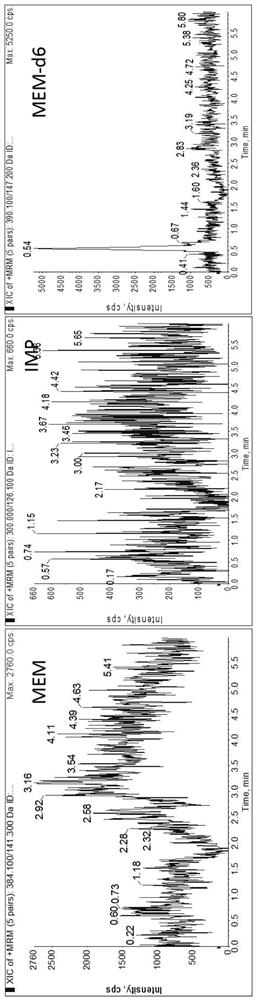
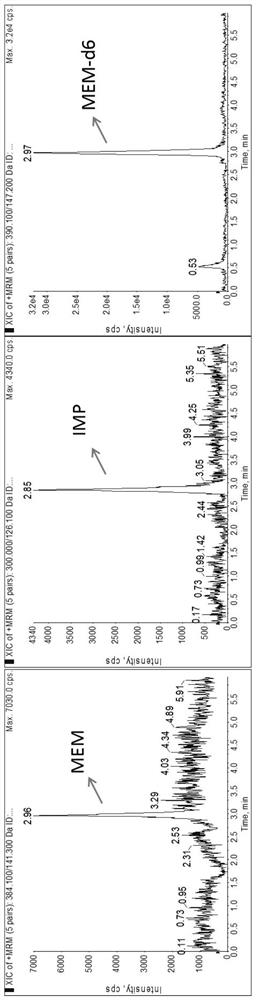
Method for detecting plasma protein binding rate of meropenem or imipenem by combining liquid chromatography-mass spectrometry technology with ultrafiltration technology
A technology of imipenem and meropenem, which can be used in measurement devices, instruments, scientific instruments, etc., can solve problems such as drug instability and inaccurate measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention. Unless otherwise specified, the technical means used in the examples are conventional means well known to those skilled in the art, and the raw materials used are all commercially available products. Example 1 Determination of plasma protein binding rate of meropenem or imipenem by liquid chromatography-mass spectrometry combined with ultrafiltration
[0045] 1 material
[0046] 1.1 Instrument
[0047] High performance liquid chromatography (DGU LC-30AD, Shimadzu Corporation); quadrupole / linear ion trap mass spectrometer (ABSciex 4000 + Mass spectrometry detector, American AB SCIEX company), equipped with electrospray ionization source (ESI); MS 3 digital vortex mixer (Germany IKA company); BT25S electronic balance (Germany Sartorius company); 3-18K centrifuge (US Sigma company); Cascada-I pure water machine (USA Pall company), HWT-10...
Embodiment 2
[0107] The optimization of embodiment 2 centrifugal force and centrifugal time
[0108] Add 200 μL and 400 μL plasma samples to the ultrafiltration tubes respectively, and centrifuge at 20°C for 20 minutes under the centrifugal force of 6000g, 8000g and 10000g for each volume of double samples (number 200-1, 200-2 and 400-1, 400-2 respectively). Or 30min, investigate the volume that obtains ultrafiltrate.The results are shown in Table 10:
[0109] The volume of ultrafiltrate under different centrifugal conditions of table 10
[0110]
[0111]
[0112] According to the standards that the ultrafiltration membrane is not damaged and the ratio of the ultrafiltrate to the total volume of plasma before ultrafiltration is 0.3-0.6, preferably 8000g is used as the centrifugal force, and the ultrafiltration time is in the range of 20-30min.
Embodiment 3
[0113] Embodiment 3 chromatographic column and the selection of mobile phase ratio
[0114] In this embodiment, the selection of the chromatographic column and the proportion of the mobile phase is carried out with the peak time and peak shape as the selection criteria.
[0115] Mobile phase: A: Acetonitrile (containing 0.1% formic acid); B: 5mM ammonium formate solution (containing 0.1% formic acid, 5% acetonitrile)
[0116] ①Symmetry C18 column (2.1×50mm, 3.5μm)
[0117] Mobile phase ratio: 90%B
[0118] Peak time: IMP: 0.38min; MER: 0.48min. IMP and MER are not retained on this column ( Figure 4 ).
[0119] ②Atlantis T3 column (2.1×50mm, 3μm)
[0120] Mobile phase ratio: 95%B
[0121] Peak time: IMP: 0.61min; MER: 2.42min. IMP is not retained on this column ( Figure 5 ).
[0122] ③HILIC Silica column (2.1mm×50mm, 3μm)
[0123] Mobile phase ratio: 15% B
[0124] Peak time: IMP: 3.68min; MER: 4.64min. Poor peak shape and poor sensitivity ( Figure 6 ).
[0125...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com