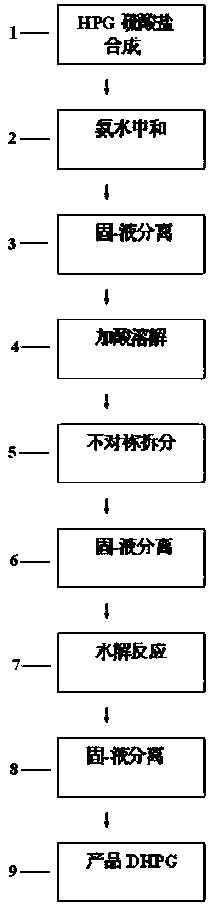
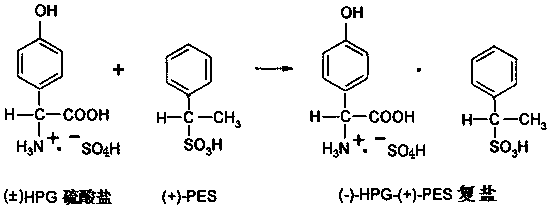
D-p-hydroxyphenyl glycine preparation process
A technology for L-para-hydroxyphenylglycine and para-hydroxyphenylglycine, which is applied in the field of organic compound synthesis, can solve the problems of waste water treatment requiring large energy consumption, unfavorable environmental protection, increased process loss and the like, and achieves a guaranteed first pass rate and production cycle The effect of shortening, energy and labor reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]In a 1000ml reaction flask, put 1 mol of phenol, 1 mol of 20% glyoxylic acid, and 1.2 mol of sulfamic acid, then add 0.2 g of composite catalyst, then heat up to synthesize p-hydroxyphenylglycine-sulfate, and monitor the HPG content by HPLC to reach 99.2%; Next, add 25g of pure water-methanol mixture to the obtained reaction solution, add 0.5g of powdered activated carbon and decolorize and remove impurities at 60-65°C for 30min to obtain pure hydroxyphenylglycine sulfate Reaction solution; then add 165g of 40% phenylethylsulfonic acid solution, make it react at 80°C for 2.5h, obtain L-p-hydroxyphenylglycine-phenylethylsulfonic acid suspension; obtain solid L-p-hydroxyphenylglycine-phenylethylsulfonic acid after centrifugation The double salt of sulfonic acid, then, make it hydrolyze with 25% sodium hydroxide solution, control the pH value to 5.5, and precipitate the crystals of L-p-hydroxyphenylglycine, and then obtain the L-p-hydroxyphenylglycine crystal through crystal...
Embodiment 2
[0039] In a 1000ml reaction flask, put 1 mol of phenol, 1 mol of 20% glyoxylic acid, and 1.2 mol of sulfamic acid, then add 0.2 g of composite catalyst, then heat up to synthesize p-hydroxyphenylglycine-sulfate, and monitor the HPG content by HPLC to reach 99.0%; Next, add 25g of pure water-ethanol mixture to the obtained reaction solution, add 0.5g of powdered activated carbon and decolorize and remove impurities for 30min at 60-65°C to obtain pure hydroxyphenylglycine sulfate Reaction solution; then add 165g of 40% phenylethylsulfonic acid solution, make it react at 80°C for 2.5h, obtain L-p-hydroxyphenylglycine-phenylethylsulfonic acid suspension; obtain solid L-p-hydroxyphenylglycine-phenylethylsulfonic acid after centrifugation The double salt of sulfonic acid, next, make it hydrolyze with 20% ammonia water, control the pH value to 5.3, and then obtain crystals of L-p-hydroxyphenylglycine through crystal growth and solid-liquid separation, and the filtrate is desalted and ...
Embodiment 3
[0041] In a 1000ml reaction flask, put 1 mol of phenol, 1 mol of 20% glyoxylic acid, and 1.2 mol of sulfamic acid, then add 0.15 g of composite catalyst, and then heat up to synthesize p-hydroxyphenylglycine-sulphate. HPLC monitors that the HPG content reaches 99.3%; Next, add 18g of pure water-methanol mixture to the obtained reaction solution, add 01g of powdered activated carbon, and decolorize and remove impurities for 30min at 60-65°C to obtain pure vortex p-hydroxyphenylglycine sulfate reaction solution; then add 165g of 40% phenylethylsulfonic acid solution and make it react at 75°C for 2.5h to obtain a suspension of L-p-hydroxyphenylglycine-phenylethylsulfonic acid; centrifuge to obtain solid L-p-hydroxyphenylglycine-phenylethylsulfonate The double salt of acid, then, make it hydrolyze with 20% ammonia water, control the pH value to 5.6, and then obtain crystals of L-p-hydroxyphenylglycine through crystal growth and solid-liquid separation, and the filtrate is desalted ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com