A method for catalytically converting cyano groups into deuterated methyl groups, prepared aromatic deuterated methyl compounds and applications thereof
A catalytic conversion and compound technology, applied in the direction of hydrocarbons, hydrocarbons, organic chemical methods, etc., can solve the problems that cannot be applied to the synthesis of deuterated methyl groups, high temperature, etc., to achieve large-scale production and maintain basic Unchanged, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
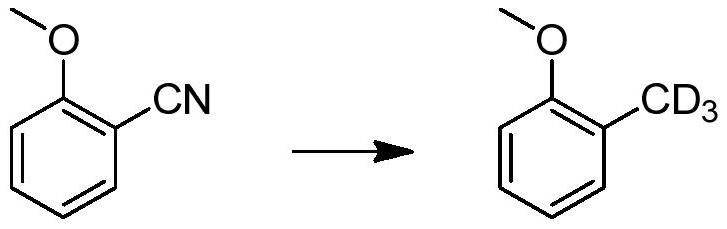
[0030] In this example, an aromatic deuterated methyl compound is prepared by the following preparation method, and the reaction formula is as follows:
[0031]
[0032] 2-Methoxybenzonitrile (1g) and deuterium-containing water palladium hydroxide on carbon (0.1g) were mixed in deuterated methanol (10mL), under 0.1MPa deuterium gas pressure, stirred at room temperature for 18 hours, after the reaction, 2-deuterated methyl anisole was obtained by filtration with a yield of 90% and a deuterated rate of 98%.
[0033] 1 H NMR (400MHz, d 6 -DMSO), δ7.15 (m, 2H), 6.92 (d, 1H), 6.84 (m, 1H), 3.77 (s, 3H); GC-MS: MS 125.
Embodiment 2
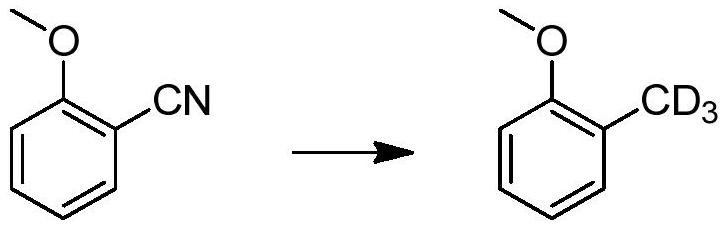
[0035] In this example, an aromatic deuterated methyl compound is prepared by the following preparation method, and the reaction formula is as follows:
[0036]
[0037] 2-Methoxybenzonitrile (1g) and anhydrous palladium carbon (0.1g) were mixed in deuterated methanol (10mL), under 0.1MPa deuterium gas pressure, stirred at room temperature for 48 hours, after the reaction was completed, filtered to obtain 2 - deuterated methyl anisole, yield 92%, deuterium rate 98.3%.
Embodiment 3
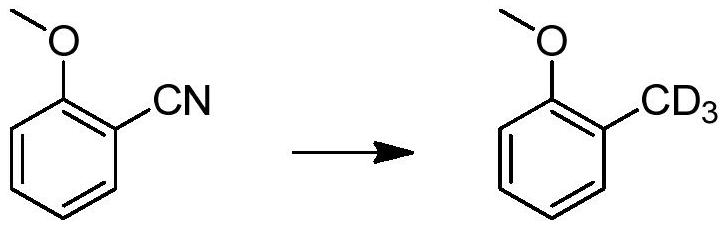
[0039] In this example, an aromatic deuterated methyl compound is prepared by the following preparation method, and the reaction formula is as follows:
[0040]
[0041] Mix 2-methoxybenzonitrile (1g) and anhydrous palladium carbon (0.1g) in deuterated methanol (10mL), under 0.1MPa deuterium gas pressure, stir at 60 degrees for 24 hours, after the reaction is completed, filter to obtain 2-Deuterated methyl anisole, the yield is 90%, and the deuterated rate is 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com