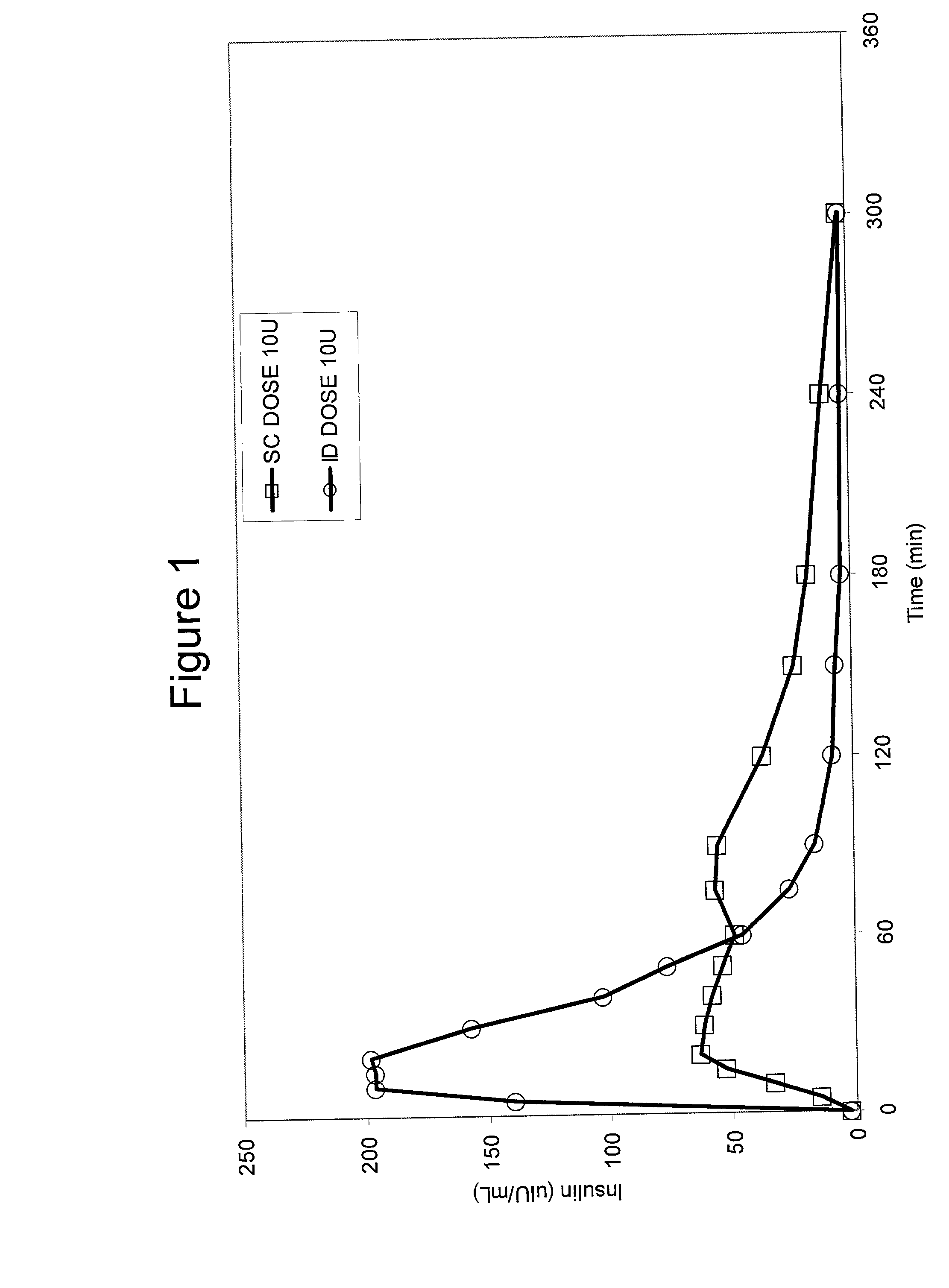
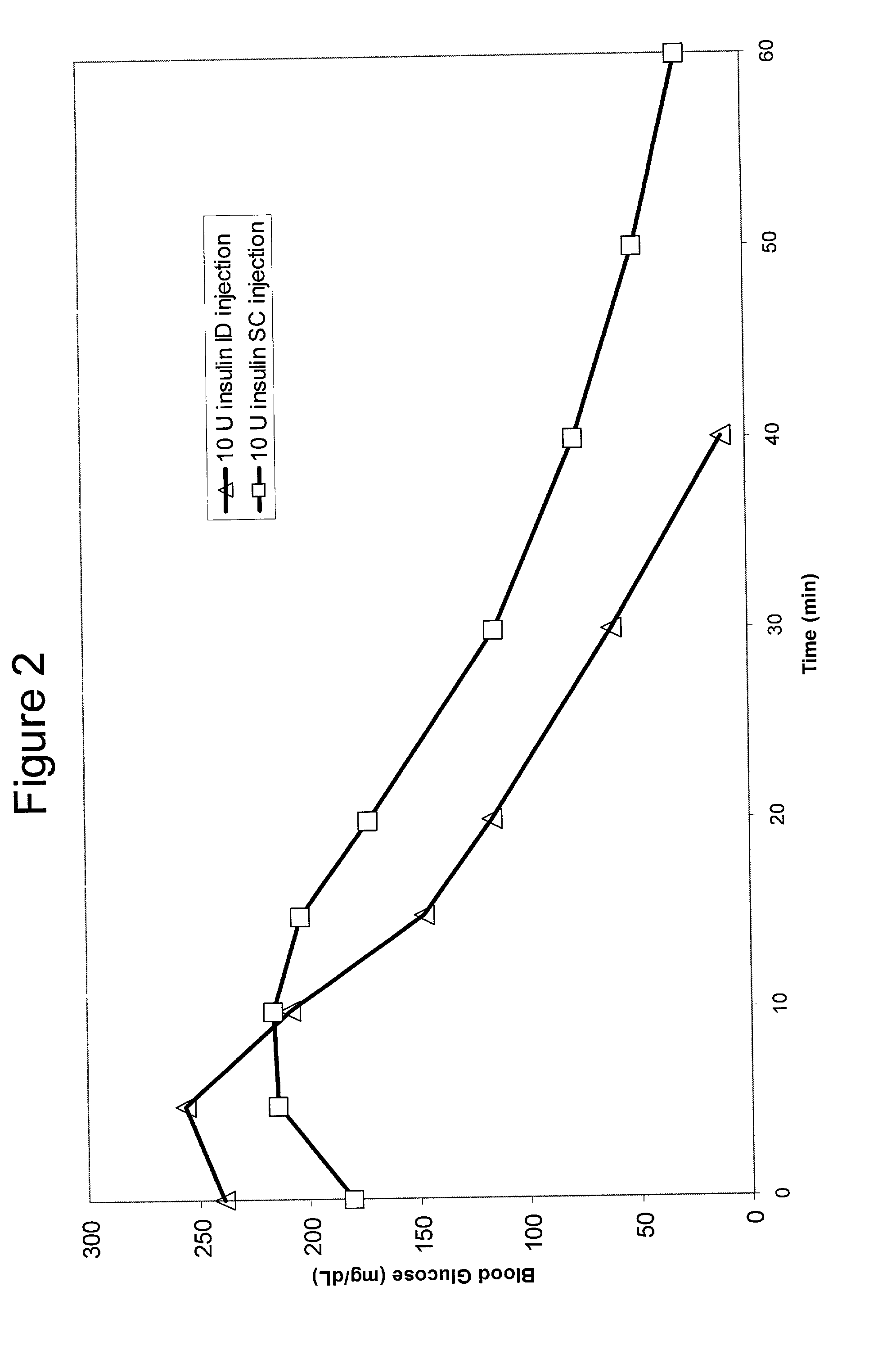
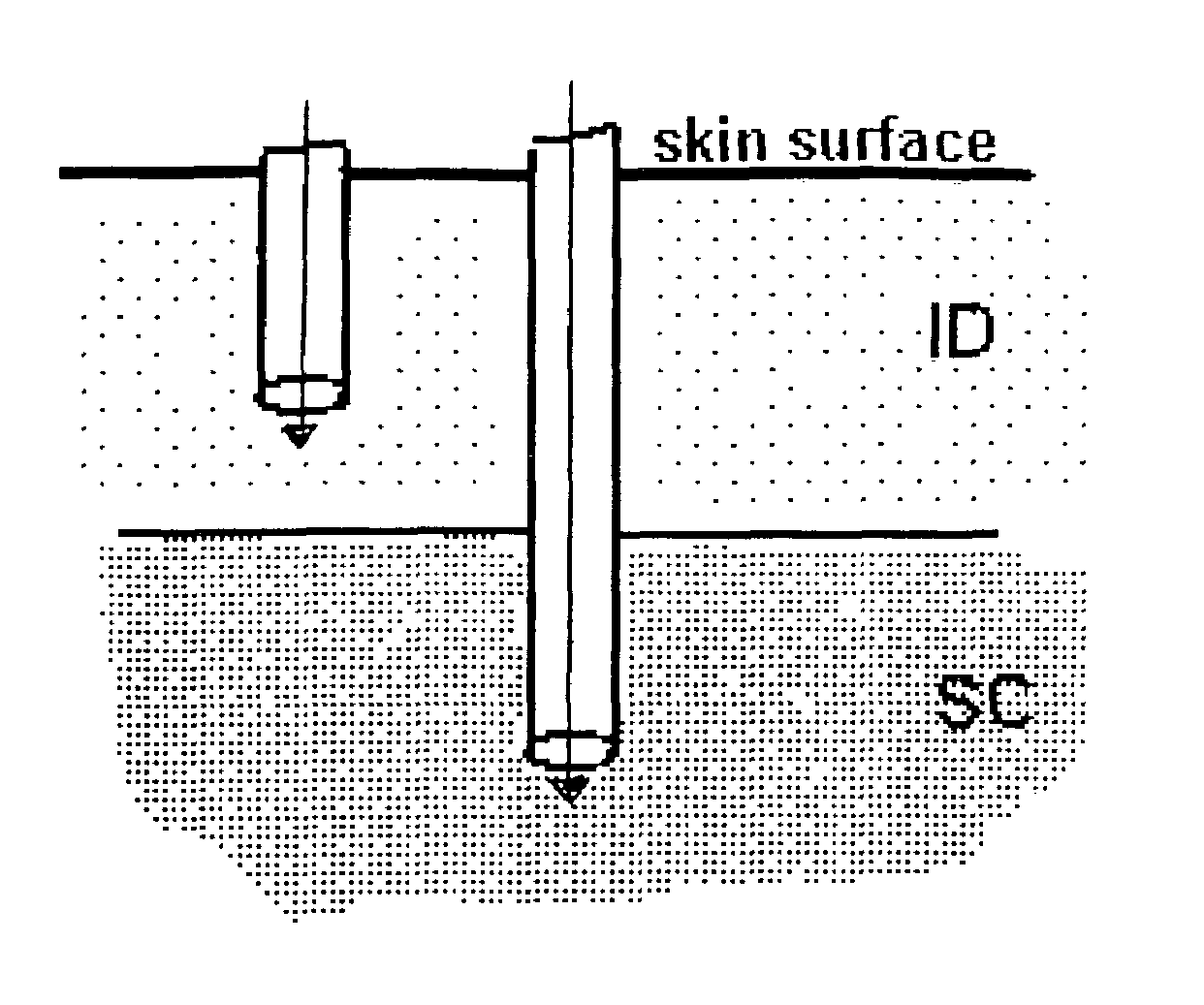
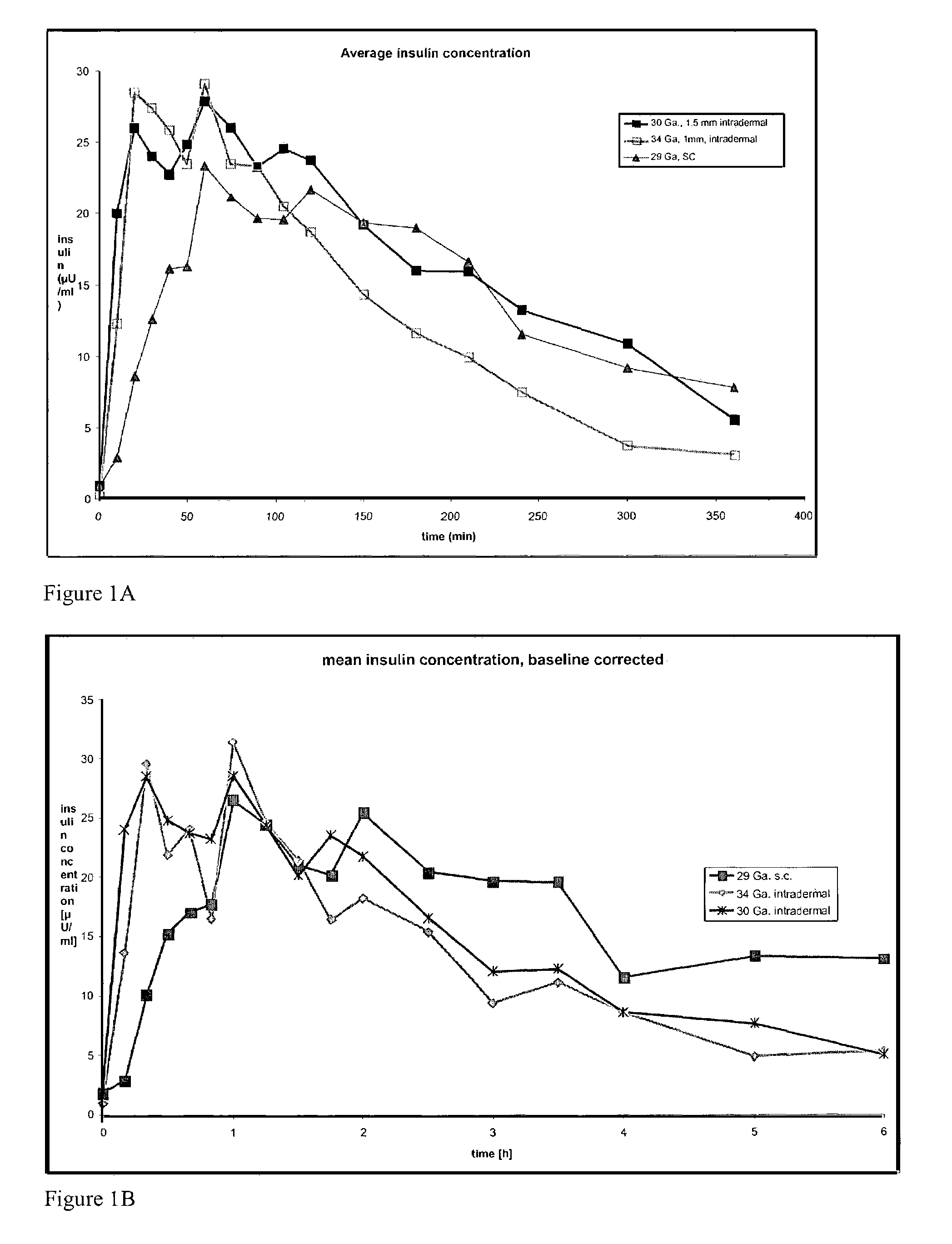
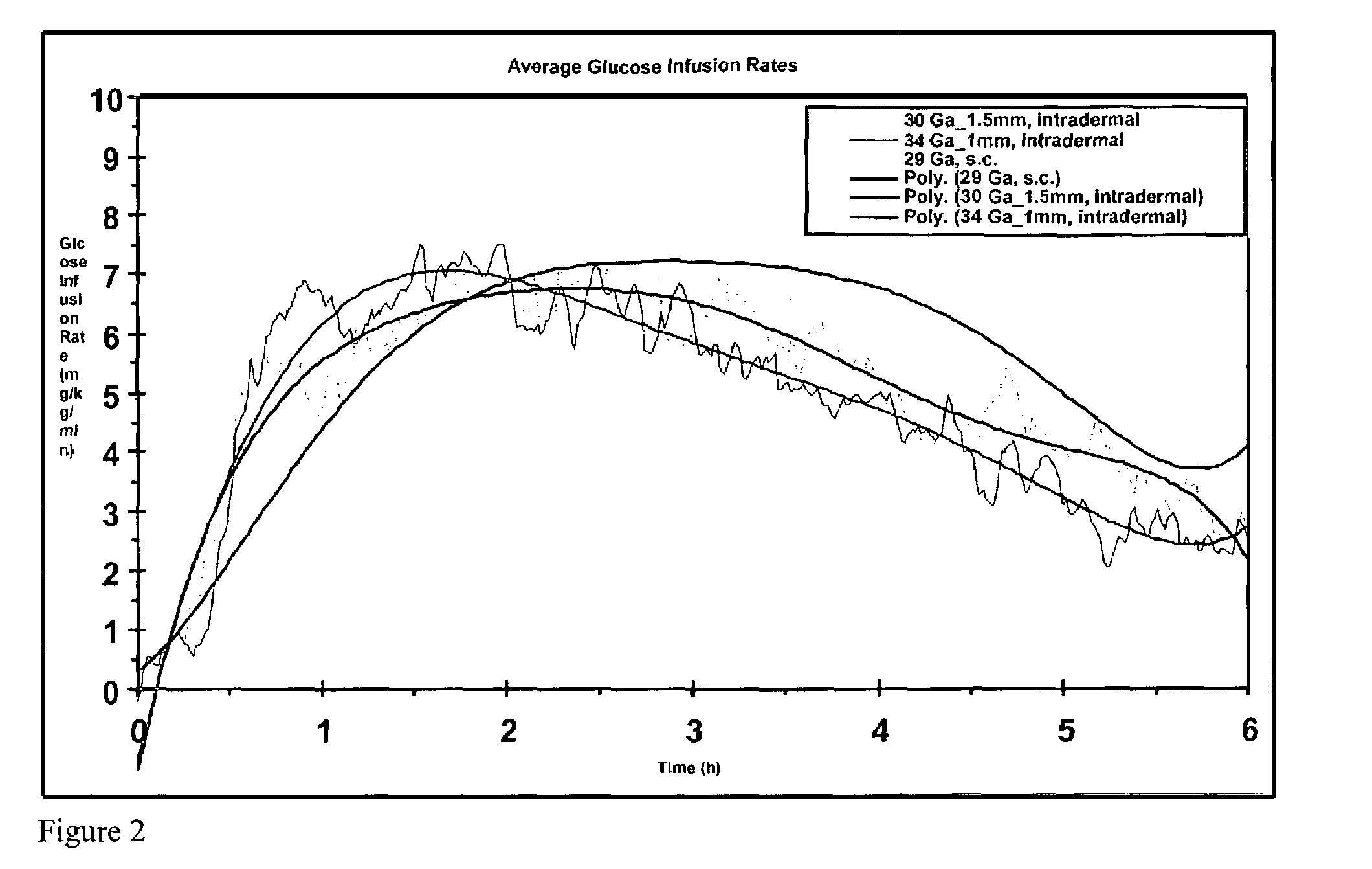
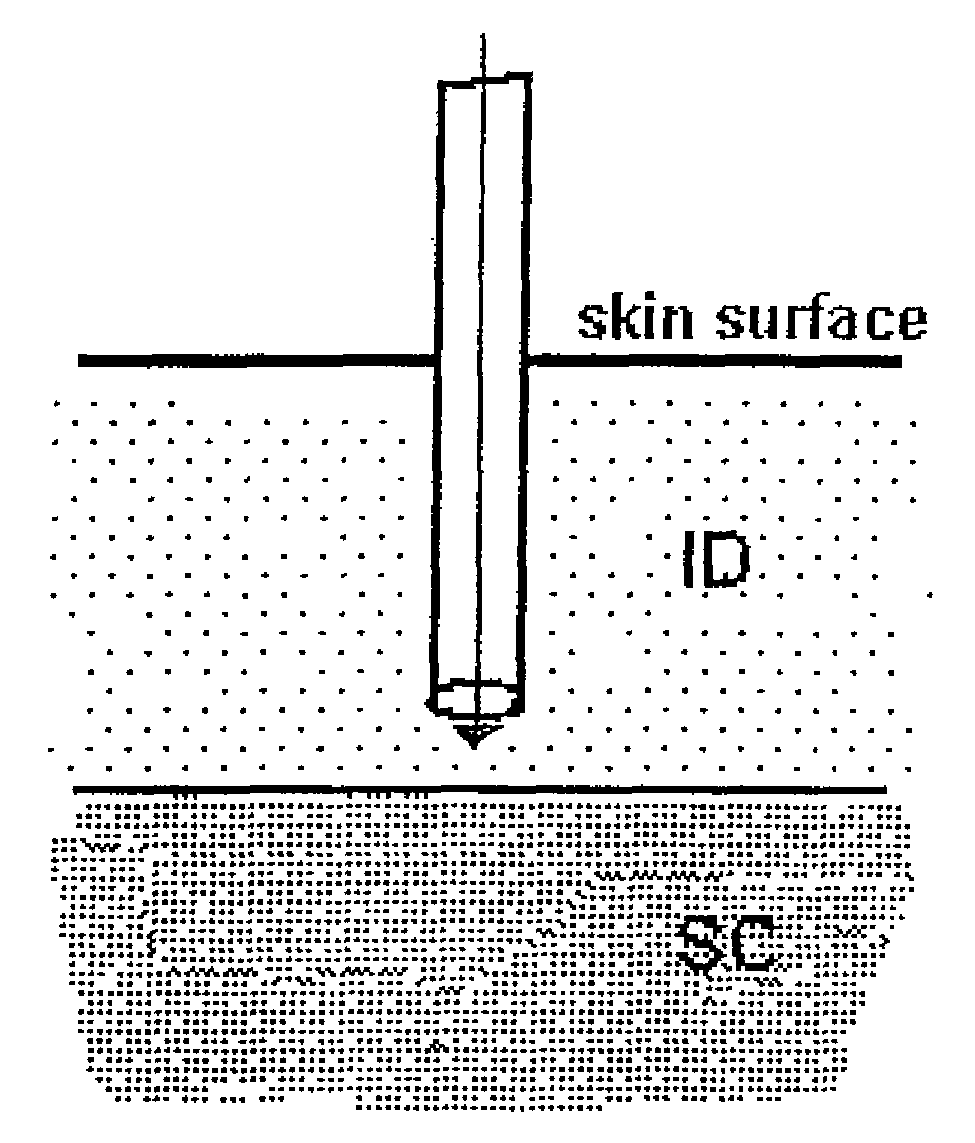
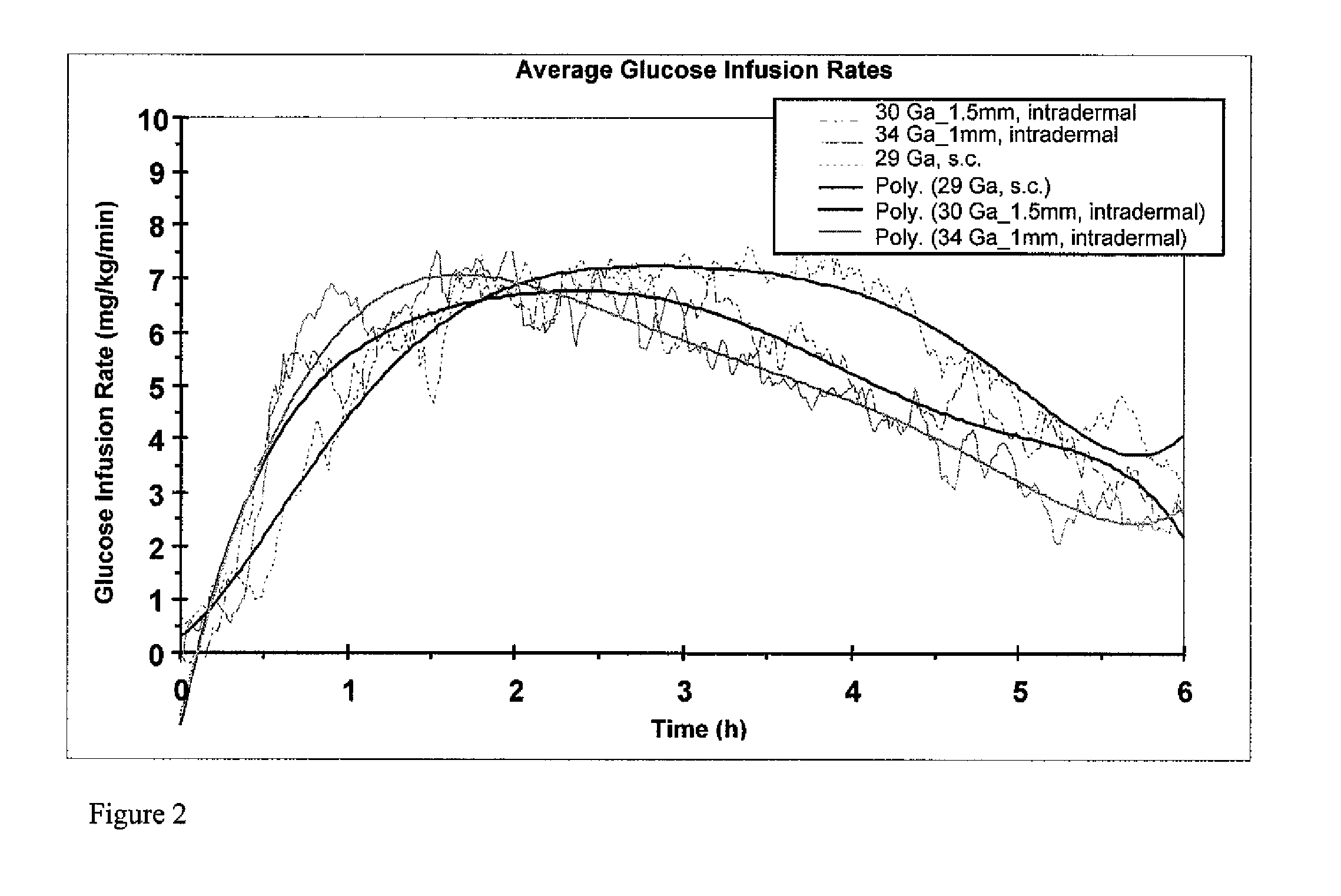
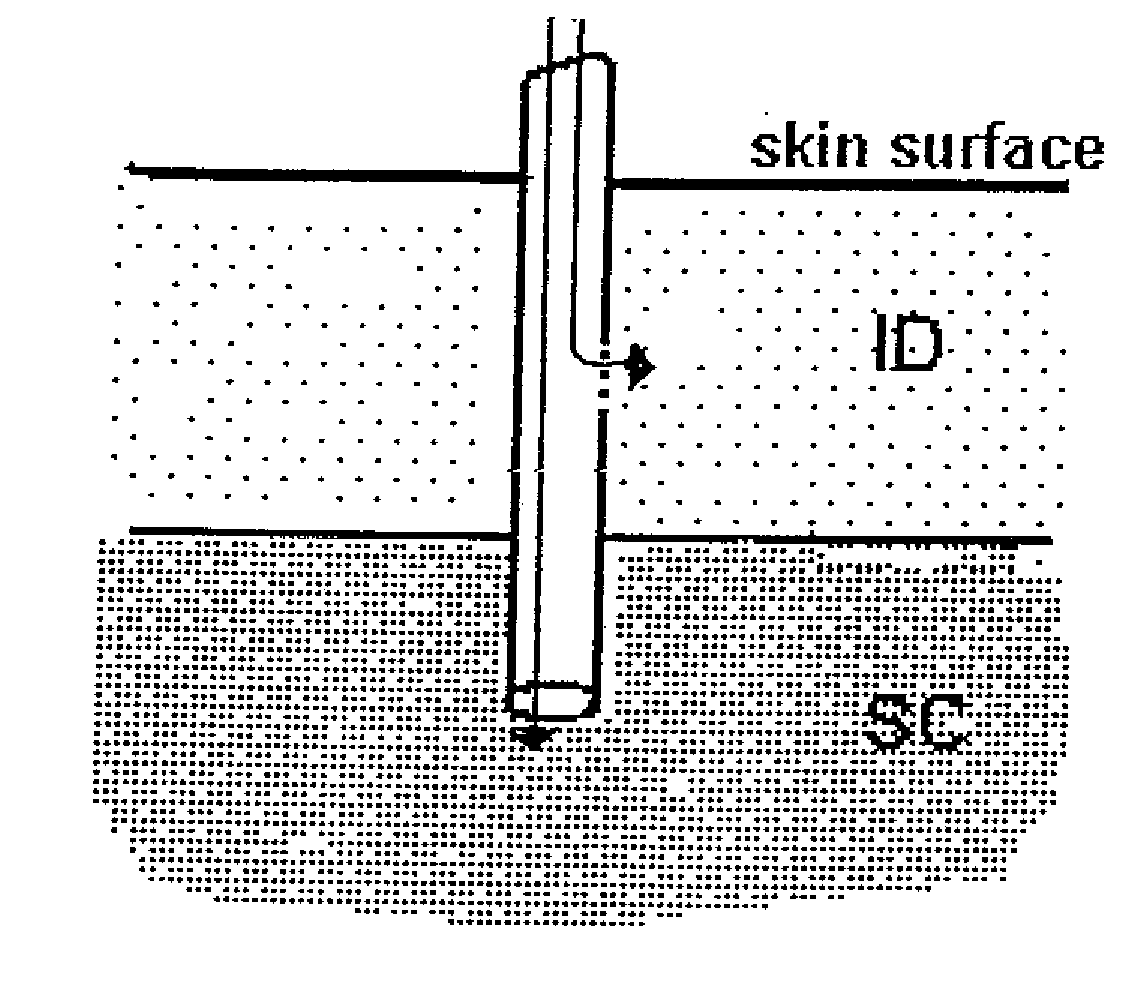
A method for directly delivering whereby a substance is introduced into an intradermal space within mammalian skin which involves administering the substance through at least one small gauge hollow needle having an outlet with an exposed height between 0 and 1 mm. The outlet is inserted into the skin to a depth of between 0.3 mm and 2 mm such that the delivery of the substance occurs at a depth between 0.3 mm and 2 mm.
Owner:BECTON DICKINSON & CO
Method and device for controlling drug pharmacokinetics
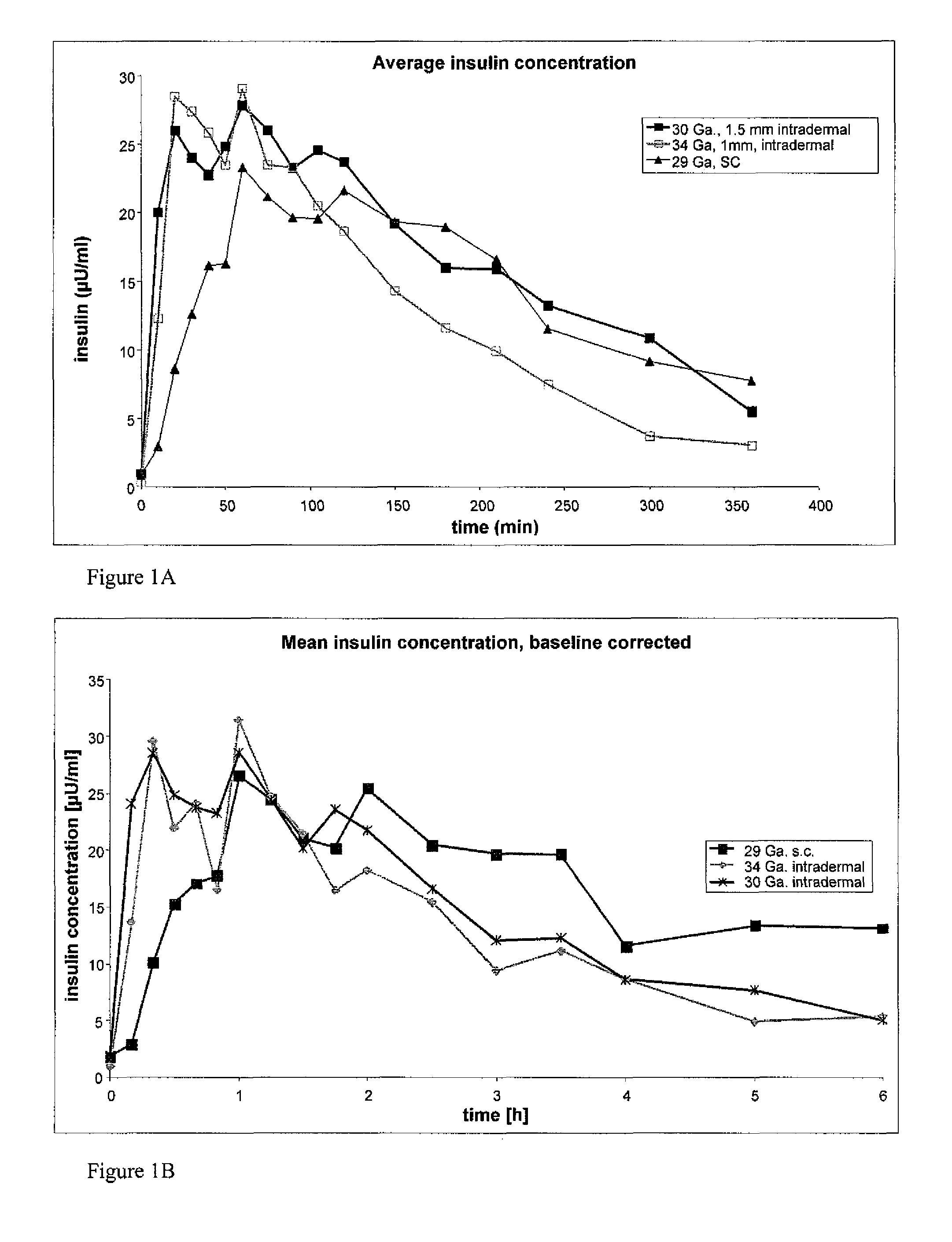
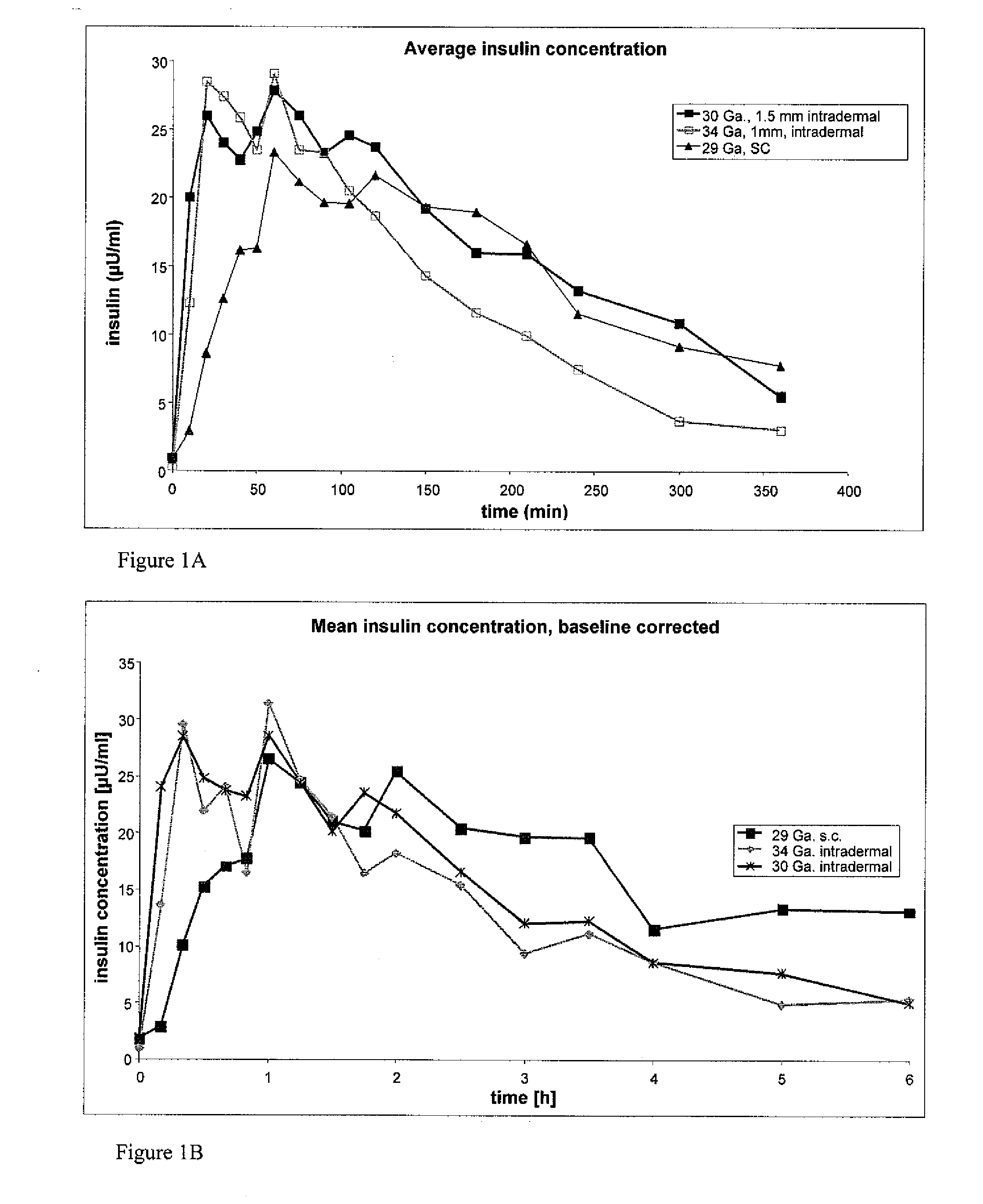
The invention pertains to methods and devices for controlling the pharmacokinetics of administered substances, particularly therapeutic substances by combining advantages of delivery to two or more compartments within the skin. The invention provides methods and devices for delivering substances to subcutaneous and intradermal compartments of the skin to achieve a hybrid pharmacokinetic profile that has a portion similar to that achieved by intradermal delivery, e.g., rapid and high peak onset levels of the substance, and a portion similar to that achieved by subcutaneous delivery, e.g., longer circulating levels of the substance.
Owner:BECTON DICKINSON & CO
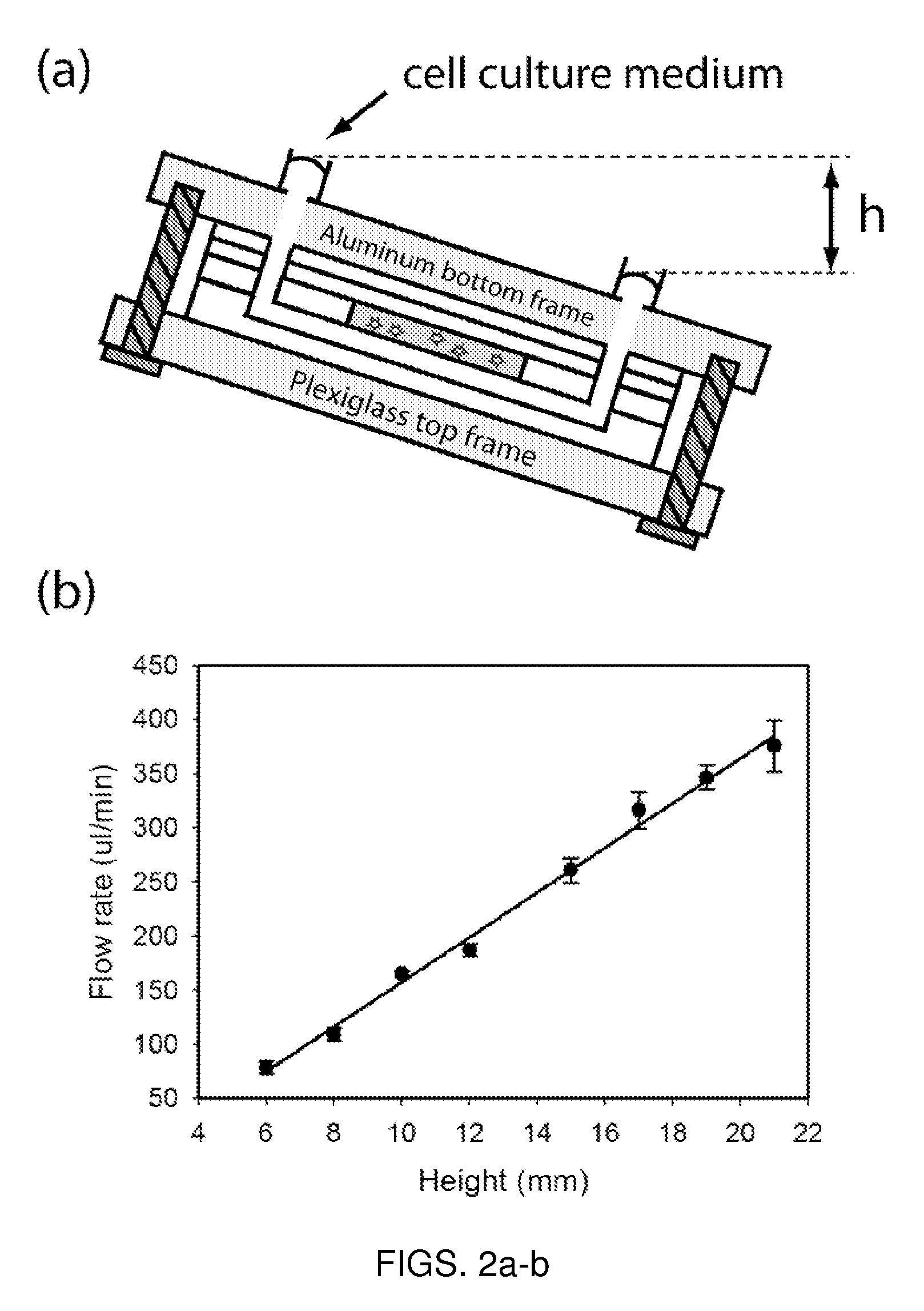
Microfluidic device for pharmacokinetic-pharmacodynamic study of drugs and uses thereof
ActiveUS8748180B2Bioreactor/fermenter combinationsCompound screeningTissues typesMicrofluidic channel
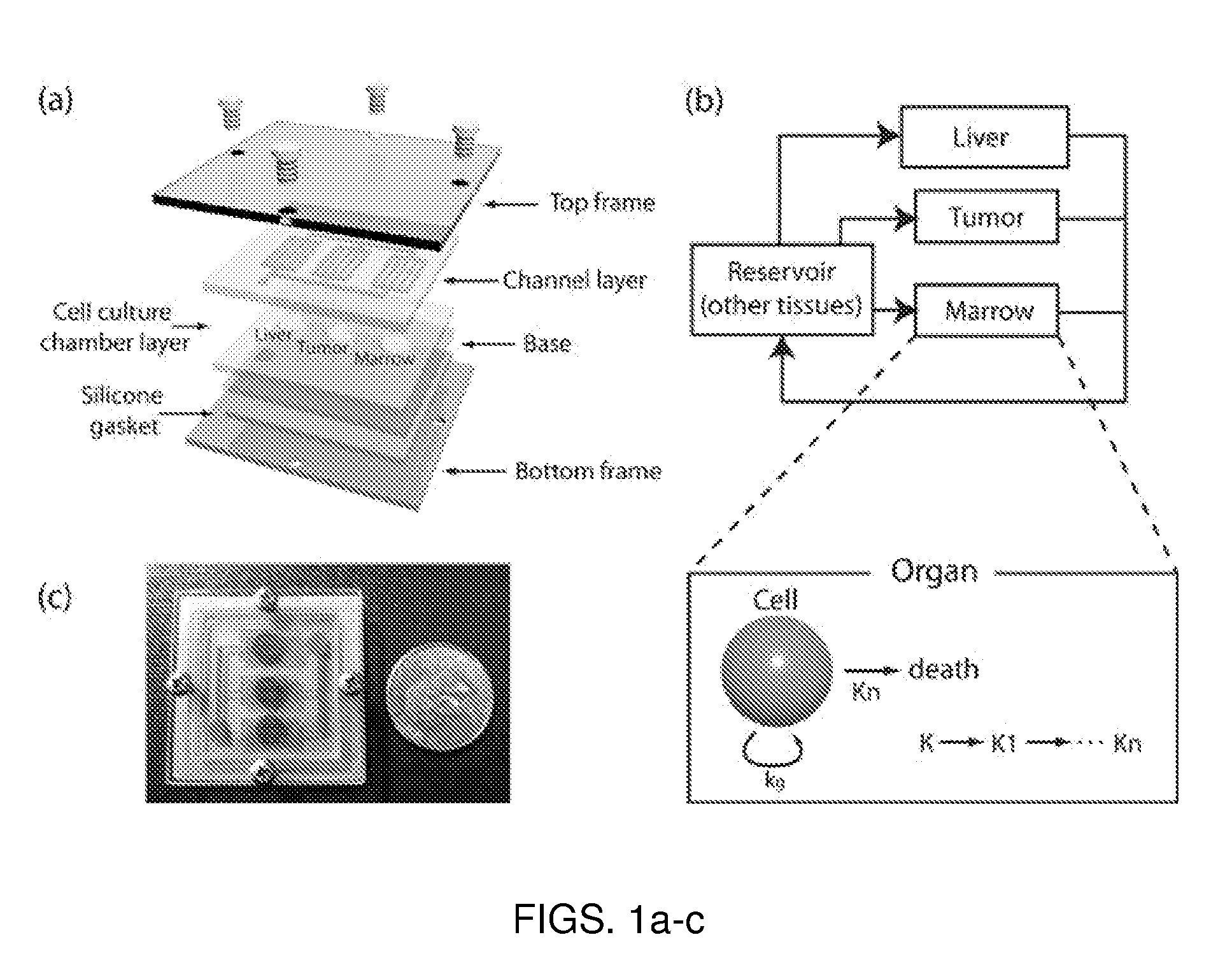
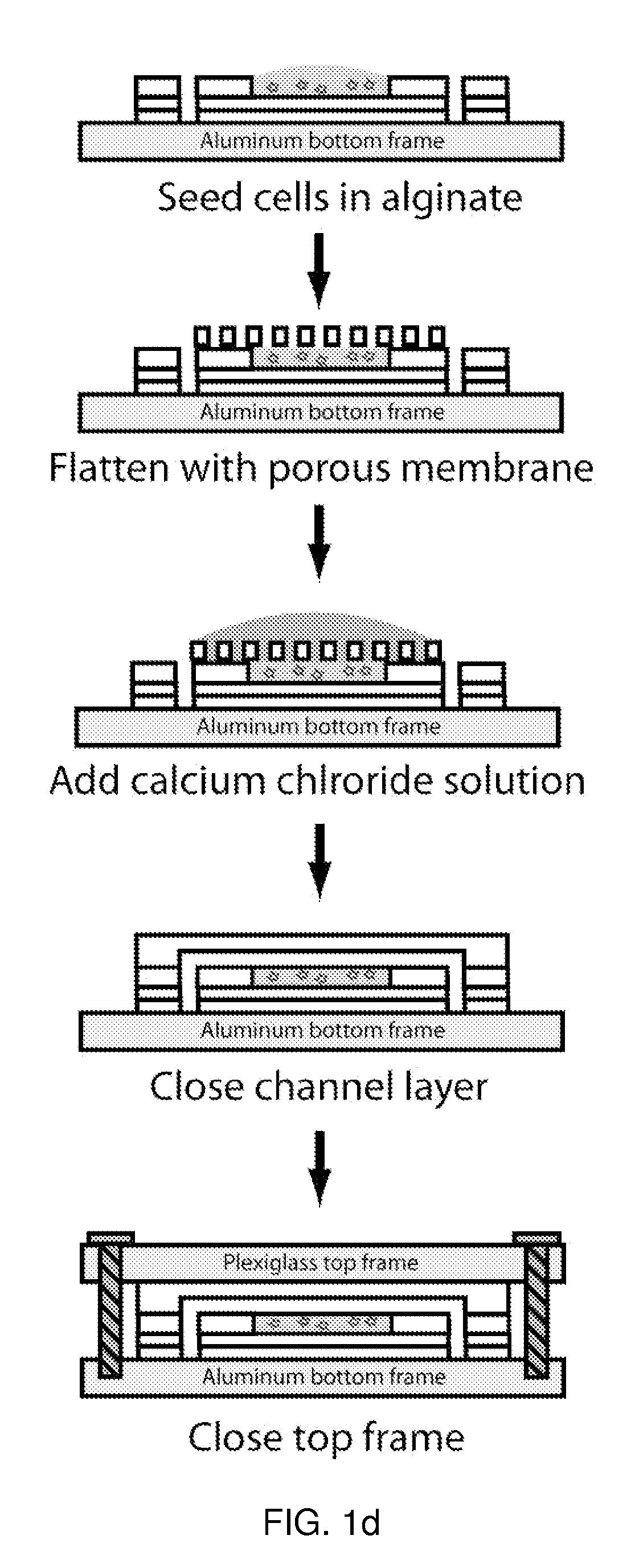
A microfluidic device for culturing cells, termed a microscale cell culture analog (μCCA), is provided. The microfluidic device allows multiple cell or tissue types to be cultured in a physiologically relevant environment, facilitates high-throughput operation and can be used for drug discovery. The microfluidic device uses gravity-induced fluidic flow, eliminating the need for a pump and preventing formation of air bubbles. Reciprocating motion between a pair of connected reservoirs is used to effect the gravity-induced flow in microfluidic channels. Bacterial contamination is reduced and high throughput enabled by eliminating a pump. The microfluidic device integrates a pharmacokinetic-pharmacodynamic (PK-PD) model to enable PK-PD analyses on-chip. This combined in vitro / in silico system enables prediction of drug toxicity in a more realistic manner than conventional in vitro systems.
Owner:CORNELL UNIVERSITY
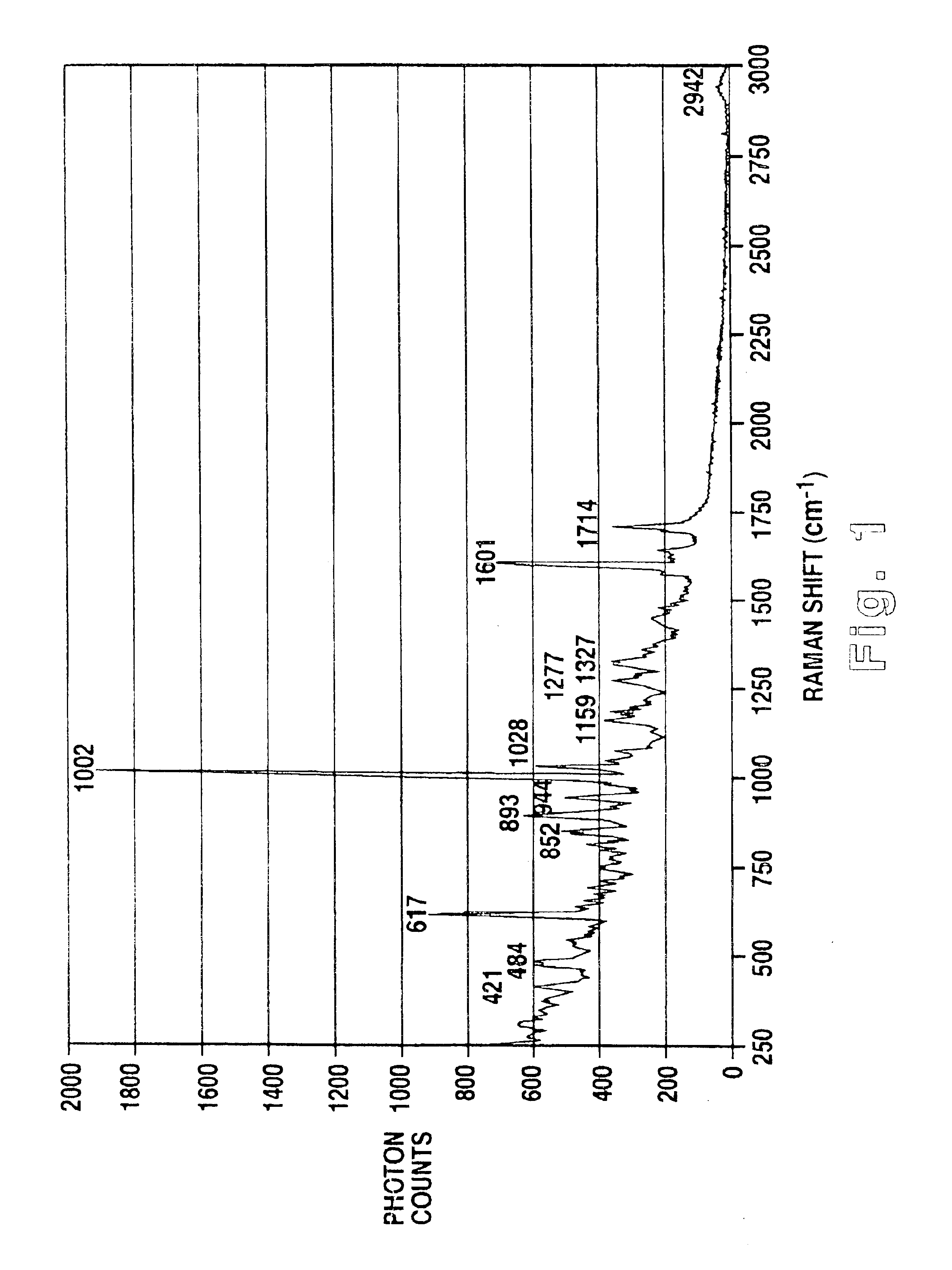
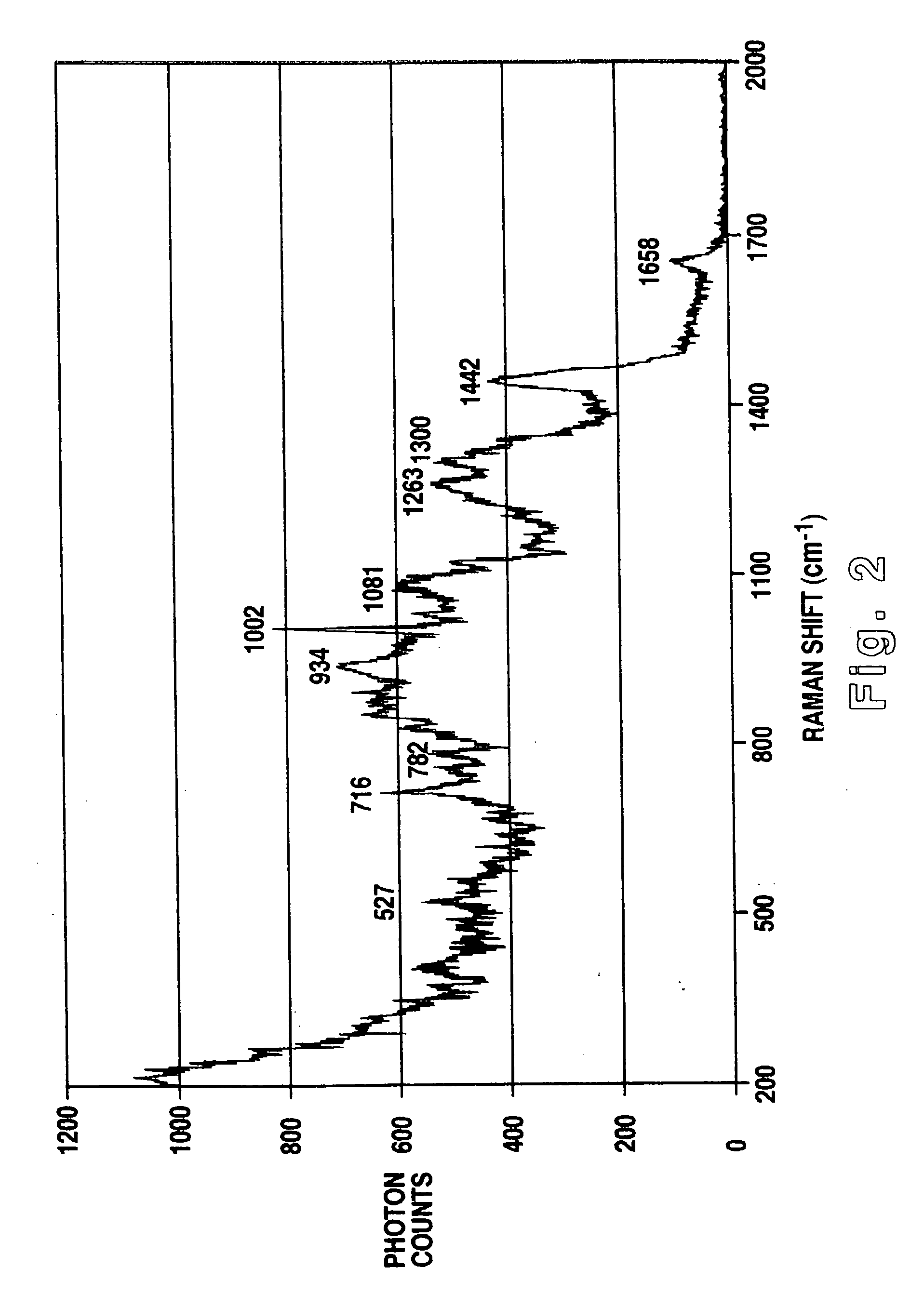
Methodology of using raman imaging microscopy for evaluating drug action within living cells
InactiveUS6939686B2Convenient and cost-effective methodRadiation pyrometryMicrobiological testing/measurementStudy drugRaman imaging
A method of using Raman imaging microscopy to evaluate drug actions in living cells is disclosed. Specifically the invention describes the methods of using Raman imaging microscopy to detect drug uptake, distribution, binding, and metabolism in a single cell, and to study drug pharmacokinetics at the cellular level. The method involves measuring the Raman image of both the drug and the cell. Control images and post-treatment images of the cell were studied. Ratio images were calculated and the requisite information was obtained from a study of the intensity of the bright areas in the ratio images.
Owner:SOUTHWEST RES INST
Lipid carrier, indissolvable pharmaceutical composition and preparation method thereof
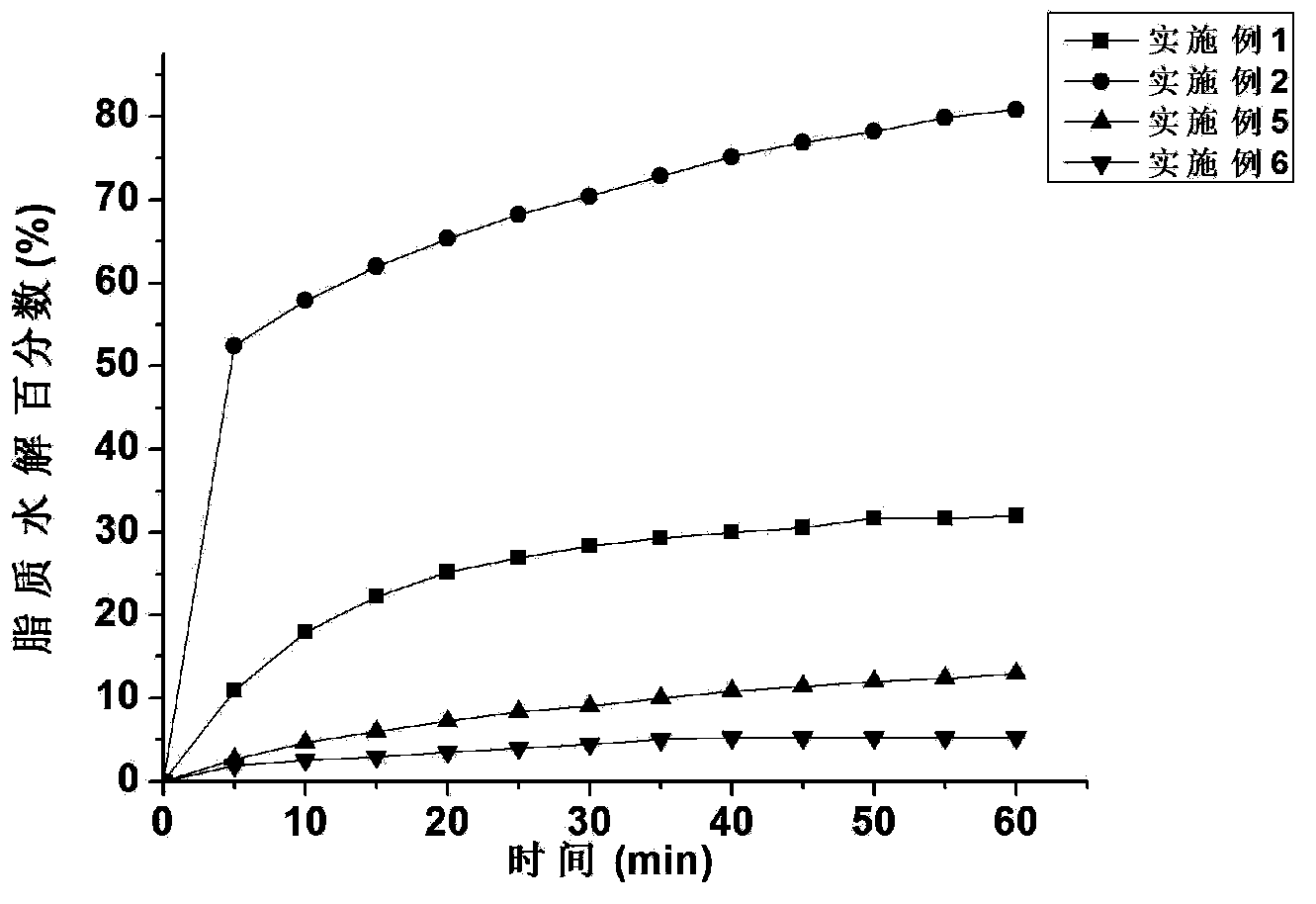
InactiveCN103877065AEvenly distributedParticle size controllableEmulsion deliveryMicrocapsulesMicroemulsionHydrolysis
The invention provides a lipid carrier, an indissolvable pharmaceutical composition and a preparation method thereof. The composition includes a self-microemulsion indissolvable pharmaceutical composition and a composition composed of a solid lipid nanoparticle carrier and an indissolvable pharmaceutical. The self-microemulsion indissolvable pharmaceutical composition comprises, by weight, 0.05%-10% of an indissolvable pharmaceutical, 5%-60% of a liquid lipid, 20%-50% of a surfactant and 10%-35% of a cosurfactant; the composition composed of a solid lipid nanoparticle carrier and an indissolvable pharmaceutical comprises, by weight, 0.05%-5% of the indissolvable pharmaceutical, 40%-60% of a solid lipid and 30%-59% of the surfactant. By changing the type and the proportion of the lipid in the composition composed of a solid lipid nanoparticle carrier and an indissolvable pharmaceutical, the degree of hydrolysis can be controlled between 3%-90%, wherein the hydrolysis is carried out with a lipase. Because that the releasing rate of the indissolvable pharmaceutical in the lipid carrier is related to the hydrolysis of the lipid carrier, the purpose of changing the pharmacokinetics of the indissolvable pharmaceutical is achieved by controlling the hydrolysis rate of the lipid carrier.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
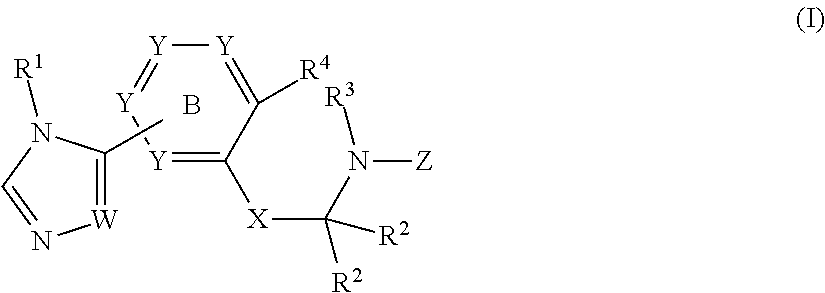
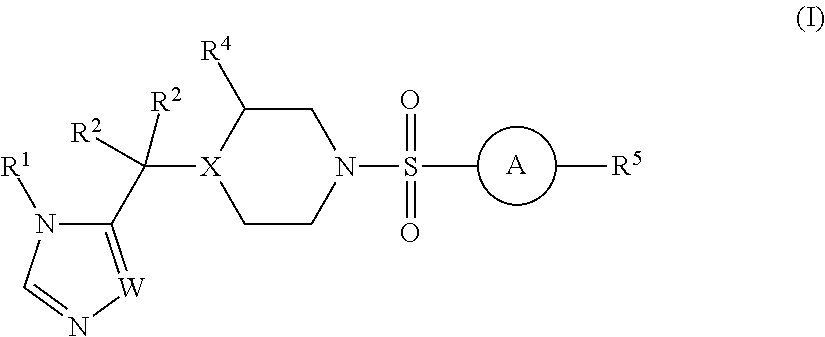
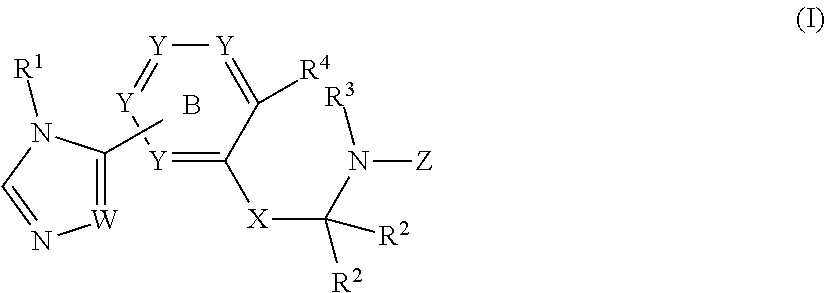
Sulfonamide derivatives and methods of use thereof for improving the pharmacokinetics of a drug
InactiveUS20140005103A1Improve pharmacokineticsBiocideOrganic chemistryDrug pharmacokineticsStereochemistry
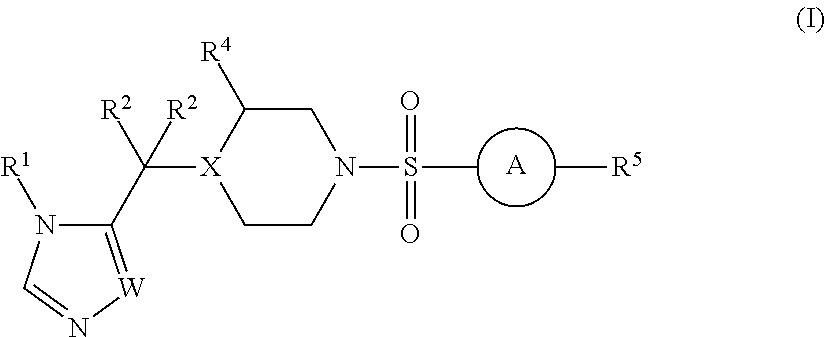
The present invention relates to Sulfonamide Derivatives of Formula (I):and pharmaceutically acceptable salts thereof, wherein A, W, X, R1, R2, R3, R4 and R5 are as defined herein. The present invention also relates to compositions comprising at least one Sulfonamide Derivative, and methods of using the Sulfonamide Derivatives for improving the pharmacokinetics of a drug.
Owner:COBURN CRAIG A +5
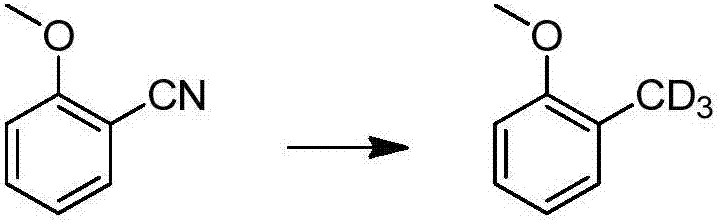
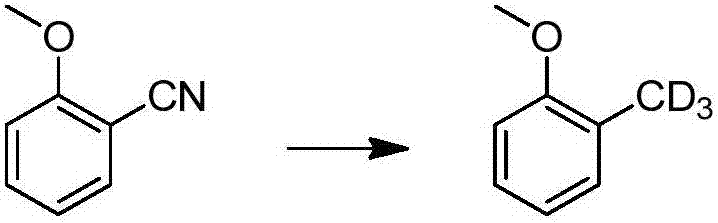
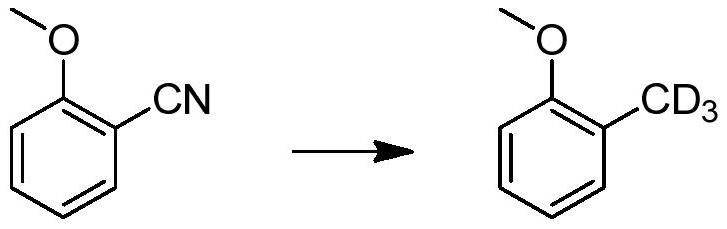
Method for catalytic conversion of cyano group into deuterated methyl, prepared aromatic deuterated methyl compound and application of compound
ActiveCN107353176AIncrease spawn rateDeuterium source increaseIsotope introduction to heterocyclic compoundsOrganic compound preparationDrug metabolismMetal catalyst
The invention provides a method for catalytic conversion of a cyano group into deuterated methyl, a prepared aromatic deuterated methyl compound and an application of the compound. The method comprises the steps as follows: an aromatic cyano compound reacts to produce the aromatic deuterated methyl compound under the action of a metal catalyst with deuterium gas serving as a deuterium source. The cyano group is directly catalyzed into deuterated methyl with the deuterium gas serving as the deuterium source, the operation is simple, the raw material is cheap and easy to obtain, the reaction yield is high, the product deuteration rate is high, and the method can be applied to mass production. The prepared aromatic deuterated methyl compound can be used as a deuterated medicine or can be used for preparation of a deuterated medicine or deuterated medicine composition, and the pharmacokinetics, pharmacodynamics or metabolism toxicity of the medicine can be reduced while the medicine molecular activity is kept unchanged basically.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Transgenic non-human animals for pharmacological and toxicological studies
InactiveUS20070101443A1Improve predictabilityHigh correlationCell receptors/surface-antigens/surface-determinantsSerum albuminHuman animalToxicology studies
Owner:GENE STREAM PTY LTD
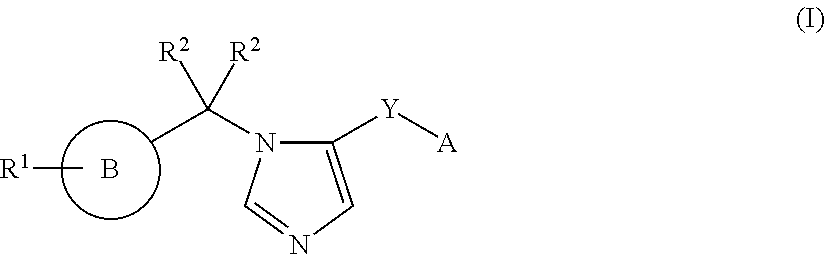
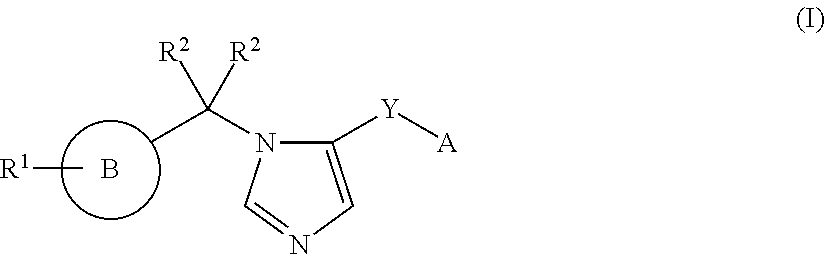
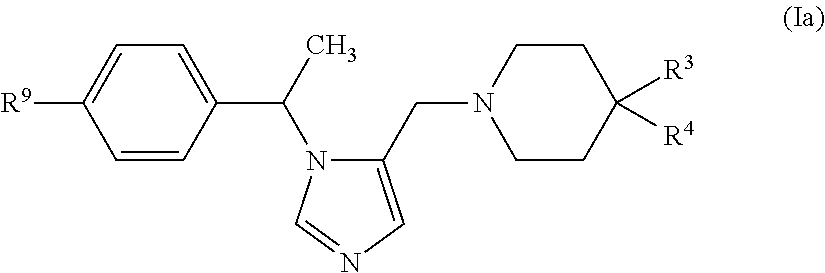
Imidazole derivatives and methods of use thereof for improving the pharmacokinetics of a drug
ActiveUS20160108048A1Improve pharmacokineticsBiocideOrganic chemistryPharmaceutical drugPharmaceutical medicine
The present invention relates to Imidazole Derivatives of Formula (I), and pharmaceutically acceptable salts thereof, wherein A, B, Y, R1 and R2 are as defined herein. The present invention also relates to compositions comprising at least one Imidazole Derivative, and methods of using the Imidazole Derivatives for inhibiting CYP450 3A. Inhibition of CYP450 3A can be used to improve the pharmacokinetics of a drug that is metabolized by CYP450 3A4.
Owner:MERCK SHARP & DOHME LLC
Methodology of using raman imaging microscopy for evaluating drug action within living cells
InactiveUS20050153274A1Convenient and cost-effectiveConvenient and cost-effective methodIn-vivo radioactive preparationsMicrobiological testing/measurementRaman imagingStudy drug
A method of using Raman imaging microscopy to evaluate drug actions in living cells is disclosed. Specifically the invention describes the methods of using Raman imaging microscopy to detect drug uptake, distribution, binding, and metabolism in a single cell, and to study drug pharmacokinetics at the cellular level. The method involves measuring the Raman image of both the drug and the cell. Control images and post-treatment images of the cell were studied. Ratio images were calculated and the requisite information was obtained from a study of the intensity of the bright areas in the ratio images.
Owner:LING JIAN +2
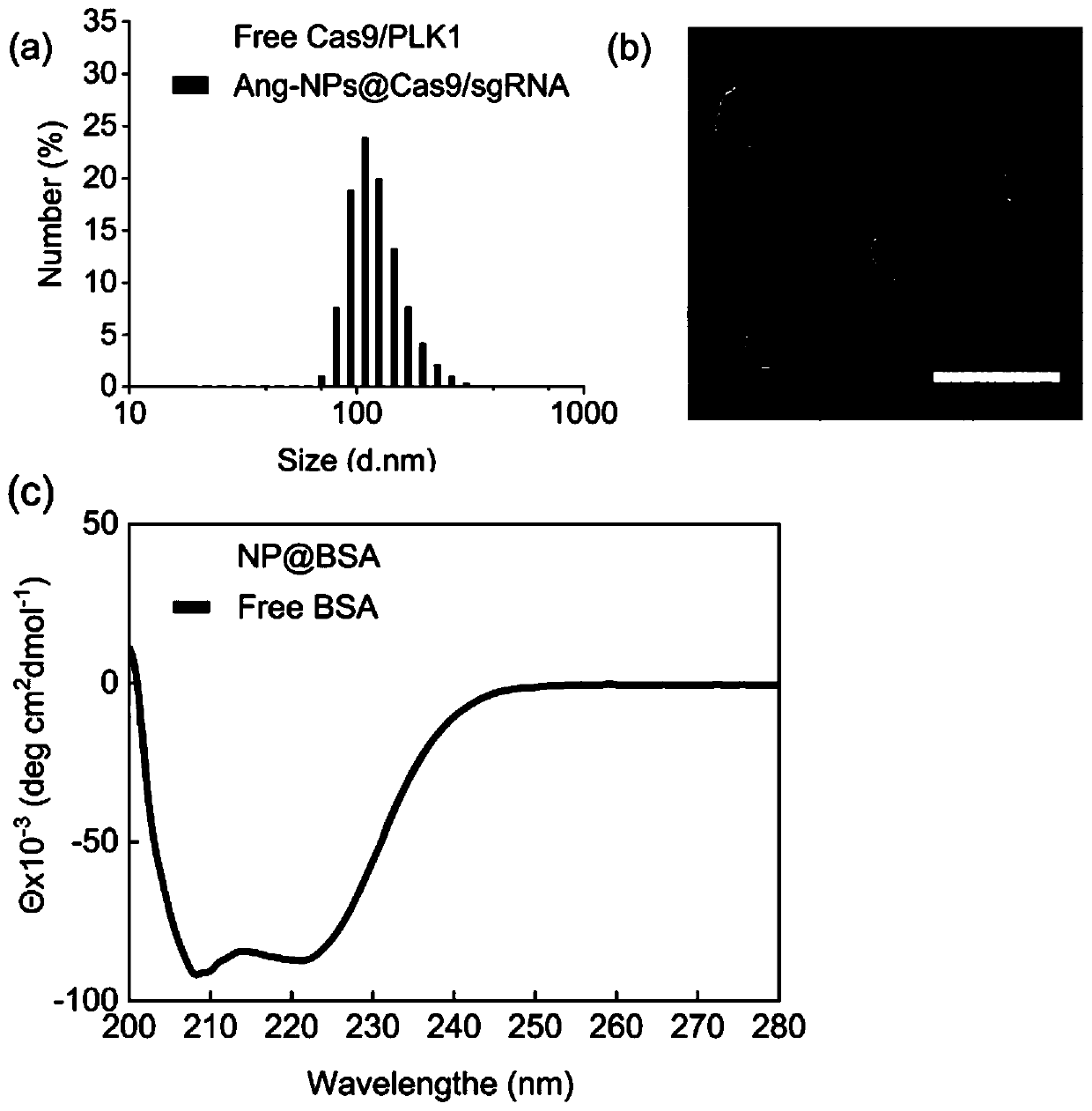
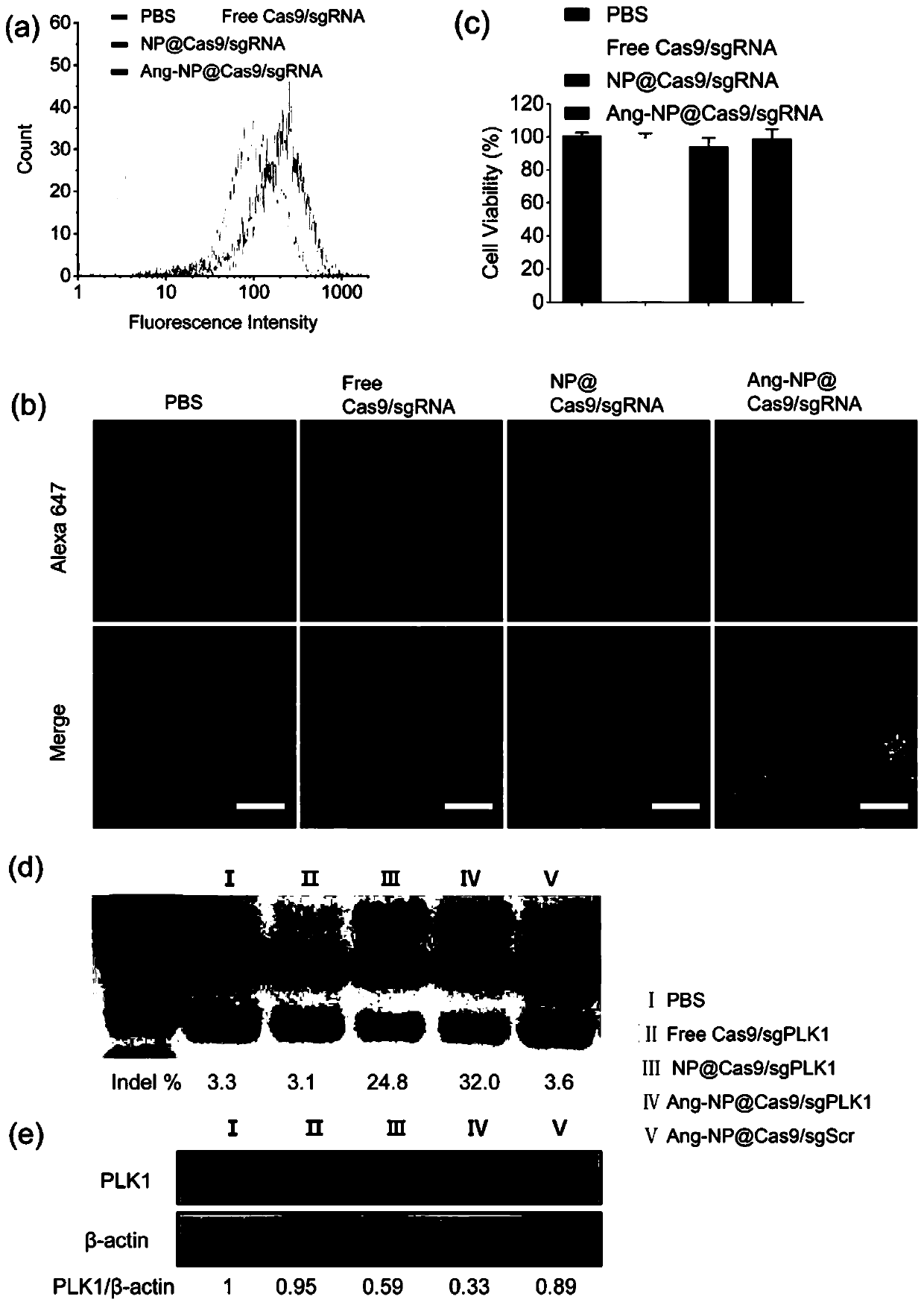
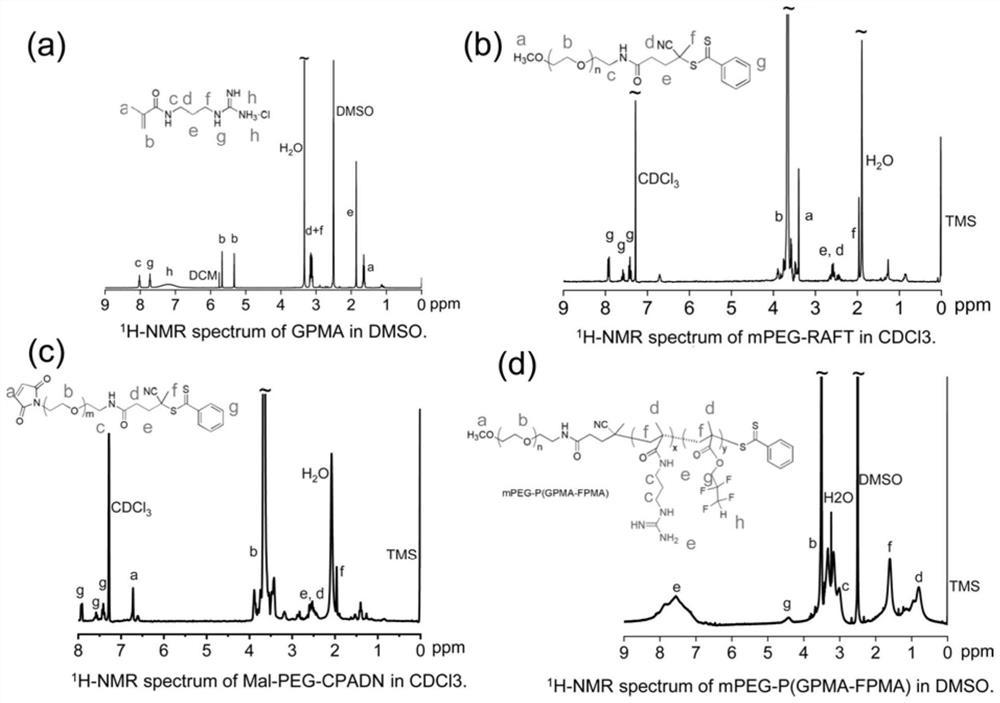
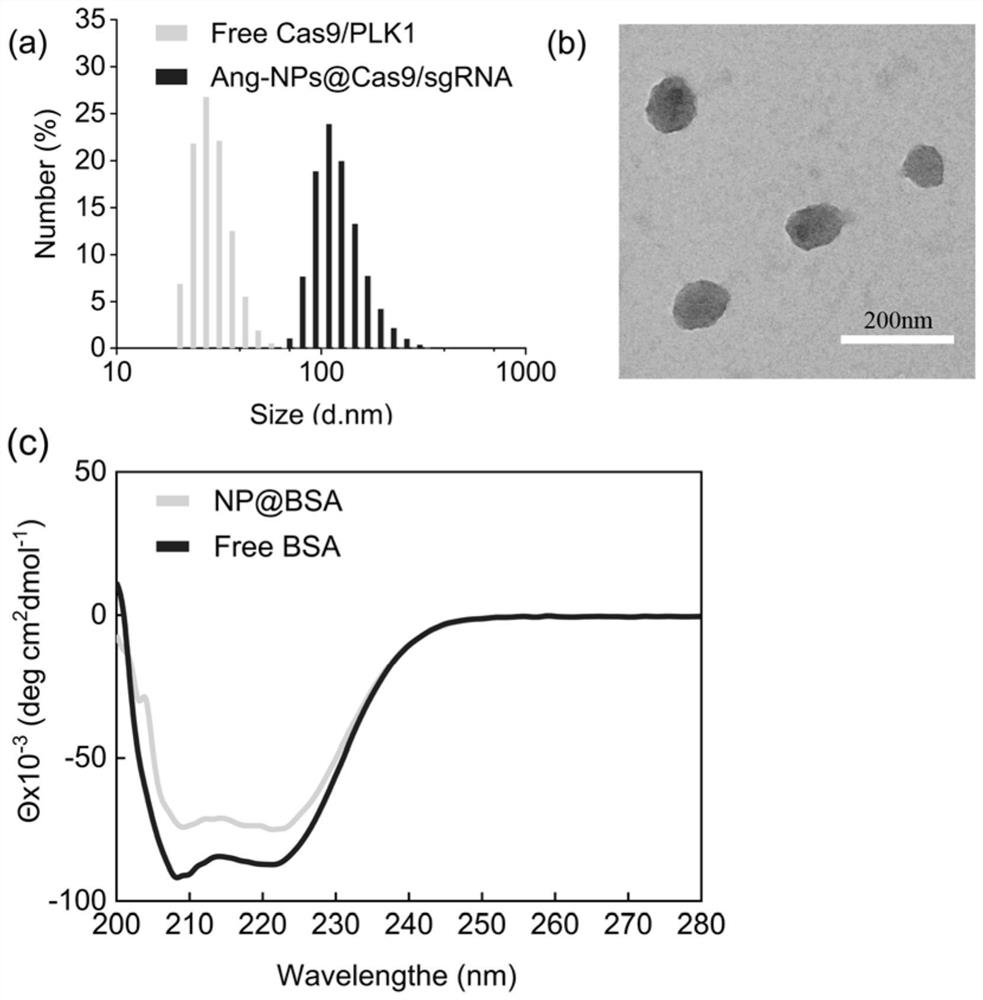
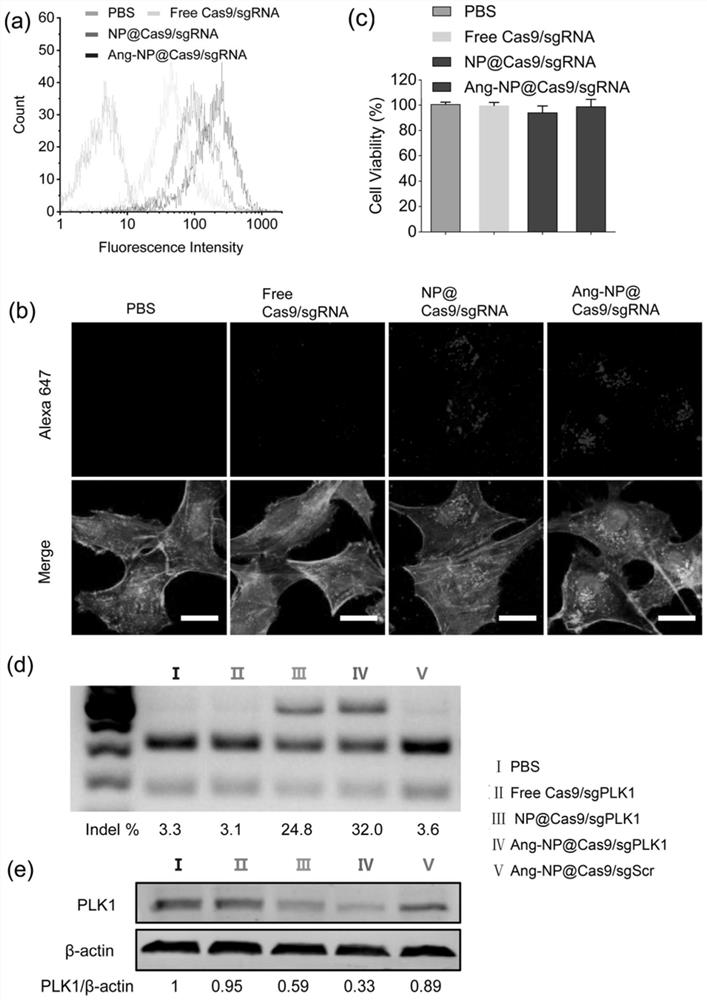
Drug carrier, brain-targeted nano drug based on CRISPR gene editing technology, and preparation method and application of drag carrier and nano drug
ActiveCN111388677AEfficient transportLong cyclePowder deliveryOrganic active ingredientsPharmacologyProtide
The invention provides a drug carrier, a brain-targeted nano drug based on CRISPR gene editing technology, and a preparation method and application of the drag carrier and nano drug. The nano drug provided in the invention contains nanoparticles prepared by coupling Cas9 / sgRNA and a drug carrier, the cutting efficiency of Cas9 / sgRNA is high, the Angiopep polypeptide in the drug carrier helps to cross the blood-brain barrier (BBB) and target to brain tumor cells, PEG can effectively prolong the blood circulation cycle and has good biocompatibility, Guanidine group Gu+ can combine riboprotein complex not only through electrostatic interaction, but also through forming salt bridges and hydrogen bonds, the stability of nano drugs can enhanced by a small amount of fluorine, and the half-life ofnano drug pharmacokinetics is greatly prolonged. The therapeutic drug can be effectively transported to the lesion site by using the drug carrier. The nano drug can perform tumor suppression and treatment at gene level.
Owner:HENAN UNIVERSITY
Method for improving the pharmacokinetics of drugs metabolized by ugt2b10
InactiveUS20100087493A1Improvement in pharmacokinetic parameterImprove efficacyBiocideCompound screeningGlucuronide metabolismActive agent
A method for modifying the pharmacokinetics of a pharmacologically active agent that undergoes direct N-glucuronidation by UDP-glucuronosyltransferase isoenzyme UGT2B10 in a human subject comprising administering an effective amount of an UGT2B10 modulator to said human subject. A method for identifying compounds which are directly metabolized by UGT2B10 or which act as UGT2B10 modulators is also disclosed.
Owner:ORION CORPORATION
Method to improve pharmacokinetics of drugs
A compound comprises a pharmacologically active agent coupled to a plasma protein binding agent. The pharmacologically active agent, in some embodiments, may be an OLAM inhibitor. The plasma protein binding agent, in some embodiments, is a compound that is pharmacologically active in inflamed / injured tissue. A pharmaceutical composition that includes these compounds may be used to treat pain, shock, inflammatory conditions, or combinations thereof in a subject comprising administering to a subject who would benefit from such treatment a therapeutically effective amount of the pharmaceutical composition.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method and device for controlling drug pharmacokinetics
Methods and devices for administration of substances into at least two compartments of skin for systemic absorption and improved pharmacokinetics, based on biphasic or bimodel kinetic profiling.
Owner:BECTON DICKINSON & CO
Capillary electrophoresis in-vivo detection method for cobra neurotoxin
InactiveCN106814049AHigh detection sensitivityLow detection limitPreparing sample for investigationFluorescence/phosphorescenceSingle labelBlood plasma
The invention provides a capillary electrophoresis in-vivo detection method for cobra neurotoxin. The method comprises the following steps of: performing efficient capillary electrophoresis detection on a to-be-detected plasma sample containing FITC-NT (fluorescent single labeled neurotoxin); performing laser induction of a fluorescence detector to detect to obtain an electrophoretogram of the to-be-detected sample; and then comparing the electrophoretogram with a standard curve and calculating concentration of FITC-NT in the to-be-detected sample, wherein the conditions of efficient capillary electrophoresis are as follows: a CEofixTMMEKC kit serves as a buffer liquid, the separating voltage is 20kV, the temperature is 24 DEG C, and the detected wavelengths as follows: the excitation wavelength is 488nm and the emitting wavelength is 520nm. The in-vivo analysis method of FITC-NT, provided by the invention, is high in accuracy, high in precision and good in stability, can quickly and accurately detect neurotoxin in in-vivo blood samples, is high in detection sensitivity and very low in detection limit, can accurately and quantitatively detect a trace amount of FITC-NT components in the blood samples, can be used for in-vivo drug pharmacokinetic research of neurotoxin, and provides accurate data support.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Pharmacokinetics-based comprehensive early druggability evaluation method and application thereof
InactiveCN112366001AFew stepsSimplify the evaluation processMolecular designDrug referencesNew medicationsBiochemical engineering
The invention belongs to the field of innovative drug research and development, and particularly discloses a pharmacokinetics-based comprehensive early druggability evaluation method and application thereof. The method comprises the following steps: firstly, calculating a molecular descriptor of a to-be-evaluated drug, and secondly, inputting the molecular descriptor of the to-be-evaluated drug into a drug pharmacokinetics model obtained by training known drugs; and finally, calculating the druggability score of the to-be-evaluated drug through the output of the drug pharmacokinetic model. Themethod has few steps, performs comprehensive rapid screening based on pharmacokinetic characteristics of new drugs, provides important decision information for early drug research and development, and saves a large amount of experiment expenditure and time.
Owner:SUZHOU LEO BIOTECH
Piperidine or piperazine linked imidazole and triazole derivatives and methods of use thereof for improving the pharmacokinetics of a drug
ActiveUS20160297792A1Improve pharmacokineticsConvenient treatmentOrganic active ingredientsOrganic chemistryPharmaceutical drugTriazole derivatives
The present invention relates to piperidine or piperazine linked imidazole and triazole derivatives, compositions comprising said compounds, alone or in combination with other drugs, and methods of using the compounds for improving the pharmacokinetics of a drug. The compounds of the invention are useful in human and veterinary medicine for inhibiting CYP3A4 and for improving the pharmacokinetics of a therapeutic compound that is metabolized by CYP3A4.
Owner:MERCK SHARP & DOHME LLC
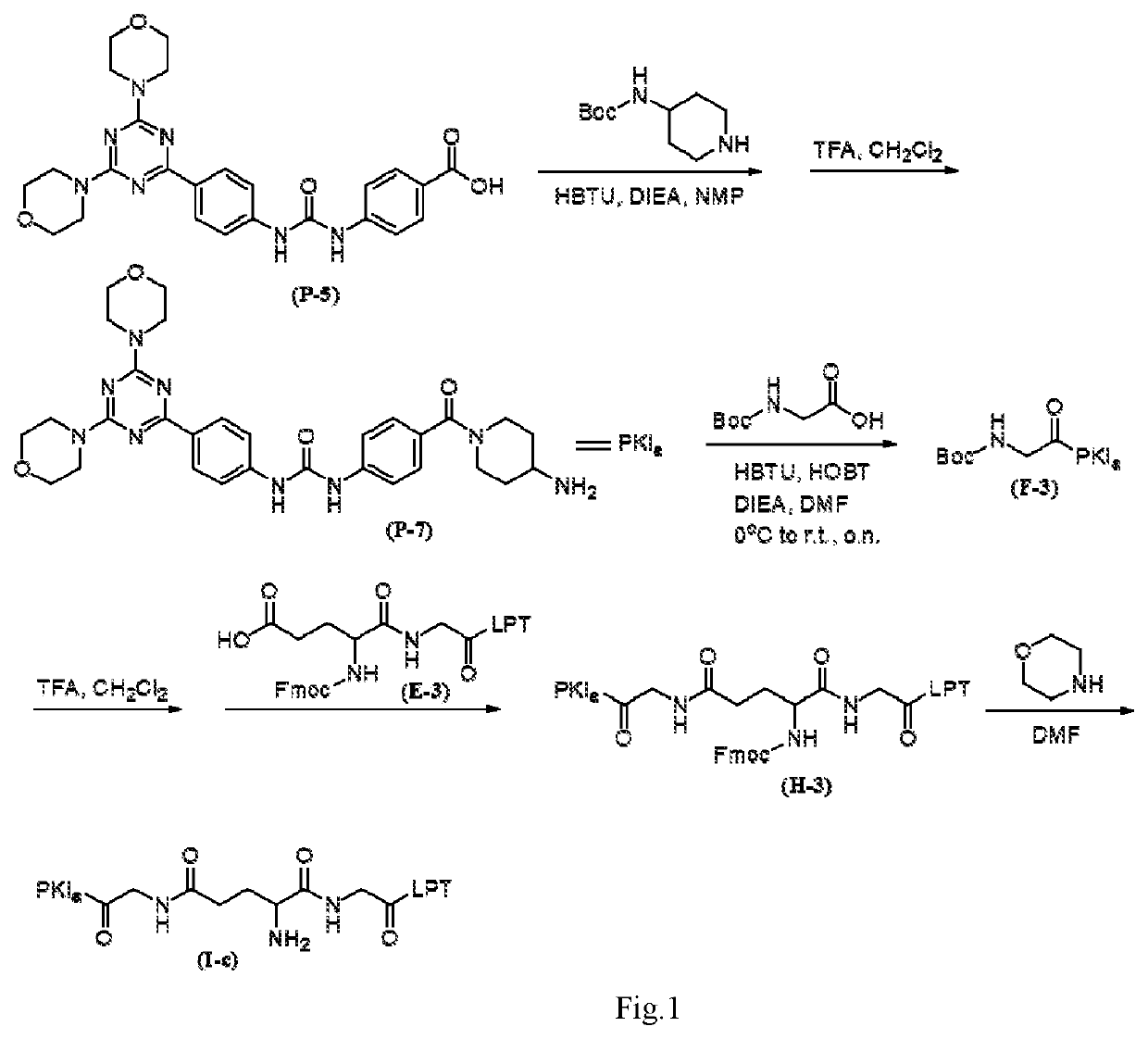
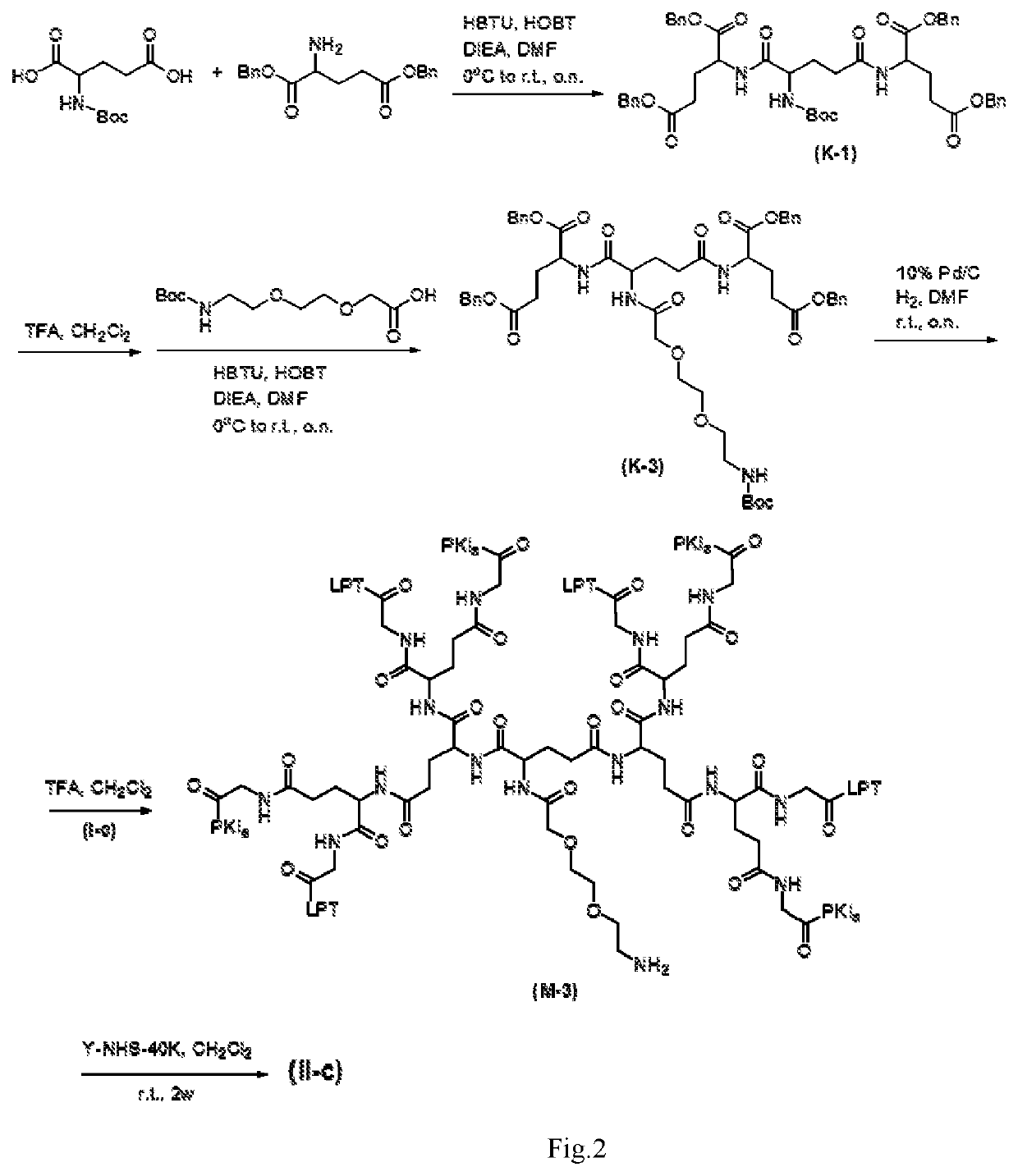
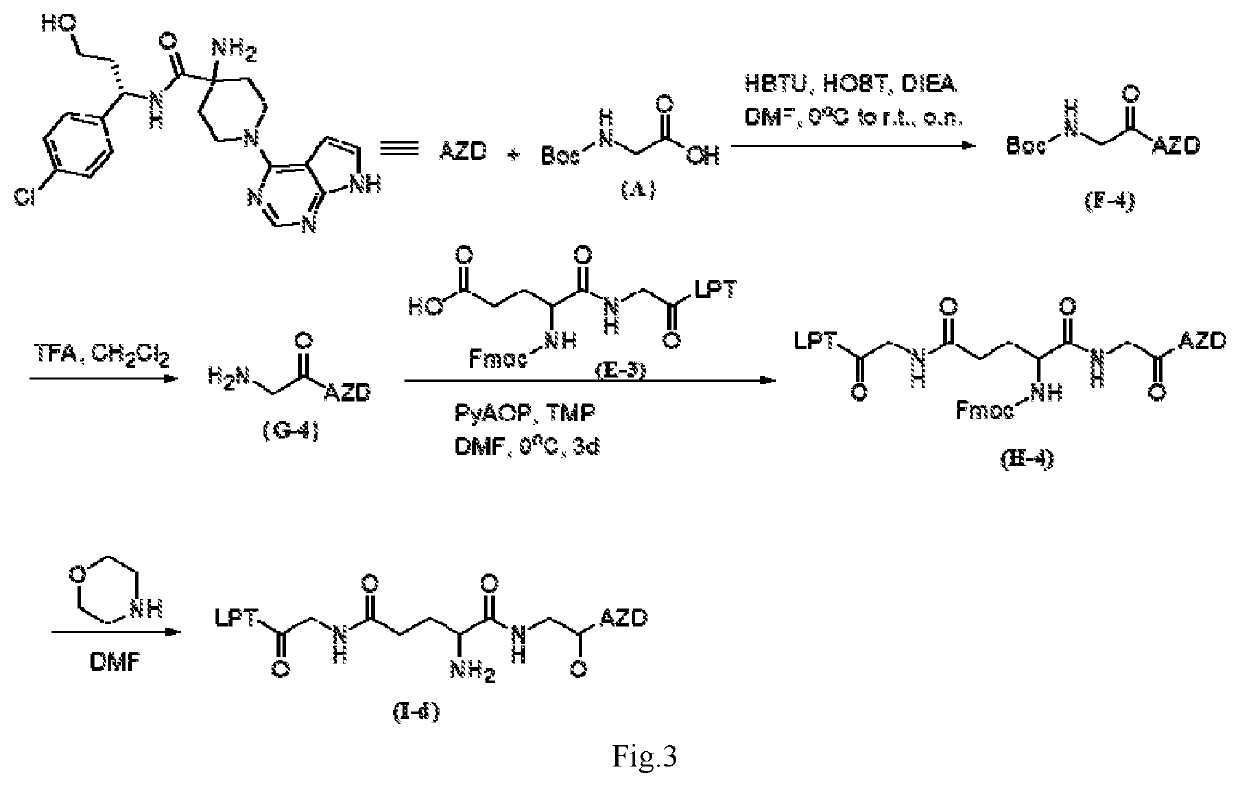
Intermediate drug with synergistic anticancer activity and polyethylene glycol-coupled synergistic anticancer drug, and preparation method therefor and use thereof
ActiveUS20200261588A1Avoid toxic reactionsUseful in preparationOrganic active ingredientsDipeptide ingredientsUse medicationPolythylene glycol
Disclosed are an intermediate drug having synergistic anticancer activity and a polyethylene glycol-coupled synergistic anticancer drug, and a method preparing therefor and use thereof. The intermediate drug has the general structural formula of (I), and the polyethylene glycol-coupled synergistic anticancer drug has the general structural formula of (II). The drugs achieve the combined medication of various anticancer drugs and avoid the toxic reaction caused by the interaction among the drugs or by the pharmacokinetics of the drugs when taking various anticancer drugs, facilitate overcoming the multidrug resistance of cancers, have a synergistic effect, and can be used for preparing anticancer medicaments and for treating cancers.
Owner:CHONGQING UPGRA BIOLOGICAL SCI & TECH LTD
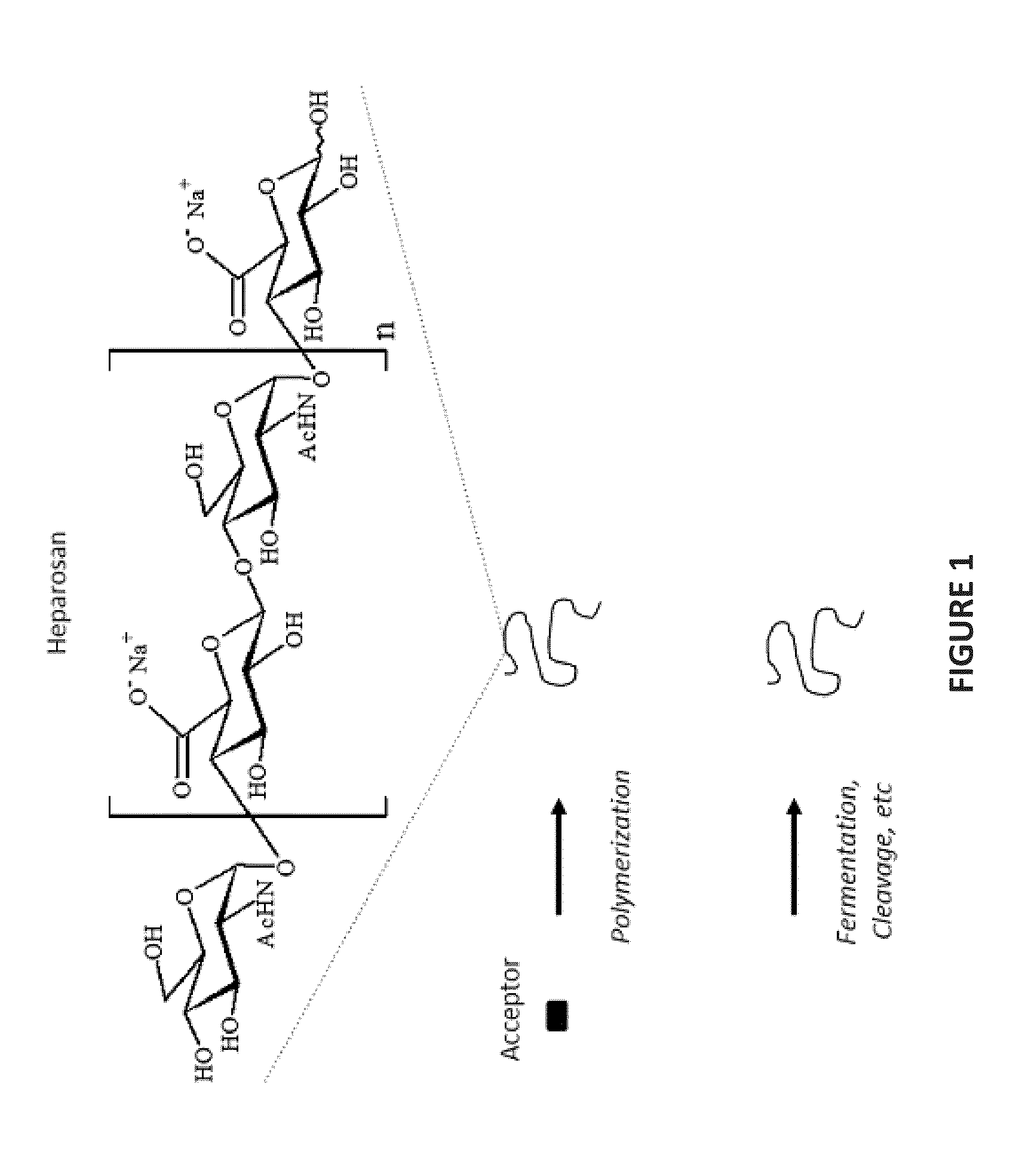
Heparosan/Therapeutic Prodrug Complexes and Methods of Making and Using Same
InactiveUS20160114050A1Organic active ingredientsHeavy metal active ingredientsSide effectNatural polymers
Compositions, methods, and systems are disclosed for the development and use of heparosan, a natural polymer related to heparin, as a new therapeutic modifying agent or complexation vehicle which can modulate drug cargo pharmacokinetics and behavior within a mammalian patient. In certain non-limiting embodiments, the use of heparosan is complexed with anti-cancer drugs and the like, thus forming a prodrug for the purposes of increasing efficacy and reducing side effects compared to the parental drug alone.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Aryl linked imidazole and triazole derivatives and methods of use thereof for improving the pharmacokinetics of a drug
ActiveUS20160297809A1Improve pharmacokineticsConvenient treatmentOrganic active ingredientsOrganic chemistryArylMedicine
The present invention relates to aryl linked imidazole and triazole derivatives, compositions comprising said compounds, alone or in combination with other drugs, and methods of using the compounds for improving the pharmacokinetics of a drug. The compounds of the invention are useful in human and veterinary medicine for inhibiting CYP3A4 and for improving the pharmacokinetics of a therapeutic compound that is metabolized by CYP3A4.
Owner:MERCK SHARP & DOHME LLC
Method and device for controlling drug pharmacokinetics
Methods and devices for administration of substances into at least two compartments of skin for systemic absorption and improved pharmacokinetics, based on biphasic or bimodel kinetic profiling.
Owner:BECTON DICKINSON & CO
Method for establishing prediction model of influence of fat emulsion on kinetics in drug body
ActiveCN114235722AIntuitive buildEasy to set upCompounds screening/testingComponent separationIn vivo pharmacokineticsHplc mass spectrometry
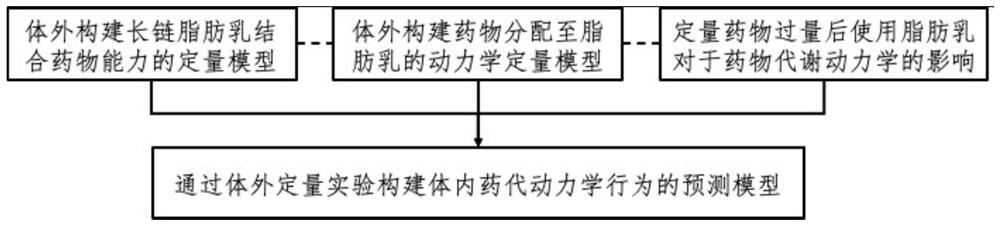
The invention discloses a method for establishing a prediction model for the influence of fat emulsion on kinetics in a drug body. Comprising the following steps: constructing a quantitative model of the drug binding capacity of the long-chain fat emulsion in vitro; constructing a kinetic quantitative model for distributing the medicine to the fat emulsion in vitro; the influence of fat emulsion on pharmacokinetics after quantitative drug excess; and constructing a prediction model of in-vivo pharmacokinetic behaviors through an in-vitro quantitative experiment. On the basis of the phenomenon that fat-soluble drug molecules diffuse to the fat emulsion, an isothermal titration calorimetry method and a high performance liquid chromatography analyzer are used for analyzing the diffusion phenomenon of the drug, and a reliable prediction model for predicting the drug binding capacity of the fat emulsion in vitro is established; an ultraviolet-visible spectrophotometer is used for quantitatively monitoring the speed of combining the fat emulsion with drug molecules in real time, an animal model is established to analyze the influence of the fat emulsion on pharmacokinetics of drugs, and then a prediction model of the influence of the fat emulsion on pharmacokinetics of the drugs is established through an in-vitro quantitative experiment.
Owner:JIANGSU PROVINCE HOSPITAL THE FIRST AFFILIATED HOSPITAL WITH NANJING MEDICAL UNIV +1
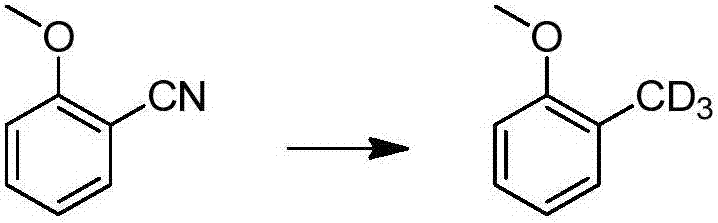
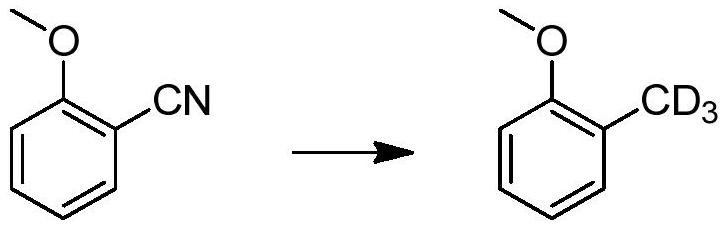
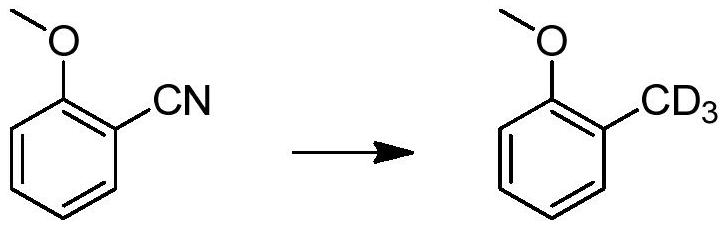
A method for catalytically converting cyano groups into deuterated methyl groups, prepared aromatic deuterated methyl compounds and applications thereof
ActiveCN107353176BImprove pharmacodynamicsReduce metabolic toxicityIsotope introduction to heterocyclic compoundsOrganic compound preparationPtru catalystMethyl palmoxirate
The invention provides a method for catalytic conversion of a cyano group into deuterated methyl, a prepared aromatic deuterated methyl compound and an application of the compound. The method comprises the steps as follows: an aromatic cyano compound reacts to produce the aromatic deuterated methyl compound under the action of a metal catalyst with deuterium gas serving as a deuterium source. The cyano group is directly catalyzed into deuterated methyl with the deuterium gas serving as the deuterium source, the operation is simple, the raw material is cheap and easy to obtain, the reaction yield is high, the product deuteration rate is high, and the method can be applied to mass production. The prepared aromatic deuterated methyl compound can be used as a deuterated medicine or can be used for preparation of a deuterated medicine or deuterated medicine composition, and the pharmacokinetics, pharmacodynamics or metabolism toxicity of the medicine can be reduced while the medicine molecular activity is kept unchanged basically.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Drug carrier, brain-targeted nanomedicine based on CRISPR gene editing technology and its preparation method and application
ActiveCN111388677BEfficient transportLong cyclePowder deliveryOrganic active ingredientsBiocompatibilityTumor cells
The invention provides a drug carrier, a brain-targeted nano drug based on CRISPR gene editing technology, a preparation method and application thereof. The nanomedicine provided in the present invention contains nanoparticles prepared by coupling Cas9 / sgRNA and a drug carrier, the Cas9 / sgRNA has high cutting efficiency, and the Angiopep polypeptide in the drug carrier helps to pass through the blood-brain barrier (BBB) and target to Brain tumor cells, PEG can effectively prolong the blood circulation cycle and has good biocompatibility, guanidino Gu + It can not only combine with riboprotein complex through electrostatic interaction but also form salt bridge and hydrogen bond. A small amount of fluorine can enhance the stability of nanomedicine and greatly prolong the pharmacokinetic half-life of nanomedicine. Using the drug carrier can effectively transport the therapeutic drug to the lesion site. The nano-medicine can suppress and treat tumors at the gene level.
Owner:HENAN UNIVERSITY
Sulfonamide derivatives and methods of use thereof for improving the pharmacokinetics of a drug
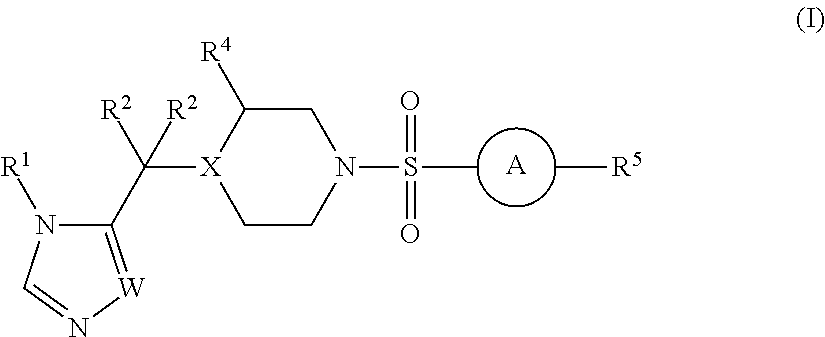
The present invention relates to Sulfonamide Derivatives of Formula (I):and pharmaceutically acceptable salts thereof, wherein A, W, X, R1, R2, R3, R4 and R5 are as defined herein. The present invention also relates to compositions comprising at least one Sulfonamide Derivative, and methods of using the Sulfonamide Derivatives for improving the pharmacokinetics of a drug.
Owner:MERCK SHARP & DOHME LLC
Reduction-responsive targeted polyethylene glycol-polycarbonate maytansinoid prodrug micelles, its preparation method and application
ActiveCN106581691BKeep aliveThe synthesis steps are simpleOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityCyclic process
The invention discloses a reduction-responsive targeted polyethylene glycol-polycarbonate maytansine prodrug micelle, a preparation method and application thereof. The amphiphilic polyethylene glycol-polycarbonate maytansinoid prodrug polymer composed of amphiphilic polyethylene glycol-polycarbonate maytansinoid prodrug polymer and the terminal-bonded targeting molecule were automatically activated in buffer. Targeted polyethylene glycol-polycarbonate maytansine prodrug micelles with reduction response were assembled; the particle size of the micelles was 30-150 nanometers, and the drug-loading capacity of maytansine was 2-60 wt .%. The reduction-responsive targeted polycarbonate maytansinoid prodrug micelles provided by the present invention have targeting properties, amphiphilicity and biodegradability, and nano-medicines can be prepared therefrom, which can significantly improve the water-solubility of drugs and enhance drug Stability in circulation process, improvement of drug pharmacokinetic behavior and improvement of drug bioavailability; it can be applied in the preparation of malignant tumors such as targeted therapy drugs for melanoma.
Owner:SUZHOU UNIV
Intermediate drug with synergistic anticancer activity and polyethylene glycol-coupled synergistic anticancer drug, and preparation method therefor and use thereof
ActiveUS11484600B2Avoid reactionUseful in preparationOrganic active ingredientsDipeptide ingredientsUse medicationPolythylene glycol
Disclosed are an intermediate drug having synergistic anticancer activity and a polyethylene glycol-coupled synergistic anticancer drug, and a method preparing therefor and use thereof. The intermediate drug has the general structural formula of (I), and the polyethylene glycol-coupled synergistic anticancer drug has the general structural formula of (II). The drugs achieve the combined medication of various anticancer drugs and avoid the toxic reaction caused by the interaction among the drugs or by the pharmacokinetics of the drugs when taking various anticancer drugs, facilitate overcoming the multidrug resistance of cancers, have a synergistic effect, and can be used for preparing anticancer medicaments and for treating cancers.
Owner:CHONGQING UPGRA BIOLOGICAL SCI & TECH LTD
Aryl linked imidazole and triazole derivatives and methods of use thereof for improving the pharmacokinetics of a drug
The present invention relates to aryl linked imidazole and triazole derivatives, compositions comprising said compounds, alone or in combination with other drugs, and methods of using the compounds for improving the pharmacokinetics of a drug. The compounds of the invention are useful in human and veterinary medicine for inhibiting CYP3A4 and for improving the pharmacokinetics of a therapeutic compound that is metabolized by CYP3A4.
Owner:MERCK SHARP & DOHME LLC
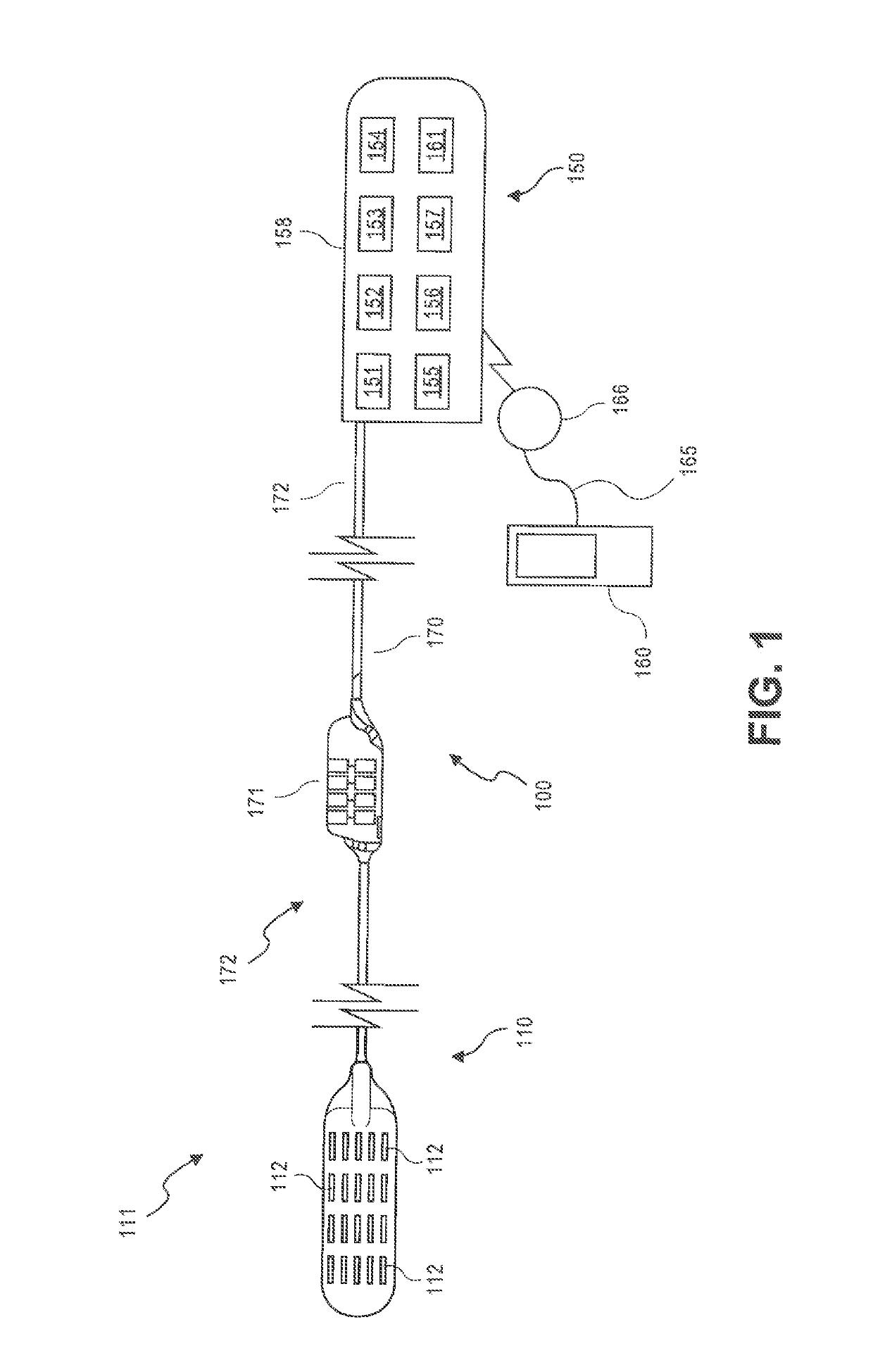
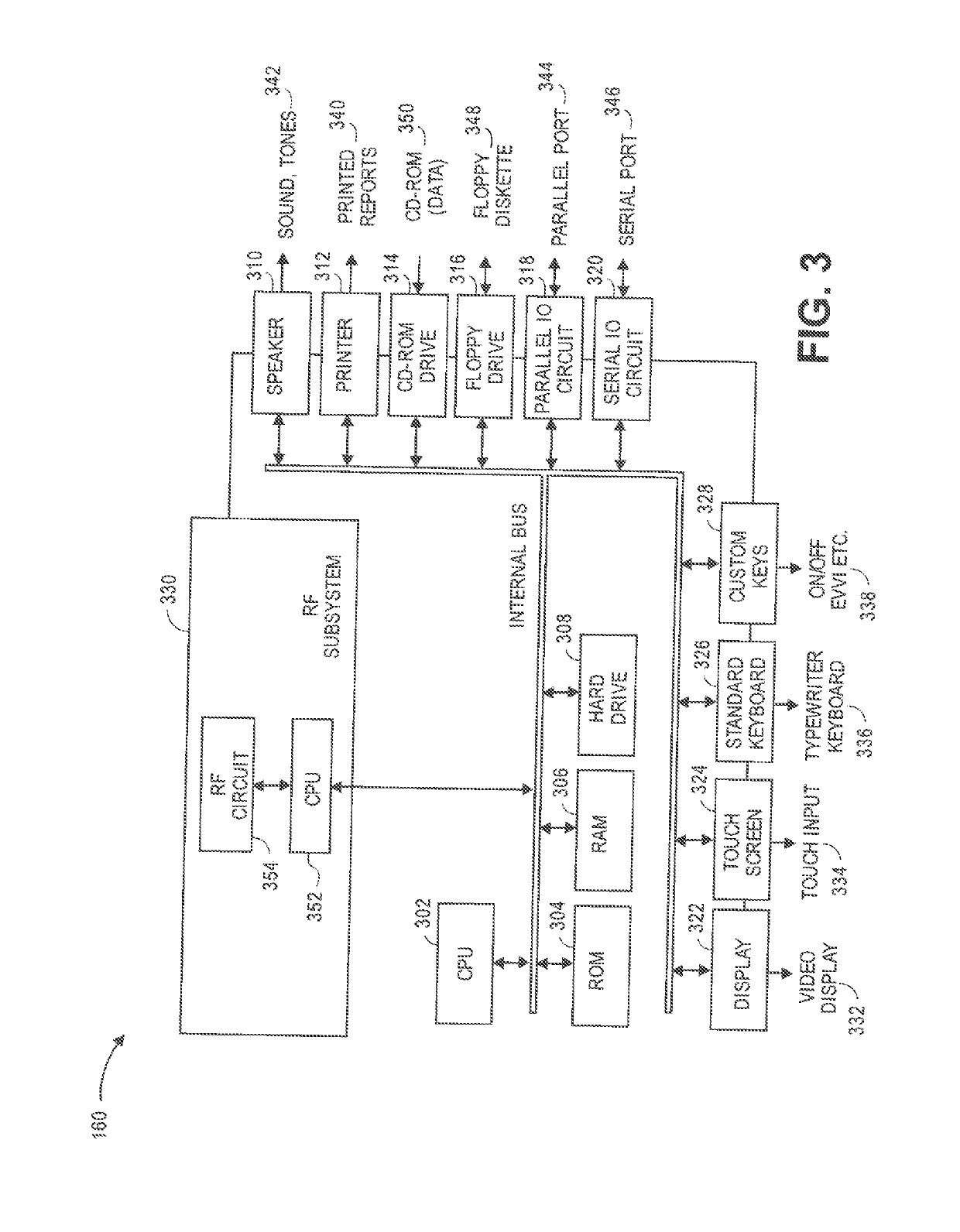
Method and system for adjusting a neurostimulation therapy
The systems and methods described herein generally relate to adjusting a neurostimulation (NS) therapy based on drug pharmacokinetics of a patient. The systems and methods deliver an NS therapy to a portion of electrodes of a lead positioned proximate to neural tissue of interest, which is associated with a target region. The NS therapy is defined by stimulation parameters. The systems and methods determine a trigger event indicative of a drug being administered to a patient. The drug is configured to affect at least one of the neural tissue of interest or the target region. The systems and methods adjust one or more of the stimulation parameters based on the PS profile.
Owner:ADVANCED NEUROMODULATION SYST INC
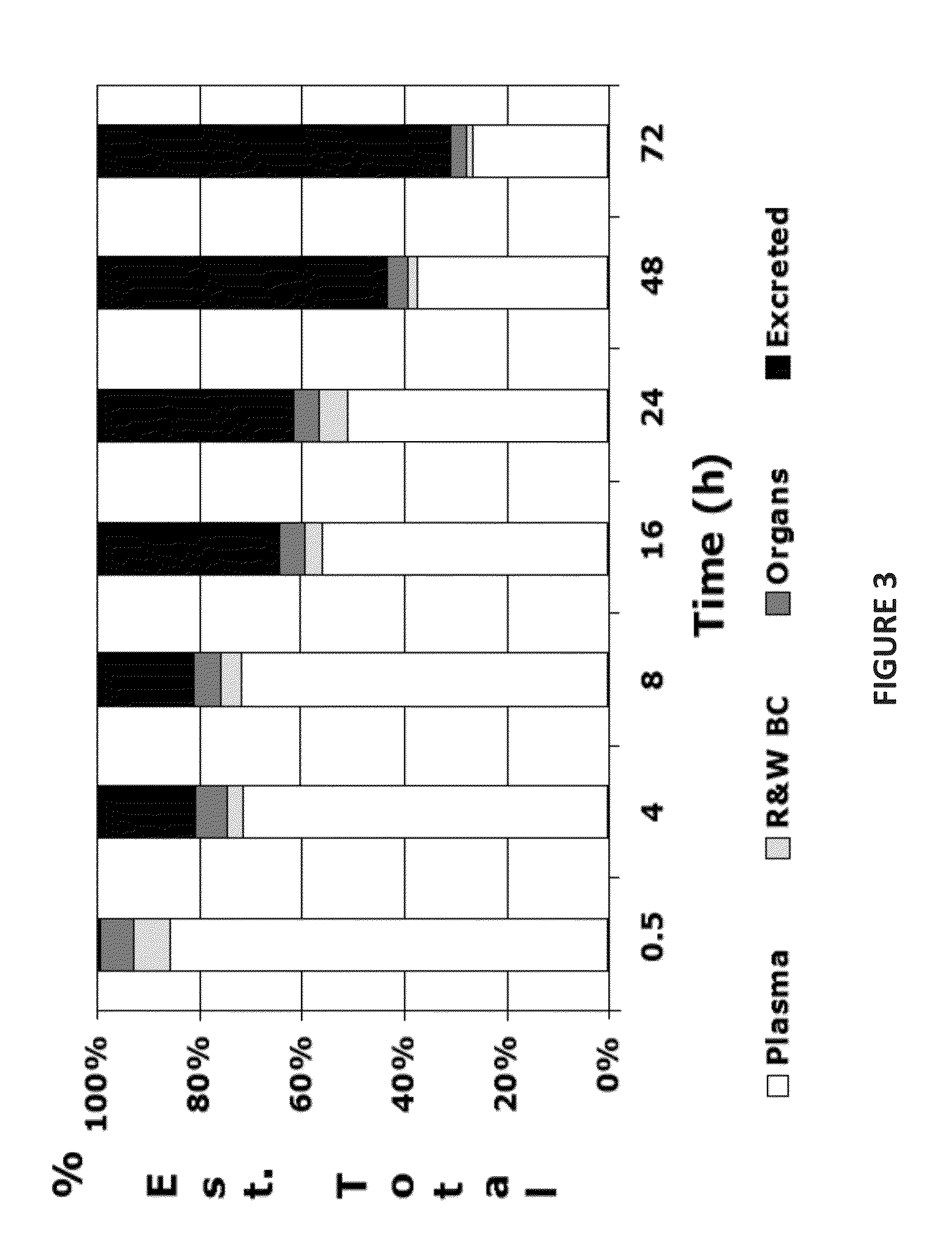
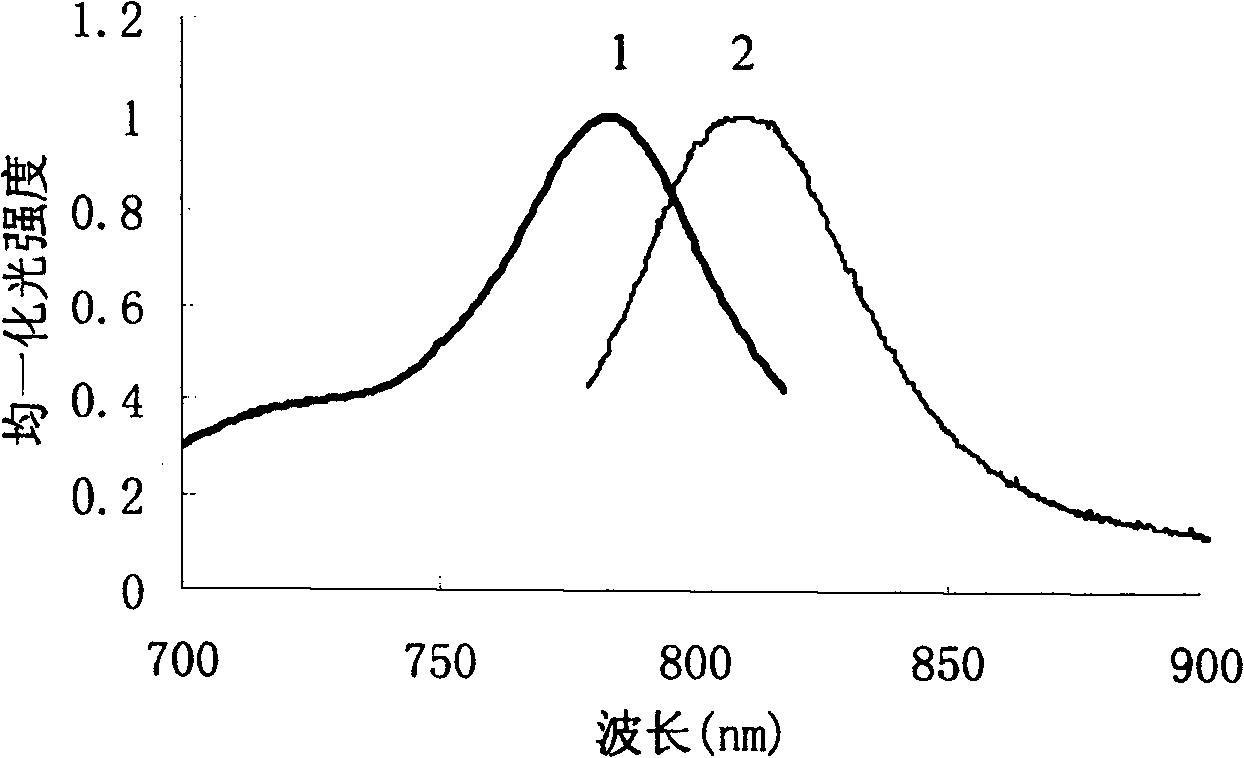
Pharmaceutical in vivo dynamics characteristic-nondestructive in situ monitoring system and monitoring method
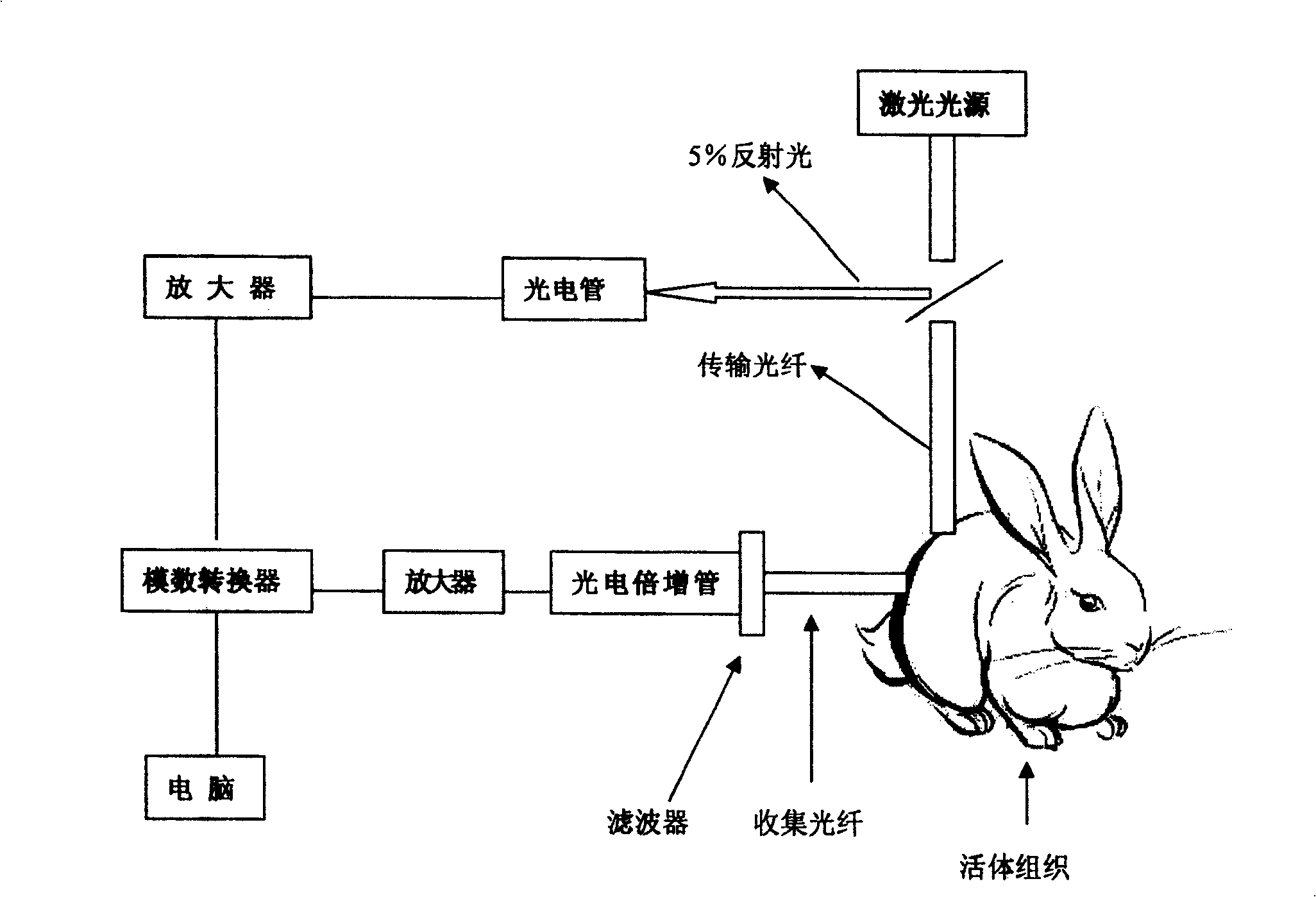
InactiveCN100435721CNo radioactivityFix damageDiagnostic recording/measuringSensorsFluorescenceIn vivo
The invention relates to a system used in the internal dynamic nondestructive online checking of protein polypeptide drug, and relative checking method. The inventive system comprises a fluorescence probe and a detecting system, while the detecting system is formed by a near-infrared light source, a transmission optical fiber, a receiving optical fiber, a near-infrared high-pass filter, and a near-infrared detector. The laser of light source and the fluorescence light received by the detector can be transmitted by the optical fiber; the near-infrared band-pass or high-pass filter is at the front of detector. The invention can avoid sampling timely in the test, avoid killing animals in the organism distribution test, to and avoid the following sample treatment.
Owner:CHINA PHARM UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com