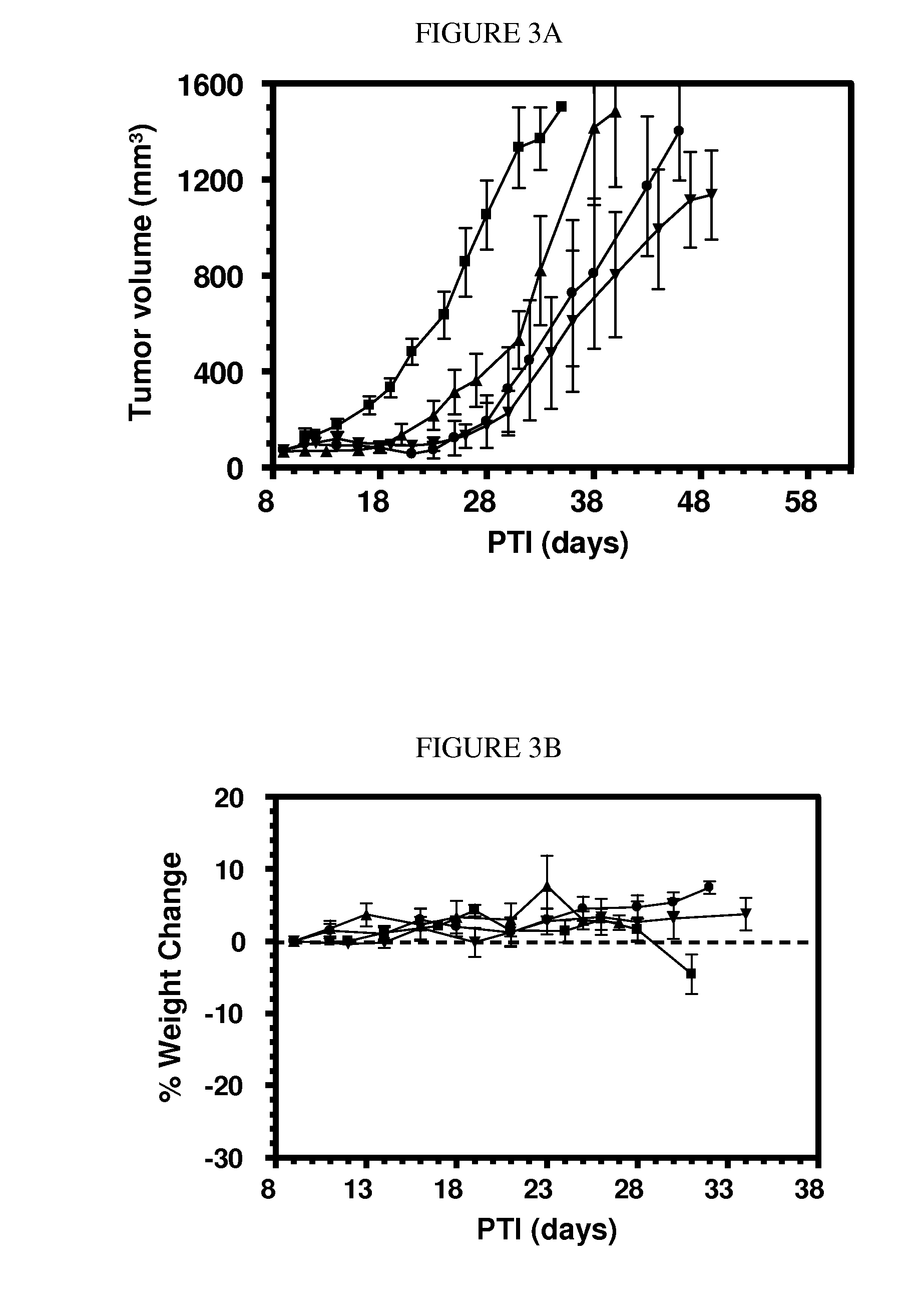
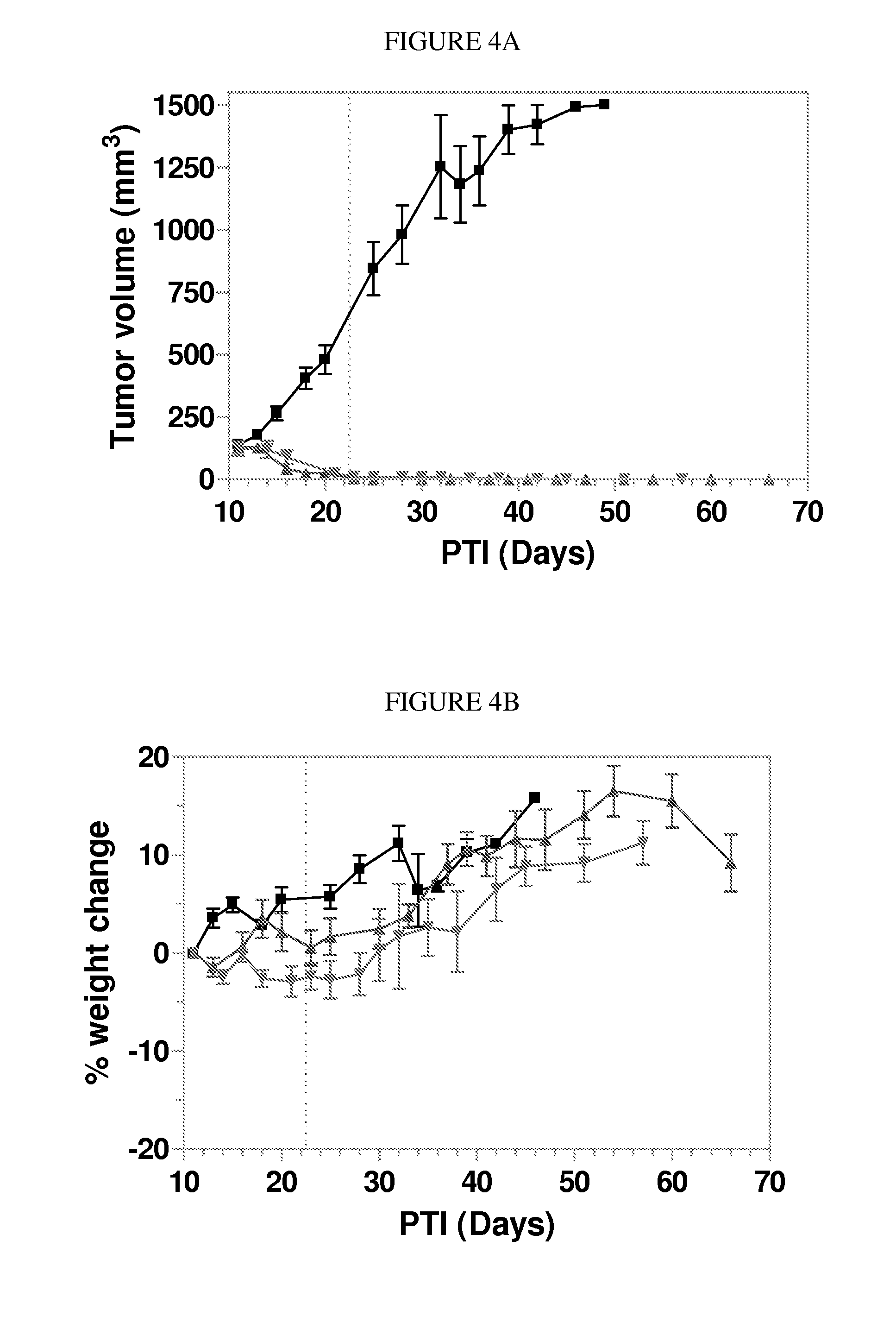
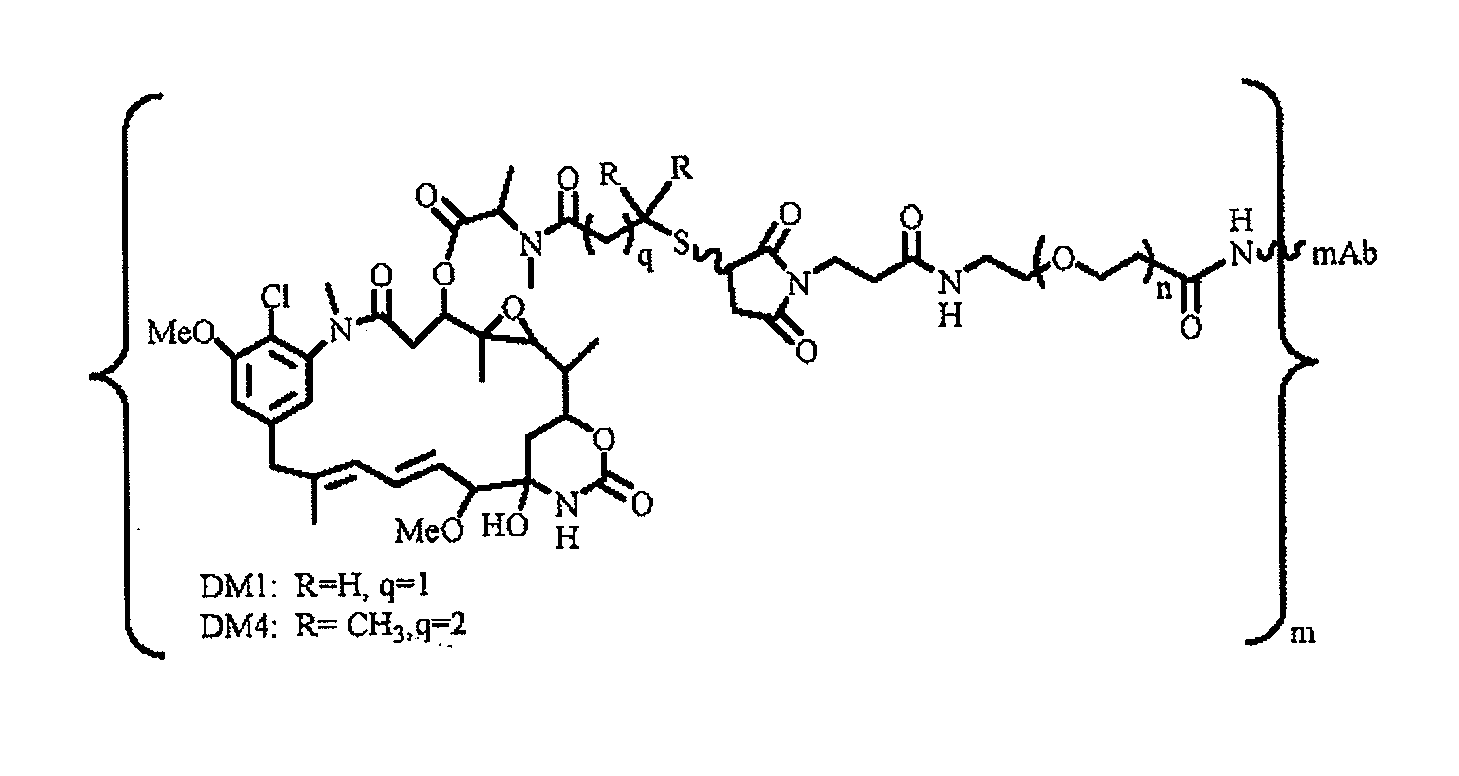
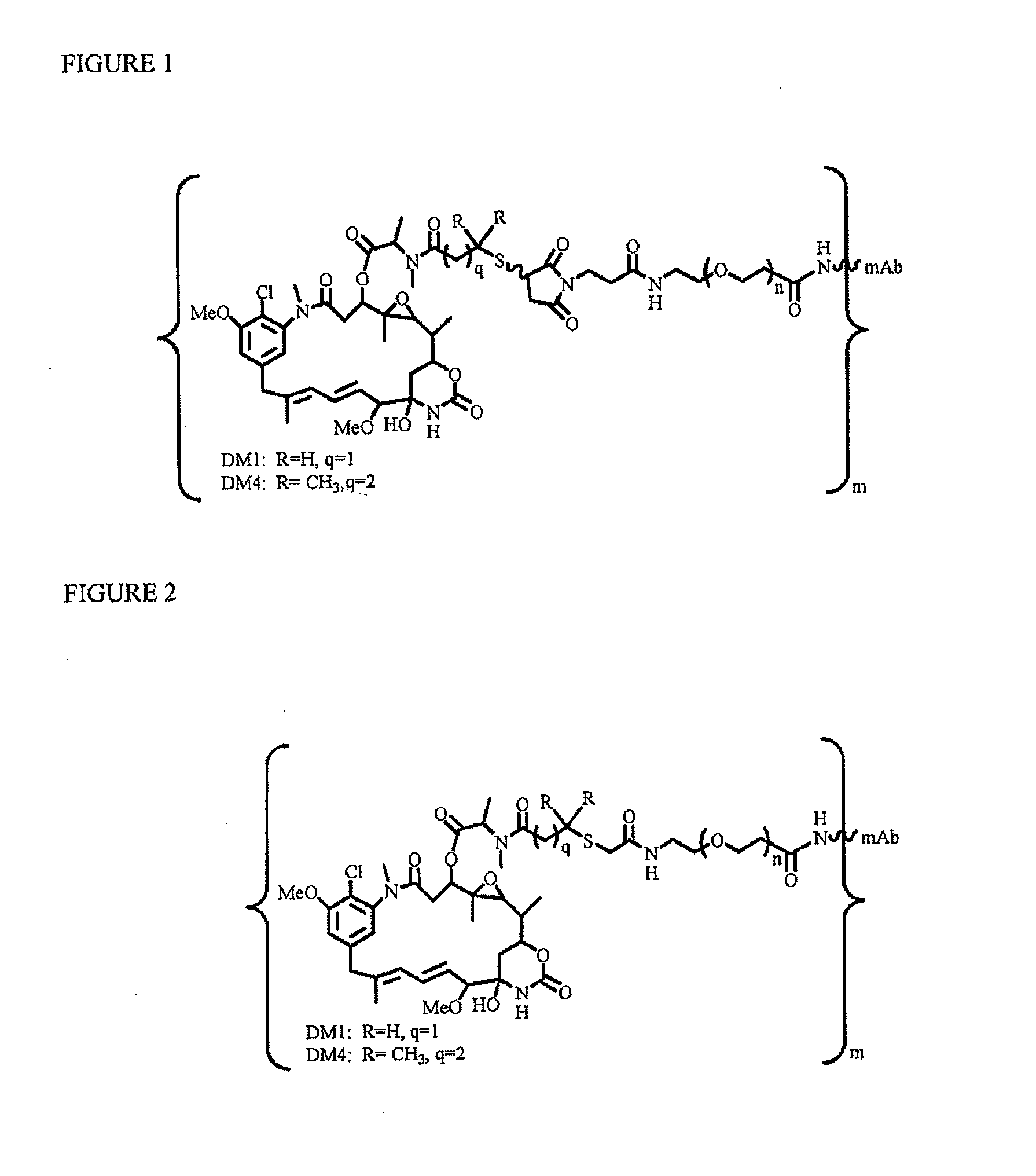
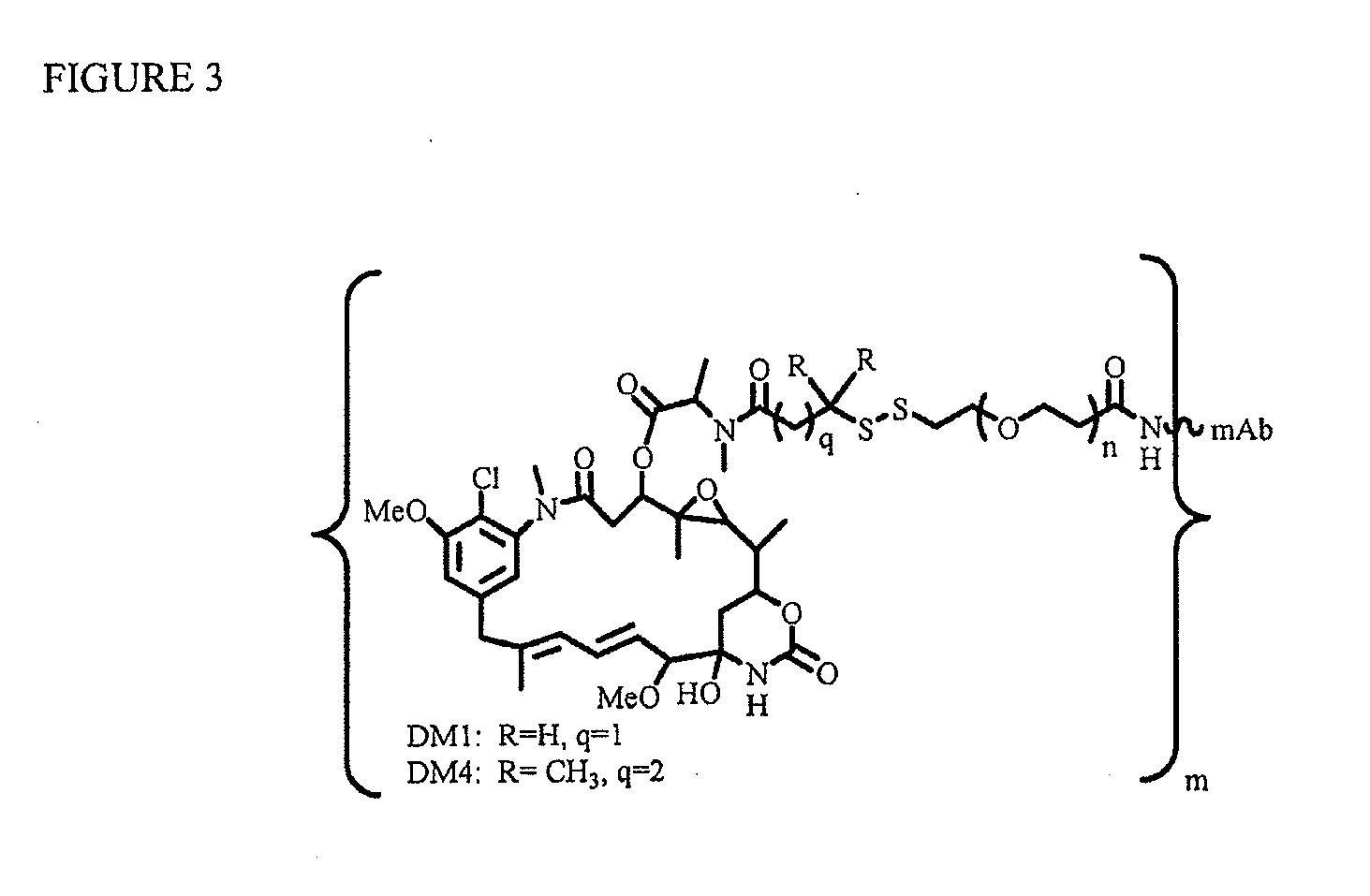
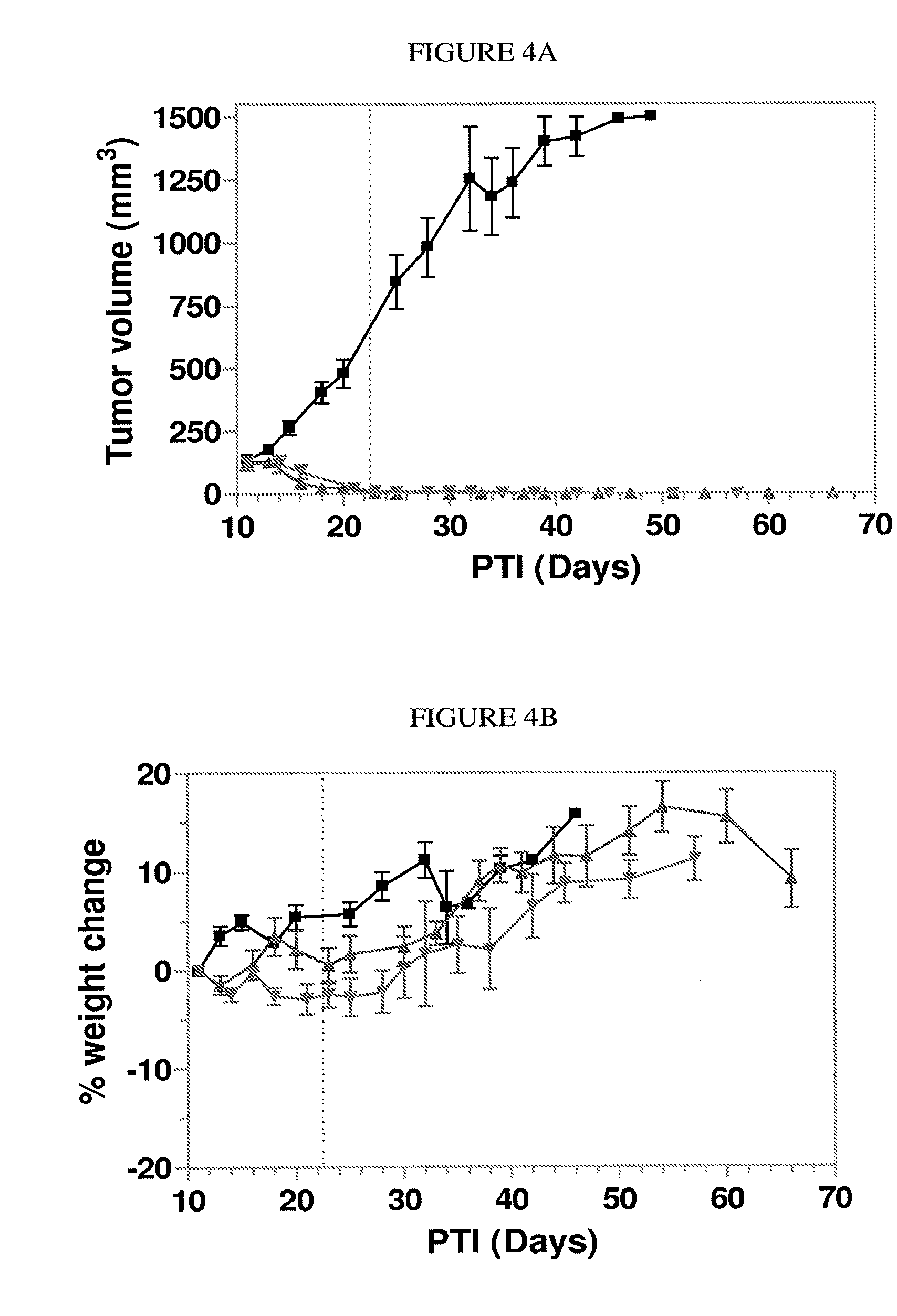
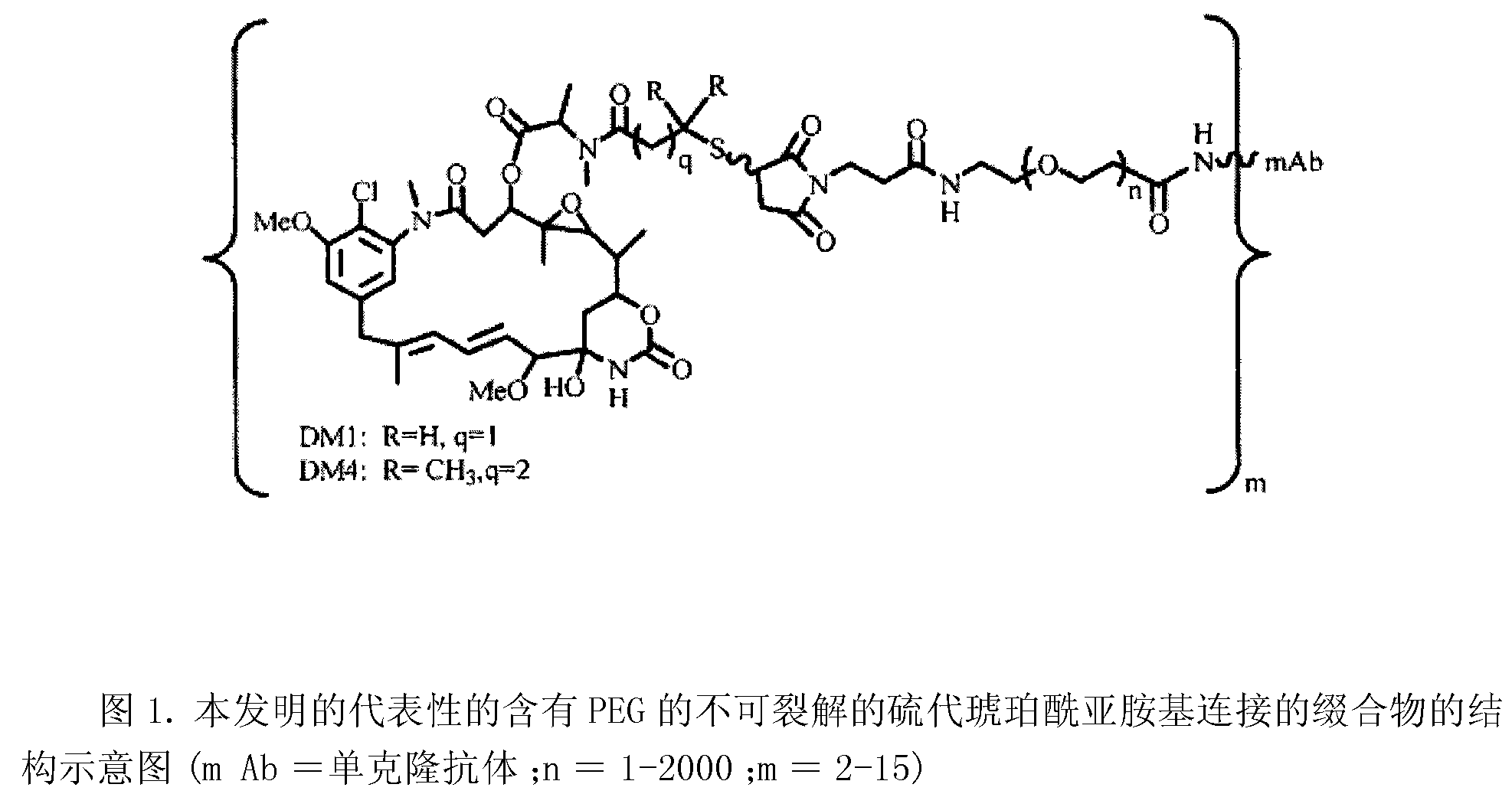
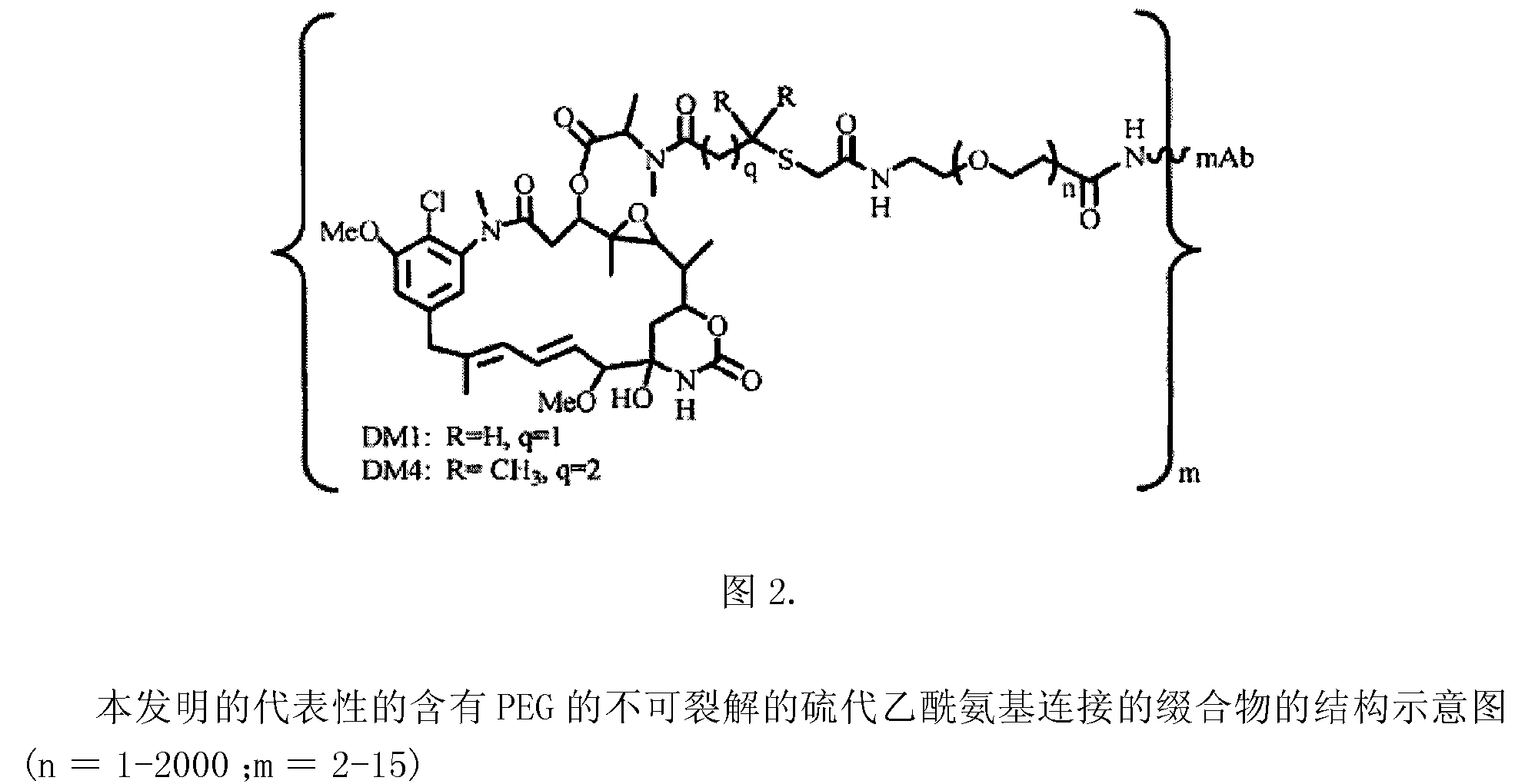
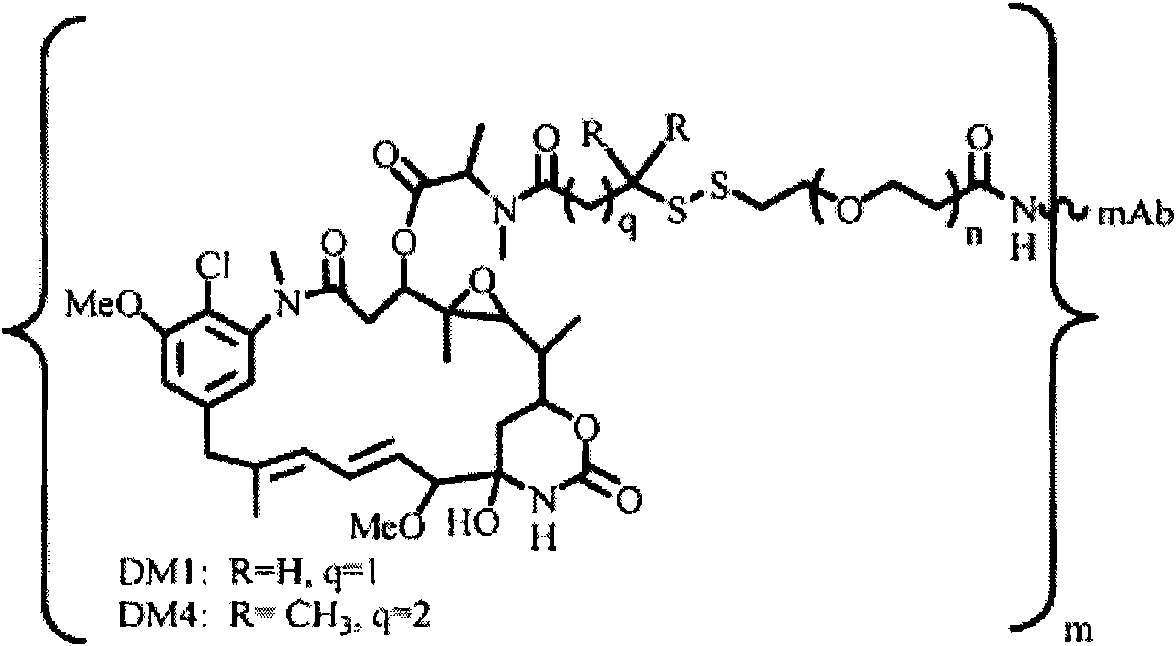
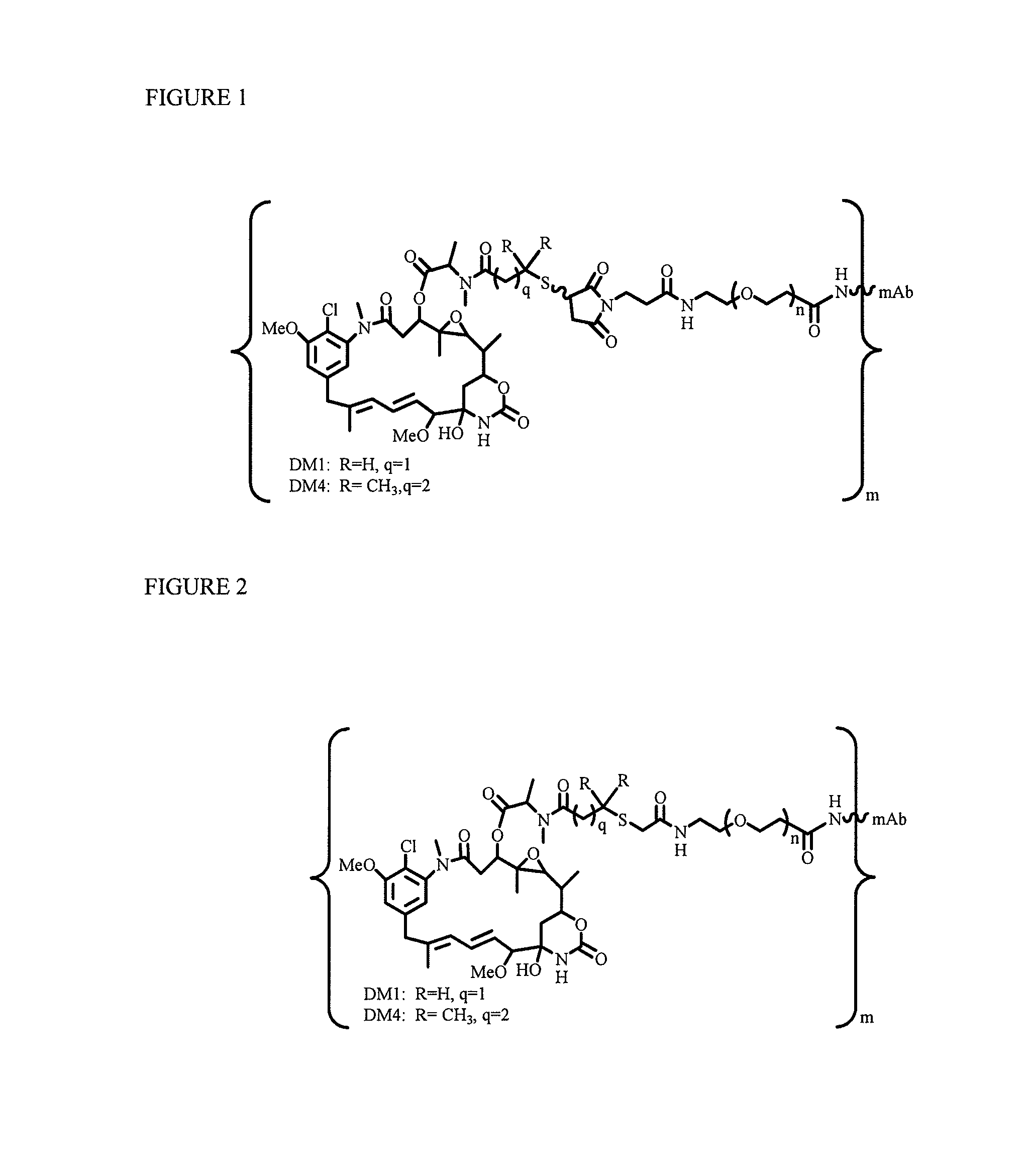
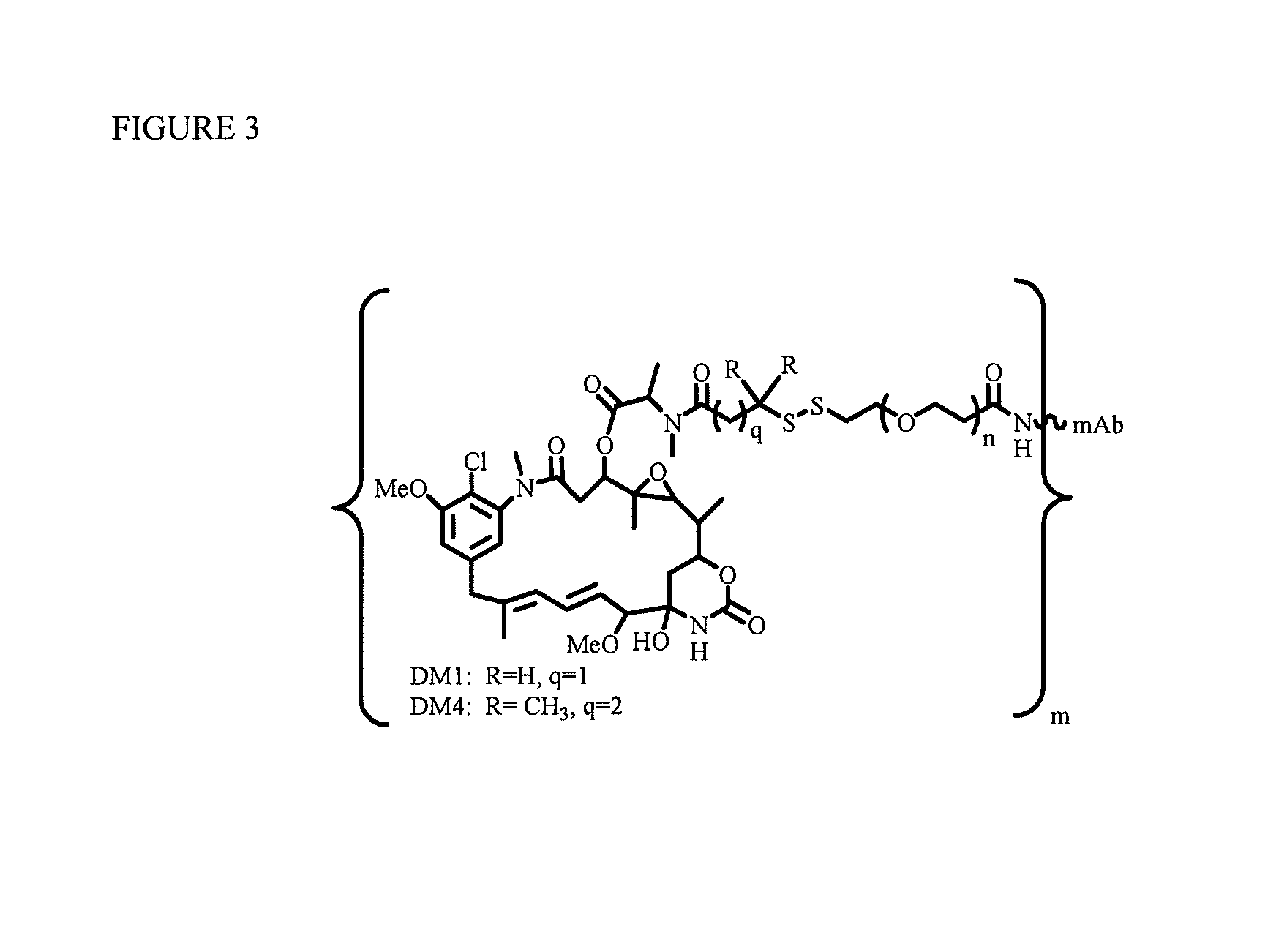
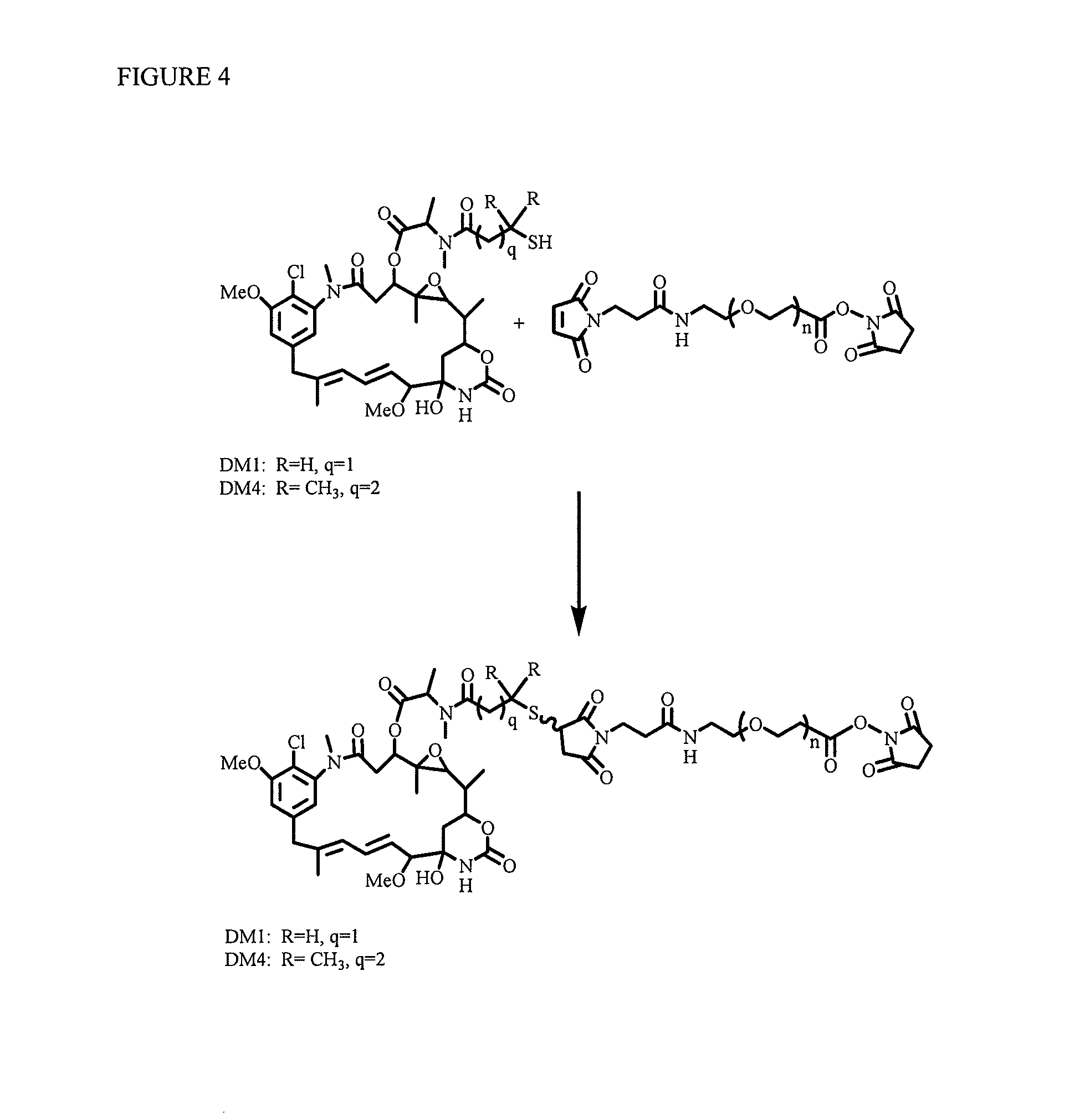
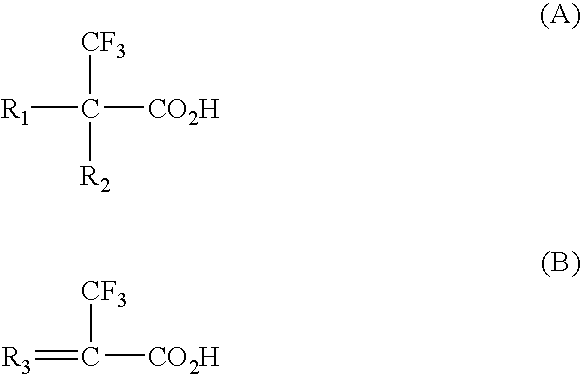Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Bound drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
For example, assume that Drug A and Drug B are both protein-bound drugs. If Drug A is given, it will bind to the plasma proteins in the blood. If Drug B is also given, it can displace Drug A from the protein, thereby increasing Drug A's fraction unbound.
Vitamin receptor binding drug delivery conjugates
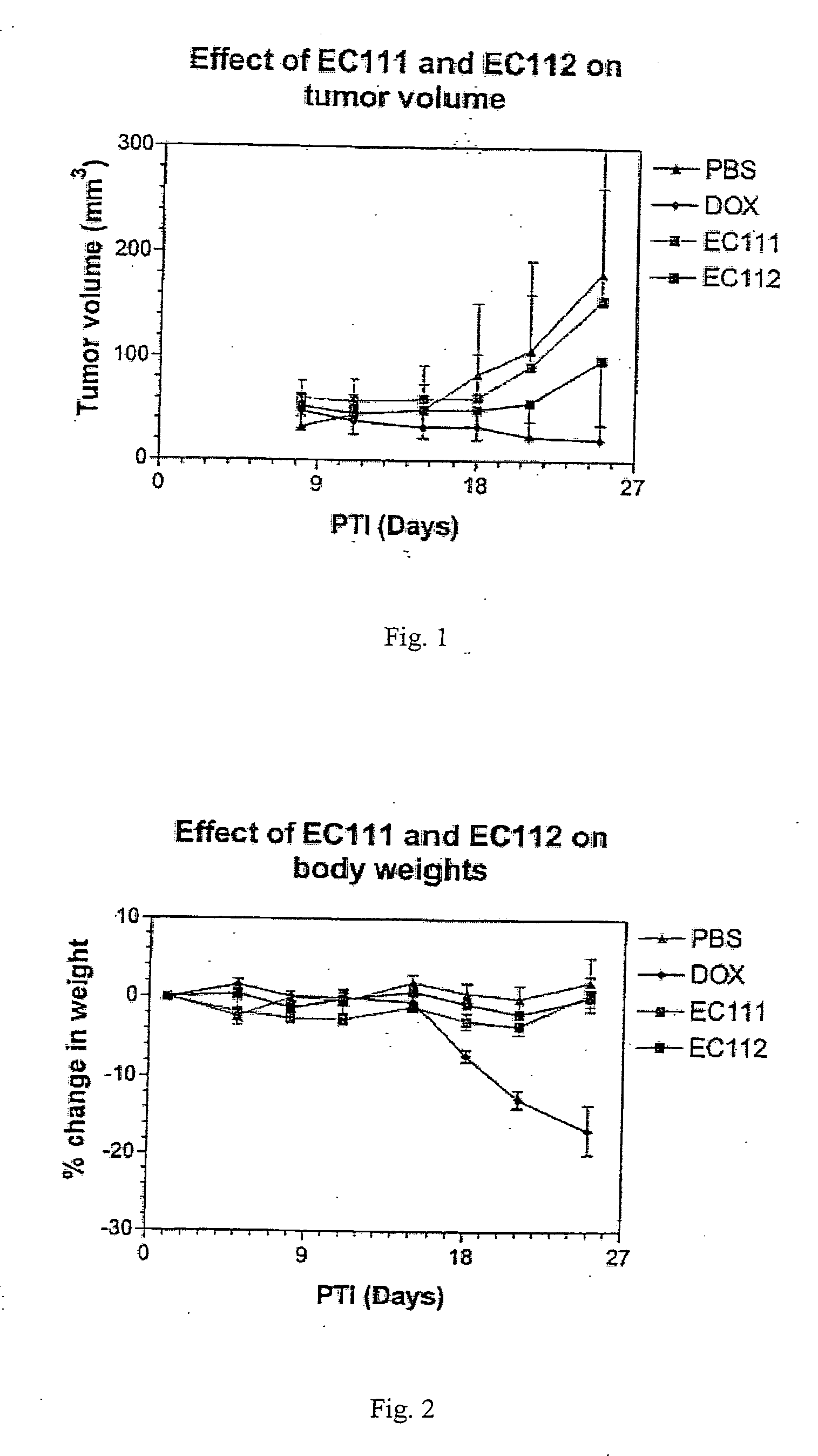
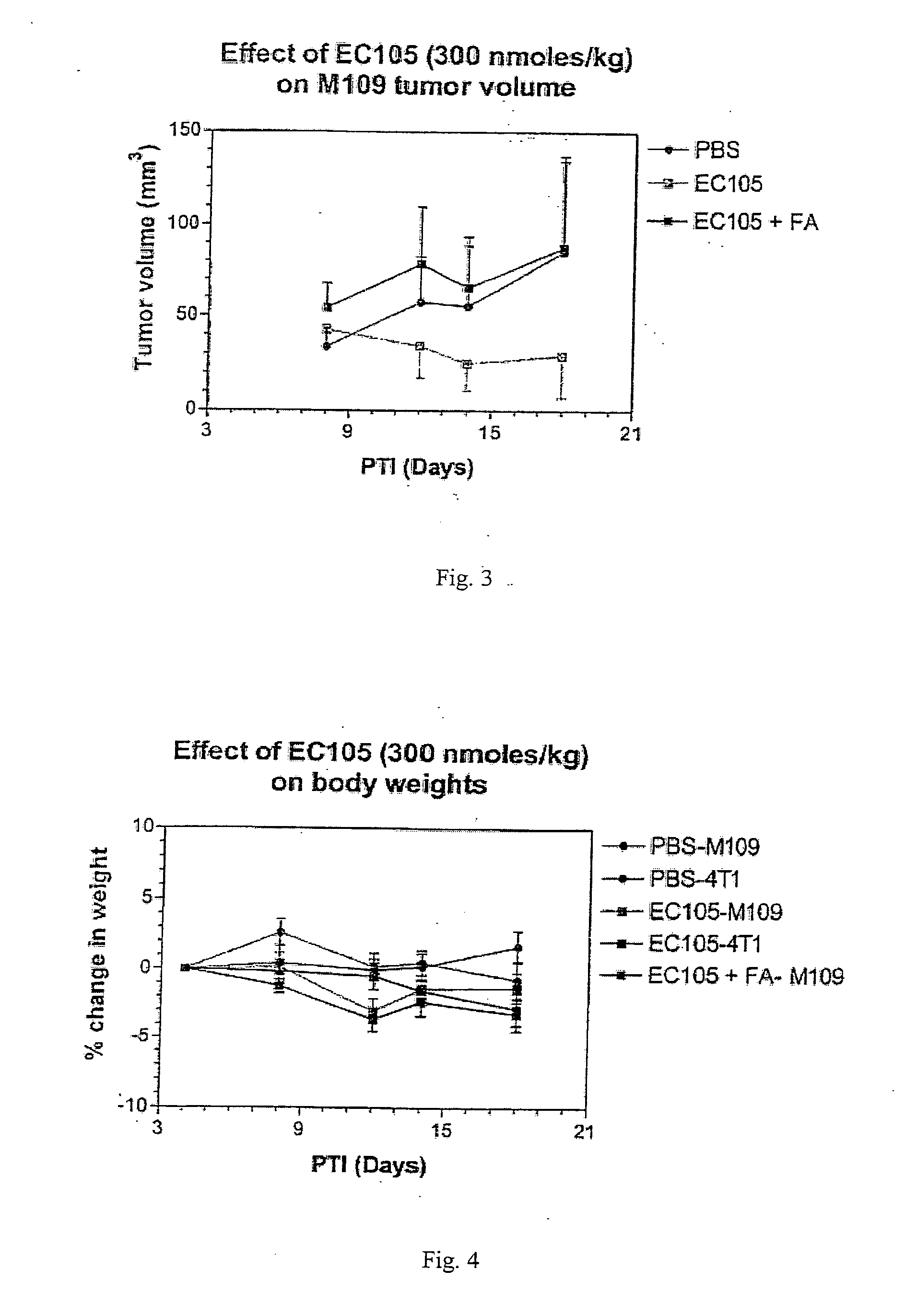
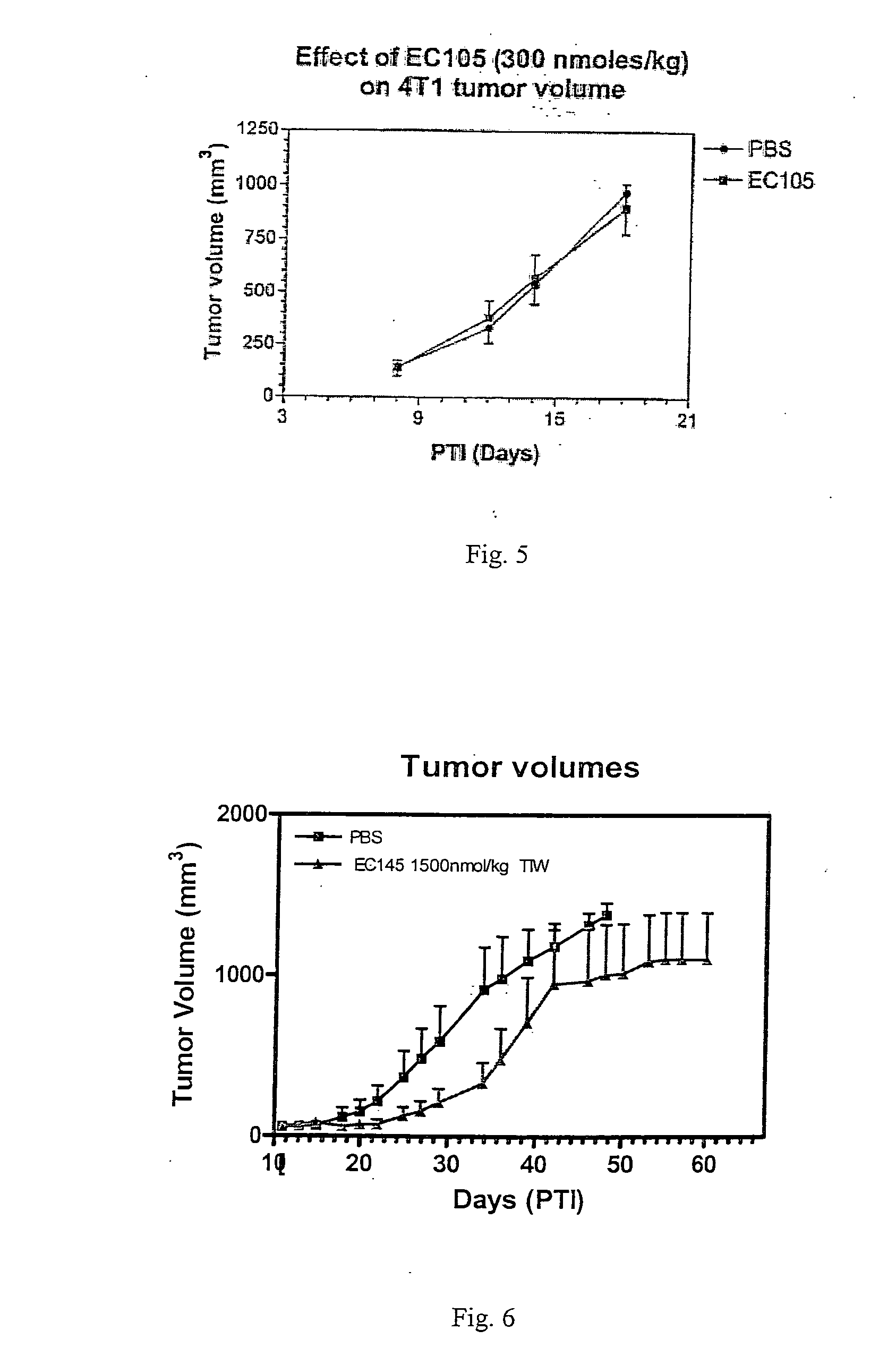
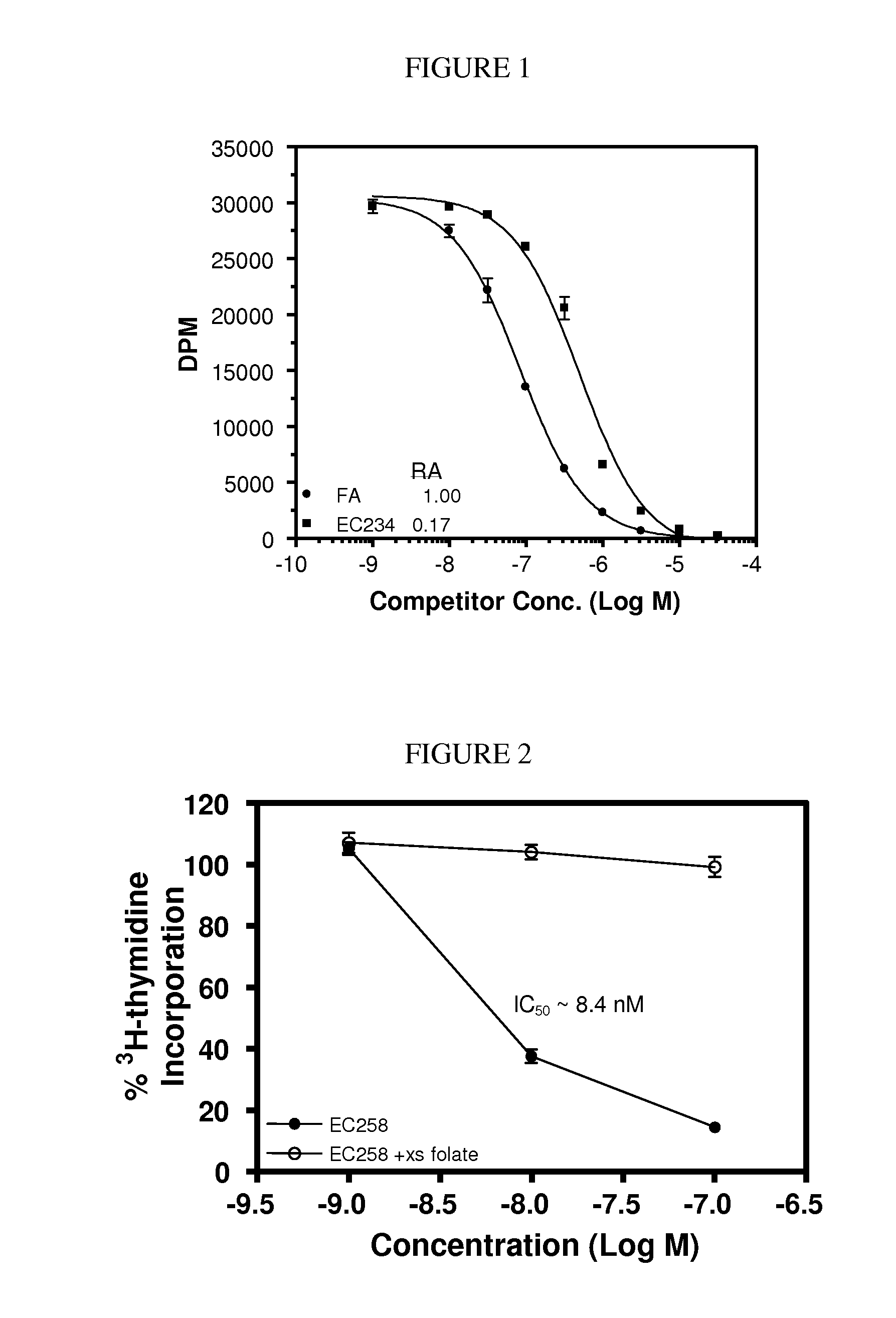
The invention describes a vitamin receptor binding drug delivery conjugate, and preparations therefor. The drug delivery conjugate consists of a vitamin receptor binding moiety, a bivalent linker (L), and a drug. The vitamin receptor binding moiety includes vitamins, and vitamin receptor binding analogs and derivatives thereof, and the drug includes analogs and derivatives thereof. The vitamin receptor binding moiety is covalently linked to the bivalent linker, and the drug, or the analog or the derivative thereof, is covalently linked to the bivalent linker, wherein the bivalent linker (L) includes components such as spacer linkers, releasable linkers, and heteroatom linkers, and combinations thereof. Methods and pharmaceutical compositions for eliminating pathogenic cell populations using the drug delivery conjugate are also described.
Owner:ENDOCTYE INC
Potent conjugates and hydrophilic linkers
InactiveUS20120226026A1Improve drug activitySimple wayAntiviralsImmunoglobulins against cell receptors/antigens/surface-determinantsBound drugAntigen
Linkers for binding drugs to cell binding agents are modified to hydrophilic linkers by incorporating a polyethylene glycol spacer. The potency or the efficacy of the cell-binding agent-drug conjugates is surprisingly enhanced several folds in a variety of cancer cell types, including those expressing a low number of antigens on the cell surface or cancer cells that are resistant to treatment. A method for preparing maytansinoids bearing a thioether moiety and a reactive group which allows the maytansinoid to be linked to a cell-binding agent in essentially a single step is also provided.
Owner:IMMUNOGEN INC
Resveratrol hydrophilic conjugate, its preparation method and application
InactiveCN102766258AImprove stabilityGood water solubilityHydroxy compound active ingredientsMetabolism disorderBound drugDisease
The invention discloses a novel conjugate composed of a hydrophilic polymer and resveratrol, a pharmaceutical composition including the conjugate, their preparation methods and applications in the preparation of drugs for treating tumor, diabetes and cardio cerebrovascular disease, wherein the hydrophilic polymer is selected from the group consisting of polyethylene glycol, polyglutamic acid, polyaspartic acid, polypropylene glycol, polyvinyl alcohol, polypropylene morpholine, polyethylene glycol-amino acid oligopeptide and their copolymer. Through modification of the hydrophilic polymer, a bound drug can be protected, stability and water solubility of the conjugate are raised, and active period of the conjugate in organisms is prolonged.
Owner:YANTAI TARGET DRUG RES +2
Potent conjugates and hydrophilic linkers
Linkers for binding drugs to cell binding agents are modified to hydrophilic linkers by incorporating a polyethylene glycol spacer. The potency or the efficacy of the cell-binding agent-drug conjugates is surprisingly enhanced several folds in a variety of cancer cell types, including those expressing a low number of antigens on the cell surface or cancer cells that are resistant to treatment. A method for preparing maytansinoids bearing a thioether moiety and a reactive group which allows the maytansinoid to be linked to a cell-binding agent in essentially a single step is also provided.
Owner:IMMUNOGEN INC
Potent conjugates and hydrophilic linkers
InactiveUS9150649B2Improve drug activitySimple wayPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenBound drug
Owner:IMMUNOGEN INC
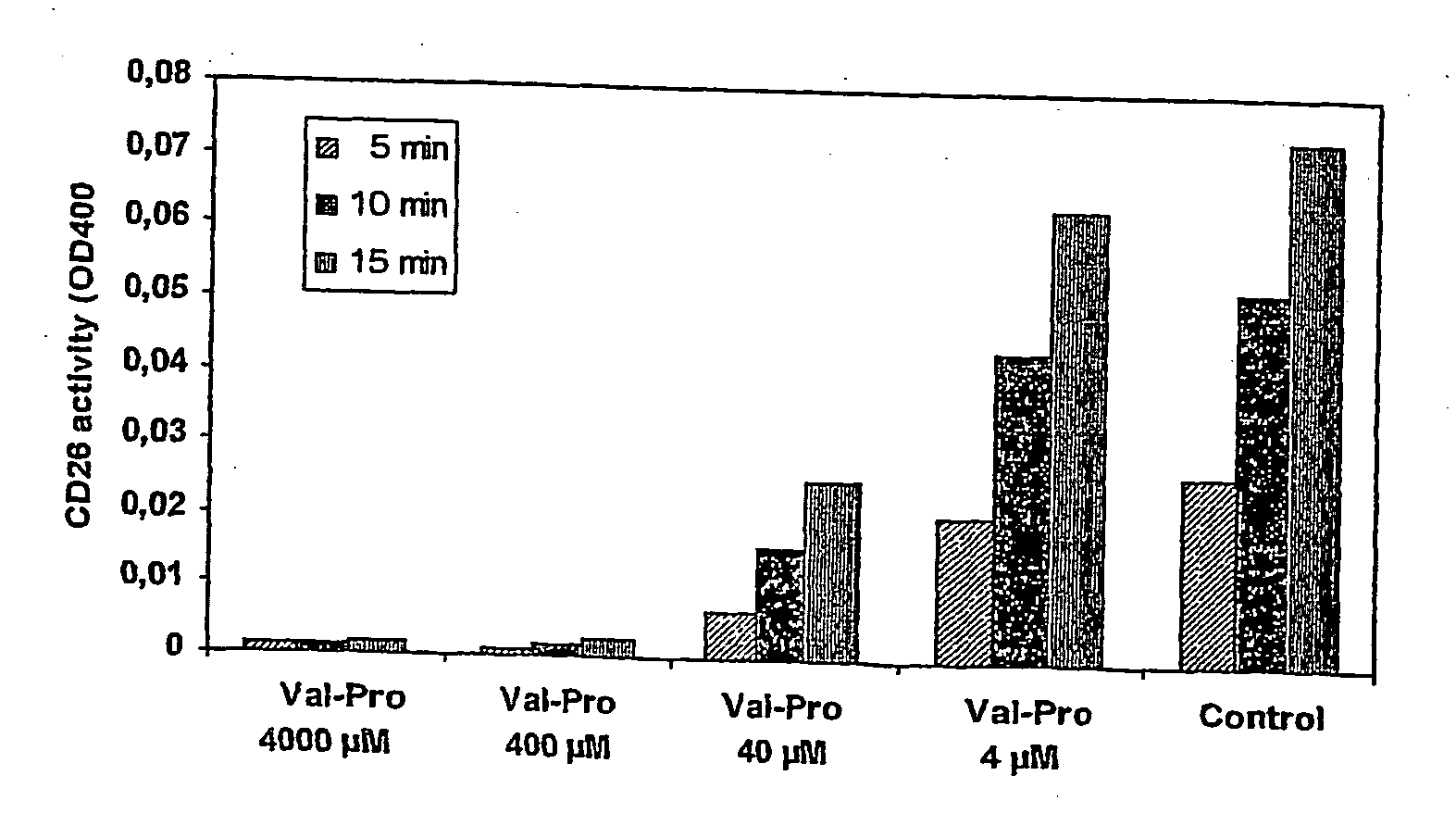
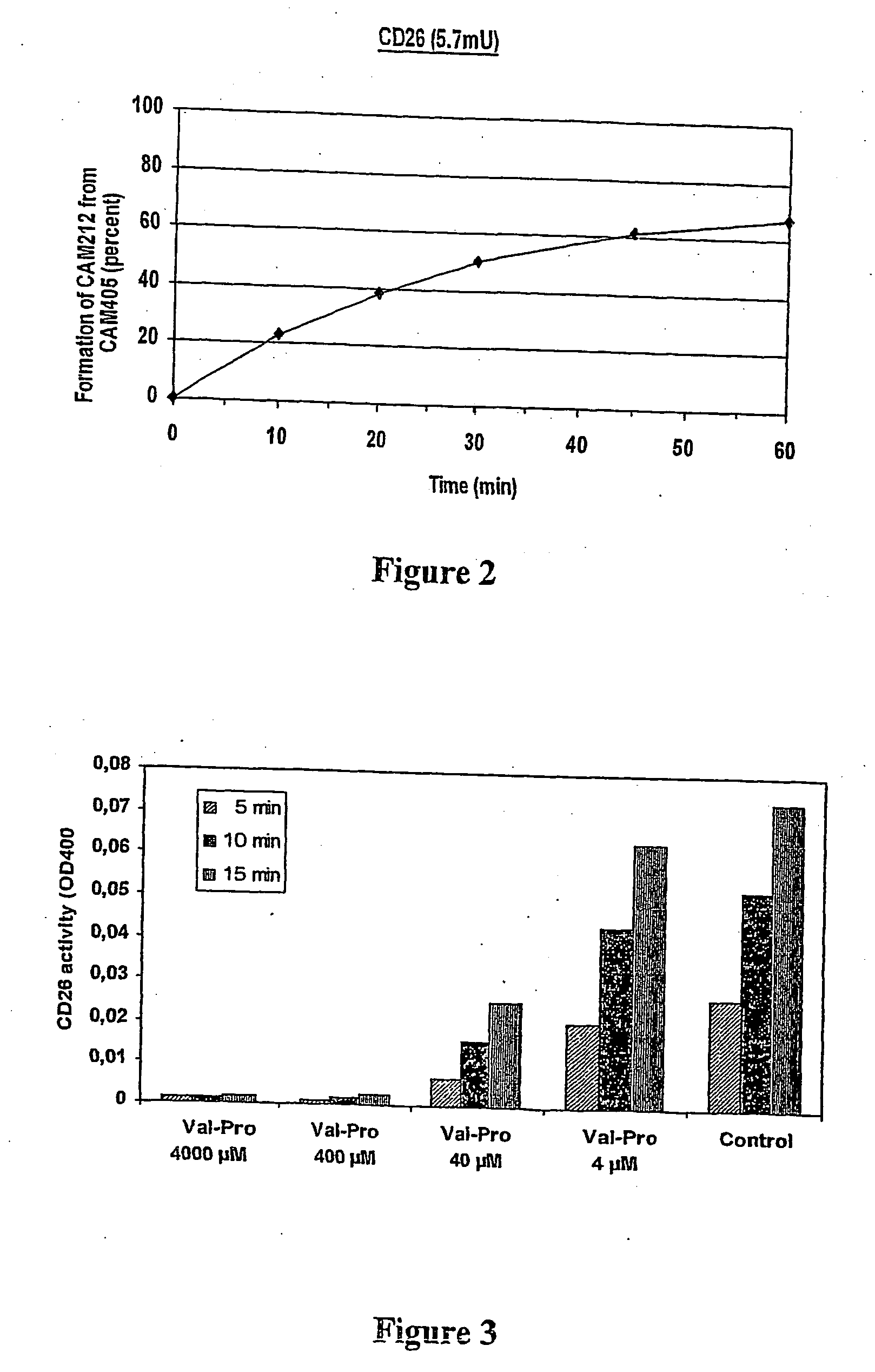
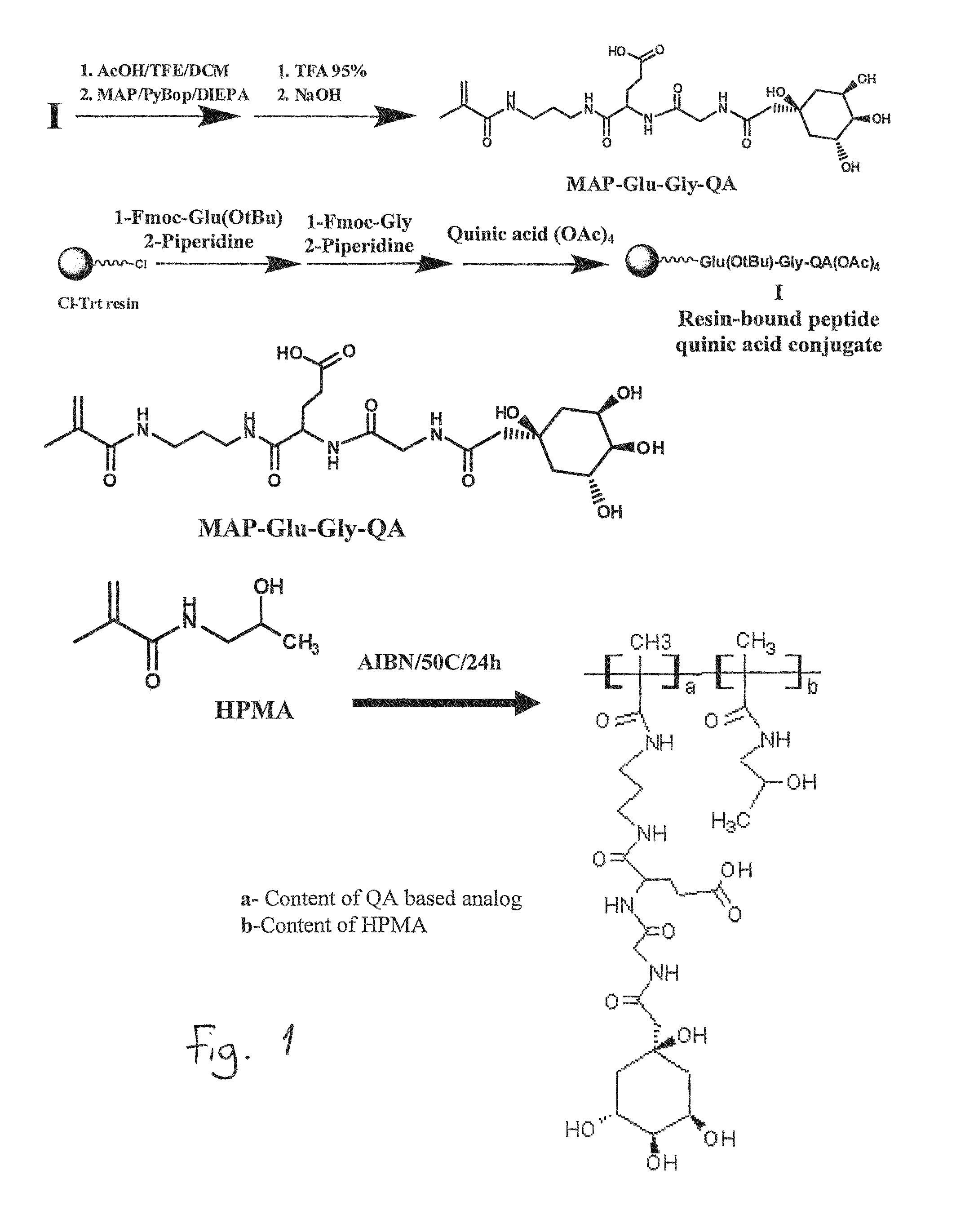
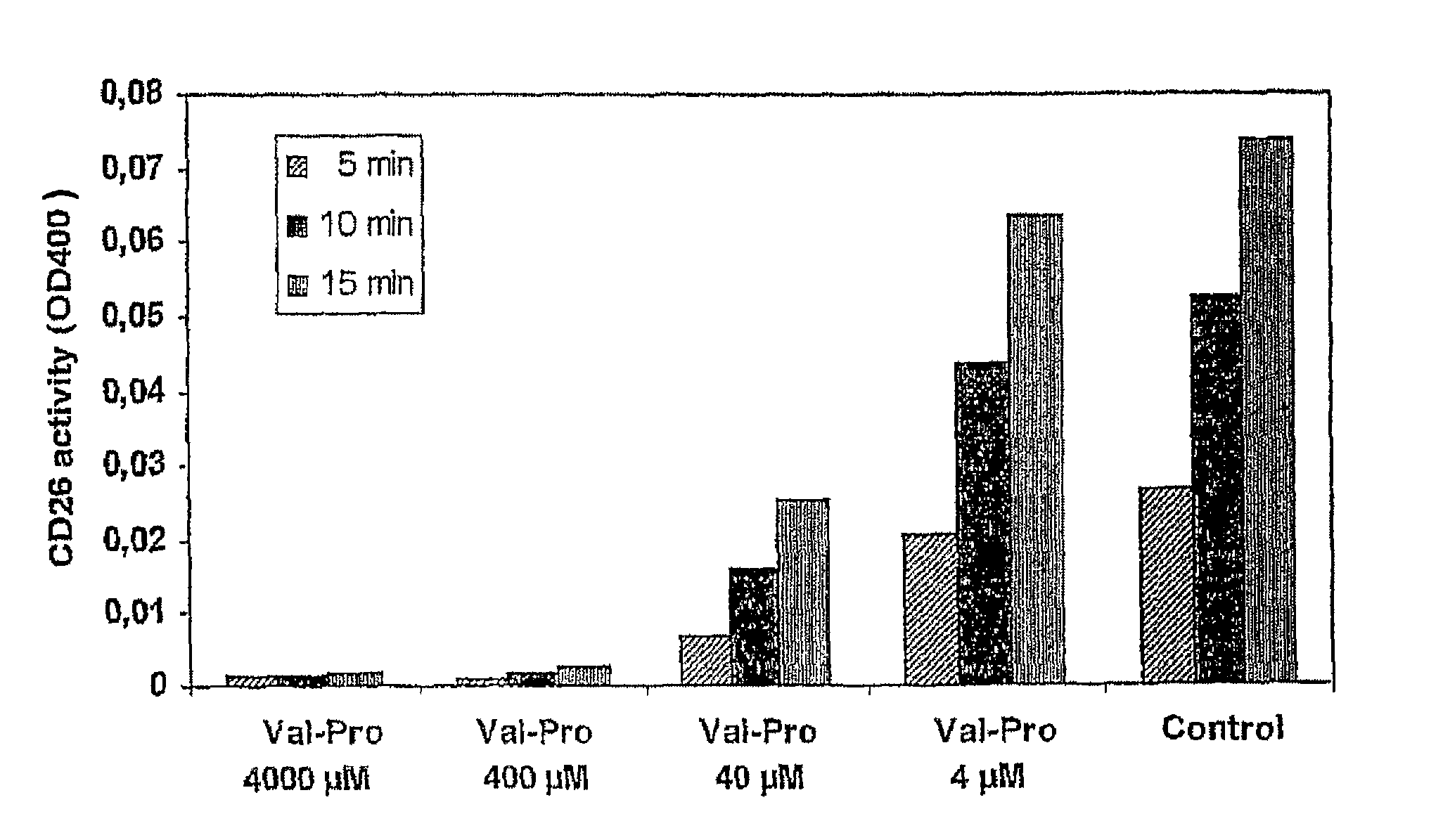
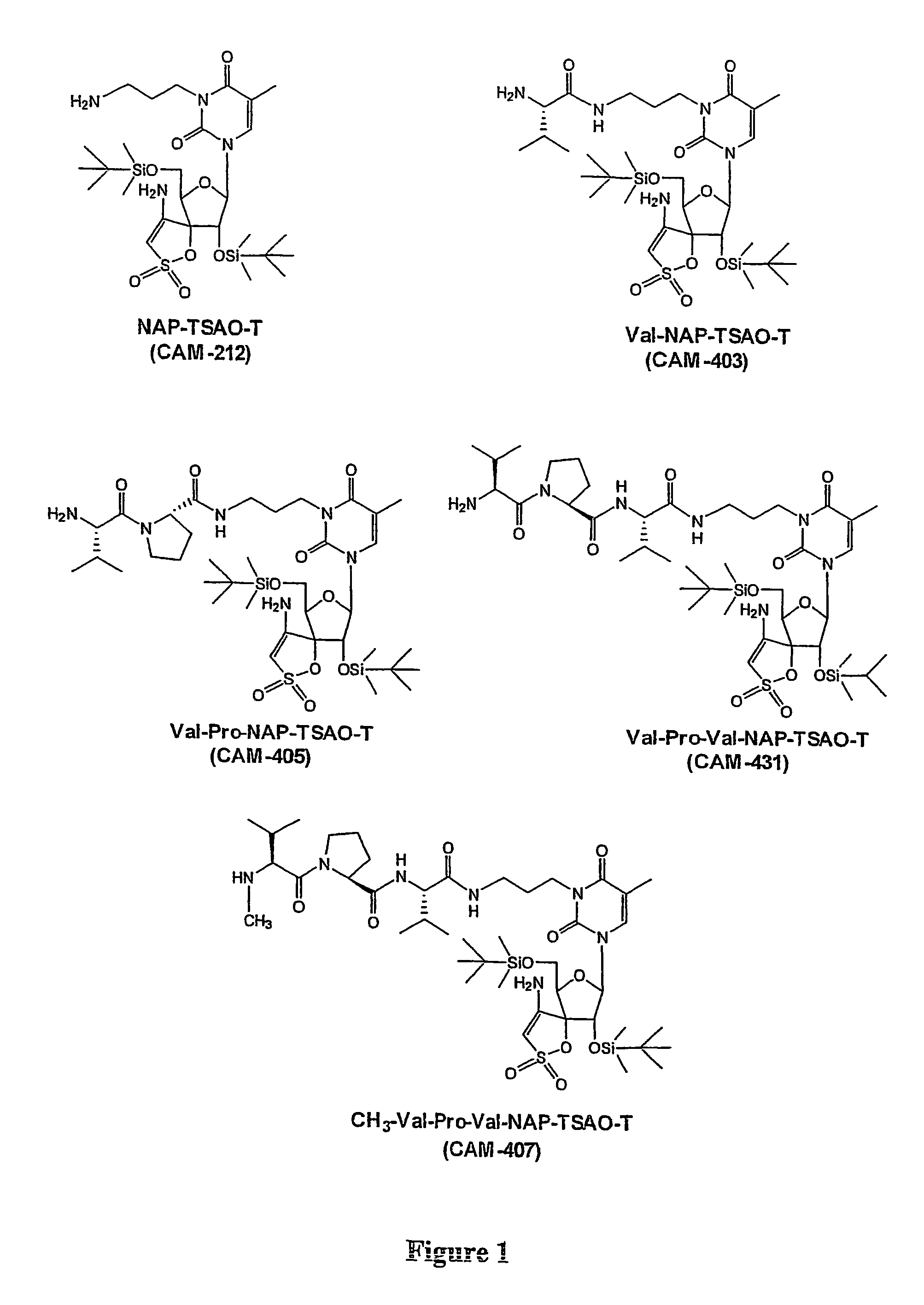
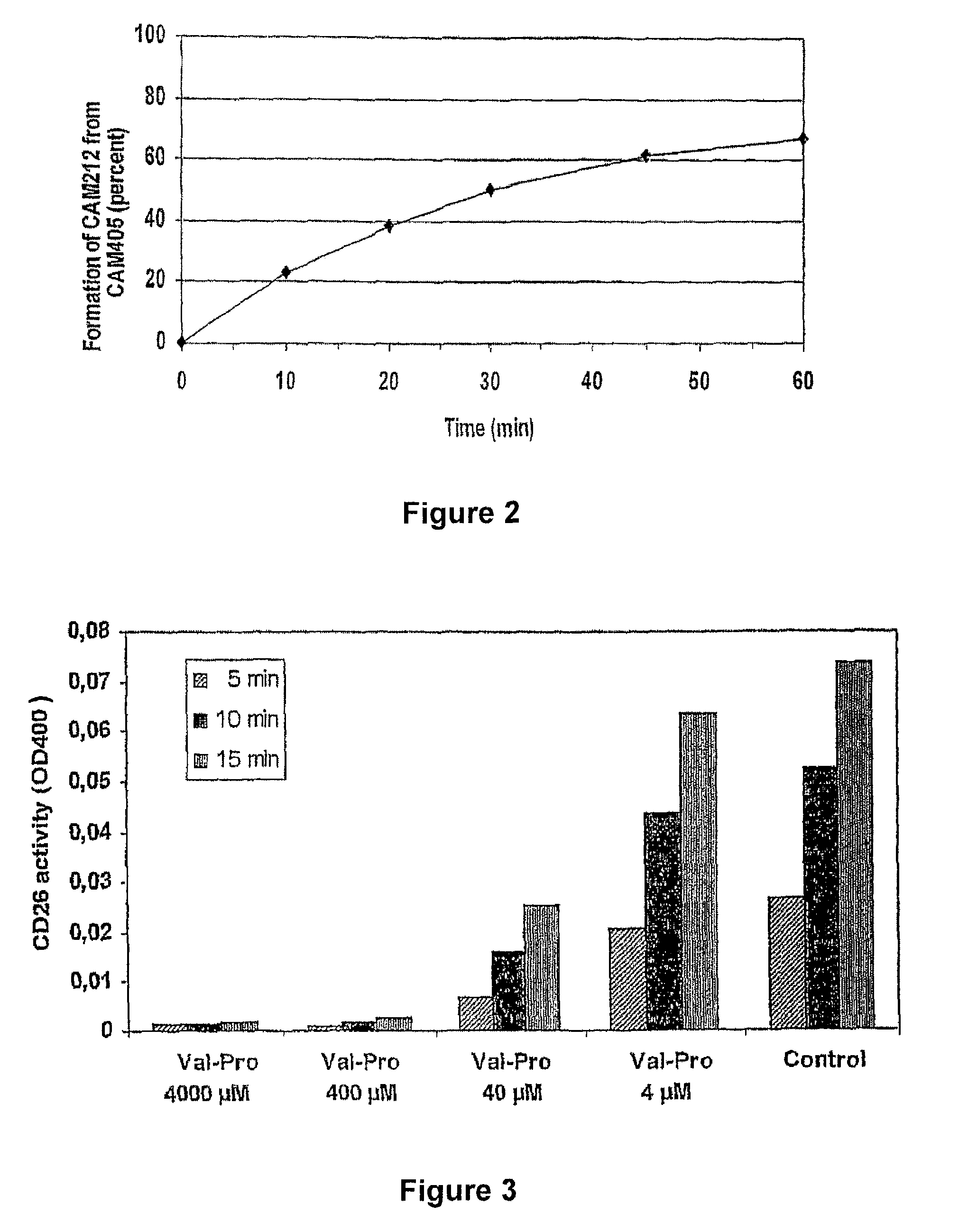
Prodrugs Cleavable by Cd26
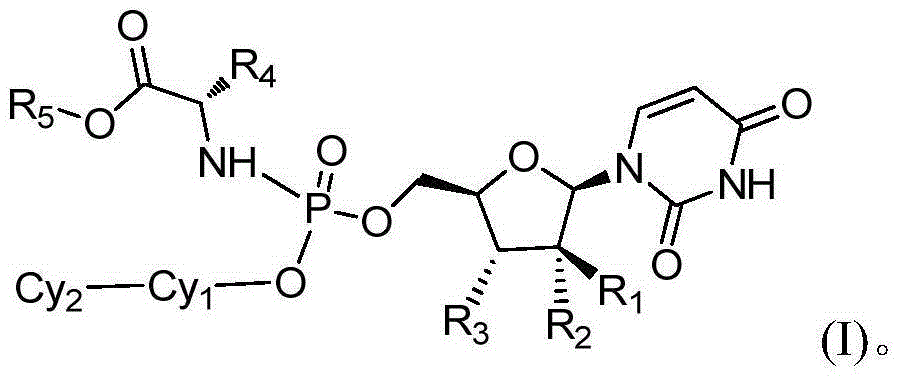
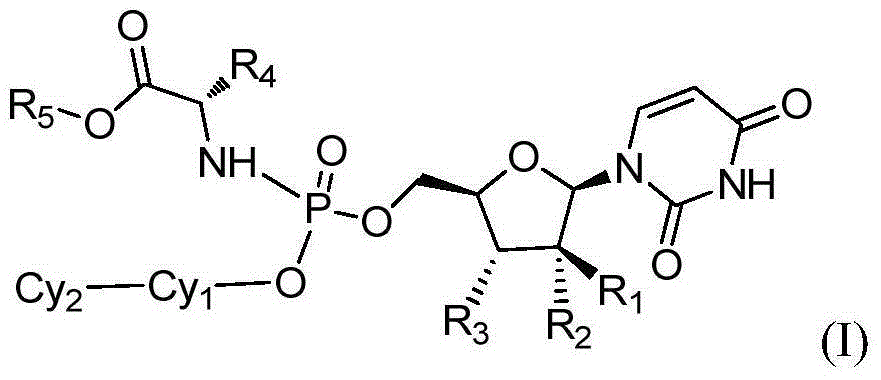
The present invention provides a new prodrug technology and new prodrugs in order to increase the solubility, to modulate plasma protein binding or to enhance the biovailability of a drug. In the present invention the prodrugs are conjugates of a therapeutic compound and a peptide (eg tetrapeptide or hexapeptide) wherein the conjugate is cleavable by dipeptidyl-peptidases, more preferably by CD26, also known as DPPIV (dipeptidyl aminodipeptidase IV). The present invention furthermore provides a method of producing said prodrugs, to enhance brain and lymphatic delivery of drugs and / or to extend drug half-lives in plasma.
Owner:K U LEUVEN RES & DEV +1
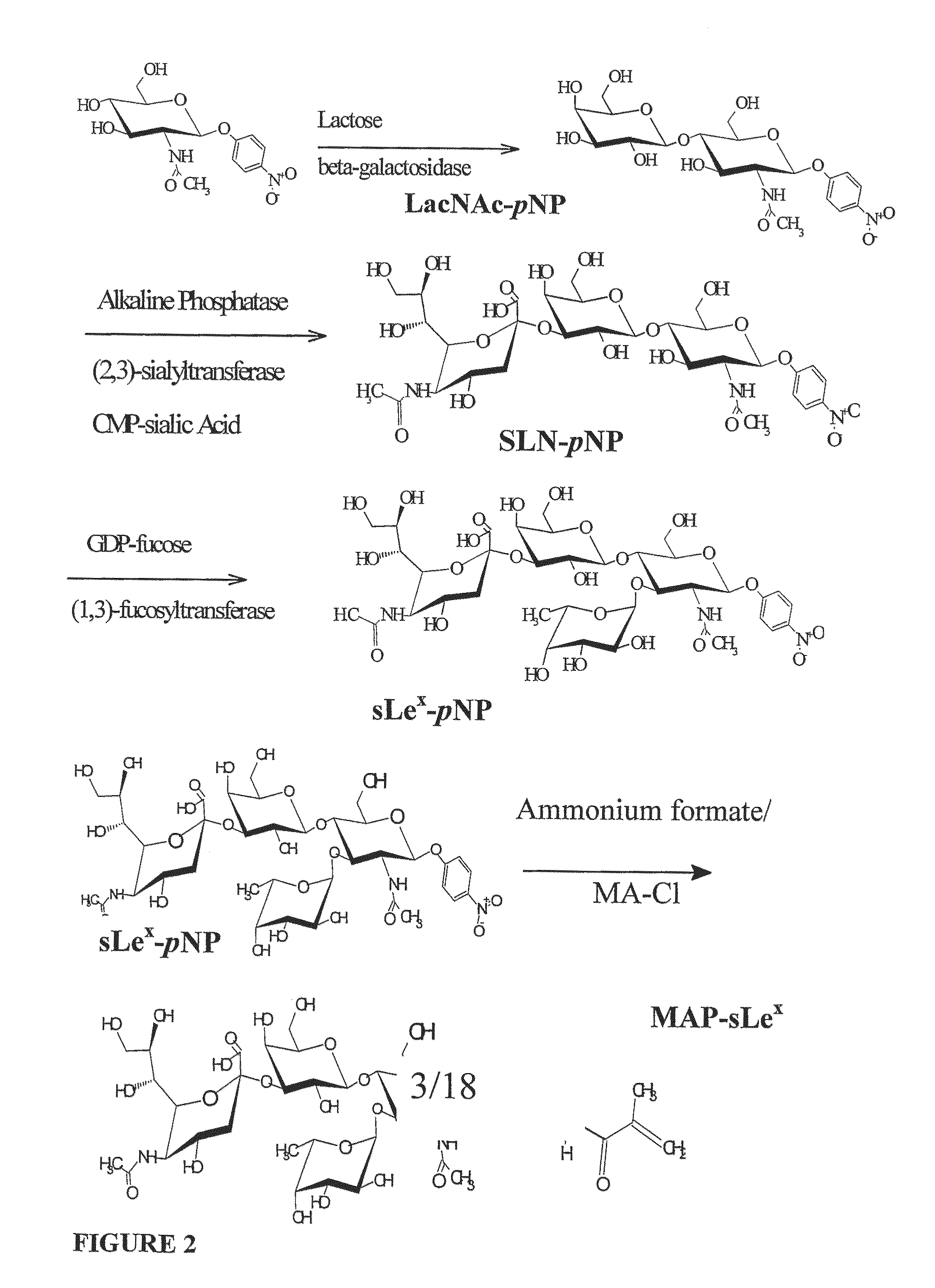
Vascular delivery systems
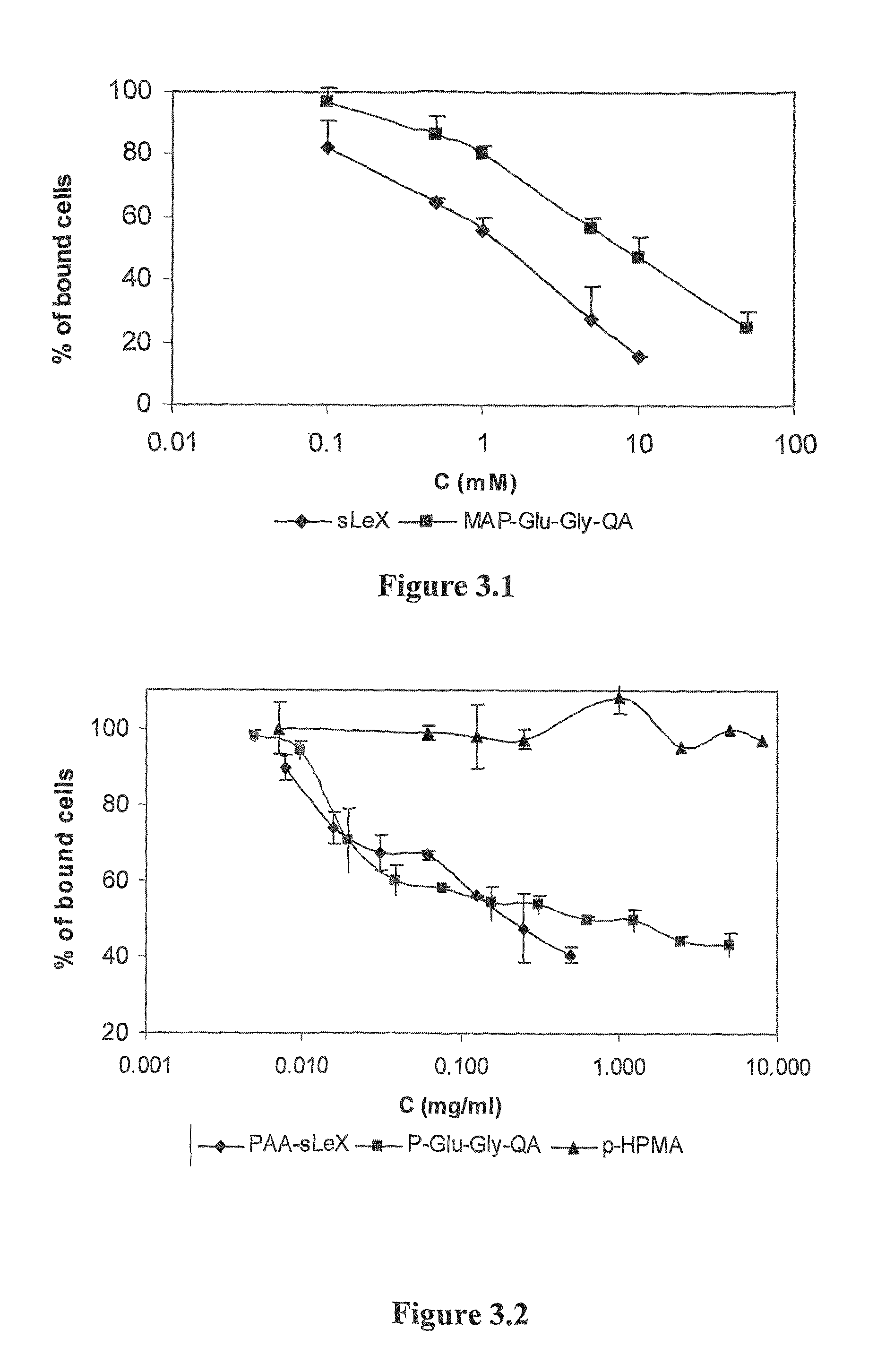
The site-specific expression of selectins on endothelial cells of blood vessels during angiogenesis provides an opportunity to target anti-cancer drugs to the vascular endothelium to extend the range of the therapeutic effect. This invention describes an innovative drug targeting strategy for the selective delivery of the anticancer drugs to endothelial cells by means of polymer-drug conjugates modified with a carbohydrate ligand for the vascular selectins. A model chemotherapeutic drug, doxorubicin, and the E-selectin ligand, sLex, are attached to a biocompatible polymer (HPMA). The selective binding, cellular uptake, intracellular fate, and cell cytotoxicity of the polymer-bound drug are investigated in human endothelial cells.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Hepatitis C virus inhibitor and application thereof
The invention belongs to the field of medical chemistry, and particularly relates to a compound with the good hepatitis C virus inhibition effect, a preparation method of the compound, a composition comprising the compound and application of the compound or the composition as a medicine for treating hepatitis C virus infectious diseases. The compound has the excellent antiviral activity, meanwhile has the low cytotoxicity, is good in safety, good in plasma protein binding effect and suitable for patent medicine and has the quite good clinical application prospect.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
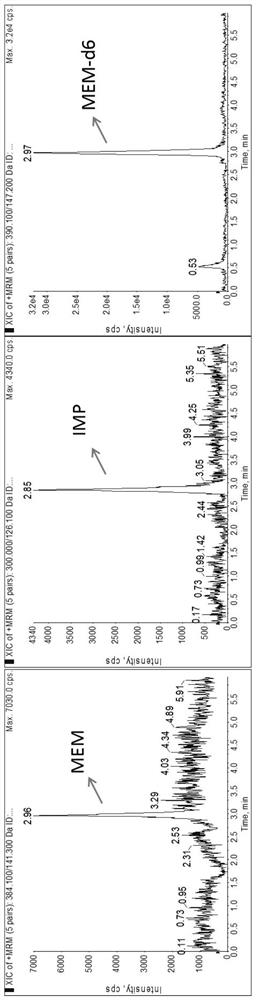
Method for detecting plasma protein binding rate of meropenem or imipenem by combining liquid chromatography-mass spectrometry technology with ultrafiltration technology
According to a method for detecting the plasma protein binding rate of meropenem or imipenem by combining a liquid chromatography-mass spectrometry technology with an ultrafiltration technology, the liquid chromatography-tandem mass spectrometry detection technology is combined with the ultrafiltration technology, and the total concentration of meropenem or imipenem in plasma and the free concentration of drugs in ultrafiltrate are respectively detected. And an MOPs stabilizer is added during sample treatment, so that the problem of poor drug stability is solved, and the determination result is more accurate. The method can be used for rapidly detecting the protein binding rate of the drug at high throughput, and is particularly suitable for unstable drug molecules.
Owner:PEKING UNIV THIRD HOSPITAL +1
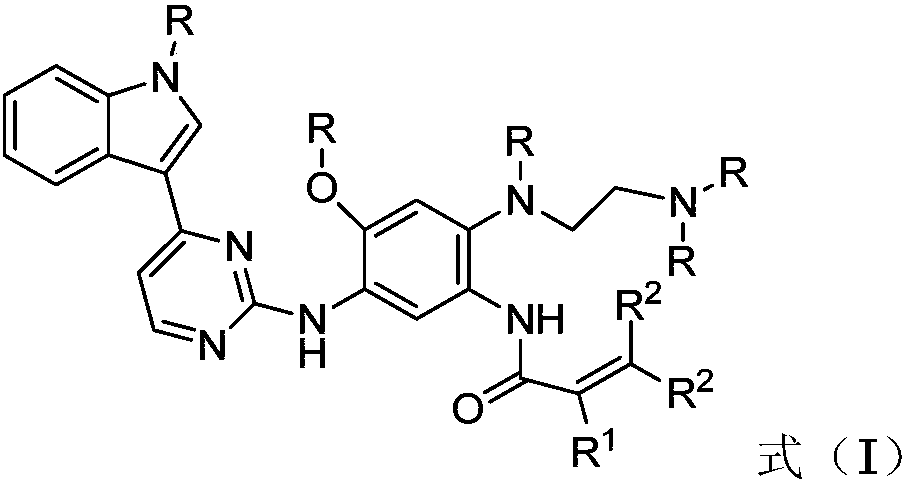
2-(2,4,5-substituted phenylamino)pyrimidine derivative as well as preparation method and application thereof to preparation of anti-tumor drug
ActiveCN108047205AGood physical propertiesImprove permeabilityOrganic chemistryAntineoplastic agentsBound drugChemical compound
The invention discloses a novel 2-(2,4,5-substituted phenylamino)pyrimidine derivative as well as a preparation method and application thereof to preparation of an anti-tumor drug and belongs to the field of medicines. The novel 2-(2,4,5-substituted phenylamino)pyrimidine derivative has a structure shown as a general formula (I): (the formula (I) is shown in the description), wherein in the formula (I), R is independently selected from methyl and deuterated (D) methyl and can be the same or different at the same time; R1 is independently selected from H, D, methyl, C2 to C5 alkyl, Cl and F, Brand CF3; R2 is independently selected from H and D and can be the same or different at the same time. The compound or pharmaceutically acceptable salt thereof or a composition thereof is applied to preparation of a drug for treating cancers, and has advantageous physical properties (such as relatively high permeability and / or relatively low plasma protein binding) and relatively high selectivityand relatively low toxic characteristics; especially, the compound has a very good application prospect in the aspect of treating non-small-cell lung cancer.
Owner:HENAN GENUINE BIOTECH CO LTD
Method for selectively manufacturing antibody-drug conjugate
ActiveUS11173213B2Improve securityImprove quality controlImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsBound drugDrug conjugation
A method for producing an antibody-drug conjugate composition, comprising: (i) a step of reacting an antibody with a reducing agent in a buffer to reduce interchain disulfides, and (ii) a step of reacting drug linker intermediates with the antibody having thiol groups obtained in the step (i), wherein the reaction temperature in the step (i) is −10° C. to 10° C., and the average number of bound drugs in the produced antibody-drug conjugate composition is 3.5 to 4.5, and the content of antibody-drug conjugates in which four drug linkers are bound to heavy-light interchain thiols, in the produced antibody-drug conjugate composition is 50% or more; and an antibody-drug conjugate composition, wherein the content of antibody-drug conjugates wherein the average number of bound drugs is 3.5 to 4.5, and the content of antibody-drug conjugates in which four drug linkers are bound to heavy-light interchain thiols, is 50% or more.
Owner:DAIICHI SANKYO CO LTD
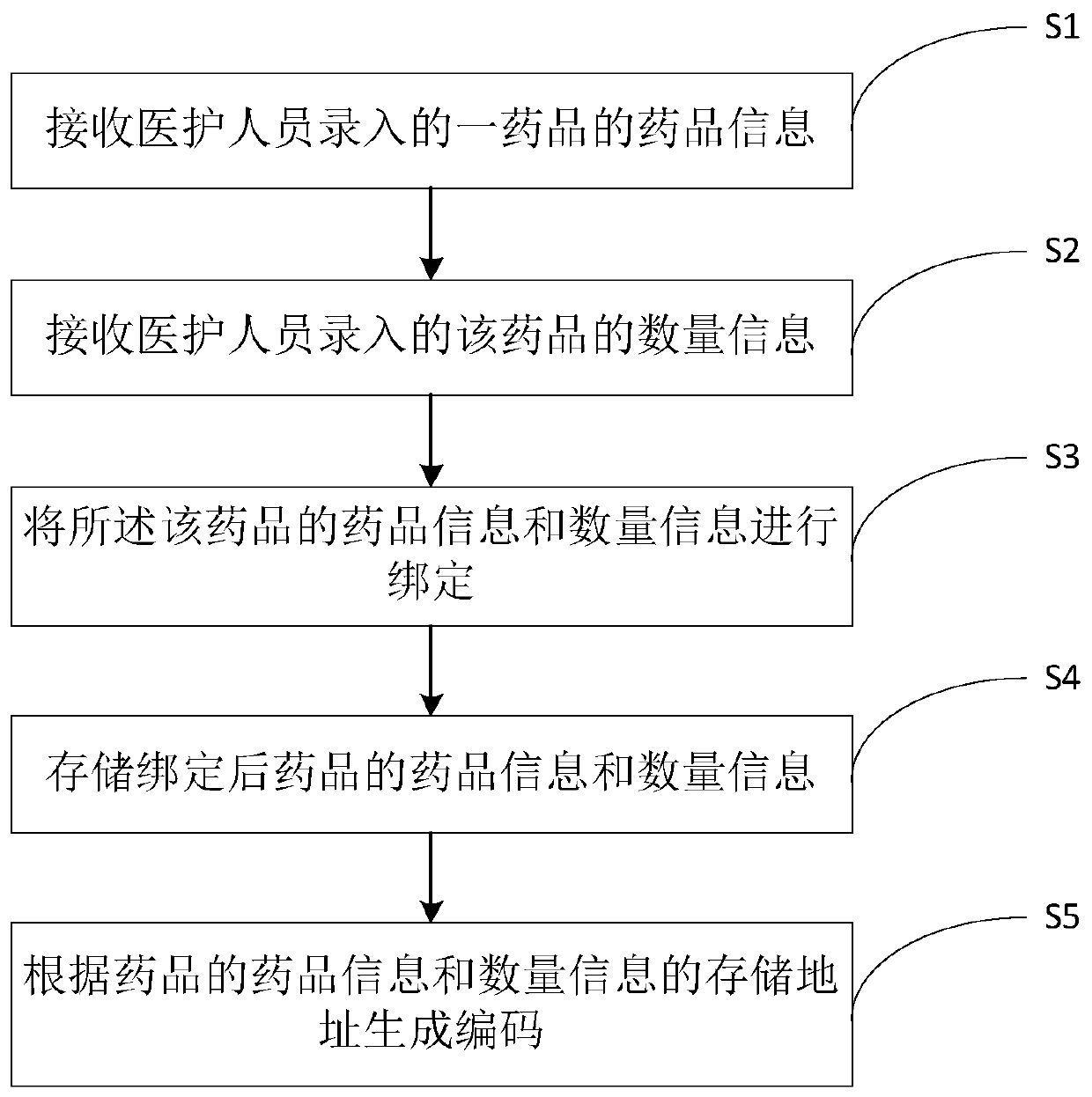
Drug code generation method and system, and drug quantity monitoring method and system
PendingCN110867235AEasy to understandDrug and medicationsCo-operative working arrangementsBound drugData mining
The invention provides a drug code generation method. The method comprises the following steps of: receiving drug information input by drug personnel; receiving quantity information and batch information, input by medical staff, of the drugs; binding the drug information, the quantity information and the batch information of the drugs; storing the drug information, the quantity information and thebatch information of the bound drugs; generating codes according to the storage addresses of the drug information, the quantity information and the batch information of the drugs, wherein the codes are pasted on drug bottles or drug boxes of the drugs and are used for identifying the drug information, the quantity information and the batch information of the drugs in the current drug bottles or the drug boxes. According to the method, the drug information, the quantity information and the batch information of drugs are bound, the codes are generated according to the storage address of the binding information and are pasted on the drug bottles or the drug boxes of the drugs, so that the medical personnel can obtain the drug information, the quantity information and the batch information inthe current drug bottles or the drug boxes when scanning the codes on the drug bottles or the drug boxes, and the medical personnel can know the inventory of the drugs conveniently.
Owner:上海林康医疗信息技术有限公司
Physically dispersed, molecularly dissolved and/or chemically bound drug(s) in an empty, hard capsule shell composition
The present invention proposes a design to incorporate drug(s) in the hard capsule shells (body and cap) composition. Drug(s) in the cap and body of the capsule shell may be the same or may be different. Other drug(s) in the form of granules, beads etc. can be filled into the capsules as a core material. The drug(s) in the capsule core material may be the same as in the shell-composition or may be different. Thus, the same capsule may contain different drug(s) as the core material and in the shell. The key advantages of incorporation of drug in the capsule shell compositions are to minimize drug-drug interaction and to obtain a desired rate of release of the drug(s), mainly for potent ones. The concept can be applied to the hard gelatin, and hard non-gelatin capsules.
Owner:JOSHI HEMANT N
Compounds, compositions and methods for reducing toxicity and treating or preventing diseases
The present invention provides compounds of Formula (I), compositions comprising an effective amount of a compound of Formula (I), optionally with chemotherapeutic drugs such as a tubulin-binding drug, and methods of their use for reducing the toxicity of cytotoxic agents, treating or preventing cancer or a neuropathic disorder, inducing a chemoprotective phase II enzyme, DNA, or protein synthesis, enhancing the immune system, treating inflammation, improving and enhancing general health or well-being, and methods for making compounds of Formula (I).
Owner:DANISHEFSKY SAMUEL J +6
Compositions of matter that reduce pain, shock, and inflammation by blocking linoleic acid metabolites and uses thereof
InactiveUS20140128588A1Organic active ingredientsSugar derivativesBound drugLinoleic acid metabolism
A method for treating and / or diagnosing pain and the source or type of pain, shock, and / or inflammatory conditions in a subject. A method of using a therapeutically effective amount of a DNA or RNA aptamer that shows high affinity for OLAMs to at least partially treat pain, shock, and / or inflammatory conditions in a subject. The DNA or RNA aptamer that shows high affinity for OLAMs may be coupled to a plasma protein binding compound or a pharmacologically active agent. A method of treating and or diagnosing pain, shock, and / or inflammatory conditions in a subject may include inactivating or preventing at least one linoleic acid metabolite to treat certain conditions (e.g., pain, shock, and / or inflammation) using a DNA or RNA aptamer that shows high affinity for OLAMs.
Owner:OTC BIOTECH +1
Compositions of matter that reduce pain, shock, and inflammation by blocking linoleic acid metabolites and uses thereof
A method for treating and / or diagnosing pain and the source or type of pain, shock, and / or inflammatory conditions in a subject. A method of using a therapeutically effective amount of a DNA or RNA aptamer that shows high affinity for OLAMs to at least partially treat pain, shock, and / or inflammatory conditions in a subject. The DNA or RNA aptamer that shows high affinity for OLAMs may be coupled to a plasma protein binding compound or a pharmacologically active agent. A method of treating and or diagnosing pain, shock, and / or inflammatory conditions in a subject may include inactivating or preventing at least one linoleic acid metabolite to treat certain conditions (e.g., pain, shock, and / or inflammation) using a DNA or RNA aptamer that shows high affinity for OLAMs.
Owner:OTC BIOTECH +1
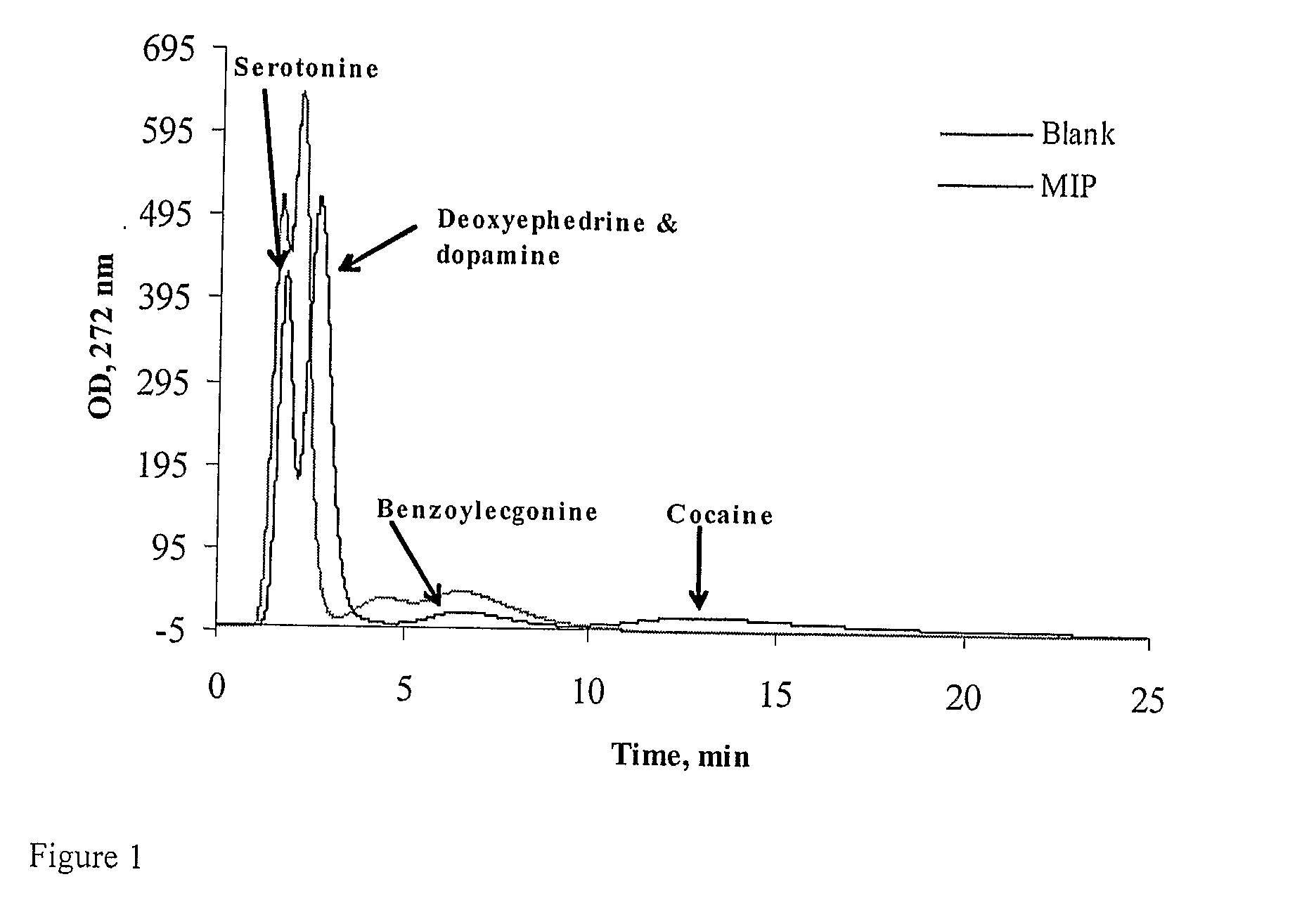
Binding drugs of abuse
InactiveUS20090298194A1High affinityIncreased selectivity and affinityComponent separationWithdrawing sample devicesTrifluoromethylOrganic acid
Drugs of abuse (e.g. cocaine) and related substances are selectively bound by an adsorbent which comprises units derived from carboxylic acids of formula A or B: (A): R1R2C(CF3)—CO2H; (B): R3═C(CF3)—CO2H or derivatives thereof. The adsorbent may be a polymer, or a solid support onto which the carboxylic acid, derivative or polymer has been grafted. The preferenced acid is 2-trifluoromethyl acrylic acid. A drug can be selectively bound from a mixture, and recovered using an eluant comprising an organic acid.
Owner:CRANFIELD UNIVERSITY
Methods and compositions for increasing the safety and efficacy of albumin-binding drugs
InactiveUS20060234960A1Improve securityImprove efficacyBiocidePeptide/protein ingredientsBound drugHypertension medications
A method is provided for increasing the safety and efficacy of albumin-binding drugs such as those used as anti-cancer, anti-infective, or anti-hypertensive drugs, or for numerous other conditions. In the preferred method, the invention modulates those drugs which bind at the IB site on human serum albumin by co-administering a compound which is highly tolerable to humans and which can bind competitively with those albumin-binding drugs at the IB binding site so as to increase the safety and efficacy of the drug. The invention is advantageous in that by administering the highly tolerable compound in a sufficient amount to compete with the targeted drug, the latter can be administered at a much lower dosage while maintaining or exceeding its potency. Compositions containing the combination of highly tolerable compound and albumin-bind drugs are also disclosed.
Owner:NEW CENTURY PHARMA INC
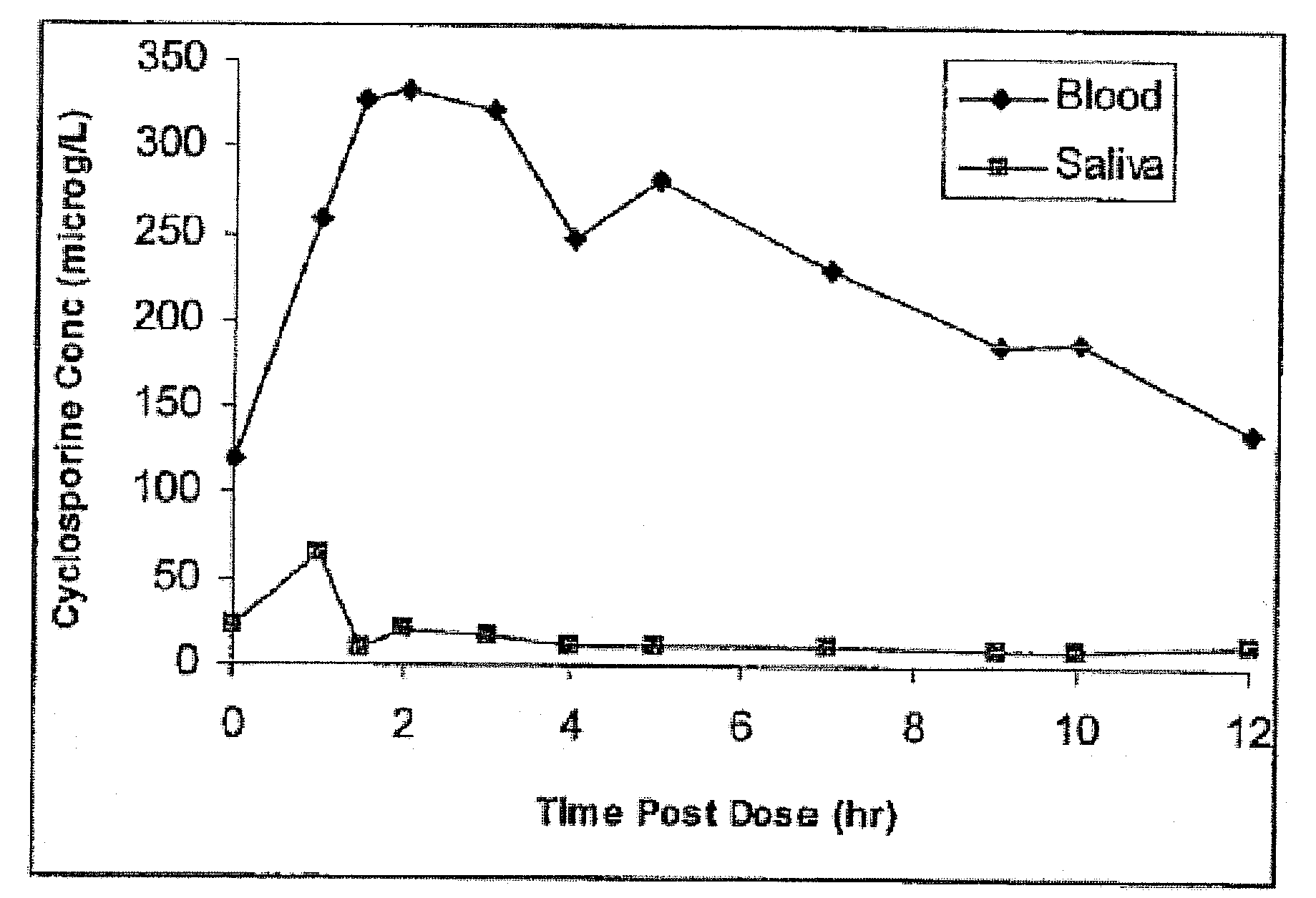
Monitoring cyclosporine in saliva
InactiveUS20080255765A1Promote recoveryImprove consistencyPreparing sample for investigationCyclic peptide ingredientsVenous accessBound drug
Saliva offers an alternative specimen for the therapeutic monitoring of cyclosporine (CsA) in children and patients with difficult venous access. For a highly protein-bound drug such as CsA, saliva provides a practical approach for measuring the unbound concentration. Liquid chromatography-tandem mass spectrometry (LC-MS / MS) is ideally suited for the measurement of drugs in saliva. A solid-phase extraction technique, analytic liquid chromatography over an Aqua Perfect column, maintained at 65° C., and electrospray tandem mass spectrometry were used to quantify CsA in saliva. The method used cyclosporine C (CsC) as the internal standard. Mobile phase comprised of a 97:3 voL mixture of methanol and 30 mmol / L ammonium acetate at a flow rate of 0.5 mL / min. Chromatograms using mass transitions of m / z 1219.9→m / z 1202.9 for CsA and m / z 1235.9→m / z 1218.9 for CsC were obtained. The calibration curve was linear from 1 to 300 μg / L with correlation coefficient values ranging from 0.9732 to 0.9968). The lower limit of quantification was 1 μg / L and limit of detection was 0.6 μg / L with an average extraction recovery of 84.7±2.6% for CsA and 93.7±4.4% for CsC from the saliva matrix. The accuracy of the method ranged from 92% to 104.7%, and the intra- and interim coefficients of variation were 6.9-12.2% and 8.3-12.1%, respectively. The correlation coefficient value between the CsA concentration measurements in 15 paired blood-saliva samples from kidney transplant recipients was 0.695 (P=0.006). The noninvasive and simple method of saliva collection coupled with the LC-MS / MS quantification technique for CsA analysis would generate novel data that could benefit patients undergoing CsA therapy.
Owner:BOARD OF GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND & PROVIDENCE PLANTATIONS
Method to improve pharmacokinetics of drugs
A compound comprises a pharmacologically active agent coupled to a plasma protein binding agent. The pharmacologically active agent, in some embodiments, may be an OLAM inhibitor. The plasma protein binding agent, in some embodiments, is a compound that is pharmacologically active in inflamed / injured tissue. A pharmaceutical composition that includes these compounds may be used to treat pain, shock, inflammatory conditions, or combinations thereof in a subject comprising administering to a subject who would benefit from such treatment a therapeutically effective amount of the pharmaceutical composition.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Traditional Chinese and western medicine combined medicine for treating atrophic gastritis and preparation method
InactiveCN107913402AUnique curative effectSignificant effectOrganic active ingredientsPeptide/protein ingredientsBound drugAdditive ingredient
The invention discloses an integrated traditional Chinese and western medicine medicine for treating atrophic gastritis and a preparation method thereof, belonging to the technical field of medicine; the raw materials of the active ingredients of the medicine integrated traditional Chinese and western medicine are composed of: pepsin, metoclopramide, rotundine, Ulmus chinensis, Kapok root, Osmanthus fragrans, Yellow bud cabbage, Shannai, White leaves, Bergamot, Longzi, Bailiang rice, Podocarpus pine fruit, Hanberry root, Orange peel and Mountain pepper root; Chinese and Western medicine of the present invention The combined medicine has a unique curative effect on the treatment of atrophic gastritis, and the preparation process is simple, the curative effect is remarkable, the absorption effect is good, it is convenient to take, safe and reliable, it is not easy to relapse after being cured, the cost is low, and it can achieve the purpose of treating both symptoms and root causes. Yin benefits the stomach, warms the middle and invigorates the spleen, relieves inflammation and relieves pain, regulates qi and activates blood circulation.
Owner:张吉新
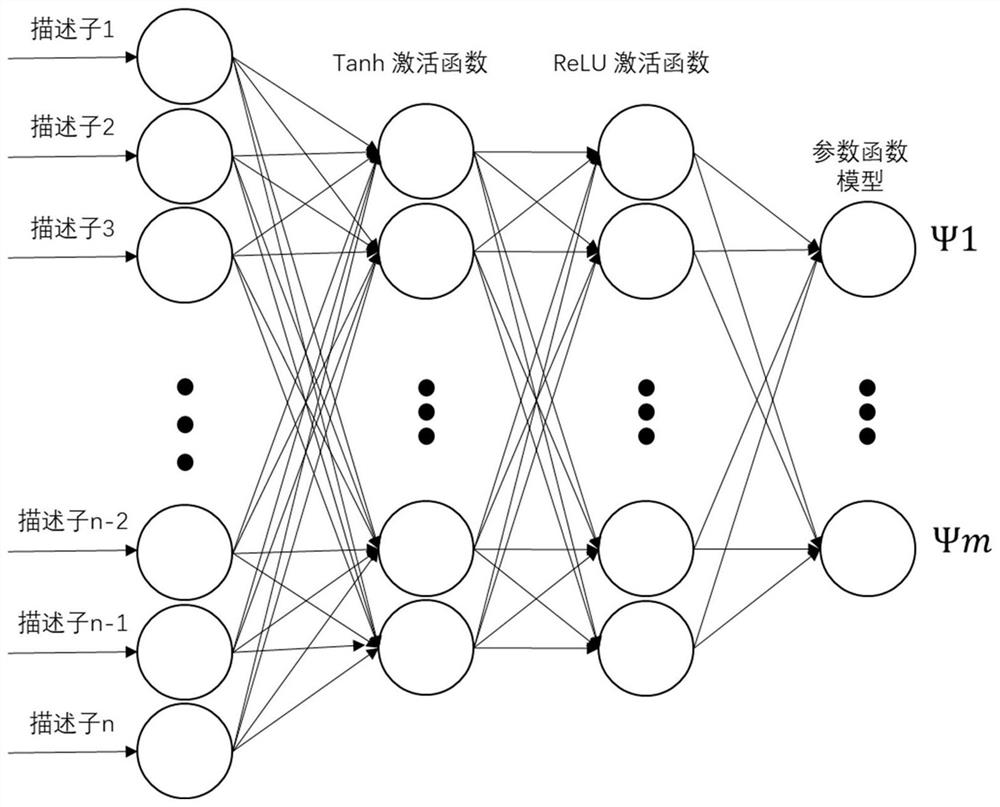
Logistic regression-based pharmacokinetic parameter prediction method for drug compound
PendingCN111833971AAccurately Predict Pharmacokinetic LipophilicityAccurately Predict SolubilityMolecular designBound drugData set
The invention discloses a logistic regression-based pharmacokinetic parameter prediction method for a drug compound in the technical field of drug research and development, and the method comprises the following steps: 1, file input: inputting a molecular three-dimensional structure file of the drug compound; 2, calculation of a descriptor: calculating the descriptor of a molecule through CDK; 3,a training process: dividing text data and label data contained in the data set into a training set and a verification set; training a model by using the training set data, and outputting a parameterprediction model; adopting an Adam optimizer to optimize the model, and optimizing the model through 20-stage training. According to the method, a neural multi-task logistic regression algorithm is utilized, and rules can be automatically designed under the condition of little or no manual intervention. Through the molecular structure of the drug compound, the pharmacokinetic lipophilicity, solubility, plasma protein binding rate, transdermal property and other parameter values of the drug compound are accurately predicted.
Owner:上海云贵信息科技有限公司
Preparation method of hydrogel for targeted treatment of cancer cells
InactiveCN110812322AEasy to makeMeet many requirements in biomedicineOrganic active ingredientsAerosol deliveryBound drugCancer cell
The invention belongs to the technical field of hydrogel preparation, and specifically discloses a preparation method of a hydrogel for targeted treatment of cancer cells. The method comprises the following steps: mixing acrylic acid and a Fe3O4 magnetic nanoparticle in mixing equipment according to a molar ratio of 1:1, then carrying out a reaction in a reactor at 35-42 DEG C for 10-15 min for combination of acrylic acid with the Fe3O4 magnetic nanoparticle to obtain a composite hydrogel, and finally wrapping the anticancer drug Adriamycin in the composite hydrogel obtained in step S2. The magnetic nanoparticle is used for a drug carrier and disease diagnosis and treatment, acrylic acid and the Fe3O4 magnetic nanoparticle are combined to form the composite hydrogel, and the anticancer drug Adriamycin is wrapped in the composite hydrogel. The hydrogel can be quickly released to achieve the role of targeted treatment of cancer cells. The nano-Fe3O4 magnetic material as a targeted drug carrier can bind drug molecules on the one hand and protect the drug molecules on the other hand.
Owner:KUNSHAN BYE MACROMOLECULE MATERIAL CO LTD
Method for detecting plasma protein binding rate of nine effect components in Sheepear Inula Herb extract
InactiveCN108508102AGood linear relationshipMeet the determination requirementsComponent separationBound drugBlood plasma
The invention discloses a method for detecting the plasma protein binding rate of nine effect components in Sheepear Inula Herb extract. The method comprises the following steps: preparing a SheepearInula Herb extract sample medicine solution; preparing a standard control solution by using the nine effect components, and preparing a buffer solution; and establishing a method for simultaneously detecting the respective content of the Sheepear Inula Herb extract nine effect components in human plasma, rat plasma and the buffer solution by ultrahigh performance liquid chromatography-mass spectrometry (UPLC-MS / MS) analysis technology; and detecting the protein binding rates of the Sheepear Inula Herb extract nine effect components in the human plasma and rat plasma by an equilibrium dialysistechnology to establish the method for detecting the protein binding rate of Sheepear Inula Herb extract nine effect components in the human plasma and rat plasma. The method for detecting the plasmaprotein binding rate of nine effect components in Sheepear Inula Herb extract provides a demonstrative research for the detection of the plasma protein binding rate of traditional Chinese medicinal multiple effect components in different species animals, and is of great significance to study the druggability of traditional Chinese medicinal active components.
Owner:GUIZHOU MEDICAL UNIV
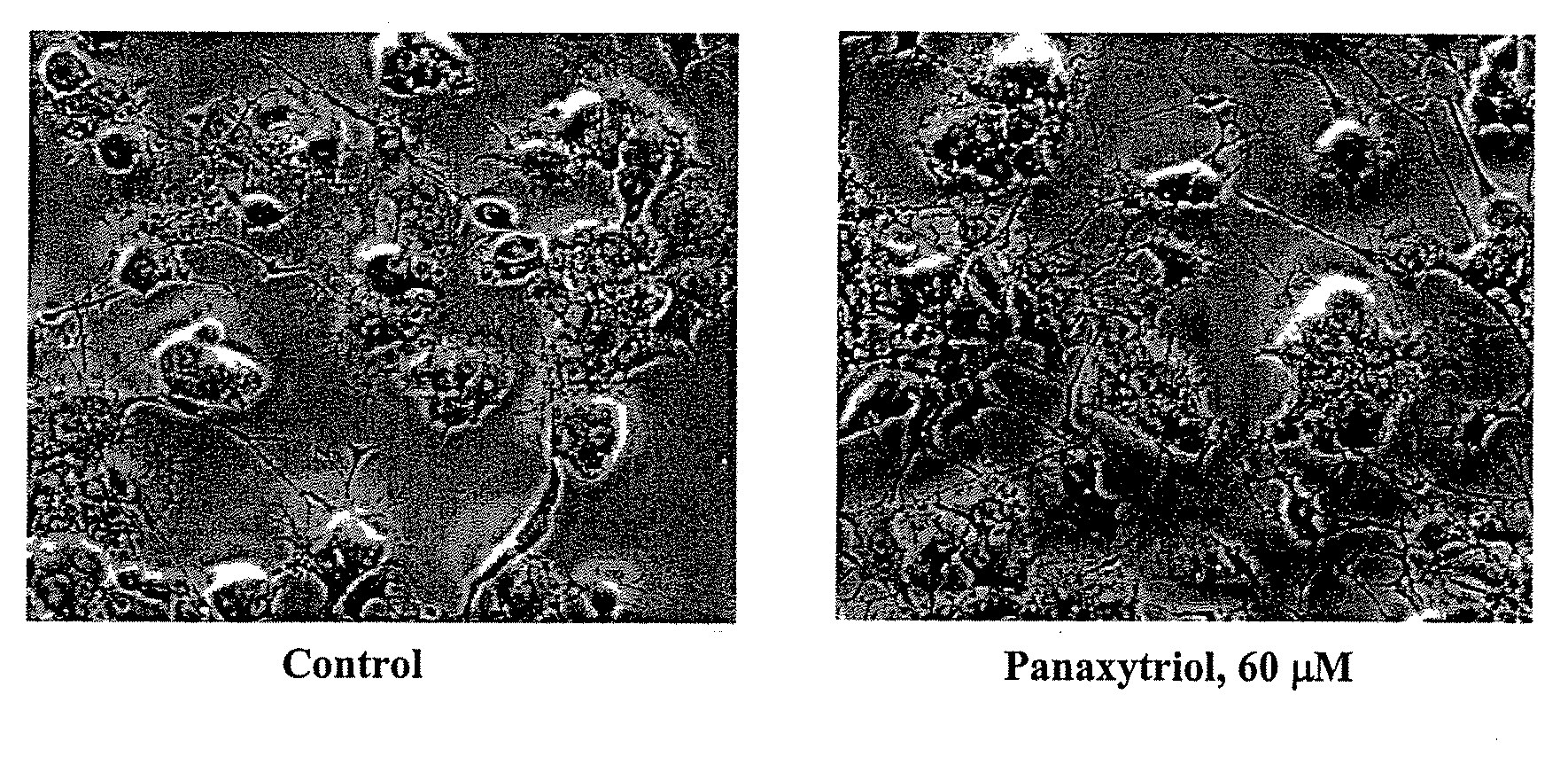
Compositions and methods for treating cancer or a neurotrophic disorder
The present invention relates to compositions comprising an effective amount of a Panaxytriol Compound and a tubulin-binding drug, methods for treating or preventing cancer or a neurotrophic disorder comprising administering to a subject in need thereof an effective amount of a Panaxytriol Compound and a tubulin-binding drug, and methods for making a Panaxytriol Compound.
Owner:DANISHEFSKY SAMUEL J +4
Vesicle monoamine transporter target bound drug and preparation method thereof
InactiveCN101537193BImprove targetingOrganic chemistryRadioactive preparation carriersBound drugDiabetes mellitus
The invention relates to a vesicle monoamine transporter target bound drug and a preparation method thereof; wherein the structure of a contained developing agent is shown as right; wherein R1=H, -CH3, -CH2CH3; R2=-(CH2)n-F, n=1-4, -(CH2CH2O)nCH2CH2-F, n=1-3. The invention adopts TBZ as lead compound, replaces 2-carbonyl group of TBZ with amino group and designs to synthetize a series of new TBZ-NH2 derivatives containing F; the series of TBZ-NH2 derivatives not only has higher targeting property and can be combined the specificity of VMAT2, but also can be used for PET development research when using F-18 to replace F-19, and is a developing agent which is used for PD diagnosis and diabetes diagnosis and has a good application prospect.
Owner:BEIJING NORMAL UNIVERSITY
Prodrugs cleavable by CD26
The present invention provides a new prodrug technology and new prodrugs in order to increase the solubility, to modulate plasma protein binding or to enhance the biovailability of a drug. In the present invention the prodrugs are conjugates of a therapeutic compound and a peptide (eg tetrapeptide or hexapeptide) wherein the conjugate is cleavable by dipeptidyl-peptidases, more preferably by CD26, also known as DPPIV (dipeptidyl aminodipeptidase IV). The present invention furthermore provides a method of producing the prodrugs, to enhance brain and lymphatic delivery of drugs and / or to extend drug half-lives in plasma.
Owner:K U LEUVEN RES & DEV +1
A kind of preparation method of oral amoxicillin pH-responsive nanocarrier and product thereof
ActiveCN108553424BEasy to shapeReduce releaseAntibacterial agentsPowder deliveryBound drugNanocarriers
The invention discloses a preparation method of oral amoxicillin pH-responsive nano-carrier and its products, which include mixing: mixing amoxicillin, polyglutamic acid and water; dropping: after mixing, amoxicillin 1. Add polyglutamic acid aqueous solution dropwise to chitosan aqueous solution; dialysis: after adding dropwise, place it in a dialysis bag for dialysis. The nanoparticles prepared by the present invention are negatively charged, and can bind drugs through surface adsorption, internal hydrogen bonding and electrostatic adsorption, and overcome the problems of complex operation and difficult release of traditional chemical drug loading, and achieve drug delivery through electrostatic complexation of drugs. The pH-responsive release realizes the ideal state of low drug release in the stomach and high intestinal release. The invention realizes that the nanoparticle loaded with amoxicillin has a low release amount under gastric acid conditions and complete release under intestinal physiological conditions, reduces the stimulation of the drug to the stomach, increases the sustained release time of the drug in the intestinal tract, and improves the drug release rate. utilization rate.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com