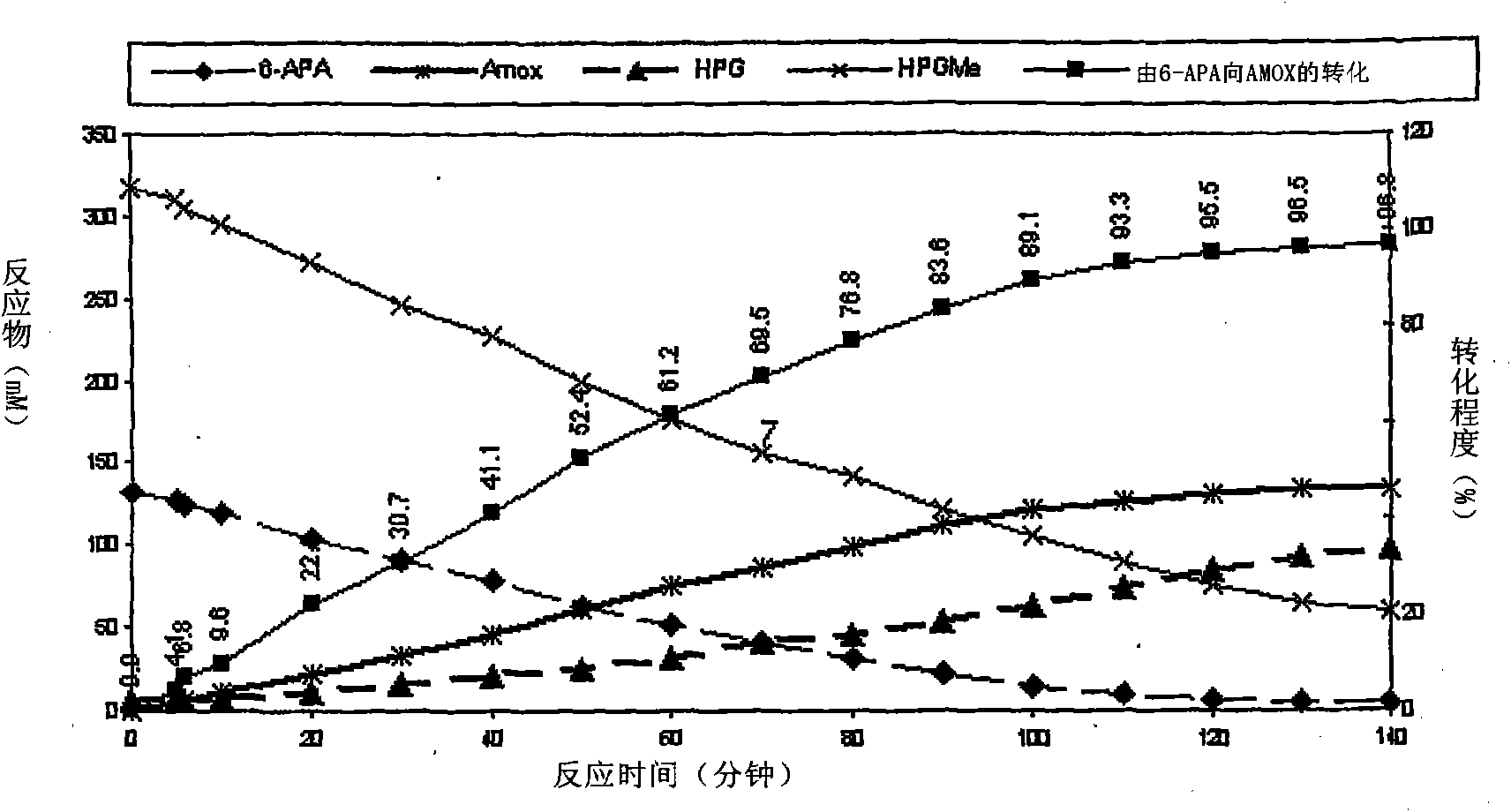
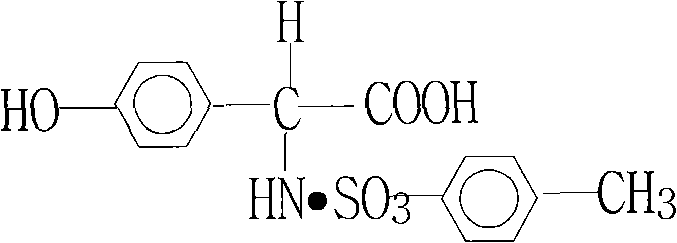
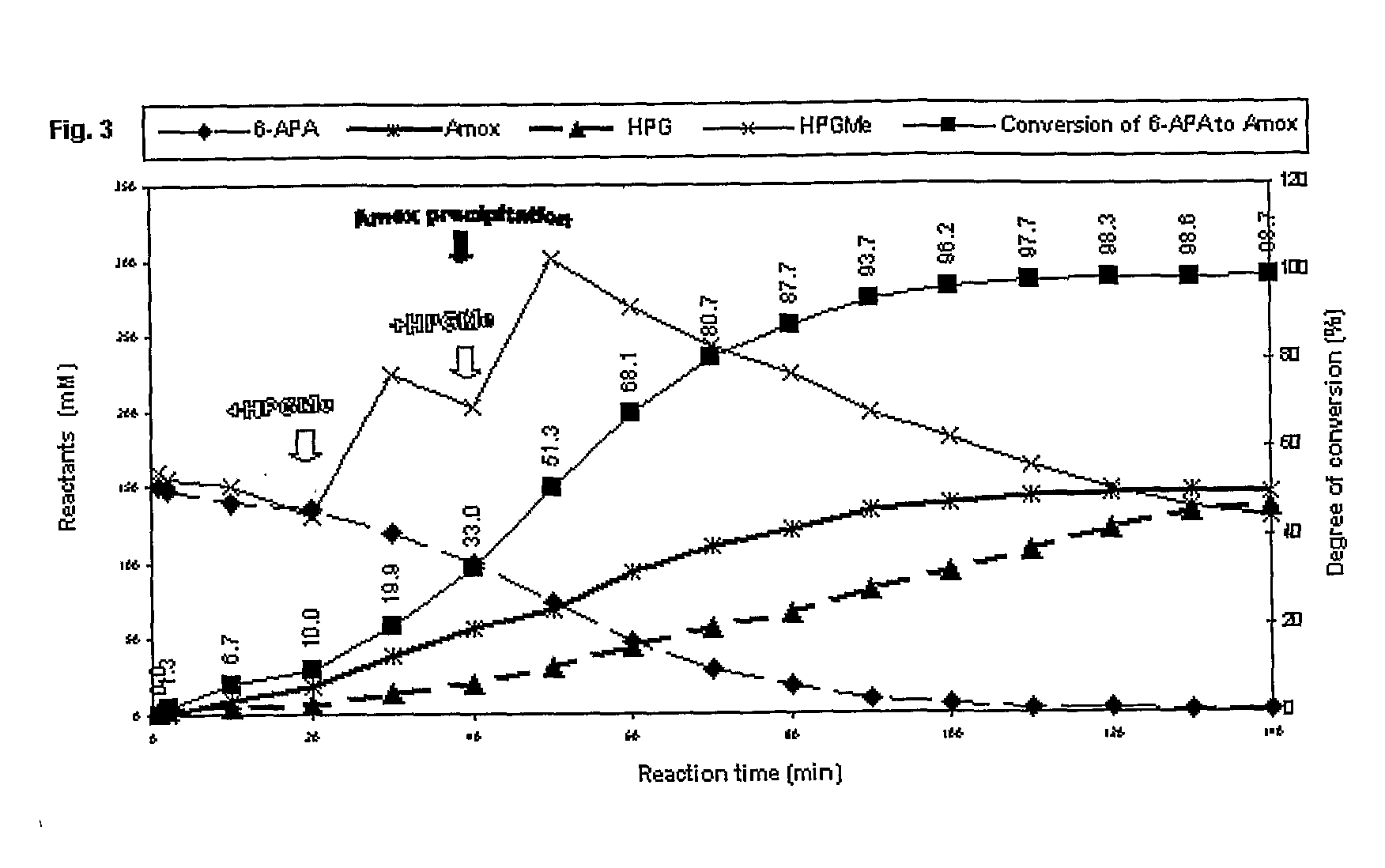
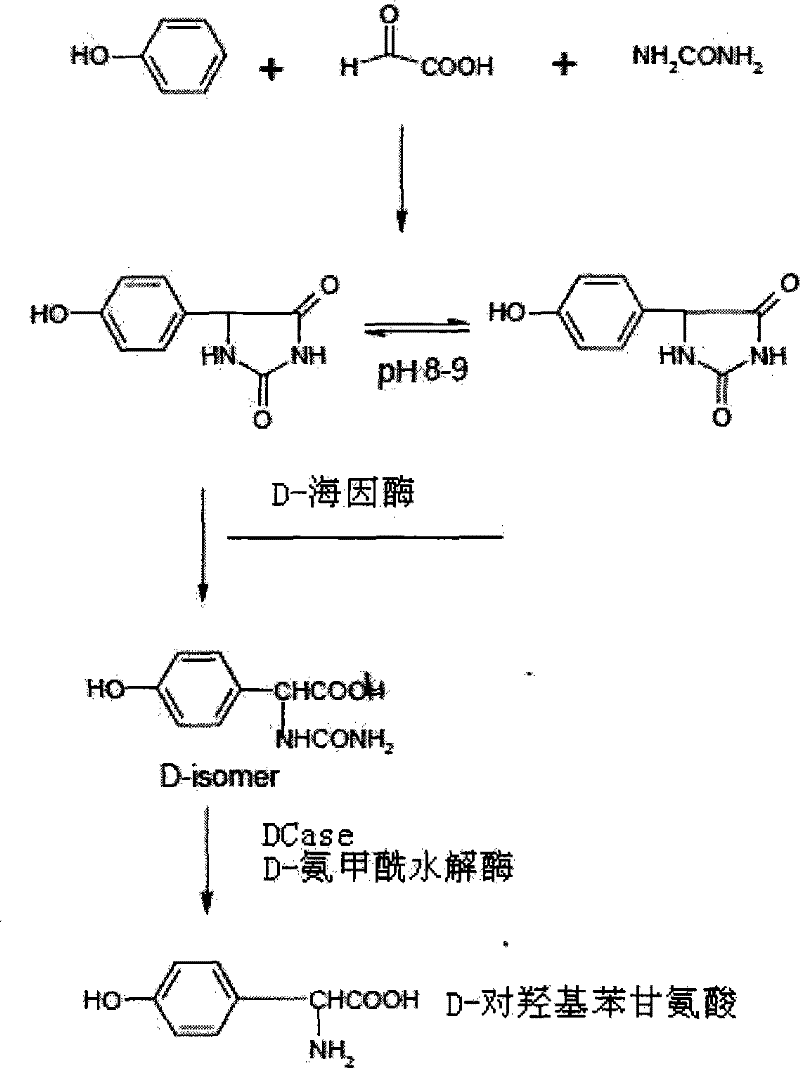
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "D-p-hydroxyphenylglycine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Penicillin G acylase immobilized with a crosslinked mixture of gelled gelatin and amino polymer
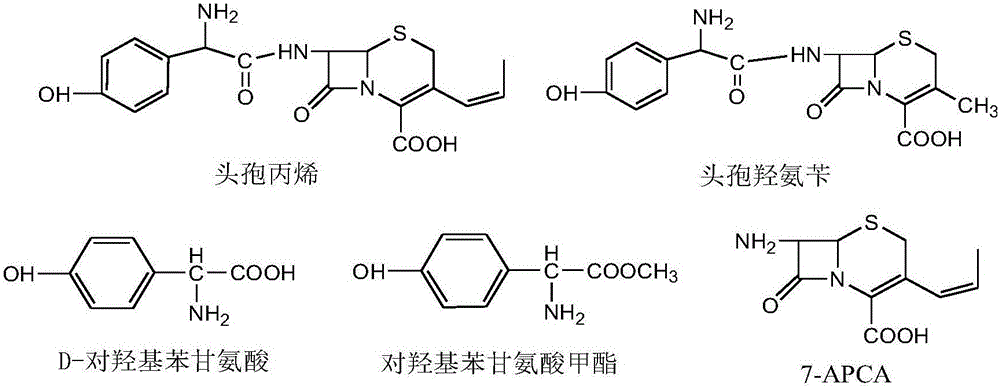
PCT No. PCT / EP96 / 03253 Sec. 371 Date Jan. 15, 1998 Sec. 102(e) Date Jan. 15, 1998 PCT Filed Jul. 16, 1996 PCT Pub. No. WO97 / 04086 PCT Pub. Date Feb. 6, 1997Penicillin G acylase is immobilized by covalent bonding to a crosslinked mixture of a gelled gelling agent such as gelatin and a polymer containing free amino groups such as alginate amine, chitosan or polyethylene imine. The immobilized penicillin G acylase provides a higher synthesis / hydrolysis ratio as compared to immobilizing with other carriers when producing beta -lactam derivatives by a condensing reaction of an amino beta -lactam with an acylating agent. The acylating agent may be a derivative of D-phenylglycine, a derivative of D-p-hydroxyphenylglycine or a derivative of D-2,5-dihydro-phenylglycine. Examples of beta -lactam derivatives that can be produced are amoxycillin, ampicillin, cephaclor, cephadroxil, cephprozil, cephalexin and cephradine.
Owner:GIST BROCADES NV
Improved method for preparing amoxicillin by enzymic method
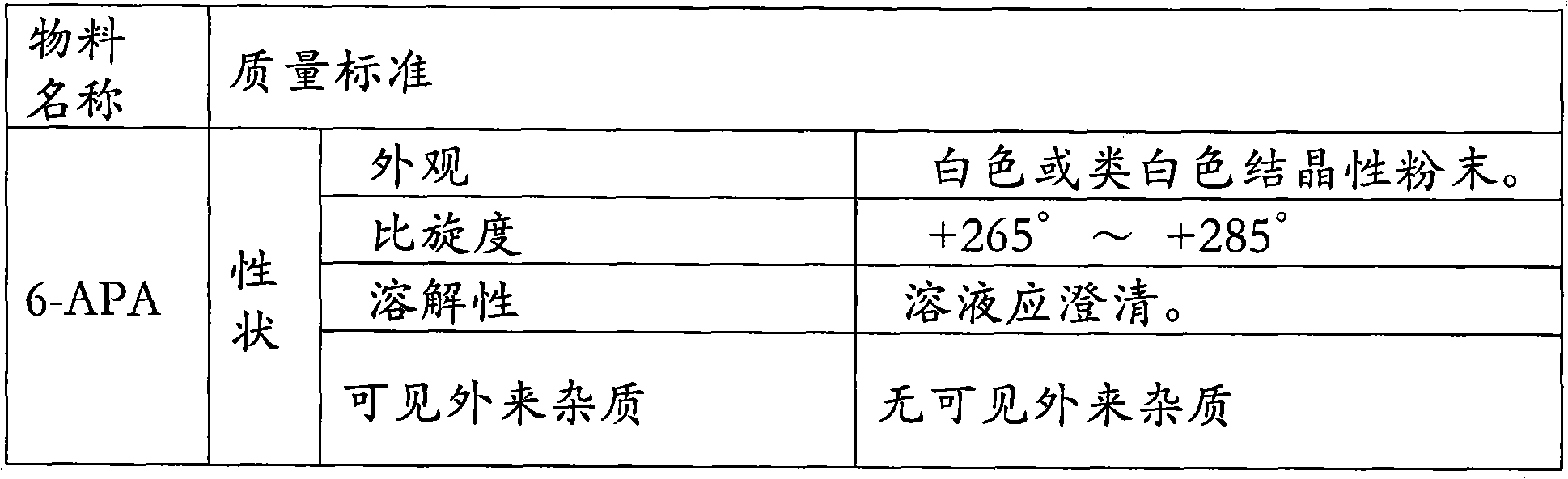
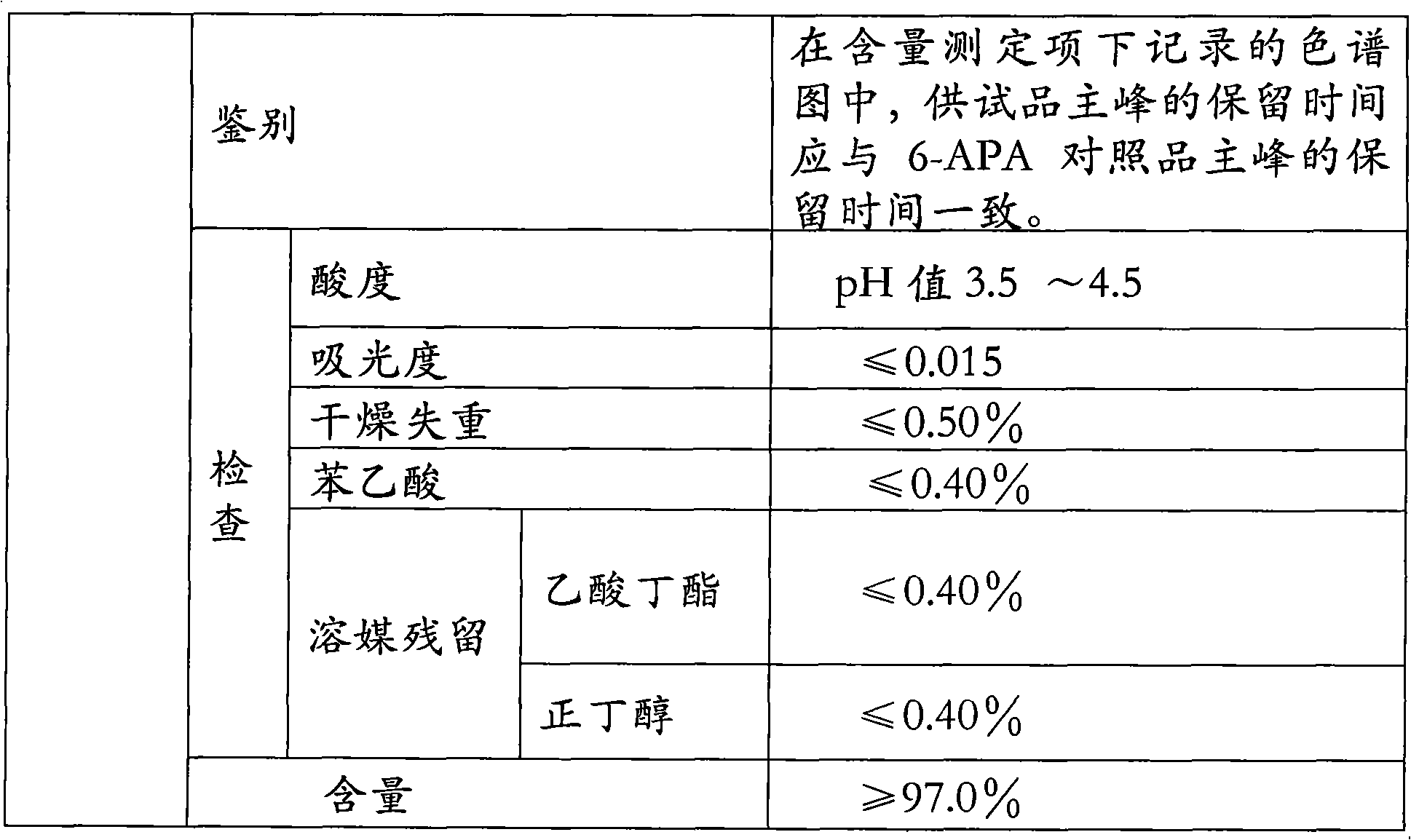
The invention relates to the field of pharmacy, and provides an improved method for preparing amoxicillin by an enzymic method, and a product obtained by the improved method for preparing amoxicillin by the enzymic method. The method comprises the following steps of: 1) dissolving 6-aminopenicillanic acid (6-APA) at the temperature of between 10 and 20 DEG C by using water or / and aqueous solution of ammonia which has the pH value of 7.0 to 8.0, and adding D-p-Hydroxyphenylglycine methyl ester hydrochlorid and penicillin G acyltransferase; 2) adjusting the pH value of a solution obtained in the step 1) to be 6.0 to 6.5, and reacting at the temperature of between 21 and 30 DEG C until the content of 6-APA is less than 5mg / ml to obtain a solution of an amoxicillin product; and 3) separating the penicillin G acyltransferase from the solution of the amoxicillin product, adjusting by using hydrochloric acid until the solution of the amoxicillin product is clarified, adding the aqueous solution of ammonia, adjusting the pH value to be 5.5 to 6.5, and crystallizing at the temperature of between 0 and 5 DEG C to obtain amoxicillin. By the improved method for preparing amoxicillin by the enzymic method, the quality of the amoxicillin product is greatly improved, and the medication safety of the amoxicillin product is further improved.
Owner:UNITED LAB INNER MONGOLIA CO LTD
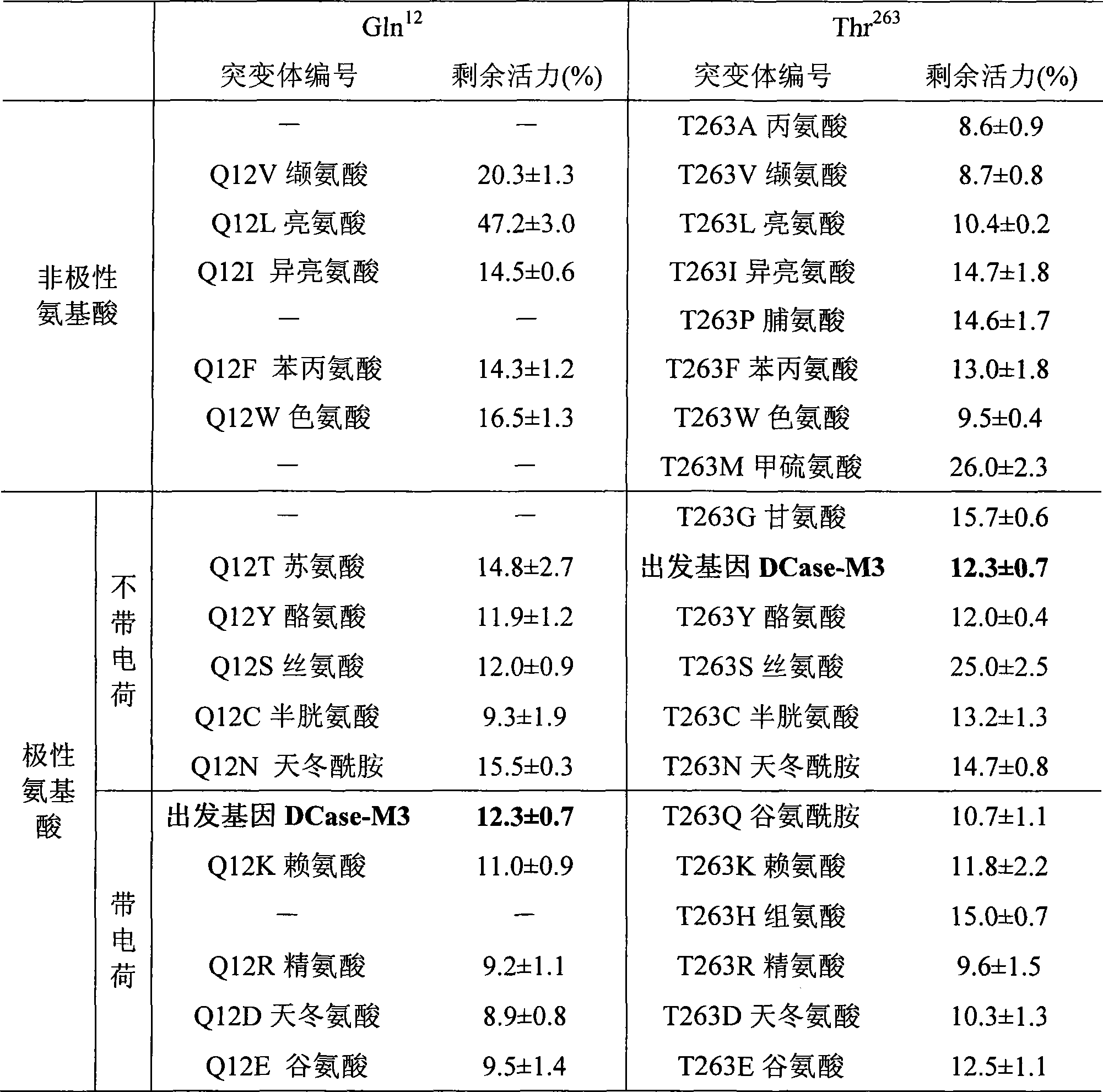
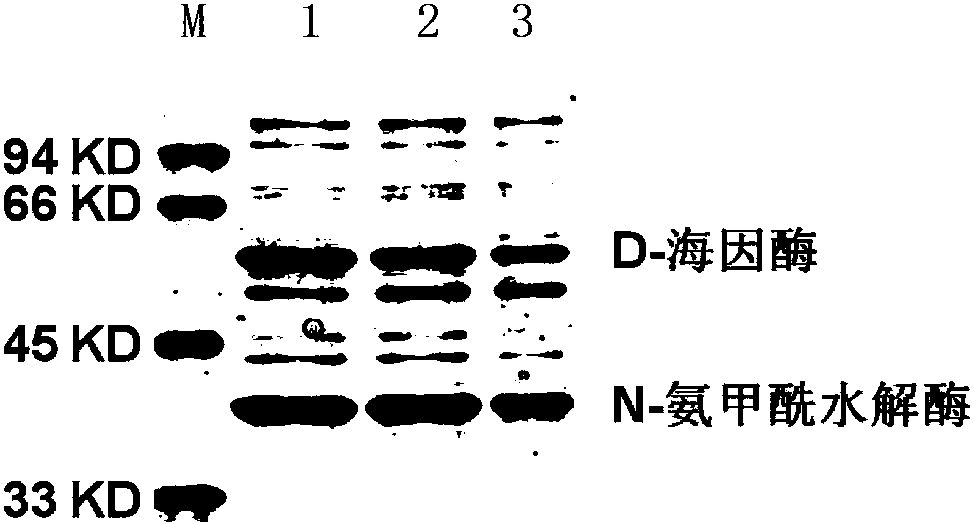
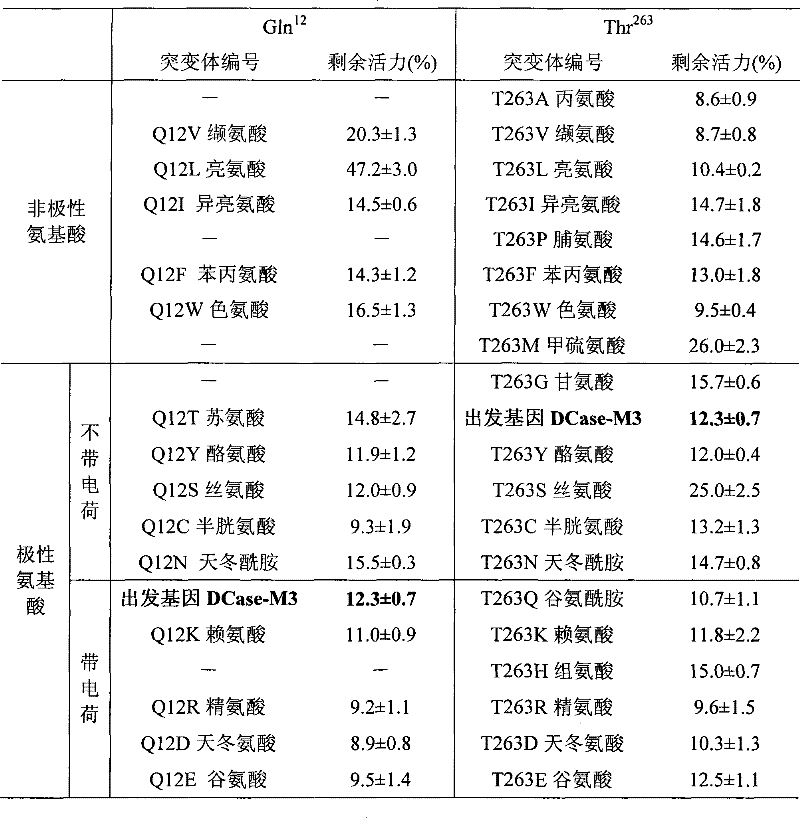
Mutant of D-carbamyl hydrolysis enzyme and application thereof
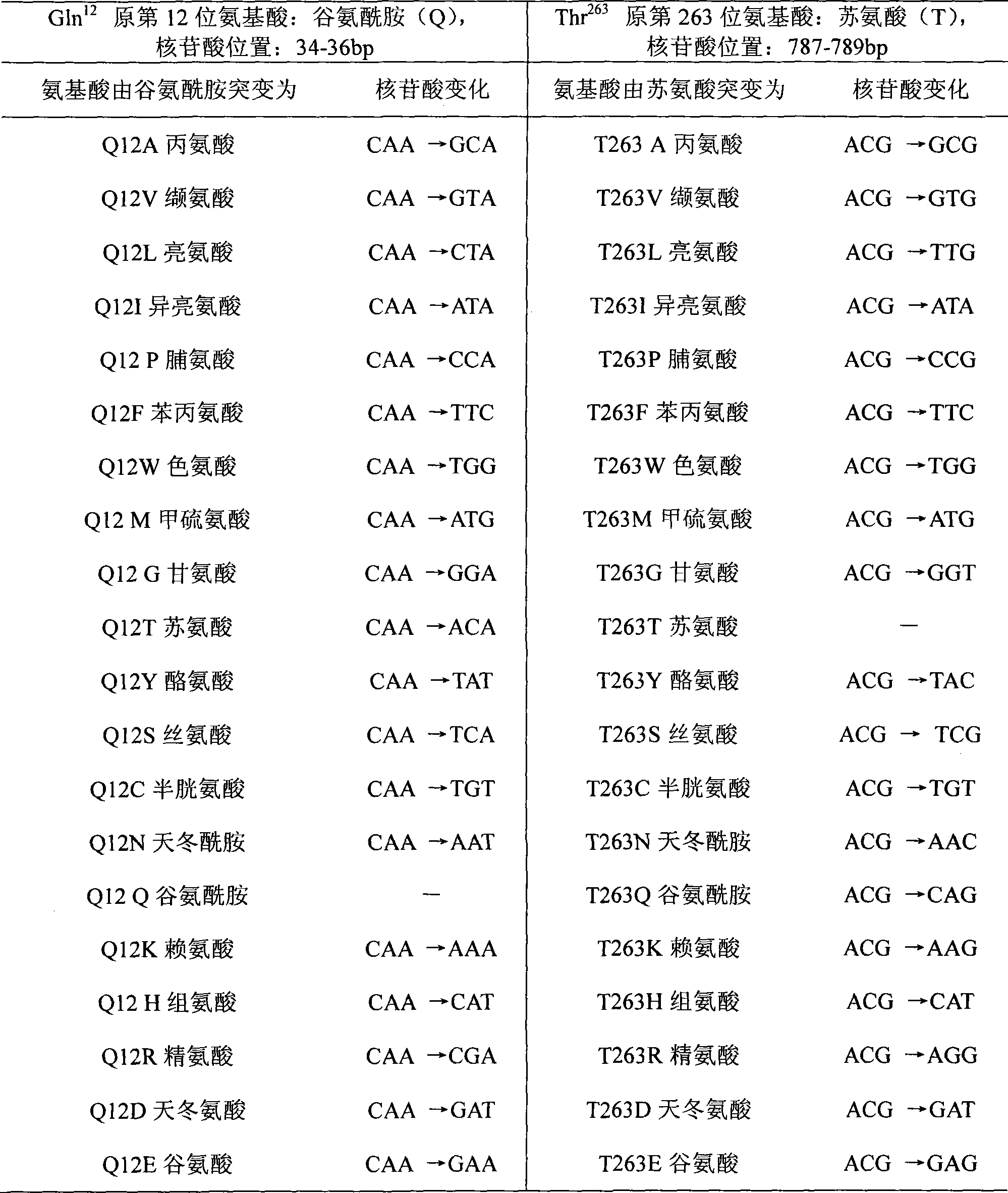
The invention discloses a mutant of D-carbamyl hydrolysis enzyme and application thereof in producing D-p-hydroxyphenylglycine. The method comprises the following steps that: a mutant is obtained by the directed evolution technology and mutates into nonpolar amino acid in 12-glutamine of the D-carbamyl hydrolysis enzyme; another mutant mutates into methionine in 263-threonine of the D-carbamyl hydrolysis enzyme; a third mutant mutates into serine in 263-threonine of the D-carbamyl hydrolysis enzyme; a fourth mutant mutates into the threonine in 12-glutamine of the D-carbamyl hydrolysis enzyme; and the 263-threonine mutates into the serine. The mutant enzyme shows higher thermal stability and enzyme activity in the production of the D-p-hydroxyphenylglycine, and indicates that using the directed evolution technology to reconstruct industrial enzyme is a quite effective method.
Owner:洛阳华荣生物技术有限公司
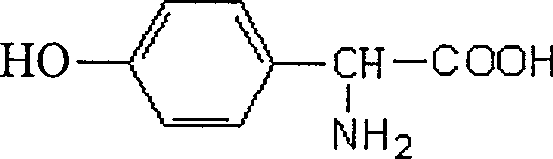
Method of preparing D-p-hydroxyphenylglycine
ActiveCN101239926AGood split effectLow costOrganic compound preparationAmino-carboxyl compound preparationRoom temperatureP-hydroxyphenylglycine
The invention provides a preparing method of D-p-hydroxyphenylglycine, which comprises adding DL-p-hydroxyphenylglycine and resolving agent beta-naphthalenesulfonic acid in water to prepare solution, rising temperature and reacting under agitating, adding induction seeds, adjusting the specific totation of the solution, performing heat-preservation reaction, cooling, sufficiently crystallizing, reducing pressure, filtering and drying, thereby obatining solid D-p-hydroxyphenylglycine beta-naphthalenesulfonic acid complex salts or L-p-hydroxyphenylglycine beta naphthalenesulfonic acid complex salts; dissolving D-p-hydroxyphenylglycine beta-naphthalenesulfonic acid complex salts in water to prepare aqueous solution, rising temperature, adding active carbon to perform decoloring treatment, adjusting the PH value by alkali liquid, cooling the sollution to room temperature, filtering, washing, and drying, thereby obatining D-p-hydroxyphenylglycine. The D-p-hydroxyphenylglycine produced by the method of the invention has good quality, short reaction time and low cost.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Method for recovering D-p-hydroxyphenylglycine in amoxicillin production waste liquid
ActiveCN104628587AReduce energy consumptionHigh purityOrganic compound preparationAmino-carboxyl compound preparationLiquid wasteDesorption
The invention discloses a method for recovering D-p-hydroxyphenylglycine in amoxicillin production waste liquid by use of ion exchange resin. The method comprises the following steps: firstly, exchanging D-p-hydroxyphenylglycine onto the resin by use of the ion exchange resin, dissolving D-p-hydroxyphenylglycin by use of a 0.5mol / L aqueous hydrochloric acid solution, and meanwhile, concentrating the solution; secondly, purifying the desorption solution by use of macroporous adsorption resin, and drying the dripping liquid to obtain a finished product. The method is characterized in that D-p-hydroxyphenylglycine is concentrated and purified by use of the ion exchange resin, the process is greatly simplified, the recovery effect is good, the energy is saved, the environment can be protected, and the subsequent production process of synthesizing amoxicillin by use of an enzyme method is further perfected.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Process for the preparation of immobilized recombinant penicillin acylase catalyst from Achromobacter sp. CCM 4824 expressed in E. coli BL 21 CCM 7394 and its use for the synthesis of beta-lactam antibiotics
ActiveCN101802212AImprove synthesis abilityExtended active timeFermentationAmpicillinBeta lactam antibiotic
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic [beta]-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
An enzymatic synthesis process of Amoxicillin
InactiveCN104830940AShort reaction timeHigh yieldOn/in organic carrierFermentationD-p-hydroxyphenylglycineP-hydroxyphenylglycine
The invention relates to a synthesis method of medicines, and particularly relates to screening of an immobilized Amoxicillin synthase and an enzymatic synthesis process of Amoxicillin. The process includes immobilizing by adoption of an amino epoxy type carrier to obtain an immobilized Amoxicillin enzyme LK218, adding the immobilized Amoxicillin enzyme LK218, 6-aminopenicilanic acid and a D-p-hydroxyphenylglycine derivative into water, stirring, mixing to obtain a mixture, adjusting the pH value of the mixture by utilization of a hydrochloric acid solution and a sodium hydroxide solution, controlling the temperature and reaction time of the mixture, and finishing the reaction until the residual concentration of the 6-APA is 0-2 mg / mL. Aiming at problems, namely difficult screening and evaluation of immobilized enzymes, tedious production steps, poor reference points, long reaction time, low conversion ratios, and the like, the immobilized Amoxicillin enzyme and the novel synthesis process of the Amoxicillin are provided.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Green method of enzymatic synthesis of cefaclor
InactiveCN106222230AHigh selectivityImprove catalytic performanceImmobilised enzymesHydrolasesChemical synthesisEnzymatic synthesis
The invention relates to a green method of enzymatic synthesis of cefaclor. The method includes the steps of: (S1) adding a parent nucleus 7-ACCA into a buffer liquid; (S2) under the pH of 5-8, adding a D-p-hydroxyphenylglycinate derivative or a salt thereof and / or D-p-hydroxyphenylglycine amide, and immobilized cefaclor synthesis enzyme, and performing a reaction for 1-3 h at 5-30 DEG C under the pH value of 6.2-7.8; after a certain reaction time, adding a seed crystal to perform crystallization, and when the reaction is finished, separating a reaction liquid and the immobilized cefaclor synthesis enzyme to obtain a cefaclor coarse product; and (S3) acid-hydrolyzing and dissolve-clarifying the coarse product, filtering and re-crystallizing the product to prepare the cefaclor. The enzymatic synthesis method, compared with a conventional chemical synthesis method, is simple in operation, is low in cost, reduces synthetic period, improves production efficiency and total yield, has strong controllability and satisfies industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Method for preparing cefprozil in pH responsive regenerative double aqueous phase system
PendingCN106939327AIncreased molar yieldEasy to separateFermentationChemical synthesisCentrifugation
The invention relates to a method for preparing cefprozil in a pH responsive regenerative double aqueous phase system. The method comprises (1) preparing two double-aqueous phase systems, (2) orderly adding 7-APRA, D-p-hydroxyphenylglycine methyl ester hydrochloride into the systems, adjusting solution pH to 5.00-6.50 and controlling a solution temperature in a range of 10-30 DEG C, (3) adding immobilized penicillin acylase into the solution obtained by the step (2), and (4) standing the mixed solution, removing the immobilized penicillin acylase, adjusting the pH of the reaction solution, recovering P<ADBA> / <PMDB> and P<ADB> / P<MDB> polymers of the double-aqueous phase systems, feeding the supernatant to a crystallization section, carrying out crystallization, and carrying out centrifugation, washing and drying to obtain a product. The method reduces a chemical synthesis cost, improves a low product conversion rate of the monohydrolase catalytic reaction, effectively improves a yield, simplifies the operation, reduces a cost and realizes easy recovery of the double-aqueous phase systems.
Owner:EAST CHINA UNIV OF SCI & TECH
Technology for treating phenol-contained wastewater in synthetic process of L-(+)-D-p-hydroxyphenylglycine
InactiveCN101759268AWater contaminantsMultistage water/sewage treatmentDistillationTherapeutic effect
The invention relates to a technology for treating phenol-contained wastewater in the synthetic process of L-(+)-D-p-hydroxyphenylglycine, which belongs to the technical field of the treatment of organic chemical wastewater. In the method, after the distillation pretreatment is carried out on synthesized wastewater, the macroporous resin adsorption treatment is carried out on the collected phenol-contained wastewater, wherein the pretreatment comprises the step of carrying out acidized pretreatment on the synthesized wastewater. In the method, because a phenol-contained substance in the wastewater is removed in advance, the recycling and processing difficulty of the subsequent inorganic salt can be greatly improved. The recycled sodium phenolate can be charged back to the synthesizing section of p-hydroxyphenylglycine. The method has stable and reliable treatment effect, low treatment cost and simple, convenient and easily-applied operation and is easy to realize industrialized application.
Owner:HENAN NEWLAND PHARMA
Method for synthesizing D-p-hydroxyphenylglycine methyl ester
ActiveCN104892444AHigh yieldOrganic compound preparationAmino-carboxyl compound preparationHydrogenEsterification reaction
The invention relates to the field of compound synthesis and discloses a method for synthesizing D-p-hydroxyphenylglycine methyl ester. The method comprises steps as follows: (1), in the presence of thionyl chloride, D-p-hydroxyphenylglycine resolving agent salt and methanol have an esterification reaction, and D-p-hydroxyphenylglycine methyl ester resolving agent salt is obtained; (2), the D-p-hydroxyphenylglycine methyl ester resolving agent salt and alkaline metal hydroxide are dropwise added to a D-p-hydroxyphenylglycine methyl ester aqueous solution at the temperature of 10-15 DEG C, the pH (potential of hydrogen) value of a system is controlled in the range from 6.5 to 7 in the dropwise adding process, after dropwise adding of the D-p-hydroxyphenylglycine methyl ester resolving agent salt is completed, alkaline metal hydroxide is continuously dropwise added until the pH value of the system ranges from 7.5 to 8, a crystal is grown at the temperature of 10-15 DEG C and under the condition of the pH value being 7.5-8, an obtained crystalline liquid is filtered, and a D-p-hydroxyphenylglycine methyl ester crystal and a mother liquor are obtained. With adoption of the method provided by the invention, D-p-hydroxyphenylglycine methyl ester with a higher yield can be produced.
Owner:山西双雁生物科技有限公司
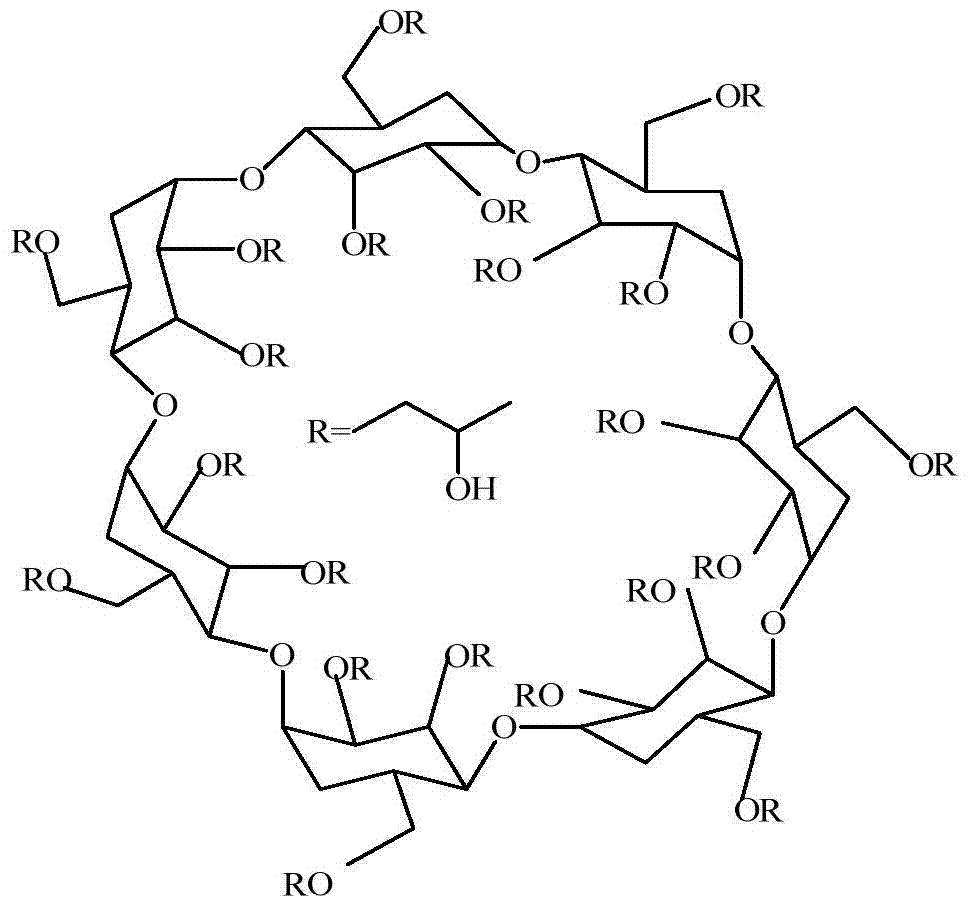
Hydroxypropyl-beta-cyclodextrin chiral composite membrane, and applications thereof
InactiveCN103752180AHigh purityNo phase changeSemi-permeable membranesOrganic compound preparationEnantiomerPolysulfone membrane
The invention discloses a hydroxypropyl-beta-cyclodextrin chiral composite membrane, and applications thereof. A preparation method of the hydroxypropyl-beta-cyclodextrin chiral composite membrane comprises following steps: a polysulfone membrane is immersed in deionized water for two days, is dried vertically in the air, is immersed in a hydroxypropyl-beta-cyclodextrin solution with a concentration of 0.02g / ml, is dried vertically in the air, and is subjected to interfacial polymerization with a 1,6-hexanedioldiisocyanate solution with a concentration of 0.012g / ml; an obtained product is collected, is washed with deionized water after volatilization of reagents on the surfaces, and is dried in that air so as to obtain a chiral composite membrane; and the chiral composite membrane is delivered into a common dialysis device, and d-p-hydroxyphenylglycine raceme solution is separated by concentration difference, wherein purity of d-p-hydroxyphenylglycine enantiomer in a permeate liquid is more than 55%. The purity of the enantiomer obtained via the preparation is relatively high; cost is low; energy is saved; environment is protected; and the preparation method is convenient for continuous operation and large-scaled industrialized production.
Owner:YUNNAN NORMAL UNIV
Method for recovering D-p-hydroxyphenylglycine (D-HPG) from cefprozil production waste liquid in enzyme synthesis process
InactiveCN105085294AEasy to industrializeReduce energy consumptionOrganic compound preparationAmino-carboxyl compound preparationIon exchangeEnzyme synthesis
The invention discloses a method for recovering D-p-hydroxyphenylglycine (D-HPG) from a cefprozil production waste liquid in an enzyme synthesis process, which comprises the following two steps: 1. separating D-HPG from the crystallization waste liquid through ion exchange by using an ion exchange resin; and 2. purifying and eluting the D-HPG by using hydrochloric acid solutions with different concentrations under the actions of ion exchange and static electricity, and drying to obtain the finished product. The method is characterized in that the process of separating and purifying the D-HPG from the cefprozil production waste liquid in the enzyme synthesis process is completed by using the ion exchange resin, and meanwhile, the subsequent waste liquid treatment technique of the enzyme-process cefprozil synthesis is completed.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
DNA molecule, recombinant plasmid and recombinant bacterium for production of D-p-Hydroxyphenylglycine
InactiveCN103993010AImprove conversion rateMild conditionsBacteriaMicroorganism based processesN-carbamoylaseHydrolase Gene
The invention discloses a DNA molecule, a recombinant plasmid and a recombinant bacterium for production of D-p-Hydroxyphenylglycine. The DNA molecule provided by the invention comprises a PL promoter, a D-hydantoinase gene and an N-carbamoylase gene, and expression of the D-hydantoinase gene and the N-carbamoylase gene is initiated by the PL promoter. The invention provides the DNA molecule able to stably and massively express D-hydantoinase and N-carbamoylase, and the recombinant plasmid and the recombinant bacterium based on the DNA molecule. At the same time, the invention also provides a method for converting DL-p-hydroxyphenylhydantoin into D-p-Hydroxyphenylglycine by the recombinant bacterium or fermented recombinant bacterium. The method only generates D-p-Hydroxyphenylglycine, has no need of splitting and separating an enantiomer, and the conversion rate is high. The whole production process has the advantages of mild conditions, simple operation, and environmental friendliness.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Construction of N-carbamoylase expression genes and engineering bacteria of N-carbamoylase expression genes
The invention provides construction of N-carbamoylase expression genes and engineering bacteria of the N-carbamoylase expression genes. A preparation method of N-carbamoylase comprises following steps: 1, screening of target bacteria is carried out; 2, extraction of bacteria total DNA is carried out; 3, amplification of the bacteria total DNA is carried out so as to obtain N-carbamoylase genes; 4, DNA segments of the N-carbamoylase genes are connected with expression vectors so as to obtain N-carbamoylase recombinant plasmids; 5, the N-carbamoylase recombinant plasmids are transferred into expression hosts, and fermental cultivation is carried out. According to the invention, amino acid sequences coded by the N-carbamoylase genes possess relatively high enzymatic activity when N-carbamoyl-D-p-hydroxyphenylglycine is taken as a substrate.
Owner:CHONGQING HONOROAD ANIMAL HEALTH
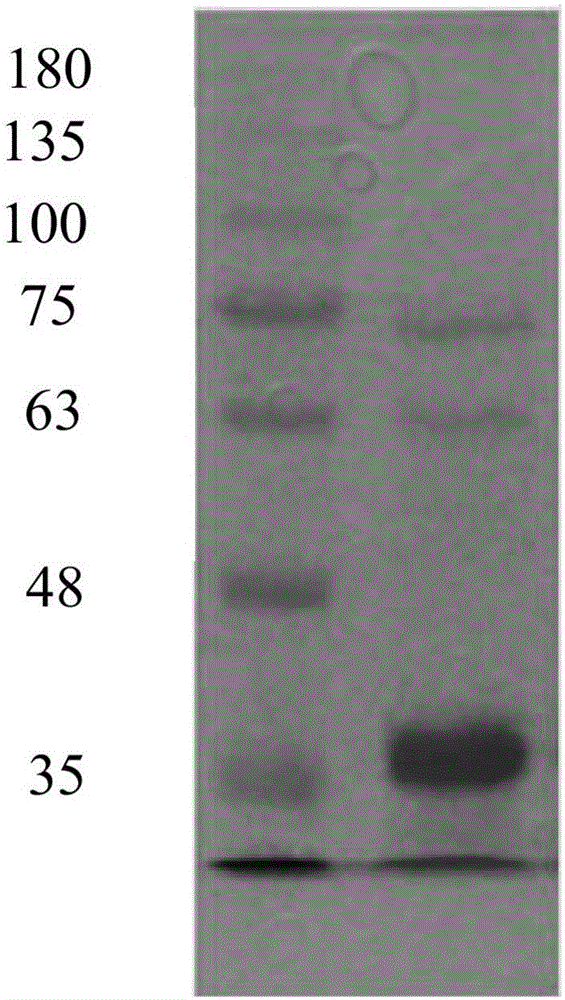
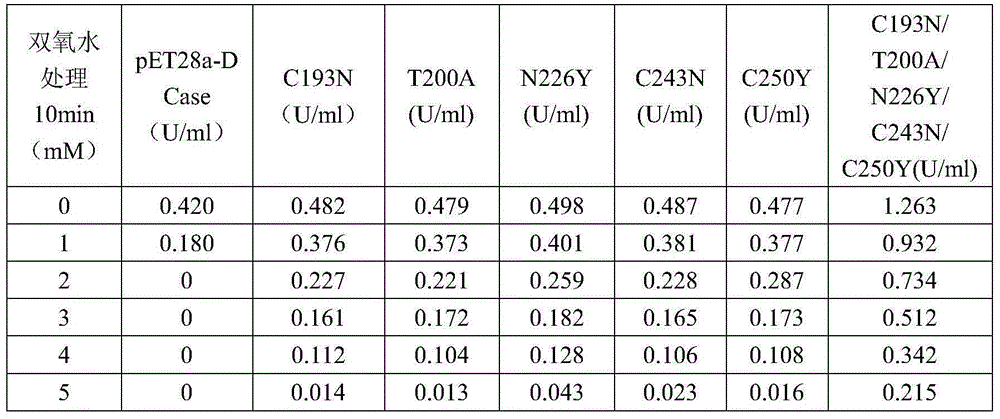
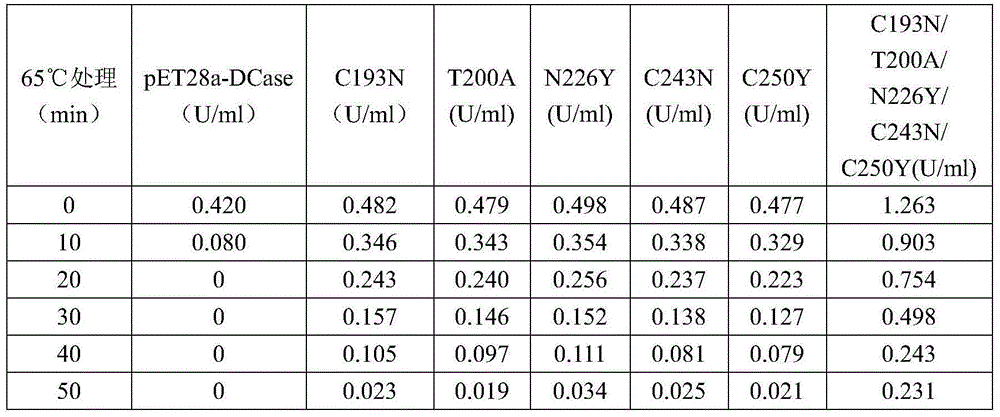
N-carbamoyl-D-p-hydroxyphenylglycine hydrolase mutants and construction of engineering bacteria thereof
ActiveCN106011117AIncrease enzyme activityImprove antioxidant capacityBacteriaHydrolasesThreonineTyrosine
The invention relates to N-carbamoyl-D-p-hydroxyphenylglycine hydrolase mutants and construction of engineering bacteria thereof. The differences between 5 mutants obtained by orthogenesis and N-carbamoyl-D-p-hydroxyphenylglycine hydrolase respectively lie in that: cysteine at position 193 in an amino acid sequence of N-carbamoyl-D-p-hydroxyphenylglycine hydrolase mutates into asparagine; threonine at position 200 mutates into alanine; asparagine at position 226 mutates into tyrosine; cysteine at position 243 mutates into asparagine; cysteine at position 250 mutates into tyrosine; and engineering bacteria construction is carried out, and the N-carbamoyl-D-p-hydroxyphenylglycine hydrolase mutants and engineering bacteria are applied in D-p-hydroxyphenylglycine production. Compared with wild enzyme, the tolerance to oxygen, tolerance to acid-base and the enzyme activity are greatly improved.
Owner:CHONGQING HONOROAD ANIMAL HEALTH
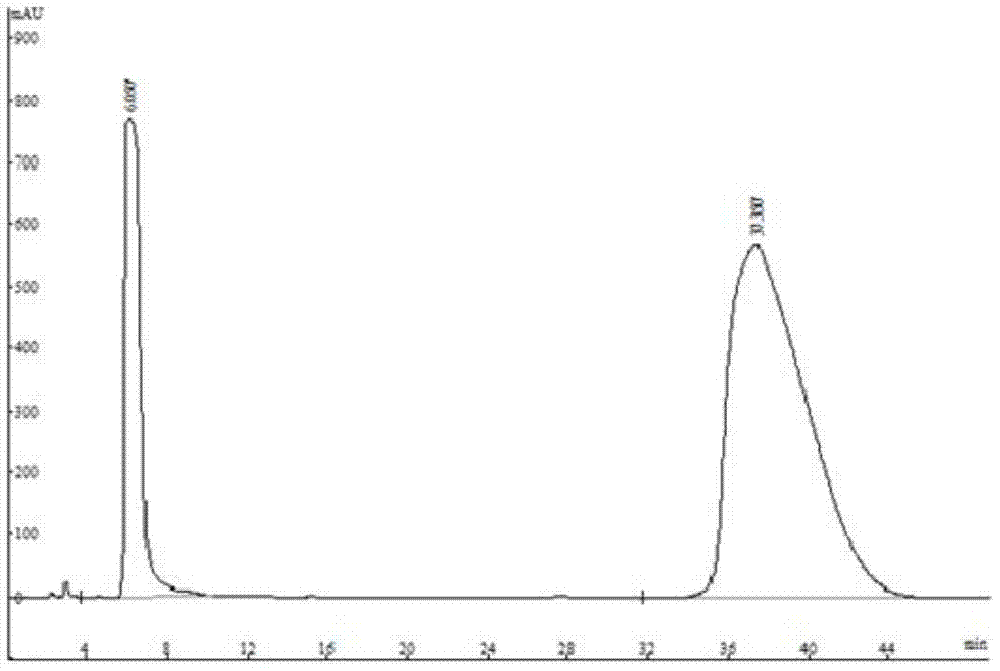
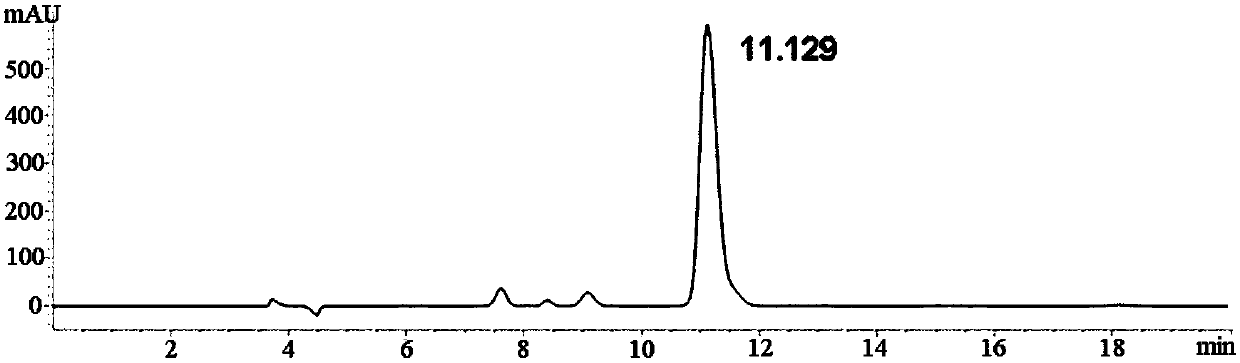
Method for separating and detecting D-p-hydroxyphenylglycine and enantiomer thereof
ActiveCN107941970AEasy to solveLow cost of analysis and detectionComponent separationBenzyl chloroformateEnantiomer
The invention discloses a method for separating and detecting D-p-hydroxyphenylglycine and an enantiomer thereof. The method comprises: step 1, carrying out a derivatization reaction through adoptionof a derivatization reagent and D-p-hydroxyphenylglycine at a certain reaction temperature in a reaction solvent to prepare a derivatized product; and step 2, analyzing the derivatized product by using normal-phase high performance liquid chromatography, and separating and detecting derivatized D-p-hydroxyphenylglycine and an enantiomer thereof. The derivatization reagent is one of di-tert-butyl dicarbonate, 9-fluorenylmethyl chloroformate, and benzyl chloroformate, and the high performance liquid chromatography takes a normal-phase chromatographic column as a separation column. According to the method, D-p-hydroxyphenylglycine and an enantiomer derivative thereof can be efficiently separated, the baseline separation is achieved, and the separation degree is more than 1.5. The baseline issmooth and steady, and the peak pattern is good. The method is great in the specialization and high in detection sensitivity and is beneficial for fast accurately detecting the content of the enantiomer in D-p-hydroxyphenylglycine.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD +1
D-p-hydroxyphenyl glycine preparation process
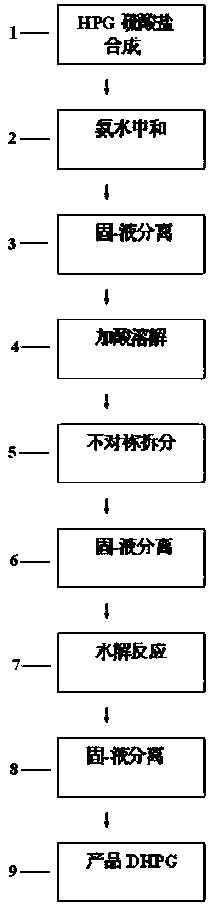
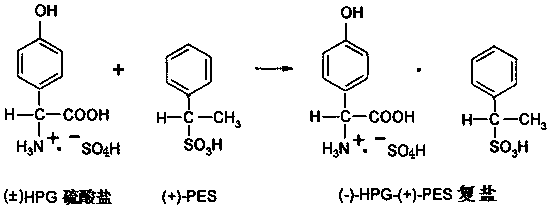
ActiveCN110467537AAccurate response orientationIncrease contentOrganic compound preparationOrganic chemistry methodsDouble saltHydrolysis
The present invention relates to the field of compound synthesis, and discloses a D-p-hydroxyphenyl glycine (short for DHPG) preparation process, which comprises: synthesizing a DL-para-hydroxyphenylglycine sulfate solution (DL-para-hydroxyphenyl glycine is short for HPG) by using phenol, glyoxylic acid and the like as raw materials; purifying, adding a resolving agent, and performing an asymmetric resolution reaction to obtain a D-p-hydroxyphenyl glycine-phenylethane sulfonic acid double salt; adding an alkali liquid to the obtained double salt in a dropwise manner, and carrying out a hydrolysis reaction and other operations to obtain a D-p-hydroxyphenyl glycine crystal; and carrying out further treatment on the mother liquor containing the resolving agent phenylethane sulfonic acid, andrecycling. According to the present invention, the preparation process is mainly characterized in that the composite catalyst is introduced, and the three steps are eliminated, such that the production cycle is shortened by 12 h; the short synthesis route is short, and the loss is low, such that the yield of D-p-hydroxyphenyl glycine is increased by 7-9%, and the water consumption is reduced by 15-16%; the technical prejudice of the previous D-p-hydroxyphenyl glycine preparation process in the prior art is objectively overcome, and the unexpected effect is achieved; and the D-p-hydroxyphenylglycine preparation process has characteristics of production cost reducing and production efficiency improving, and easily achieves water resource saving and environment protection.
Owner:HENAN NEWLAND PHARMA
Fermentative production of d-hydroxyphenylglycine and d-phenylglycine
A new fermentative process for the preparation of D-p-hydroxyphenylglycine (D-HPG) or D-henylglycine (D-pG) in enantiomerically pure form is disclosed. Precursors for the formation of D-HPG and D-pG are withdrawn form the common aromatic amino acid pathway, converted to p-hydroxyphenylglyoxylate or phenylglyoxylate, and are finally converted to D-HPG or D-pG by the action of a stero-inverting D-aminotransferase.
Owner:DSM BIOTECH +2
Process for the preparation of immobilized recombinant penicillin acylase catalyst from Achromobacter sp. CCM 4824 expressed in E. coli BL 21 CCM 7394 and its use for the synthesis of beta-lactam antibiotics
ActiveCN101802212BImprove synthesis abilityExtended active timeFermentationAmpicillinBeta lactam antibiotic
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic &bgr;-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
Method for producing D-p-hydroxyphenylglycine
InactiveCN102452949ASimple processHigh yieldOrganic compound preparationAmino-carboxyl compound preparationChemical industrySolvent
The invention relates to the technical field of pharmacy and chemical industry, in particular to a method for producing D-p-hydroxyphenylglycine. The method for producing the D-p-hydroxyphenylglycine comprises the following steps of: a, preparing a 5 to 10 percent aqueous solution of sodium chloride, and regulating the pH to be 0-3 by using acid for later use; b, adding DL-p-hydroxyphenylhydantoin in an amount which is 30 to 40 percent based on the mass of the solvent, and stirring at the temperature of between 60 and 70 DEG C for 2 to 3 hours; and c, filtering to obtain the D-p-hydroxyphenylglycine. The method for producing the D-p-hydroxyphenylglycine has the advantages of high yield and short reaction time and is simple.
Owner:HENAN NEWLAND PHARMA
Technology for hydrolyzing D-(-)-p-hydroxyphenylglycine-p-toluenesulfonate
InactiveCN101759579AOrganic compound preparationAmino-carboxyl compound preparationP-hydroxyphenylglycineHydrolysis
The invention discloses a technology for hydrolyzing D-(-)-p-hydroxyphenylglycine-p-toluenesulfonate, which belongs to the field of the production of pharmaceutical and chemical intermediates and relates to a technology for hydrolyzing a compound having the following structure. The traditional D-p-hydroxyphenylglycine obtained by hydrolysis has low yield (not more than 38 percent) and low quality (the content of not more than 98 percent and the light absorption of not smaller than 0.10), therefore, the cost is higher, and the quality is low without market competitive force. The invention overcomes the defects, improves the yield (not smaller than 45 percent) and the production quality (the content of not smaller than 99.5 percent, the light absorption of not more than 0.050, the optical rotation of minus156-minus 161 degrees and single impurities of not more than 1ppm), has easy acquisition of needed raw materials, lower production cost and simple and easily-applied operation and meets the requirement of enterprise development.
Owner:HENAN NEWLAND PHARMA
Process for the preparation of immobilized recombinant penicillin acylase catalyst from achromobacter sp. ccm 4824 expressed in e. coli bl 21 ccm 7394 and its use for the synthesis of beta-lactam antibiotics
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic β-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
Mutant of D-carbamyl hydrolysis enzyme and application thereof
Owner:洛阳华荣生物技术有限公司
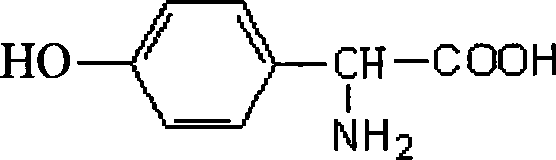
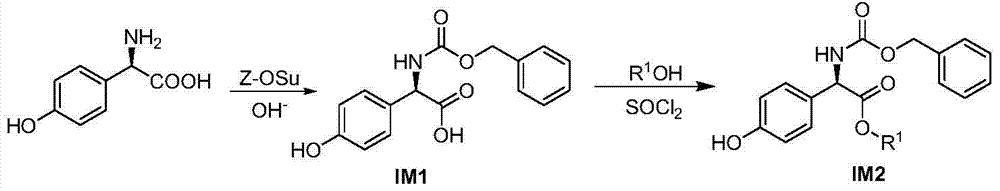
D-p-hydroxyphenylglycine derivatives and their preparation methods and applications
ActiveCN105152977BEasy to prepareNervous disorderCarbamic acid derivatives preparationP-hydroxyphenylglycineD-p-hydroxyphenylglycine
The invention discloses a D-hydroxy phenylglycine derivative. Please see the formula of the D-hydroxy phenylglycine derivative in the specification. D-hydroxy phenylglycine serves as a parent body, amino groups and carboxyl groups of the D-hydroxy phenylglycine are reasonably modified, a phenolic hydroxyl group is connected with an aromatic nucleus type hydrophobic group through a connector, the D-hydroxy phenylglycine derivative which is novel in structure is constructed, compounds capable of promoting the secreting activity of GLP-1 and / or inhibiting the activity of Nav1.7 are screened out, the preparation method of the compounds is simple and can be used for preparing GLP-1secernent and / or Nav1.7 inhibitors, and the D-hydroxy phenylglycine derivative has potential application prospects in the fields of diabetes treatment drugs and / or neuropathy pain treatment drugs. Please see the formula in the specification.
Owner:SOUTHWEST UNIV
The recovery method of d-p-hydroxyphenylglycine in enzymatic synthesis of amoxicillin crystallization mother liquor
ActiveCN105254520BImprove product qualityEfficient ConcentrationOrganic compound preparationAmino-carboxyl compound preparationEnzymatic synthesisRecovery method
Owner:SHANXI WEIQIDA PHARMA IND
Method of preparing D-p-hydroxyphenylglycine
ActiveCN100519514CGood split effectLow costOrganic compound preparationAmino-carboxyl compound preparationActivated carbonGlycine
A preparation method of D-p-hydroxyphenylglycine, which is to add DL-p-hydroxyphenylglycine and resolving agent β-naphthalenesulfonic acid in water to prepare a solution, raise the temperature under agitation to react, add induced seed crystals, and adjust the solution Specific rotation value, heat preservation reaction, cooling and sufficient crystallization, filtration under reduced pressure and drying to obtain solid D-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt or L-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt; D-p-Hydroxyphenylglycine β-naphthalenesulfonic acid double salt is dissolved in water to make an aqueous solution, heated up and added with activated carbon for decolorization, adjusted the pH value with lye, cooled to room temperature, filtered, washed and dried to obtain D-p-Hydroxyphenylglycine . The D-p-hydroxyphenylglycine produced by the invention has good quality, short reaction time and low cost.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Alcaligenes and method for preparing D-p-hydroxyphenylglycine by using same
InactiveCN102399709ABreeding method is simpleEasy to manufactureBacteriaMicroorganism based processesPollutant emissionsP-hydroxyphenylglycine
Owner:SHANGHAI NORMAL UNIVERSITY
A method for recovering d-p-hydroxyphenylglycine in amoxicillin production waste liquid
ActiveCN104628587BReduce processing costsOrganic compound preparationAmino-carboxyl compound preparationLiquid wasteDesorption
The invention discloses a method for recovering D-p-hydroxyphenylglycine in amoxicillin production waste liquid by use of ion exchange resin. The method comprises the following steps: firstly, exchanging D-p-hydroxyphenylglycine onto the resin by use of the ion exchange resin, dissolving D-p-hydroxyphenylglycin by use of a 0.5mol / L aqueous hydrochloric acid solution, and meanwhile, concentrating the solution; secondly, purifying the desorption solution by use of macroporous adsorption resin, and drying the dripping liquid to obtain a finished product. The method is characterized in that D-p-hydroxyphenylglycine is concentrated and purified by use of the ion exchange resin, the process is greatly simplified, the recovery effect is good, the energy is saved, the environment can be protected, and the subsequent production process of synthesizing amoxicillin by use of an enzyme method is further perfected.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Novel synthesis method of cefprozil
InactiveCN108440567AResidue reductionReduce cost pressureOrganic chemistrySynthesis methodsCefprozil
The invention belongs to the technical field of medicine synthesis and preparation and in particular relates to patent application of a novel cefprozil synthesis and preparation method. The method hasthe technical thought as follows: alkali substitution reaction is carried out on beta-lactam parent nucleus(7-amin-3-(Z-prop-1-enyl)-4-cephalosporanic acid) in an aprotic solvent; then a branched-chain D-p-hydroxyphenylglycine derivative is added to carry out condensation reaction, so that cefprozil is prepared. Totally, the preparation method provided by the invention has the advantages of easiness for obtaining reaction raw materials, moderate reaction conditions and easiness for controlling; after the reaction is finished, the residue of main raw materials is relatively low, the cost pressure caused by the main raw materials can be greatly reduced and the yield of the cefprozil is relatively high; meanwhile, the method has a relatively simple process route and a high-quality cefprozilproduct can be obtained through two-step reaction; the defects of an existing cefprozil preparation method that the yield is relatively low and the quality is not stable can be totally overcome relatively well; the method has relatively good practical value and production and popularization application meaning.
Owner:TOPFOND PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com