Method for separating and detecting D-p-hydroxyphenylglycine and enantiomer thereof
A technology of p-hydroxyphenylglycine and enantiomers, which is applied in the field of separation and detection of D-p-hydroxyphenylglycine and its enantiomers, can solve the problems of expensive chromatographic columns, high cost of analysis methods, and system equilibrium time Long-term problems, to achieve the effect of strong specificity, strong operability, and stable baseline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation, separation and detection of D,L-p-hydroxyphenylglycine derivatized products
[0047] Take 1.0g (5.982mmol) of D,L-p-hydroxyphenylglycine, dissolve it in 10mL of a mixture of 1,4-dioxane and water (v / v, 1:1), stir at room temperature for 10min, then slowly add 1.2 g (11.859mmol) of triethylamine and 1.60g (1.225mmol) of di-tert-butyl dicarbonate, the reaction solution was placed at room temperature and stirred for 2h, and TLC monitored that the reaction was complete. Add 6 mol / L hydrochloric acid solution to the reaction solution to adjust the pH to 7, extract with ethyl acetate, concentrate under reduced pressure and evaporate to dryness to obtain D,L-p-hydroxyphenylglycine derivative product.
[0048] The D,L-p-hydroxyphenylglycine derivatized product was dissolved in ethanol and then detected and analyzed by high performance liquid chromatography.
[0049] Liquid chromatography conditions: Daicel ChiralPak IA (250mm×4.6mm, 5μm) chromatographic...
Embodiment 2
[0050] Example 2: Preparation, separation and detection of D-p-hydroxyphenylglycine derivatized products
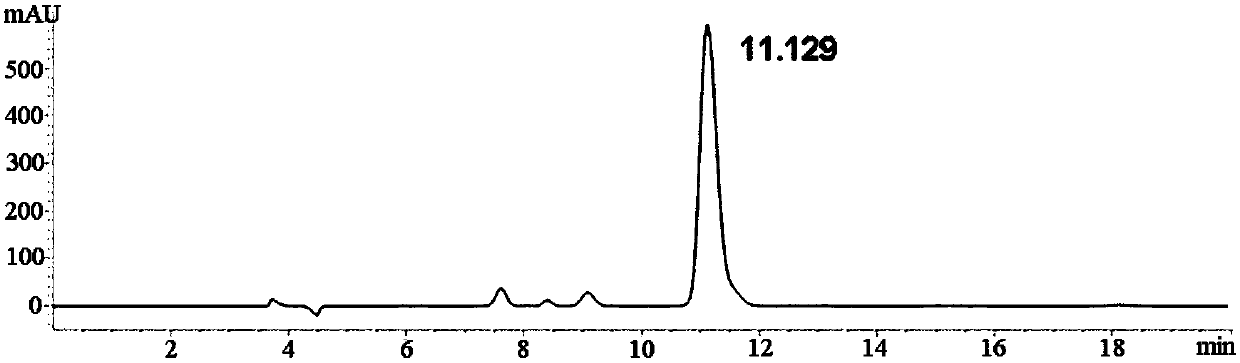
[0051] Take the D-p-hydroxyphenylglycine reference substance, and prepare the derivatized product according to the method of Example 1. The derivatized product was dissolved in ethanol and then detected and analyzed by high performance liquid chromatography according to the method of Example 1. see results figure 2 , the retention time of 11.129min is D-p-hydroxyphenylglycine derivatized product.
Embodiment 3
[0052] Embodiment 3: the selection of mobile phase
[0053] The D,L-p-hydroxyphenylglycine derivative product prepared in Example 1 was dissolved in ethanol and then detected and analyzed by high performance liquid chromatography. Liquid chromatography conditions: Daicel ChiralPak IA (250mm×4.6mm, 5μm) chromatographic column, the mobile phase is shown in Table 1, the ultraviolet detection wavelength is 245nm, the flow rate is 0.8mL / min, the column temperature is 25°C, the injection volume is 5μL, and the chromatogram is recorded.
[0054] Table 1D, Selection of mobile phase conditions in the detection of L-p-hydroxyphenylglycine derivatized products
[0055]
[0056] As can be seen from Table 1, when the mobile phase is n-hexane: ethanol: trifluoroacetic acid = 90:10:0.1 (V / V / V), in the obtained high performance liquid phase chromatogram D-p-Hydroxyphenylglycine derivatized product The peak shape is good, the retention time is appropriate, and the separation between the ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com