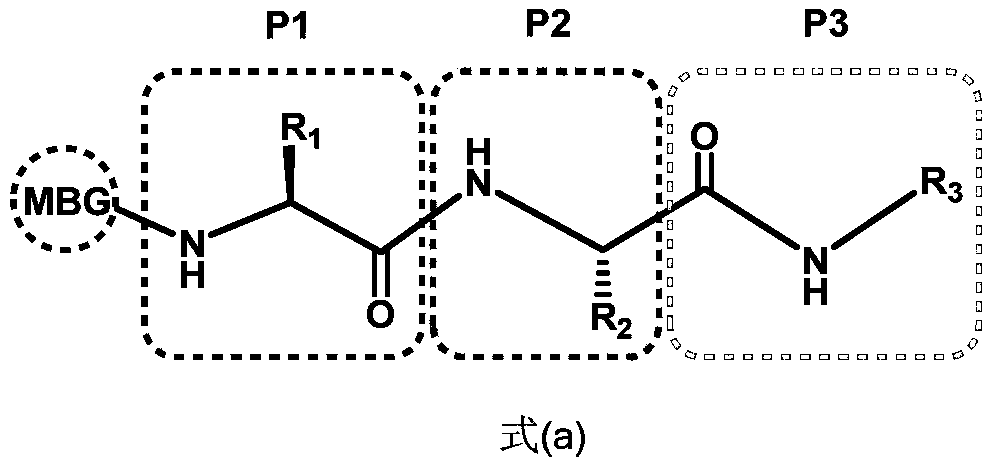
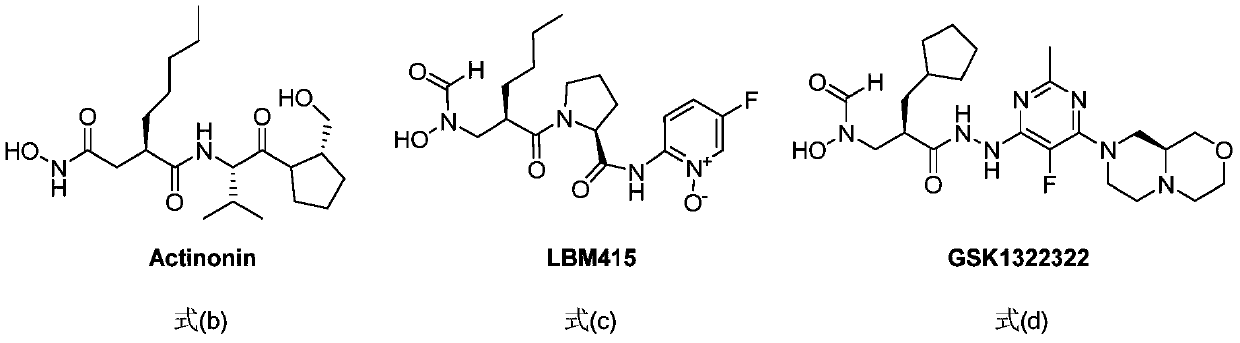
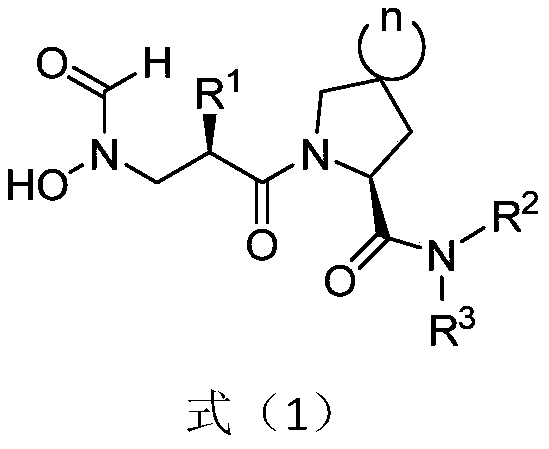
Spiro three-membered ring, spiro five-membered ring peptidyl deformylase inhibitor
A peptide deformylase and inhibitor technology, applied in the field of spiro five-membered ring peptide deformylase inhibitors and new spiro three-membered rings, can solve the problems of metabolic instability, body toxicity, poor activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] (S)-5-((R)-2-((N-hydroxycarboxamido)methyl)caproylamido)-N-(1H-pyrazol-3-yl)-5-azaspiro[2.4 ]Synthesis of heptane-6-amide
[0123]
[0124] Step 1: Add 3-aminopyrazole (1.50g, 18.0mmol), trimethylamine (4.5g, 20.6mmol), 4-(dimethylamino)pyridine (0.15g, 1.2mmol) in 60mL dioxane, Add Boc after stirring to dissolve 2 O, heated to reflux for 8h, the reaction was completed, the solvent was spun out, then diluted with EA and extracted, washed with 10% citric acid and saturated brine successively, the organic phase was concentrated to obtain an oil, and the column (PE / DCM=2 / 1) The product was obtained as a white solid (1.6 g, yield 48%).
[0125]Step 2: the operation is as in step 1 in the synthetic general formula (X1). Add 20mL DMF to the acid (2.05g, 8.5mmol) to dissolve, add N-methylimidazole (1.54g, 18.7mmol) under ice cooling, then slowly add MsCl (1.07g, 9.4mmol), stir for 15min and then add Boc Protected amine (1.56g, 8.5mmol), the reaction was detected by TLC...
Embodiment 2
[0136] 5-Fluoro-2-((S)-5-((R)-2-((N-hydroxycarboxamido)methyl)hexylcarbonyl)-5-azaspiro[2.4]heptane-6-amide base) synthesis of pyridine N-oxygen compounds
[0137]
[0138] Step 1: The operation is as in Step 1 in the synthesis of general formula (X1).
[0139] Step 2: The operation is as in step 2 in the synthesis of general formula (X1) (2.1 g, white solid, two-step yield 68%).
[0140] Step 3: The operation is as in step 3 in the synthesis of general formula (X2).
[0141] Step 4: The obtained oil (242mg, 0.46mmol) was dissolved in ethyl acetate (3mL), hydrogen peroxide-urea complex (133mg, 1.40mmol) and phthalic anhydride (207mg, 1.40mmol) were added. The mixture was stirred at room temperature for 2 hours. After the reaction was completed, the reaction was quenched with sodium thiosulfate and extracted with ethyl acetate. Drying and concentration of the organic phase gave crude product.
[0142] Step 5: The operation is as in step 4 in the synthesis of general for...
Embodiment 3
[0148] (S)-N-(5-(tert-butyl)isoxazol-3-yl)-5-((R)-2-((N-hydroxycarboxamido)methyl)hexylcarbonyl)-5- Synthesis of Azaspiro[2.4]heptane-6-amide
[0149]
[0150] Step 1: The operation is as in Step 1 in the synthesis of general formula (X1).
[0151] Step 2: The operation is as in step 2 in the synthesis of general formula (X1) (1.4 g, white solid, two-step yield 47%).
[0152] 1 H NMR (400MHz,D 2 O)δ6.14(s,1H),4.44-4.41(m,1H),3.12-2.93(m,2H),2.19(dd,J=13.4,8.9Hz,1H),1.86(dd,J=13.4 ,6.1Hz,1H),0.52-0.29(m,4H).
[0153] 13 C NMR (101MHz,D 2 O) δ183.22, 167.90, 157.06, 93.24, 60.23, 52.67, 37.13, 32.51, 27.67, 20.09, 9.86, 8.49.
[0154] Step 3: The operation is as in step 3 in the synthesis of general formula (X2).
[0155] Step 4: The operation is as in step 4 in the synthesis of general formula (X2), column chromatography (DCM:MeOH=10:1) gives a white solid, and the two-step yield is 28%.
[0156] LC-MS(ESI):[M+1] + =435.24,t R =2.25min.
[0157] 1 H NMR (400MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com