Lipid carrier, indissolvable pharmaceutical composition and preparation method thereof
A technology for insoluble drugs and lipid carriers, which is used in pharmaceutical formulations, medical preparations containing active ingredients, and emulsion delivery, etc., can solve problems such as instability of lipid carriers, avoid drug precipitation, and increase dissolution specific surface area. , promotes the effect of entering the lymphatic system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of self-microemulsion insoluble pharmaceutical composition
[0040] Weigh 4g of medium-chain fatty acid glyceride (MCT), 404g of Cermophor CO and 2g of 1,2-propanediol, mix well, stir at room temperature for 3h, and obtain microemulsion. Take 30 mg of hydroxymecoumarin, add it to the above self-microemulsion, and stir it magnetically for 2 hours to obtain the insoluble pharmaceutical composition of self-microemulsion
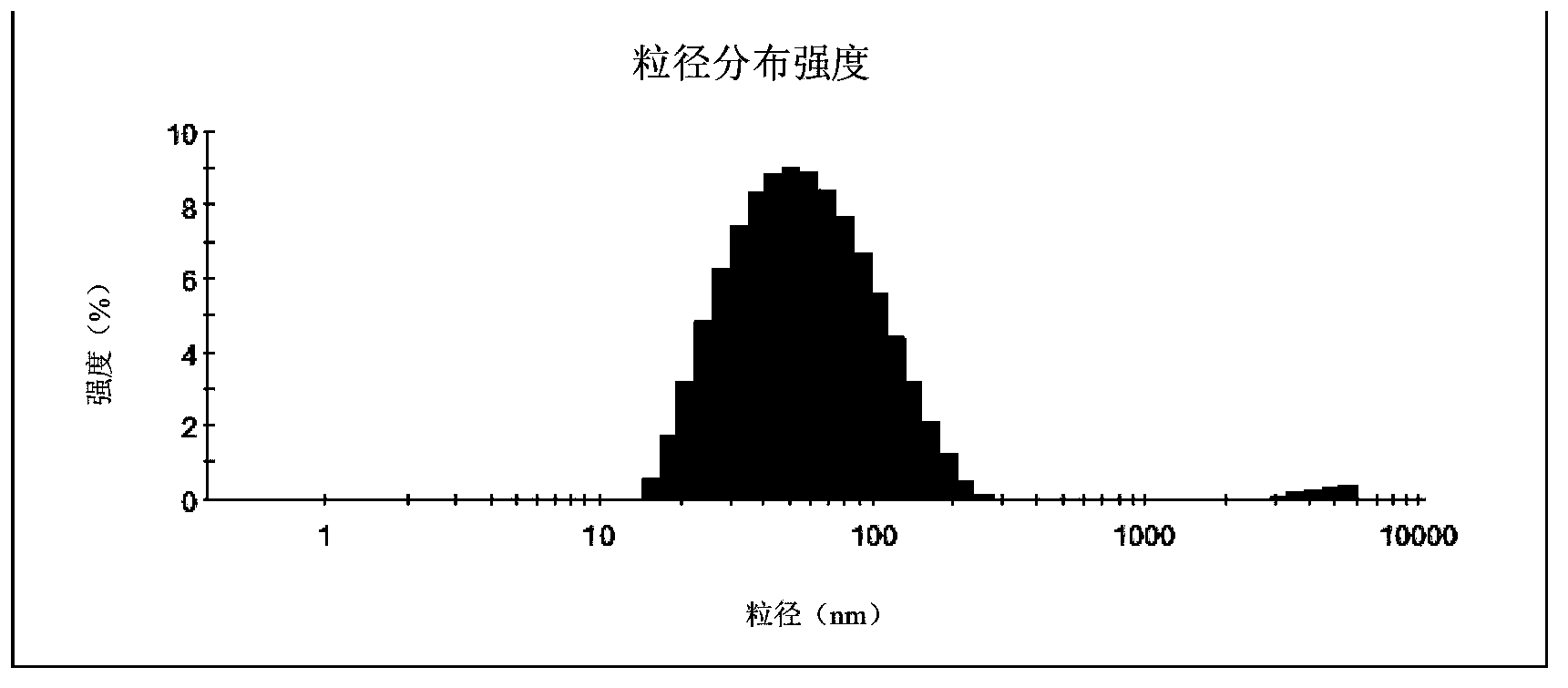
[0041] The compound has no obvious layering phenomenon after standing for half a year at room temperature. It is diluted 20-1000 times with deionized water, and the particle size is measured by a laser particle size analyzer. The particle size distribution is as follows: figure 1 As shown, the average particle size is 54nm.
Embodiment 2
[0042] Embodiment 2: Preparation of self-microemulsion insoluble pharmaceutical composition
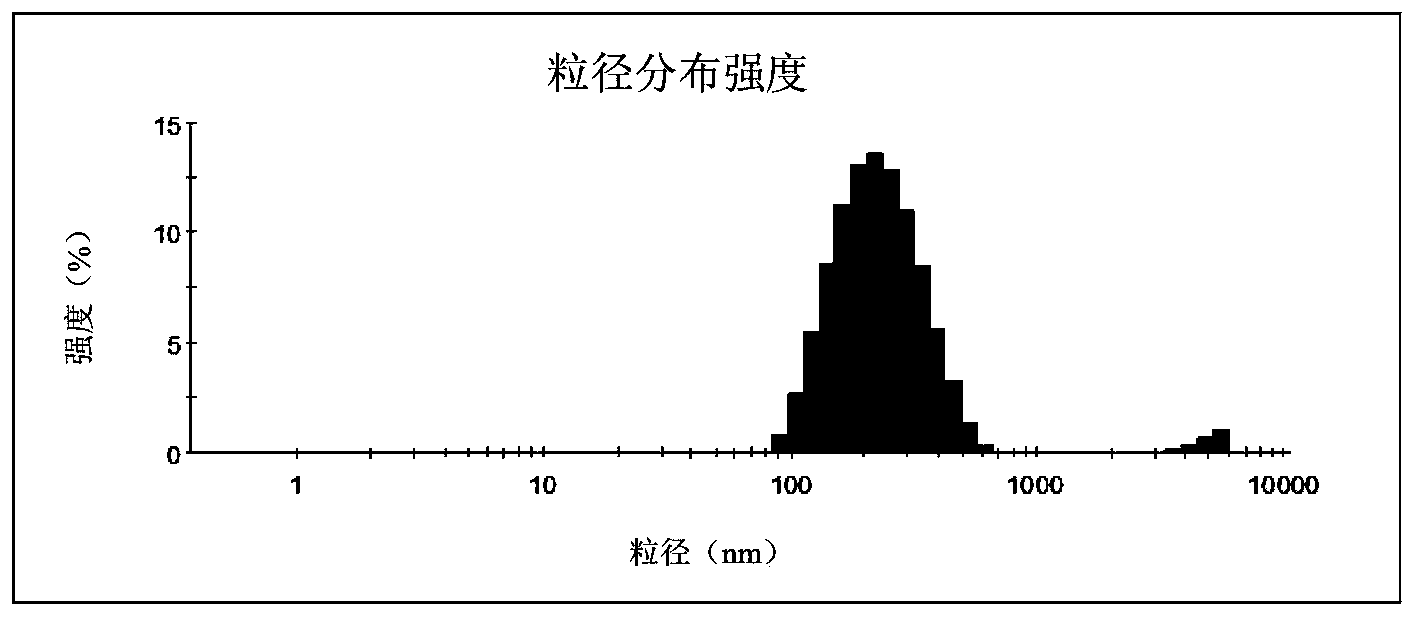
[0043] Weigh 6g of C8-C10 medium-chain fatty acid glycerides (MCT), 2.67g of Cermophor CO40, 1.33g of 1,2-propanediol and 30mg of hymecoumarin, mix well, and stir at room temperature for 3h, which is obtained from microemulsion insoluble pharmaceutical composition. After the complex was diluted 100 times, its particle size was 129nm.
Embodiment 3
[0044] Embodiment 3: Preparation of self-microemulsion insoluble pharmaceutical composition
[0045]Weigh 4g of ethyl oleate, 404g of Cermophor CO and 2g of 1,2-propanediol, mix well, stir at room temperature for 3h, and obtain microemulsion. Take 30 mg of resveratrol, add it into the self-microemulsion, and stir it magnetically for 2 hours to obtain the insoluble pharmaceutical composition from the microemulsion.
[0046] After the complex was diluted 100 times, its particle size was 59nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com