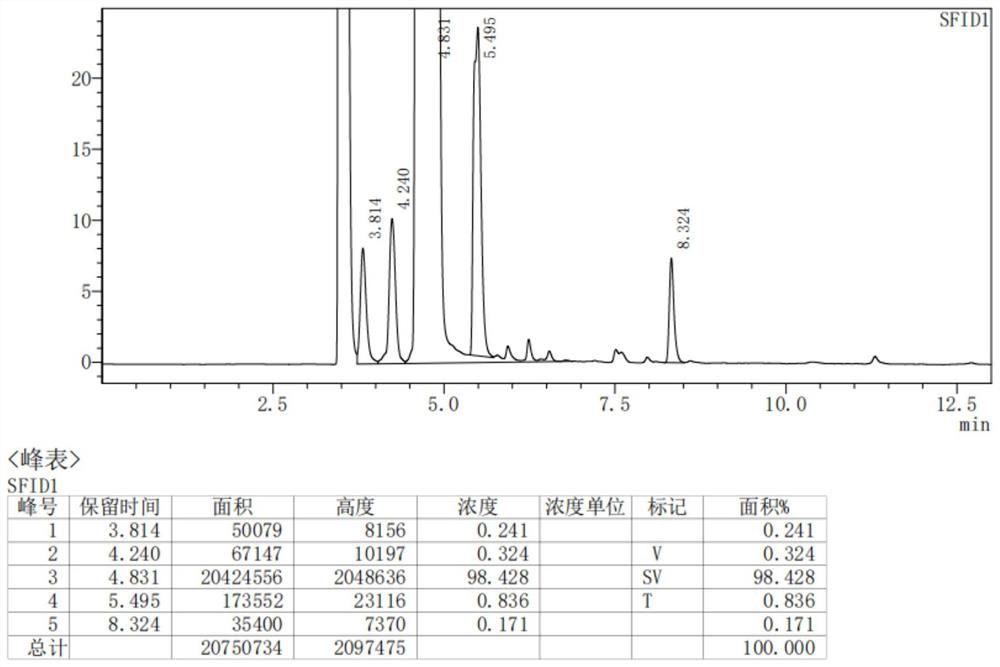
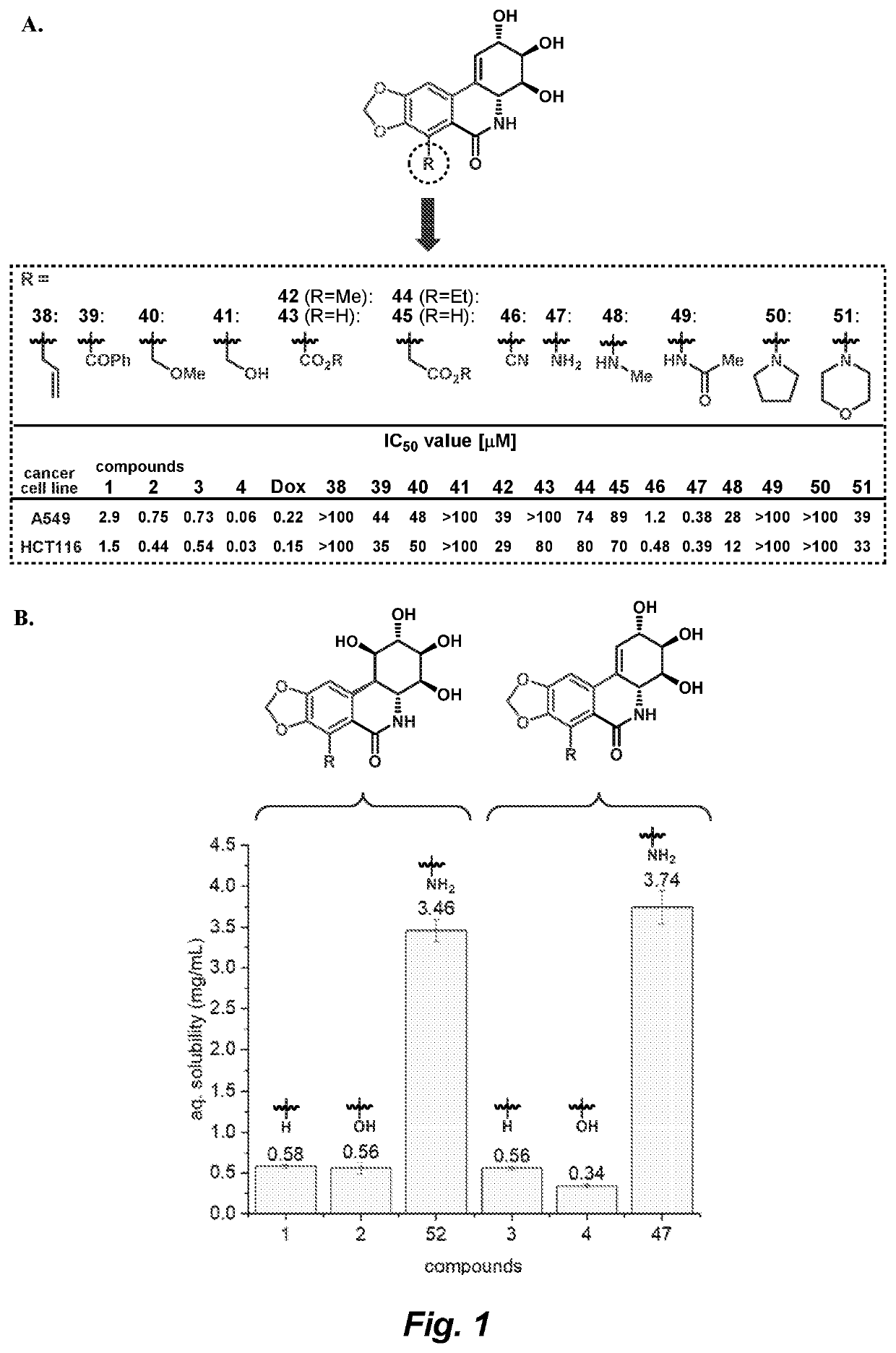
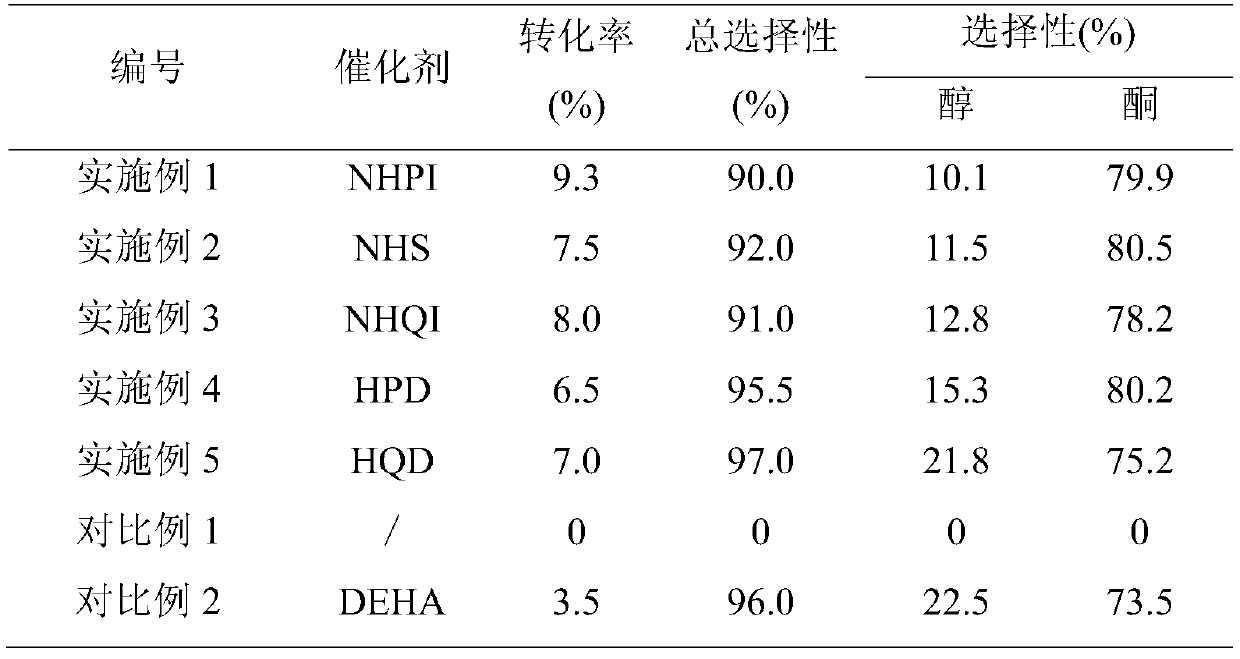
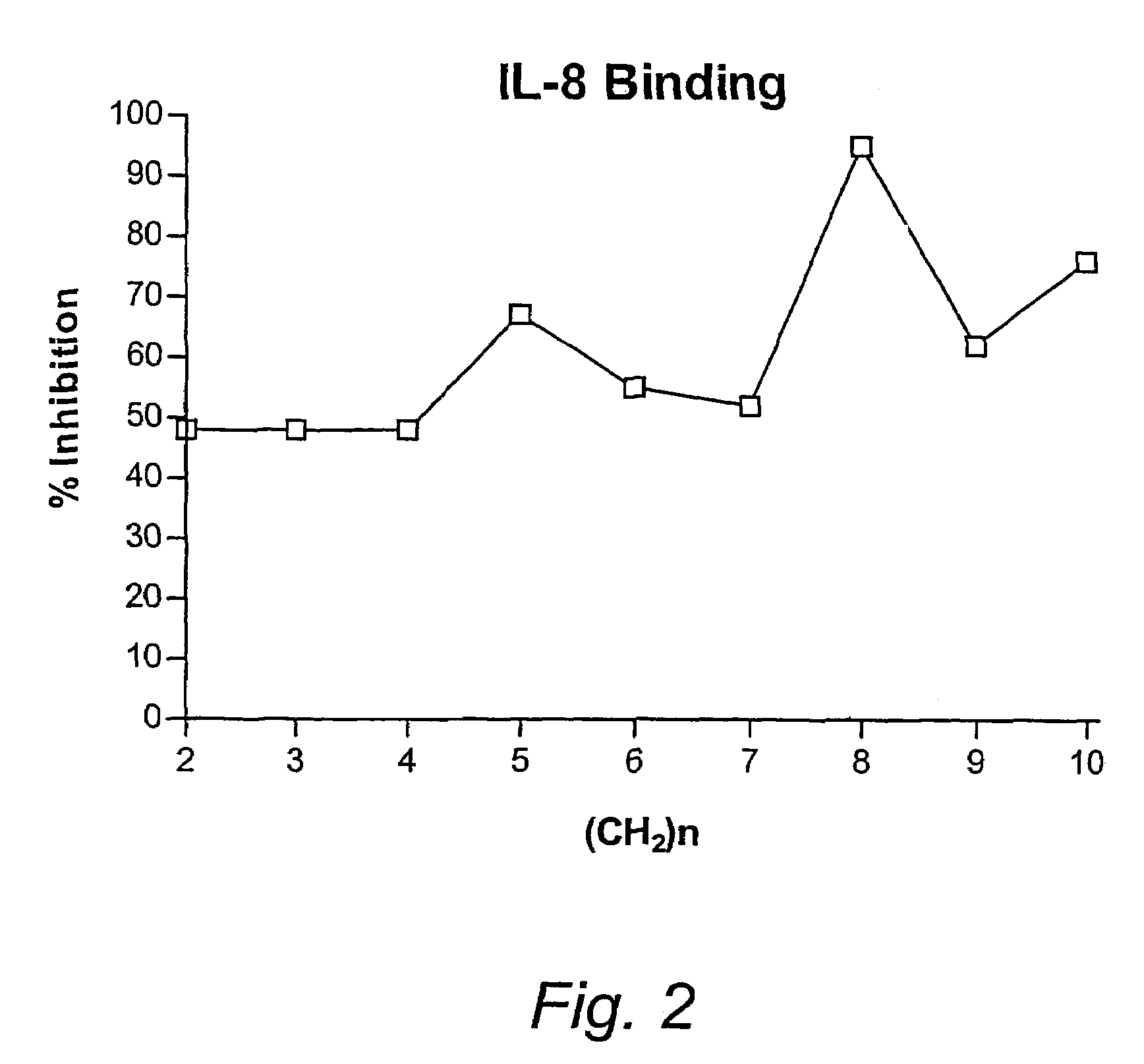
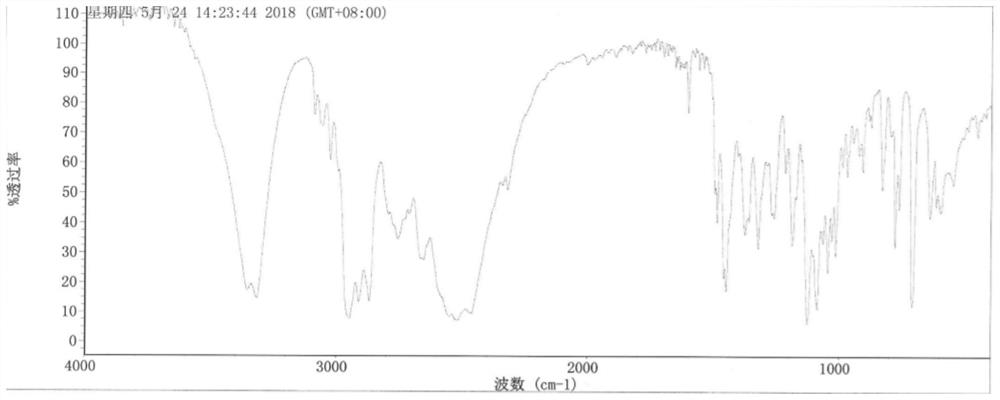
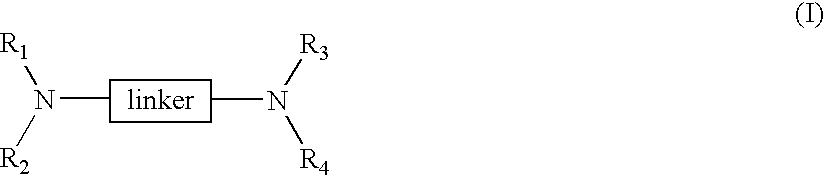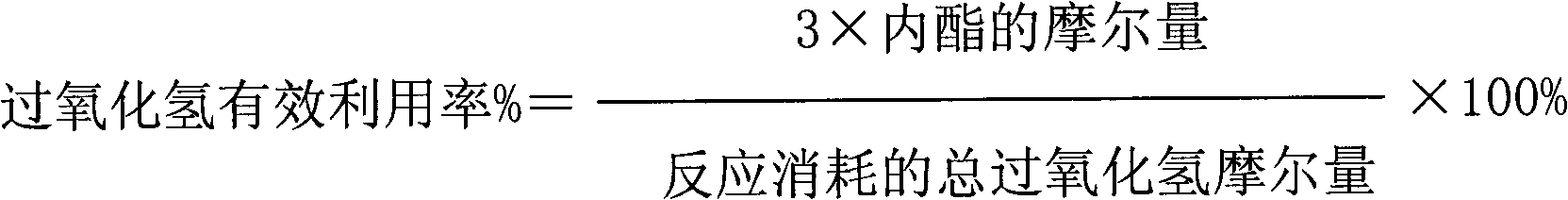Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Cyclitol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
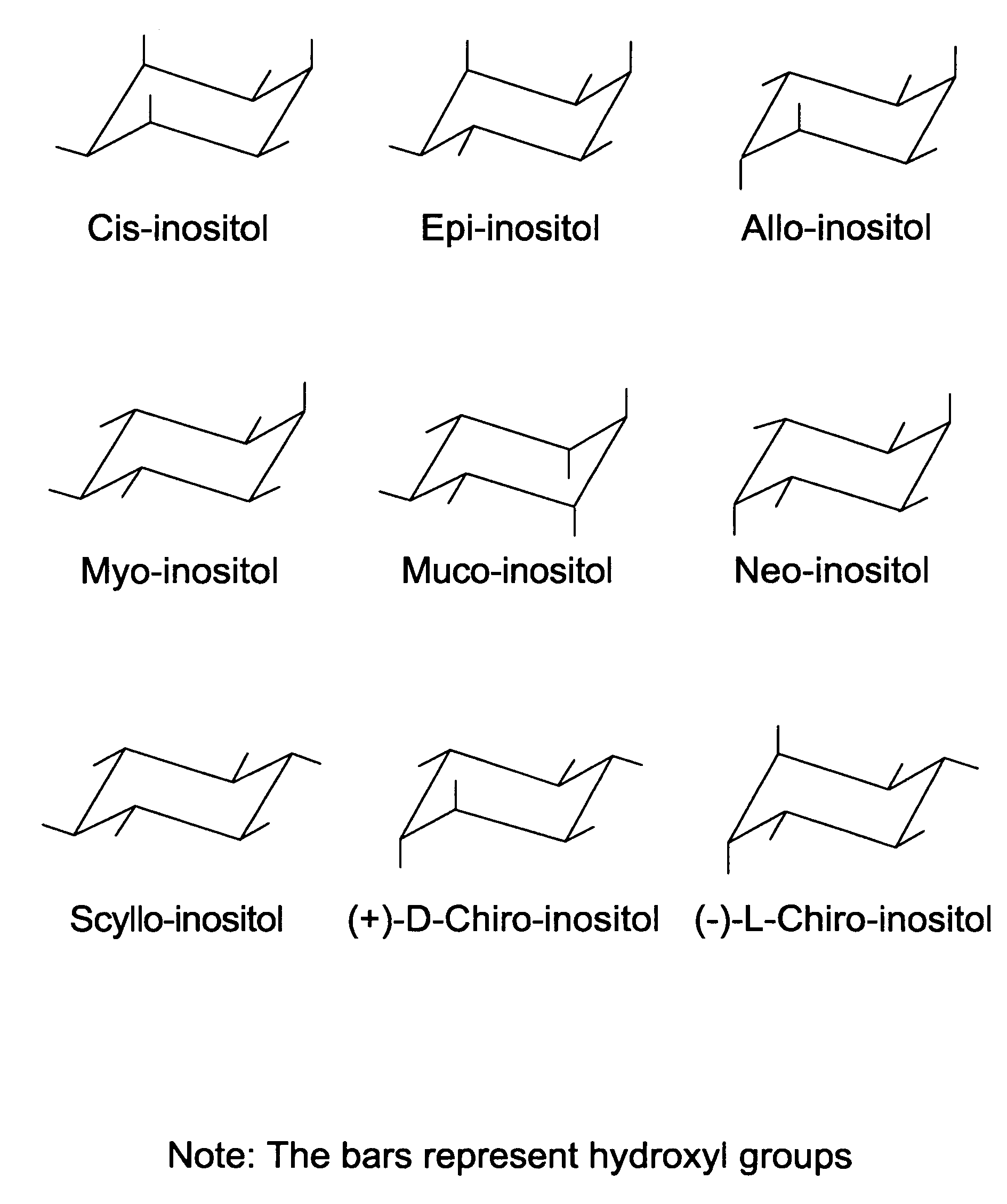
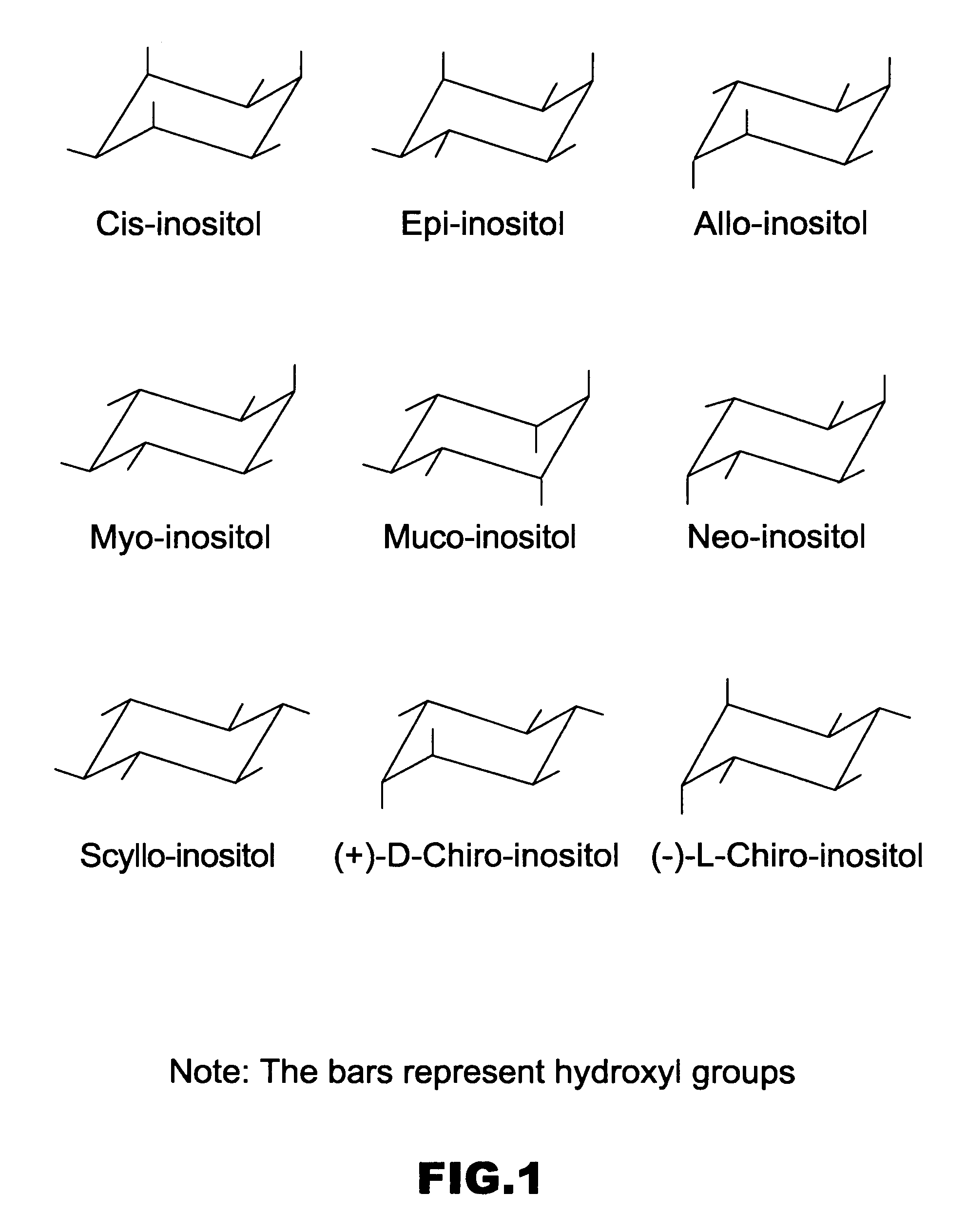
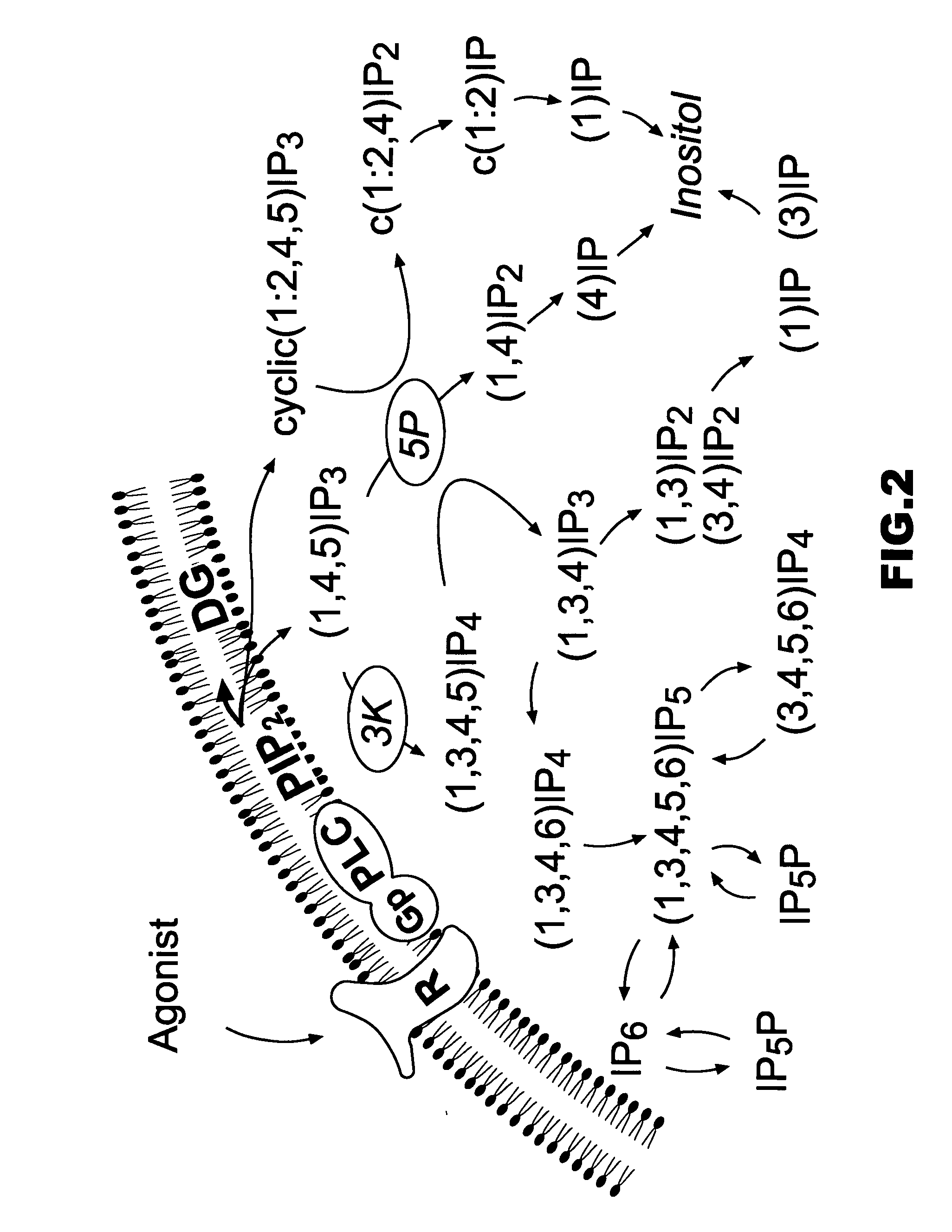
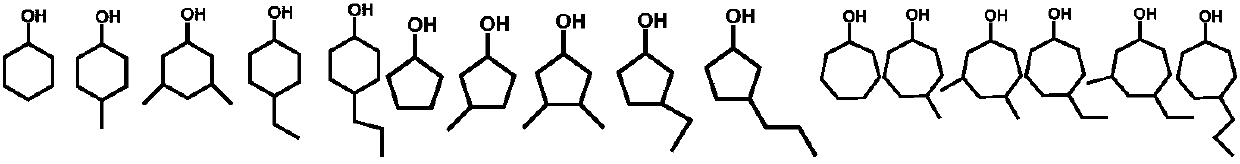
In organic chemistry, a cyclitol is a cycloalkane containing at least three hydroxyl, each attached to a different ring carbon atom. The general formula for an unsubstituted cyclitol is CₙH₂ₙ₋ₓ(OH)ₓ or CₙH₂ₙOₓ where 3 ≤ x ≤ n.
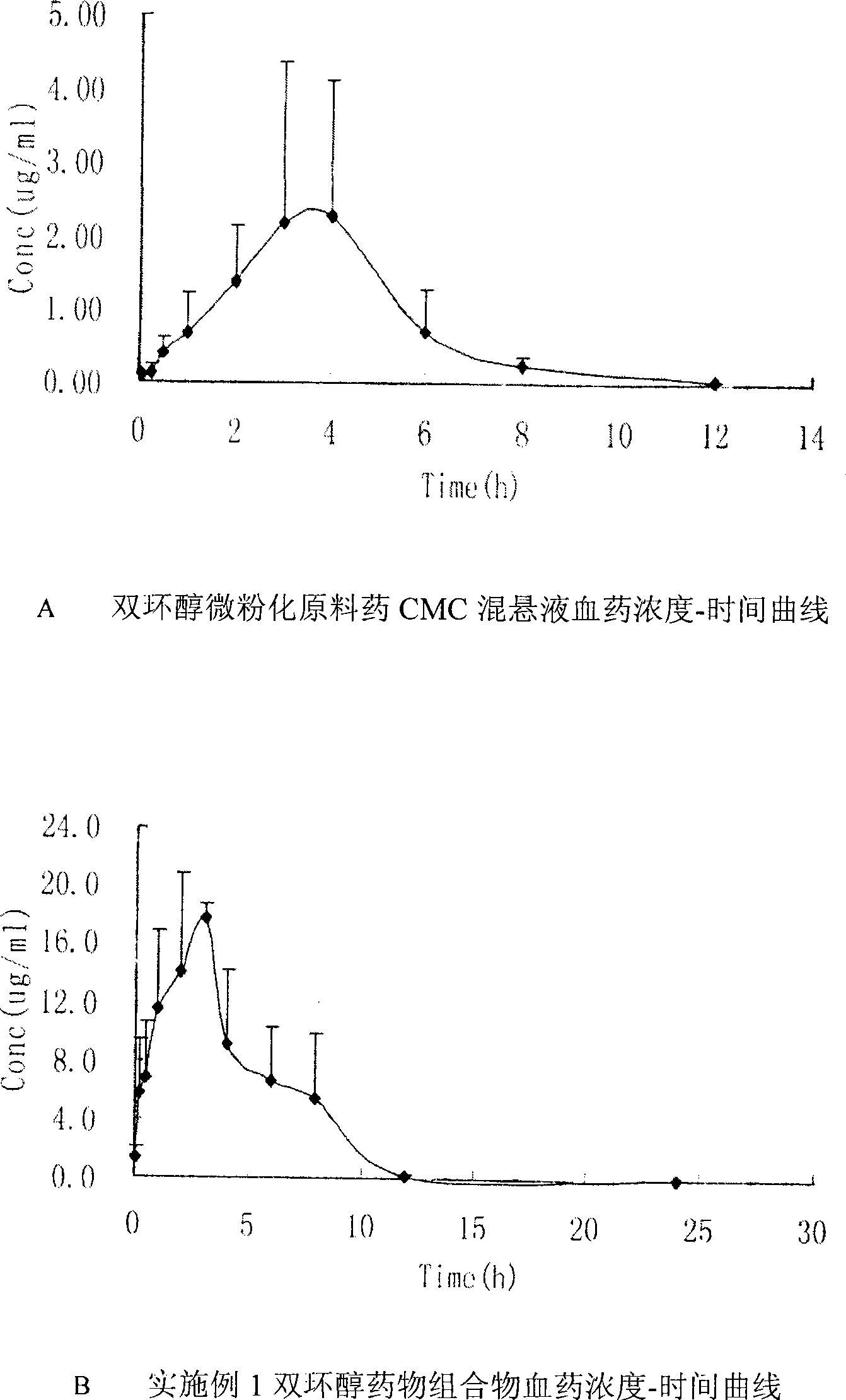
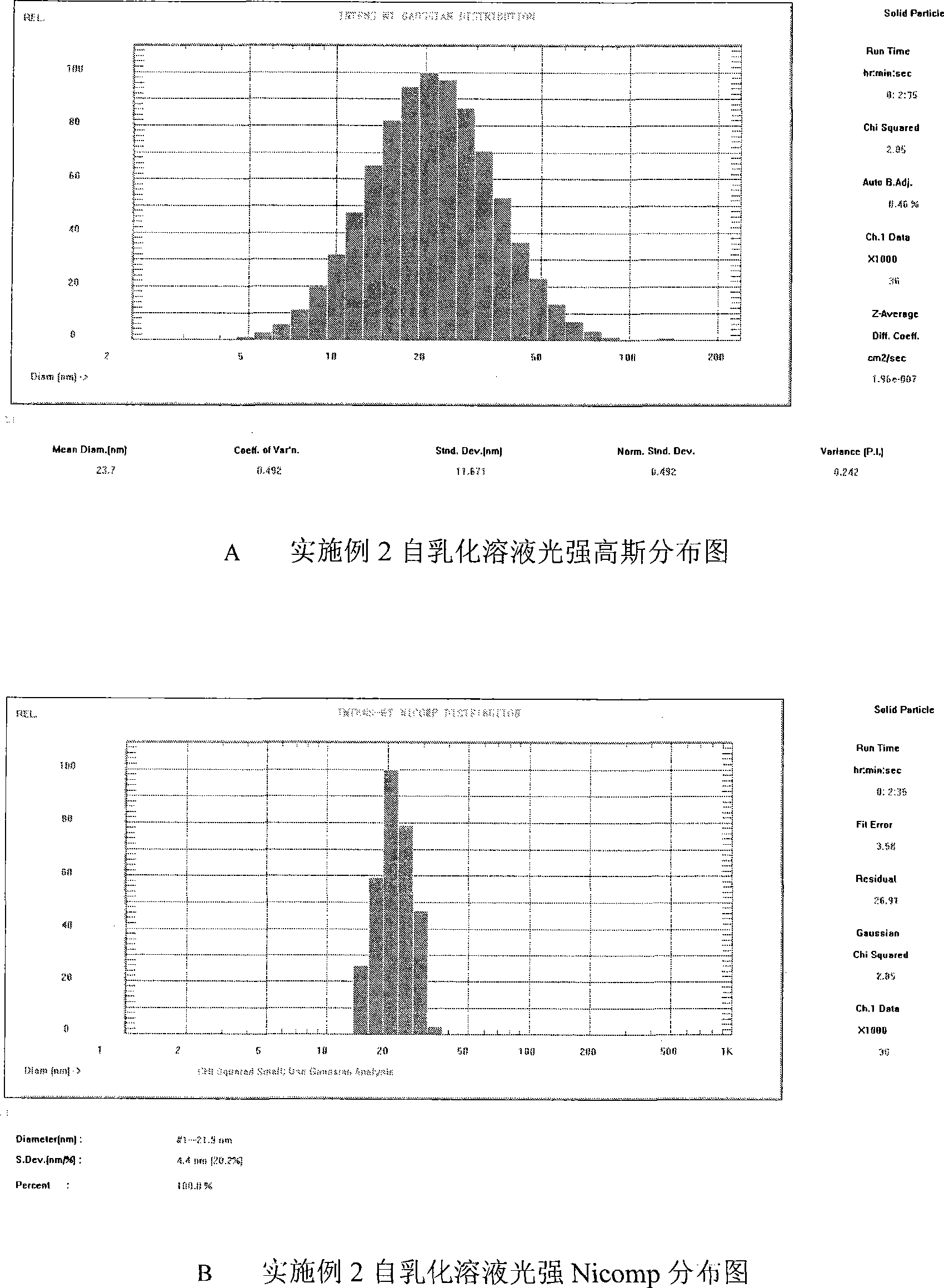
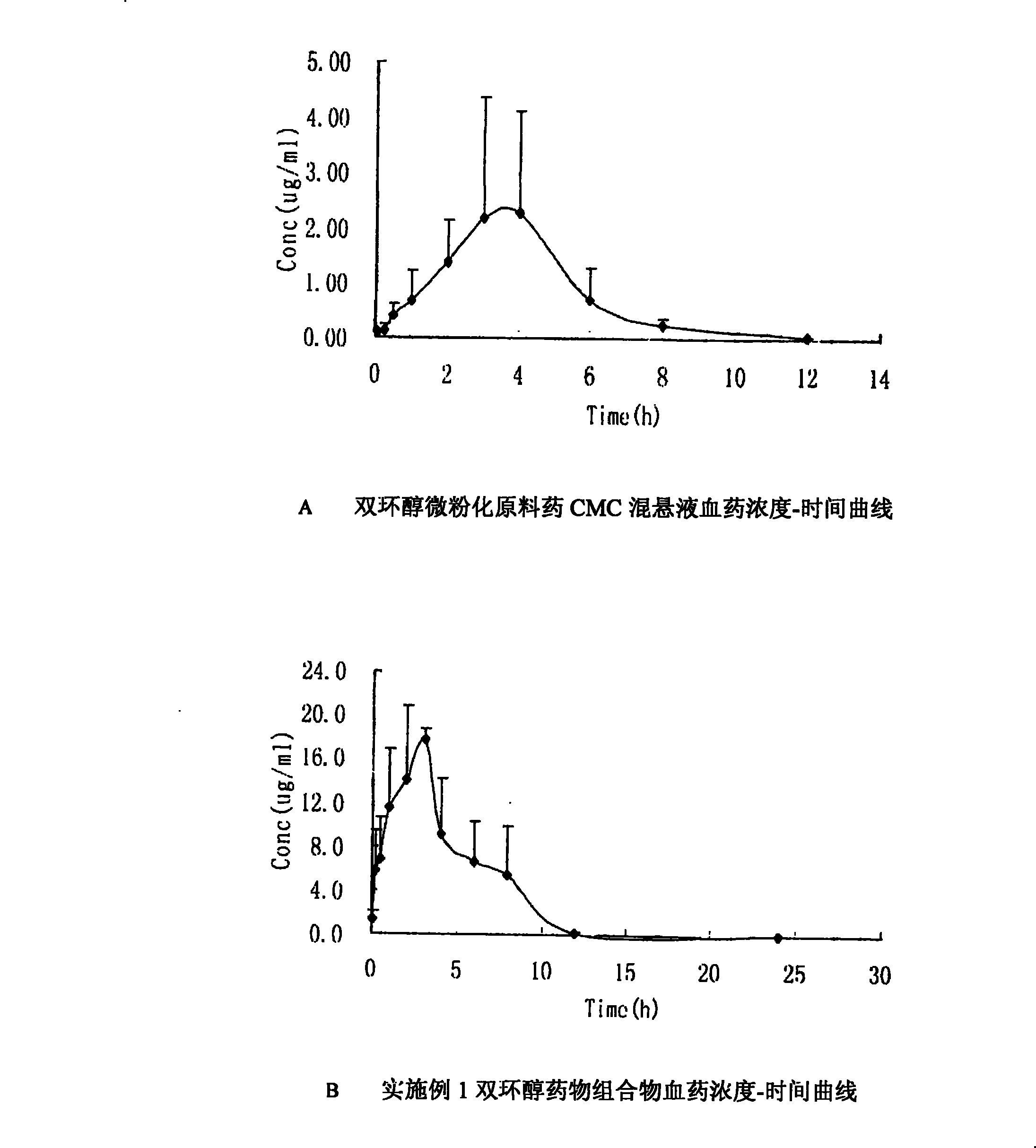
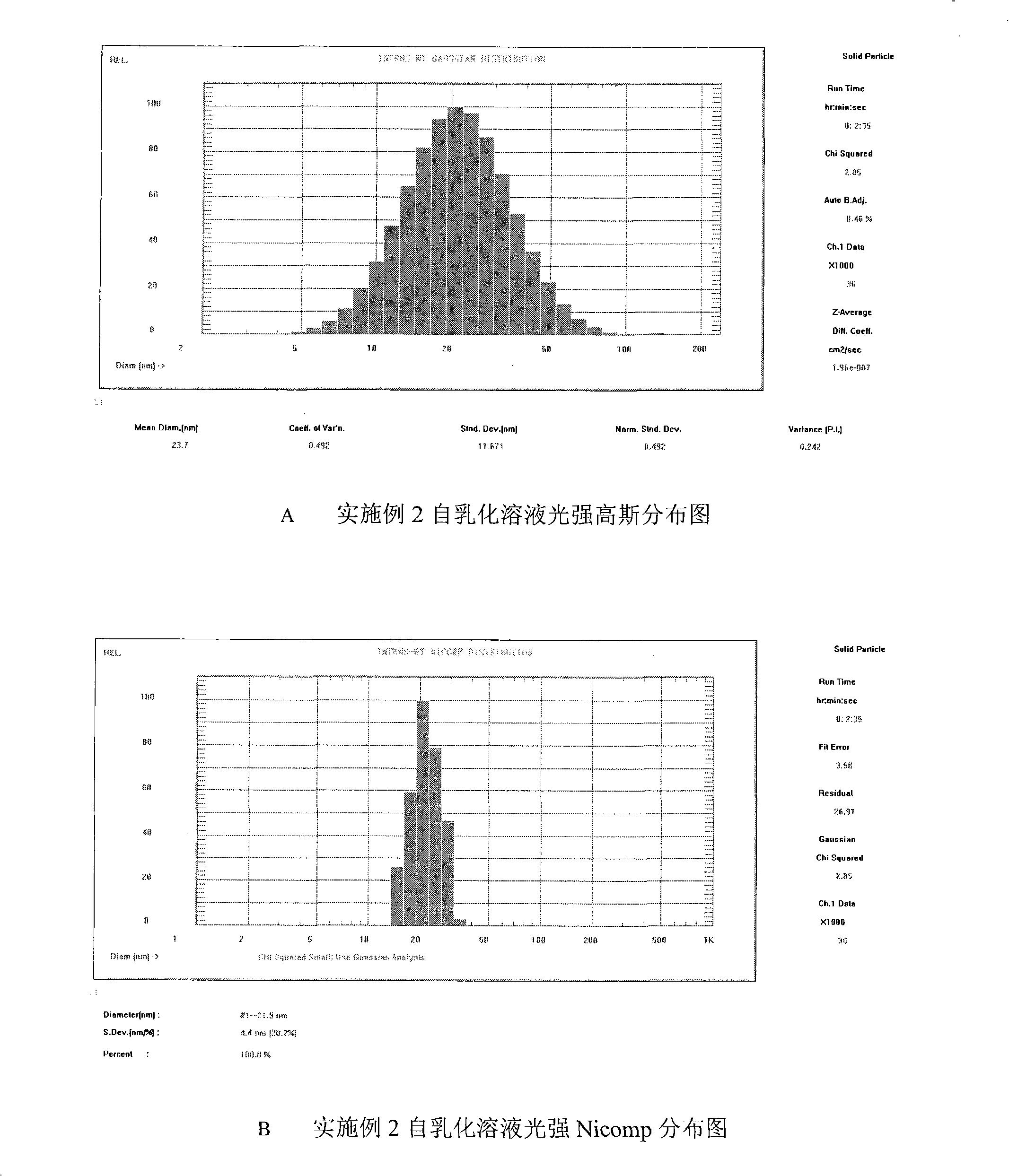
Double-cyclitol medicine composition containing surfactant and preparation method thereof
ActiveCN101390851AImprove bioavailabilityImprove clinical efficacyDigestive systemAntiviralsClinical efficacyAdditive ingredient
The invention discloses a bicyclo-ethanol medicine combination which contains surfactant, and the preparation thereof. Bicyclo-ethanol is dissolved in medicine loading substrate which is composed of one or more types of the following ingredients, surfactant, cosurfactant and oil, so as to form the medicine combination which is oily solution or semisolid. The medicine combination in different formulations can be added with water to form emulsion, microemulsion or transparent rapidly. When the medicine combination is taken orally, the absorption of difficult soluble bicyclo-ethanol is enhanced through self-emulsification, self micro-emulsification or solubilization, so as to reduce the individual difference and improve the bioavailability and clinical treatment effect of bicyclo-ethanol. The medicine combination has simple preparation process and is applicable to preparing various preparations. The invention also includes capsules, soft capsules, controlled-release capsule, osmotic pump capsules or other proper types of preparation which are prepared through the medicine combination.
Owner:BEIJING UNION PHARMA FACTORY
Cyclitols and Their Derivatives and Their Therapeutic Applications
The present invention is directed to polyphosphorylated and pyrophosphate derivatives of cyclitols. More particularly, the invention relates to polyphosphorylated and pyrophosphate derivatives of inositols. The invention also relates to compositions of the polyphosphorylated and pyrophosphate derivatives of inositol and other similar, more lipophilic derivatives, and their use as allosteric effectors, cell-signaling molecule analogs, and therapeutic agents.
Owner:NORMOXYS +1
Alkyl-substituted naphthalene alkane preparation method and purpose of naphthalene alkane as jet fuel
ActiveCN107628920AWide variety of sourcesLowering the freezing pointHydrocarbon from oxygen organic compoundsLiquid carbonaceous fuelsAlkaneAlkyl transfer
The invention discloses an alkyl-substituted naphthalene alkane preparation method and a purpose of the naphthalene alkane as a jet fuel. The method comprises the following steps: under existence of an acid catalyst, one or more of cycloolefin, cyclitol and / or cyclic ketone is subjected to an alkylation reaction and a hydrogen transfer reaction to obtain the alkyl-substituted naphthalene alkane. The prepared alkyl-substituted naphthalene alkane is taken as the jet fuel. According to the method, a complex operation flow is not required, production equipment is simple, investment cost is low, external hydrogen supplying is not required, strong acid is used for substituting the expensive catalyst, and the production cost is reduced.
Owner:TIANJIN UNIV
Method of preparing aldehyde or ketone by alcohol liquid phase selection oxidization accelerated by micro-wave
InactiveCN101054341AReduce dosageImprove economyOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by oxidationKetoneOxygen
The present invention belongs to technology field for preparing aldehyde or ketone by oxidizing alcohol, particularly which is microwave-promoted method for preparing aldehyde or ketone by alcohol liquid-phase selective catalytic oxidation. The present invention prepares aldehyde or ketone by catalytic oxidation of alcohol under microwave irradiation, which takes sodium tungstate and tungstic heteropolyacid salts as catalyst, takes bidentate ligand containing nitrogen and oxygen as additive and takes hydrogen peroxide as preparing oxidant. The catalyst system used in the present invention uses very small quantity compared with raw materials alcohol and oxidant, and it has high atomic economy and will not pollutes environment.There is not any organic solvent and phase transfer catalyst used in entire procedure, which is easy to separate for obtaining product by simple operational, whose reaction conditional is mild and easy to operate. The present invention is suitable for the reaction for generating aldehyde by oxygenizing various alcohols which include aliphatic alcohol, ester cyclitol, aromatic alcohol and diatomic alcohol, and which have high conversion rate to alcoholic and high selectivity to aldehyde or ketone.
Owner:FUDAN UNIV
Oxidizing method of cycloalkane compound
ActiveCN109232209AImprove conversion rateHigh selectivityPreparation by oxidation reactionsOrganic compound preparationCeriumCatalytic oxidation
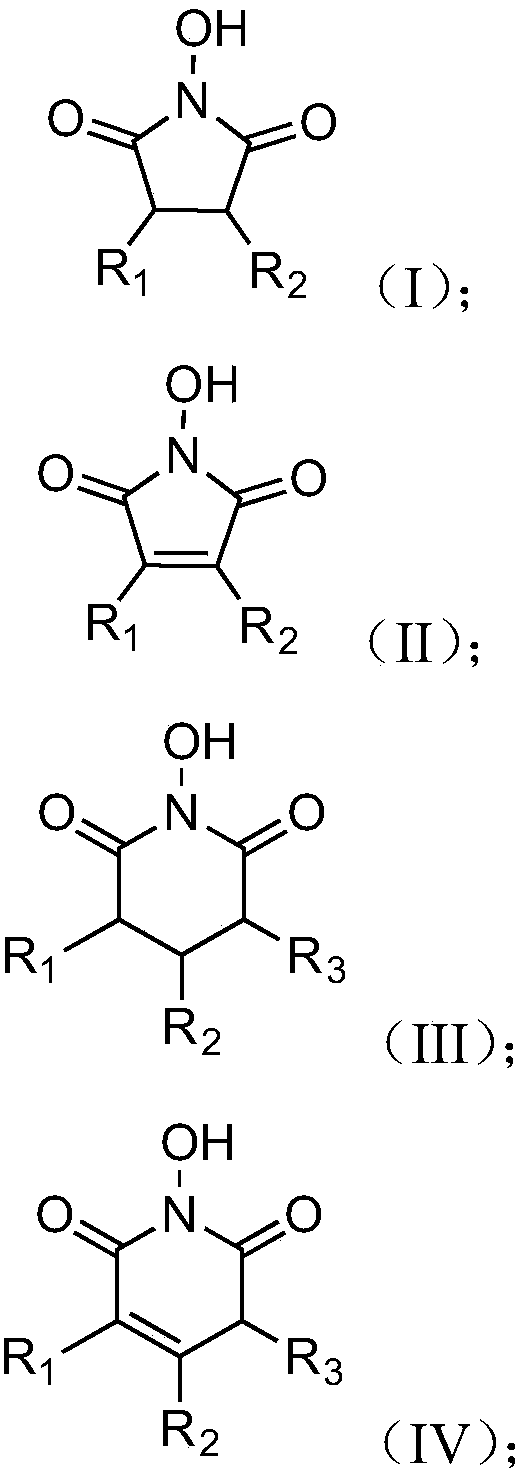
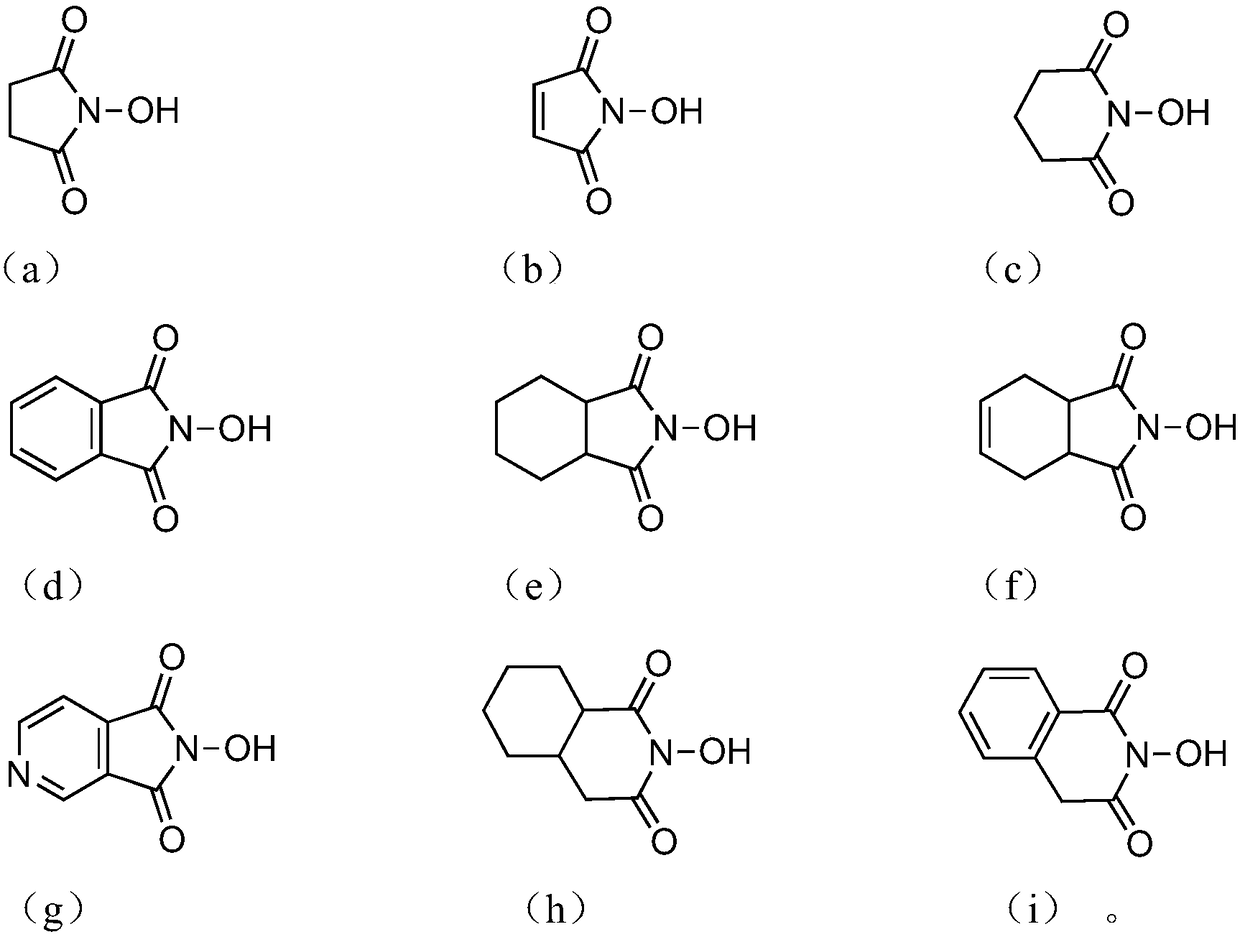
The invention discloses an oxidizing method of a cycloalkane compound. The oxidizing method comprises the following steps of using the cycloalkane compound as the raw material, and catalyzing by a catalysis system to oxidize under the oxygen-containing atmosphere, so as to obtain the corresponding cycloketone compound and cyclitol compound, wherein the catalysis system comprises a cyclic organic nitrogen and oxygen free radical precursor and a metal cerium salt; the cyclic organic nitrogen and oxygen free radical precursor is selected from the following four structures; in the formulae, R1, R2and R3 are independently selected from hydrogen atom, alkyl, cycloalkyl, aryl, heterocycle, hydroxyl, nitryl, or halogen, or at least two of R1, R2 and R3 form loops; the metal cerium salt is selected from soluble salt of trivalent cerium and / or soluble salt of tetravalent cerium. The oxidizing method has the advantages that the conditions are mild, and the safety is high; on the basis of higherconversion rate of cycloalkane compound, the selectivity of the target products, such as cycloketone compound and cyclitol compound, is improved. The formulae are shown in the attached figure.
Owner:ZHEJIANG UNIV +1
Double-cyclitol medicine composition containing surfactant and preparation method thereof
The invention discloses a bicyclo-ethanol medicine combination which contains surfactant, and the preparation thereof. Bicyclo-ethanol is dissolved in medicine loading substrate which is composed of one or more types of the following ingredients, surfactant, cosurfactant and oil, so as to form the medicine combination which is oily solution or semisolid. The medicine combination in different formulations can be added with water to form emulsion, microemulsion or transparent rapidly. When the medicine combination is taken orally, the absorption of difficult soluble bicyclo-ethanol is enhanced through self-emulsification, self micro-emulsification or solubilization, so as to reduce the individual difference and improve the bioavailability and clinical treatment effect of bicyclo-ethanol. The medicine combination has simple preparation process and is applicable to preparing various preparations. The invention also includes capsules, soft capsules, controlled-release capsule, osmotic pumpcapsules or other proper types of preparation which are prepared through the medicine combination.
Owner:BEIJING UNION PHARMA FACTORY
Ink
The invention discloses ink which is prepared from the following ingredients in percentage by weight: 0.1 to 20.0 percent of inorganic nanometer materials and 80.0 to 99.9 percent of a solvent, wherein the solvent comprises at least one kind of cyclitol organic solvents. The print ink provided by the invention has proper viscosity and surface tension, and simultaneously has the advantages of gooddispersion performance, good volatilization performance and storage stability.
Owner:SHENZHEN TCL IND RES INST
Preparation method of baloxavir key intermediate and intermediate thereof
ActiveCN111808069AHarm reductionHigh yieldOrganic chemistryBulk chemical productionBenzaldehydeThio-
The invention discloses a preparation method of a baloxavir key intermediate and the intermediate thereof. The method includes subjecting 3,4-difluoro-2-methyl benzaldehyde shown as a formula (I) as araw material to a bromination reaction to obtain 3,4-difluoro-2-bromomethyl benzaldehyde shown in a formula (II), carrying out a substitution reaction to obtain 3,4-difluoro-2-(((2-bromophenyl) thio)methyl)-benzaldehyde shown in a formula (III), and finally, carrying out a nucleophilic addition reaction to synthesize a target product, namely 7,8-difluorodibenzo[b, e]thiepin-11(6H)-ol shown in aformula (IV). The specific reaction process is shown in the specification. According to the method, 2-bromothiophenol is used for replacing foul and virulent thiophenol, the reaction steps are reducedto three steps, and the preparation method is high in yield, low in cost, environmentally friendly and easy to operate.
Owner:CHINA PHARM UNIV
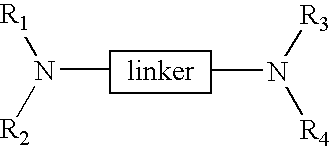
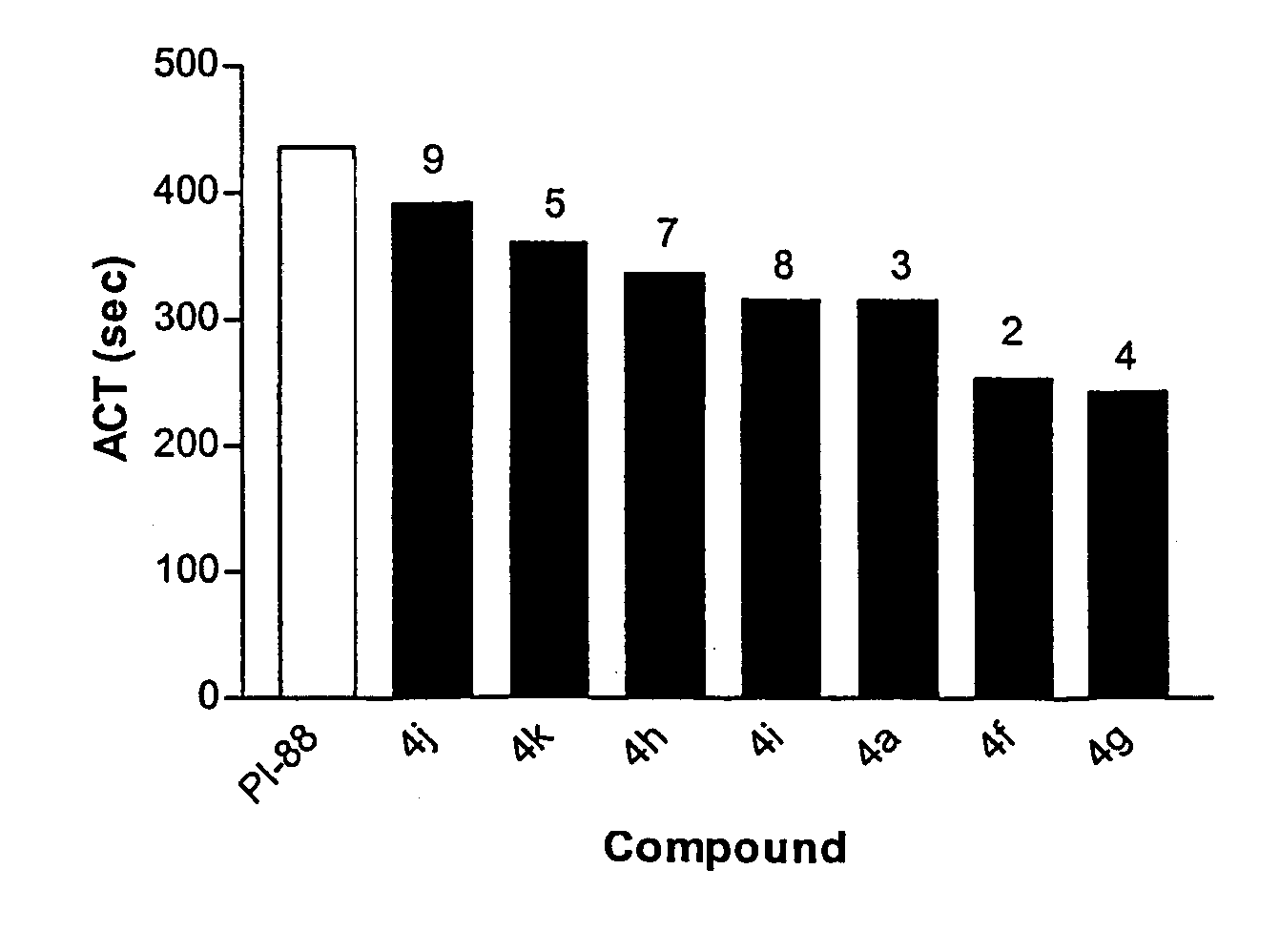
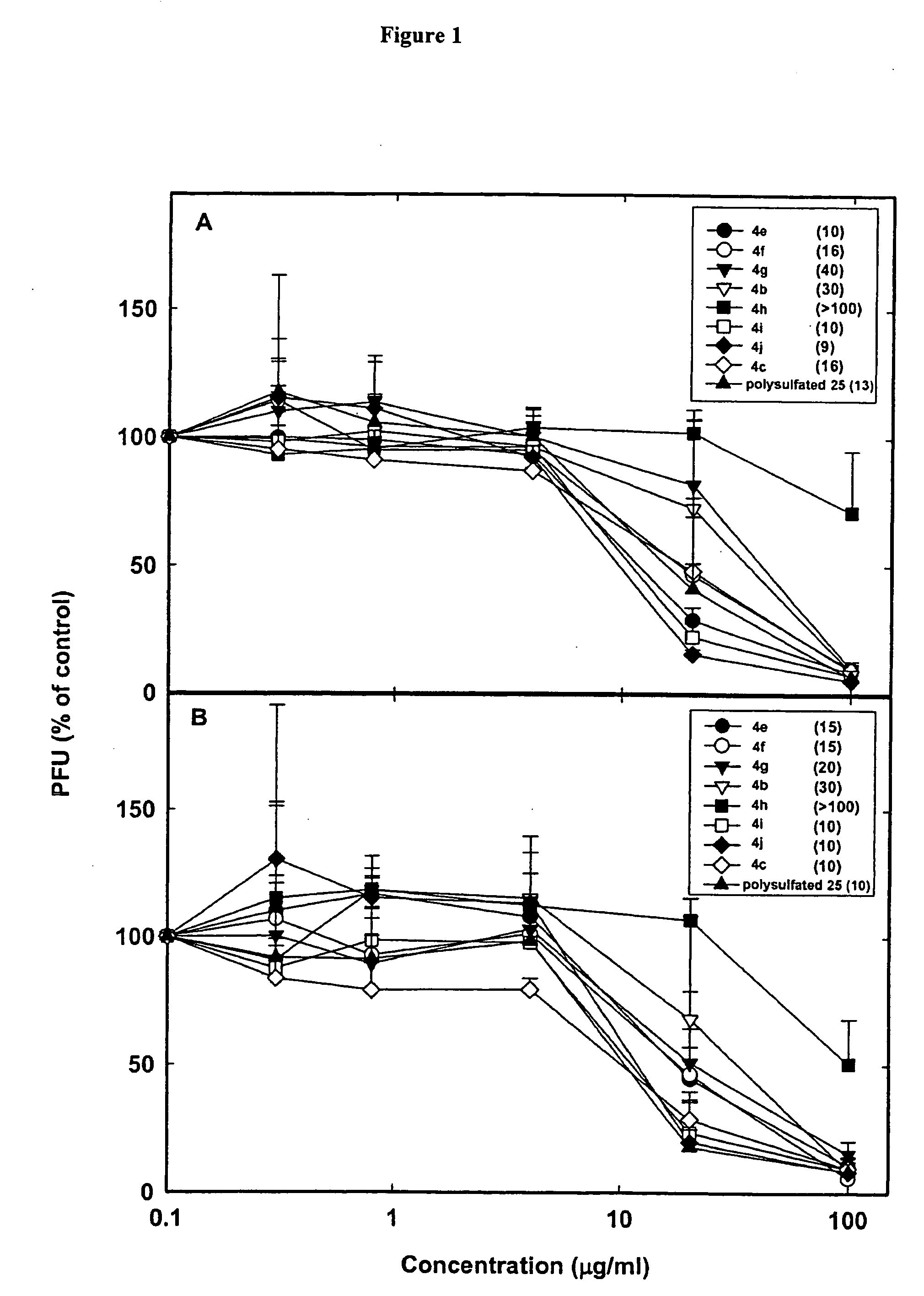
Linked cyclitols and their polysulfated derivatives
InactiveUS20040019021A1Severe side effectSuitable pH and isotonicity and stabilityBiocideOrganic chemistryArylLymphatic Spread
The invention relates to compounds of the following formula (I). In these compounds, R1, R2, R3 and R4 are each independently a substituted or unsubstituted cyclitol with a ring comprising six carbon atoms, or hydrogen, substituted or unsubstituted alkyl, cycloalkyl, aryl, acyl, alkyloxycarbonyl, or alkylaminocarbonyl. At least two of R1, R2, R3 and R4 comprise the substituted or unsubstituted cyclitol. The linker can be any one of the following: -(CH2)w-, -(CH2)x-C6H4-(CH2)x-, -(CH2)y-NR5-(CH2)y-, and -(CH2)z-HCR6-(CH2)z-; wherein: w, x, y and z are independently an integer having a value of 0-10; R5 is a substituted or unsubstituted cyclitol with a ring comprising six carbon atoms; and, R6 is -OH, -OSO3Na, -OSO3Na substituted with alkyl, cycloalkyl or aryl, or substituted or unsubstituted alkyl, cycloalkyl or aryl. The compounds can also include substituted or unsubstituted cyclitol carbamides with the linker bond at the carbamide nitrogen. In other embodiments, the invention provides pharmaceutical compositions which include the compounds, and use of the compounds in the prevention or treatment in a mammalian subject of a disorder resulting from angiogenesis, metastasis, inflammation and / or coagulation / thrombosis.
Owner:AUSTRALIEN NAT UNIV
Compounds and methods for the treatment of pain
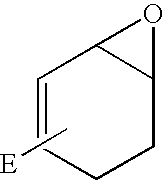
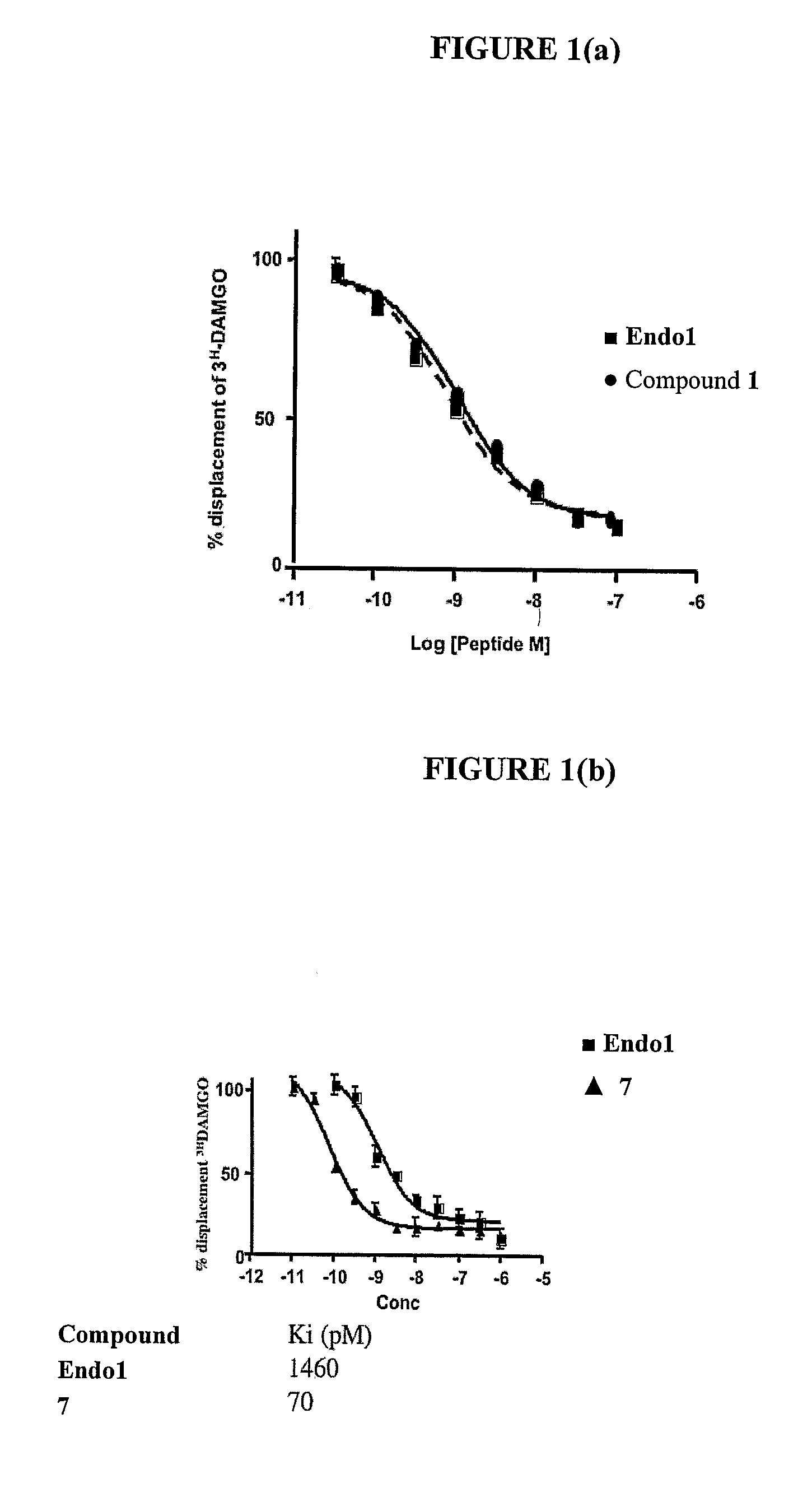
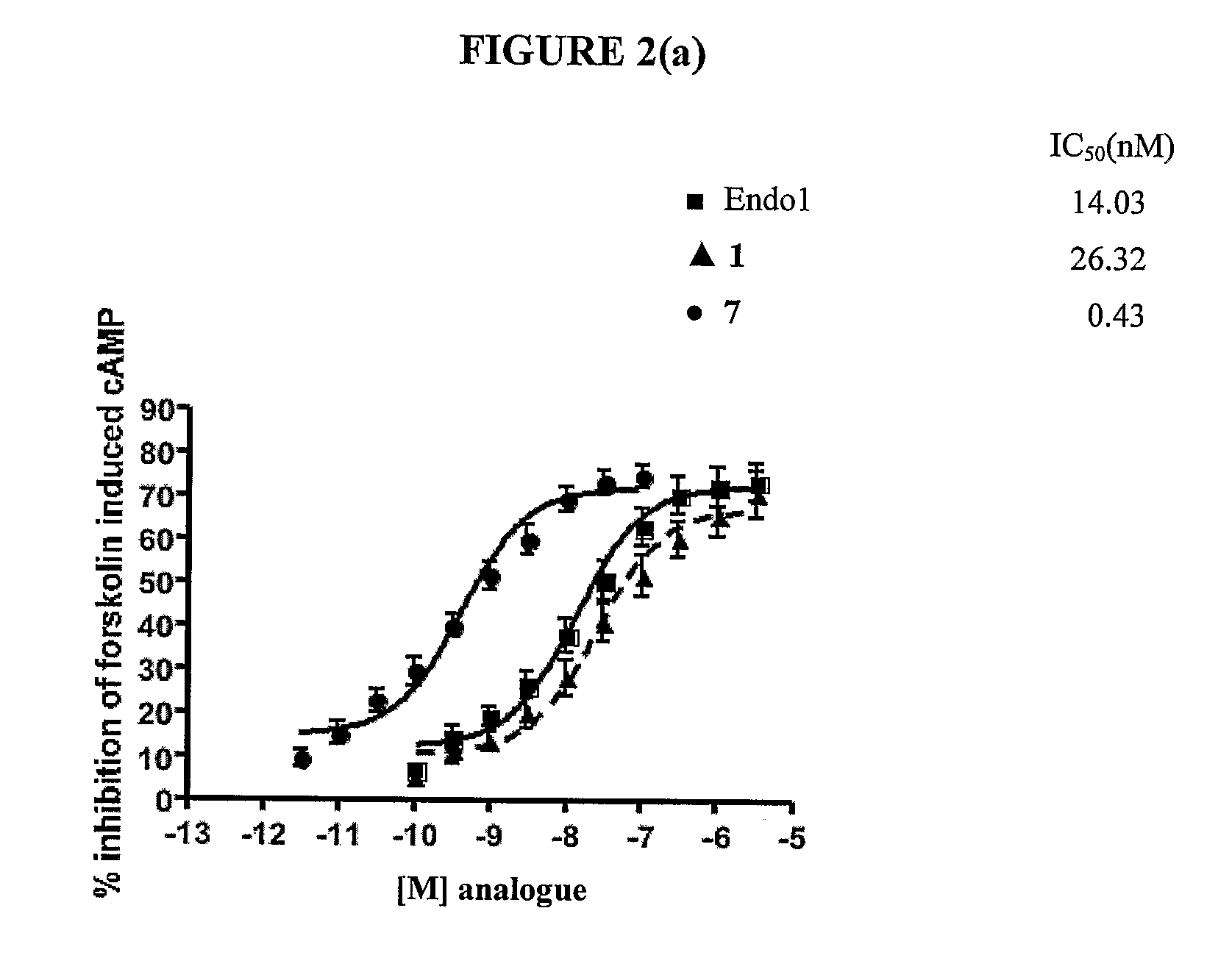
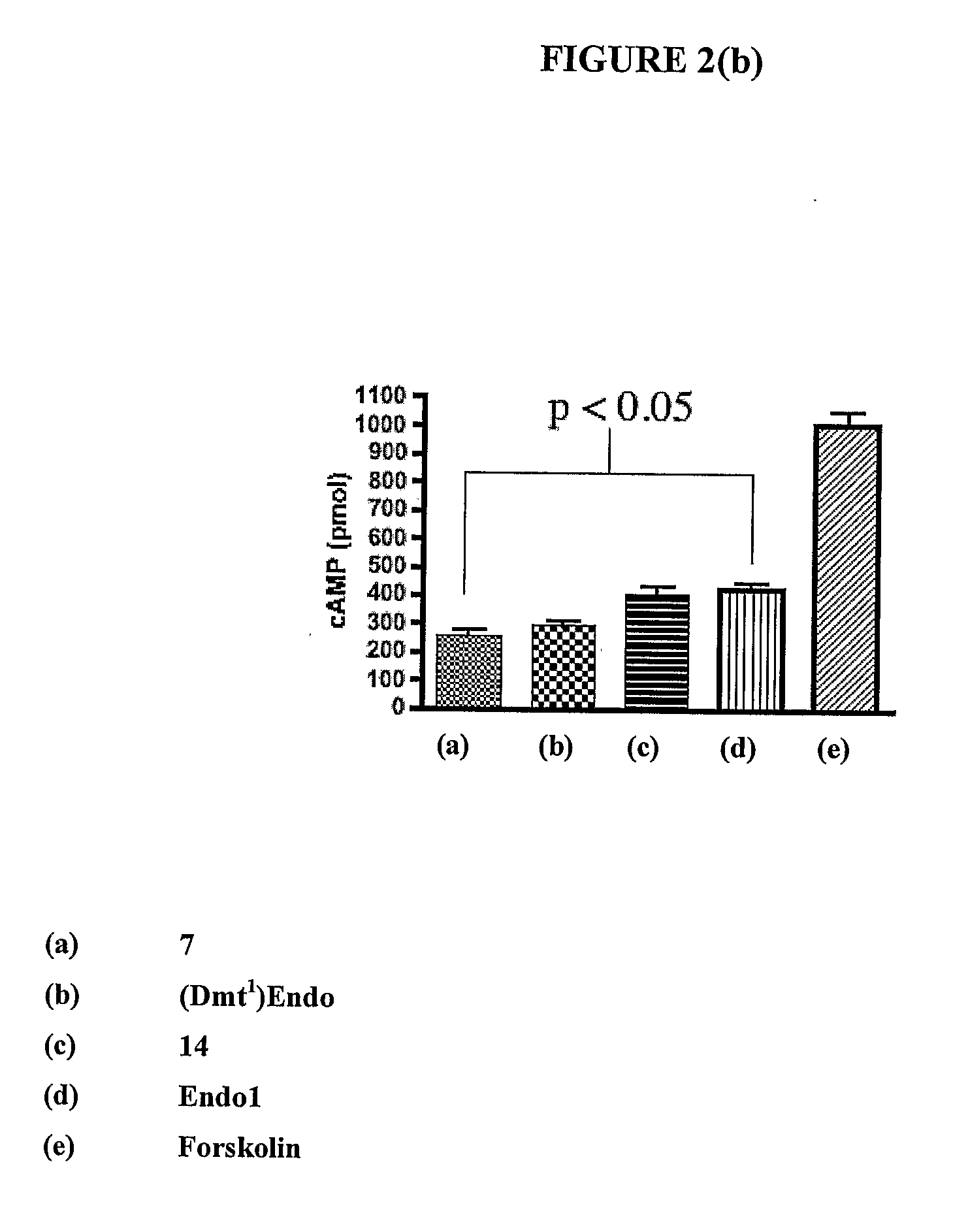
InactiveUS20100130581A1Good cell permeabilityImprove stabilityBiocidePeptide/protein ingredientsLipid moietyCyclitol
This invention relates to derivatives of an endomorphin, or of an endomorphin analog, comprising at least one moiety selected from a lipid moiety, a cyclitol moiety and a saccharide moiety.
Owner:THE UNIV OF QUEENSLAND
Method for catalytic oxidation preparation of lactone from cycloalkane
ActiveCN102336734BSimple processImprove effective utilizationOrganic chemistryMolecular sieve catalystsCatalytic oxidationSolvent
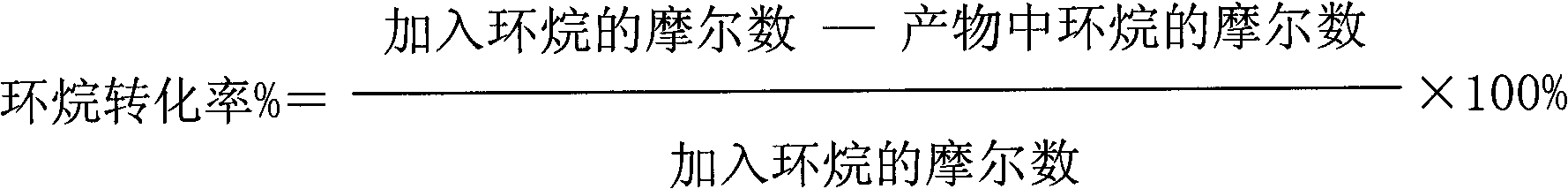
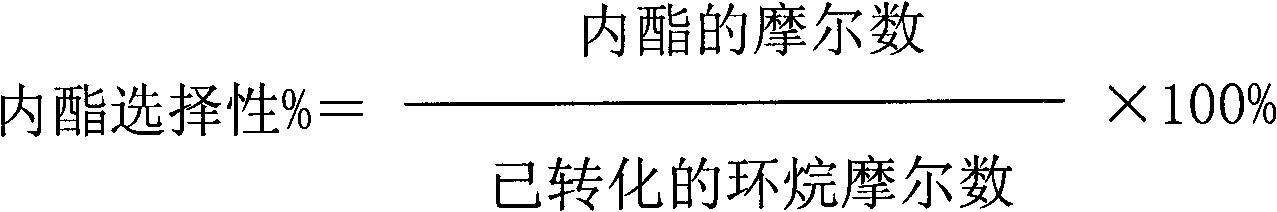
The invention discloses a method for catalytic oxidation preparation of lactone from cycloalkane, under the condition that the temperature is 0-180 DEG C and the pressure is 0.1-3 MPa, cycloalkane, an oxidant hydrogen peroxide, a solvent and a catalyst are reacted according to a mol ratio of 1:1-20 of cycloalkane to oxidant and a mass ratio of 0-100:1 of solvent to catalyst, the product is separated to obtain lactone, unreacted cycloalkane and intermediate by-product cyclitol and cyclic ketone are circularly used, wherein the catalyst is a composite composed of a zinc-containing compound and titanium silicon molecular sieve, the mass ratio of zinc-containing compound to titanium silicon molecular sieve in the composite is 0.01-10, the ratio of mol ratio of zinc in the zinc-containing compound to the mol ratio of titanium in the titanium silicon molecular sieve is 0.05-50, the mol ratio of silicon to titanium in titanium silicon molecular sieve is 5-250. The method has high catalytic oxidation activity and selectivity, especially has good catalytic activity stability. According to the method, no inhibitor or initiator is required and no special production equipment requirement is provided, the production process is simple which is beneficial to industrial production and application.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalyst for preparing dinitrile compound from alicyclic hydrocarbon and synthetic method of dinitrile
PendingCN113996322AHigh reactivityGood choiceHeterogenous catalyst chemical elementsPreparation by hydrocarbon ammoxidationPtru catalystBiochemical engineering
The invention provides a catalyst for preparing a dinitrile compound from alicyclic hydrocarbon, which can realize ring opening of alicyclic alcohol, alicyclic ketone, cycloolefin and cycloalkane and complete ammoxidation reaction at the same time to obtain the dinitrile compound. The catalyst is high in reaction activity and good in selectivity, the high-quality dinitrile compound can be prepared, the technological process is easy to control, and industrial production is facilitated.
Owner:ANSHAN HIFICHEM CO LTD
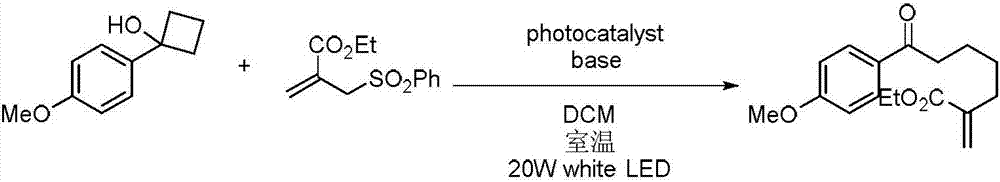
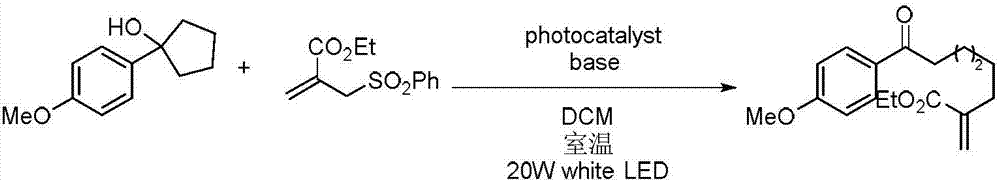
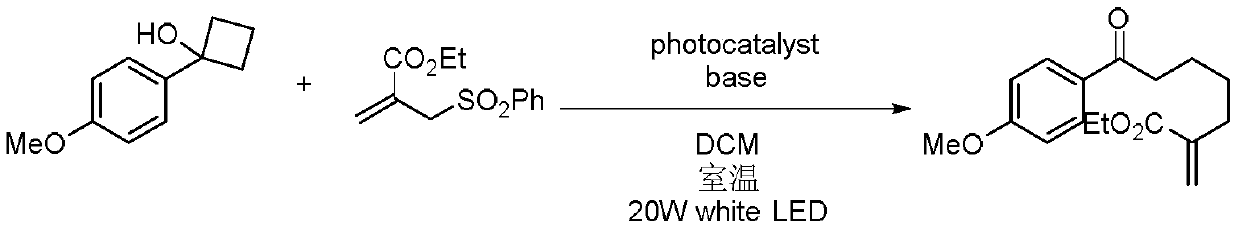
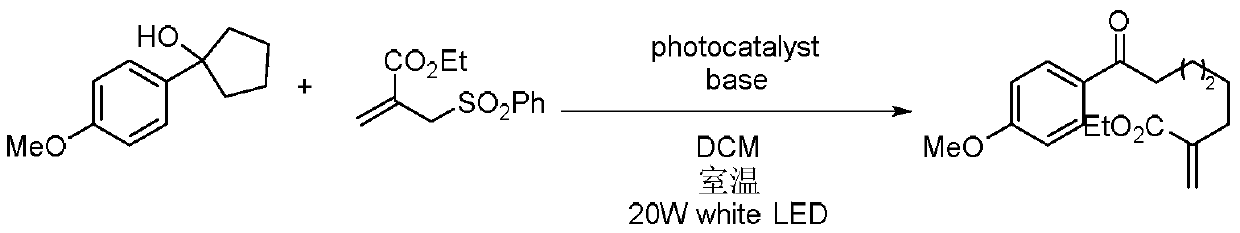
Method for preparing polysubstituted distal-end allyl ketone derivative
ActiveCN107973715AVarious conversion pathwaysHigh research valueOrganic compound preparationCarboxylic acid esters separation/purificationChromatographic separationOrganic synthesis
The invention relates to a method for preparing a polysubstituted distal-end allyl ketone derivative. An object of the invention is to provide the method for preparing the polysubstituted distal-end allyl ketone derivative. The method comprises the steps of dissolving a third-grade cyclitol derivative, an unsaturated olefin compound and a photocatalyst in an organic solvent in a nitrogen gas atmosphere at room temperature, carrying out uniform mixing, carrying out a lighting reaction under a blue LED (light emitting diode) lamp, carrying out rotary evaporation to remove the solvent, then, carrying out silica-gel column chromatographic separation and purification, thereby obtaining a product, i.e., the polysubstituted distal-end allyl ketone derivative. According to the method provided by the invention, the reaction can be carried out at normal temperature under normal pressure the reaction conditions are mild, and the yield can reach 80%; and the method has the advantages of simplicityin operation, no pollution, safety, environment-friendliness and low cost. The invention is applied to the field of organic synthesis.
Owner:HARBIN INST OF TECH
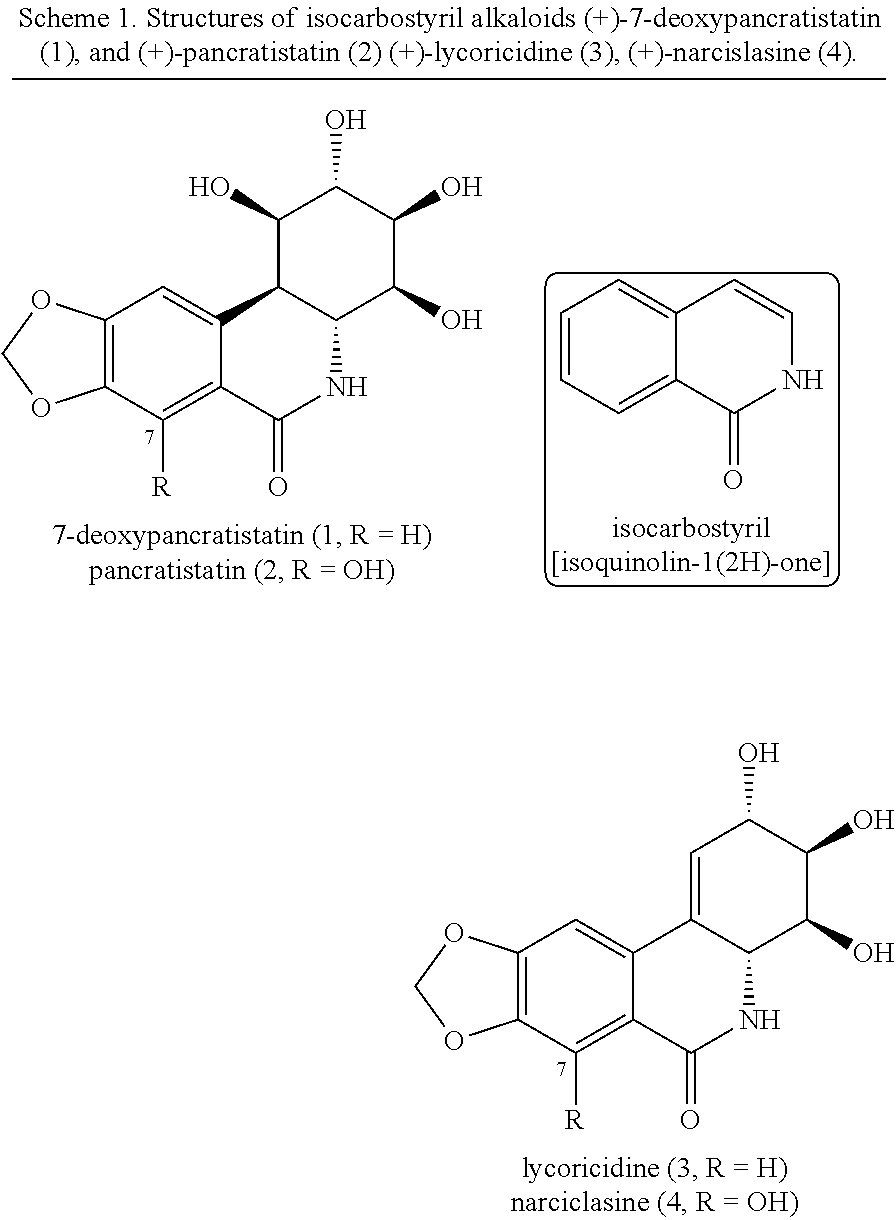
Isocarbostyril alkaloids and functionalization thereof
PendingUS20220064176A1Good metabolic stabilityImprove property stabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPancratistatinEnantio selectivity
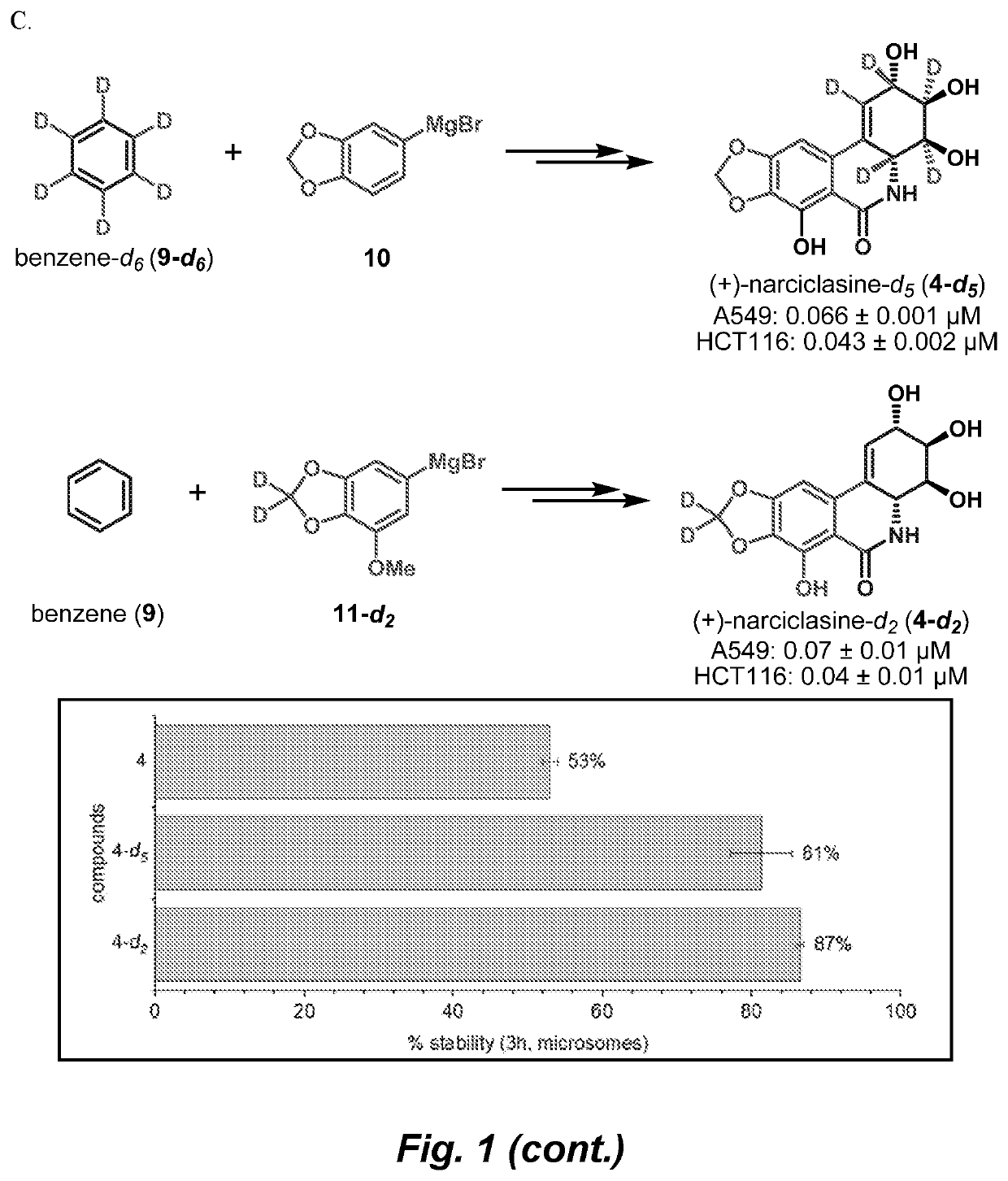
Enantioselective total syntheses of the anticancer isocarbostyril alkaloids (+)-7-deoxypancratistatin, (+)-pancratistatin, (+)-lycoricidine, and (+)-narciclasine are described. Our strategy for accessing this unique class of natural products is based on the development of a Ni-catalyzed dearomative trans-1,2-carboamination of benzene. The effectiveness of this dearomatization approach is notable, as only two additional olefin functionalizations are needed to construct the fully decorated aminocyclitol cores of these alkaloids. Installation of the lactam ring has been achieved through several pathways and a direct interconversion between natural products was established via a late-stage C-7 cupration. Using this synthetic blueprint, we were able to produce natural products on a gram scale and provide tailored analogs with improved activity, solubility, and metabolic stability.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
A kind of oxidation method of cycloalkane compound
ActiveCN109232209BImprove conversion rateHigh selectivityPreparation by oxidation reactionsOrganic compound preparationArylCatalytic oxidation
The invention discloses an oxidizing method of a cycloalkane compound. The oxidizing method comprises the following steps of using the cycloalkane compound as the raw material, and catalyzing by a catalysis system to oxidize under the oxygen-containing atmosphere, so as to obtain the corresponding cycloketone compound and cyclitol compound, wherein the catalysis system comprises a cyclic organic nitrogen and oxygen free radical precursor and a metal cerium salt; the cyclic organic nitrogen and oxygen free radical precursor is selected from the following four structures; in the formulae, R1, R2and R3 are independently selected from hydrogen atom, alkyl, cycloalkyl, aryl, heterocycle, hydroxyl, nitryl, or halogen, or at least two of R1, R2 and R3 form loops; the metal cerium salt is selected from soluble salt of trivalent cerium and / or soluble salt of tetravalent cerium. The oxidizing method has the advantages that the conditions are mild, and the safety is high; on the basis of higherconversion rate of cycloalkane compound, the selectivity of the target products, such as cycloketone compound and cyclitol compound, is improved. The formulae are shown in the attached figure.
Owner:ZHEJIANG UNIV +1
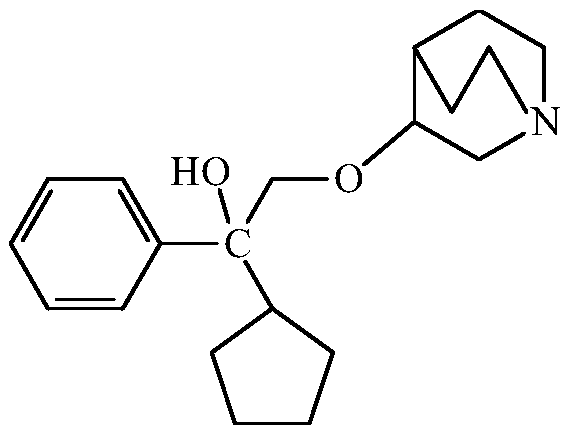
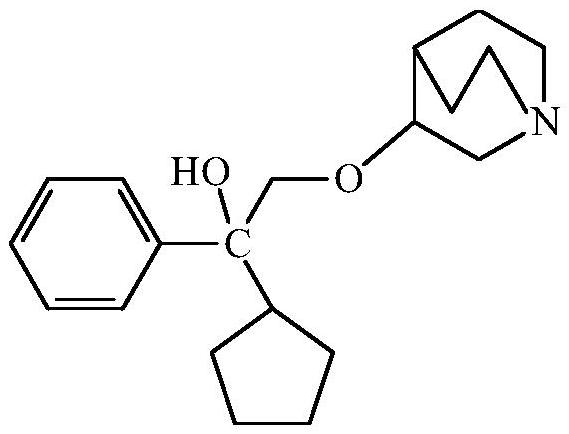
Method for purifying 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy) quinuclidine and preparing penehyclidine hydrochloride
The invention provides a method for purifying 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy) quinuclidine and preparing penehyclidine hydrochloride. The method comprises the following steps: (1) mixing 3-quinuclidinol with a dimethyl sulfoxide solution, and reacting with sodium hydride and a 1,1-phenyl amyl oxirane solution; (2) adding purified water and ethyl acetate to carry out extraction, collecting the organic phase, washing, carrying out back extraction with a hydrochloric acid solution, and collecting the water layer; (3) adjusting the pH value of the water layer to 5-7, washing the water layer by ethyl acetate, adjusting the pH value to 9-12 again, extracting the water layer by ethyl acetate, and collecting the organic phase; and (4) filtering, and carrying out reduced pressure concentration to obtain 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy) quinuclidine oil. According to the method, by separating and purifying 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy) quinuclidine, the yield and quality of a target product are improved, and a penehyclidine hydrochloride finished product with a high yield and high purity is obtained through one-step salifying crystallization.
Owner:北京鑫开元医药科技有限公司
Denatured carob flour (dcf) with a low content of soluble tannins and sugars, meant for human consumption and process to obtain it
The present invention provides an improved denatured carob flour, which includes 2 to 15% sugars, 0.2 to 1.5% cyclitols (pinitol), 2 to 10% lignins, 10 to 30% celluloses, 3 to 20% hemicelluloses, 1 to 6% pectins, 25 to 55% condensed tannins, 3 to 9% protein and less than 8% water. The invention further provides a process to obtain a denatured carob flour that includes the following steps: a. cleaning the whole fruit; b. crushing the carob fruits; c. separating the carob seeds and kibbled carob pulp, d. toasting between 130 to 200° C.; e. an extracting process; f. separating; g. milling 90% of particles below 250 pm; h. separating; i. drying below 8%; and j. classifying (sieving).
Owner:INVESTIGACION Y NUTRICION +1
Method for establishing radix stellariae tissue culture systems
InactiveCN107691219AHigh reproductive coefficientImprove seedling ratePlant tissue cultureHorticulture methodsPinitolLateral root
The invention discloses a method for establishing radix stellariae tissue culture systems. Radix stellariae is a caryophyllaceae stem plant, the roots of the radix stellariae need to be dug out from August to October when the stems and leaves of the radix stellariae wither, rhizomes and fibrous roots and sediment on the roots need to be removed, and the radix stellariae needs to be dried in the sun. The medicinal properties of the radix stellariae include that the cylindrical roots of the radix stellariae occasionally have lateral roots, the surfaces of the roots are light yellow or yellowishwhite, the root tips have a plurality of tuberculate stem residues, the roots are light and fragile and are easy to break, breaking faces are uneven and loose and have crack, and radial texture is formed by xylem bundles and rays in a yellow-white alternative manner. The radix stellariae has light smell and slightly sweet taste. The radix stellariae has uniform root lengths and light yellow cortex. The radix stellariae mainly contains saponin and cyclitols such as di-pinitol. Effects of clearing deficient heat can be realized by the radix stellariae. The radix stellariae can be used for feverdue to yin deficiency, fever due to infantile malnutrition and the like. The method has the advantages that stems with buds are used as explants, radix stellariae tissue culture regenerated plants canbe successfully obtained by the aid of steps of induction, proliferation, rooting, seedling exercising, transplanting and the like, and the systematic radix stellariae tissue culture plant regeneration systems can be established by the aid of the method; the radix stellariae tissue culture systems are high in reproduction coefficient, seedling rate and earning.
Owner:李正美
Water-soluble photochromic compound
A diarylethene compound represented by formula (I)wherein, Sg is a monovalent sugar residue formed by removing one hydroxyl group from a sugar compound or a monovalent protected sugar residue formed by removing one hydroxyl group from a sugar compound in which one or more other hydroxyl groups are protected, and wherein the sugar is selected from the group consisting of a six-membered ring sugar, a five-membered ring sugar, cyclitol, and oligosaccharides containing a six-membered ring sugar, a five-membered ring sugar, or cyclitol;U is —(CH2)n—, —CH2—U′—, or —C(═O)—whereinn is an integer of 1 to 5,U′ is a C2-C10 branched alkylene group binding to Ar); andAr is a group represented by formula (A1) or (A2);wherein,X is S, SO2, NR3 in which R3 is a C1-C3 alkyl group, or O,R is C1-C4 alkyl group,R1 and R2 are independently a C1-C3 alkyl group,a is 0 or 1,b is an integer of 0-3, and* represents a bond with U;Y is a hydrogen atom or a halogen atom; andm is an integer of 3 to 5.
Owner:RIKKYO EDUCATIONAL +1
Kinase mimic catalysts for asymmetric synthesis of phosphorylated inositols and cycloalkanols
InactiveUS7037691B2High enantiomeric purityHigh yieldPeptide/protein ingredientsHydrolasesPhosphorylationPhosphoric acid
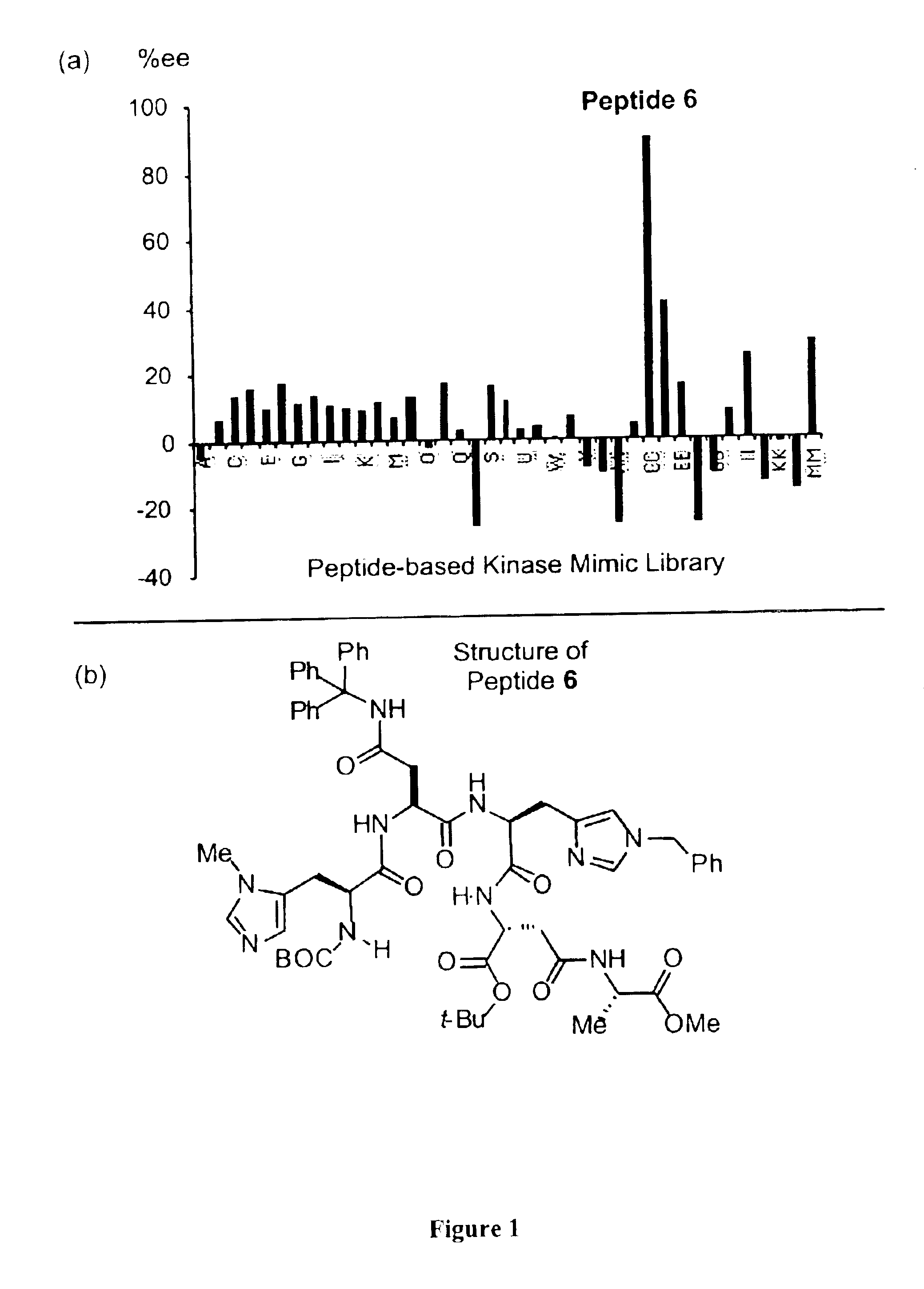
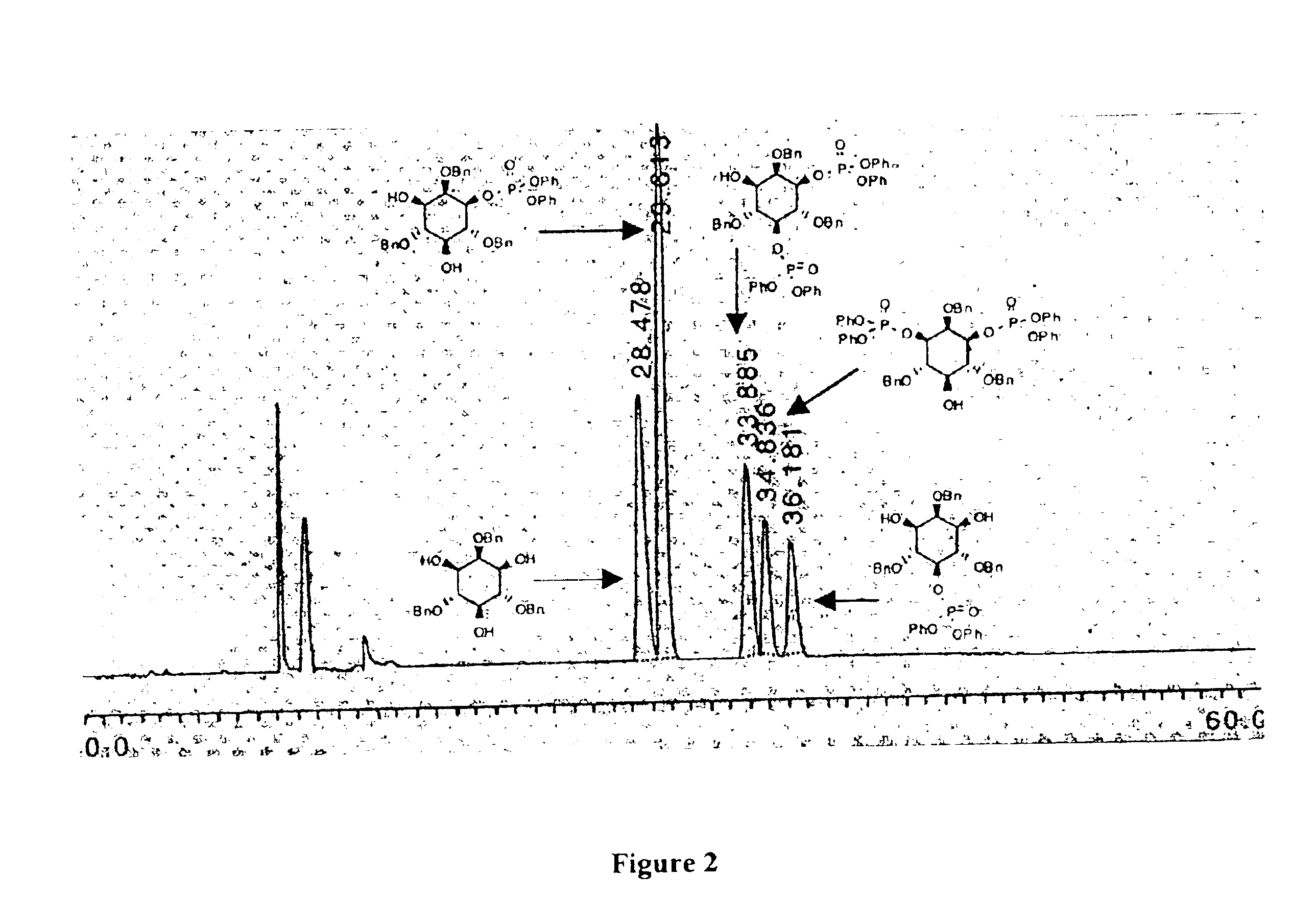
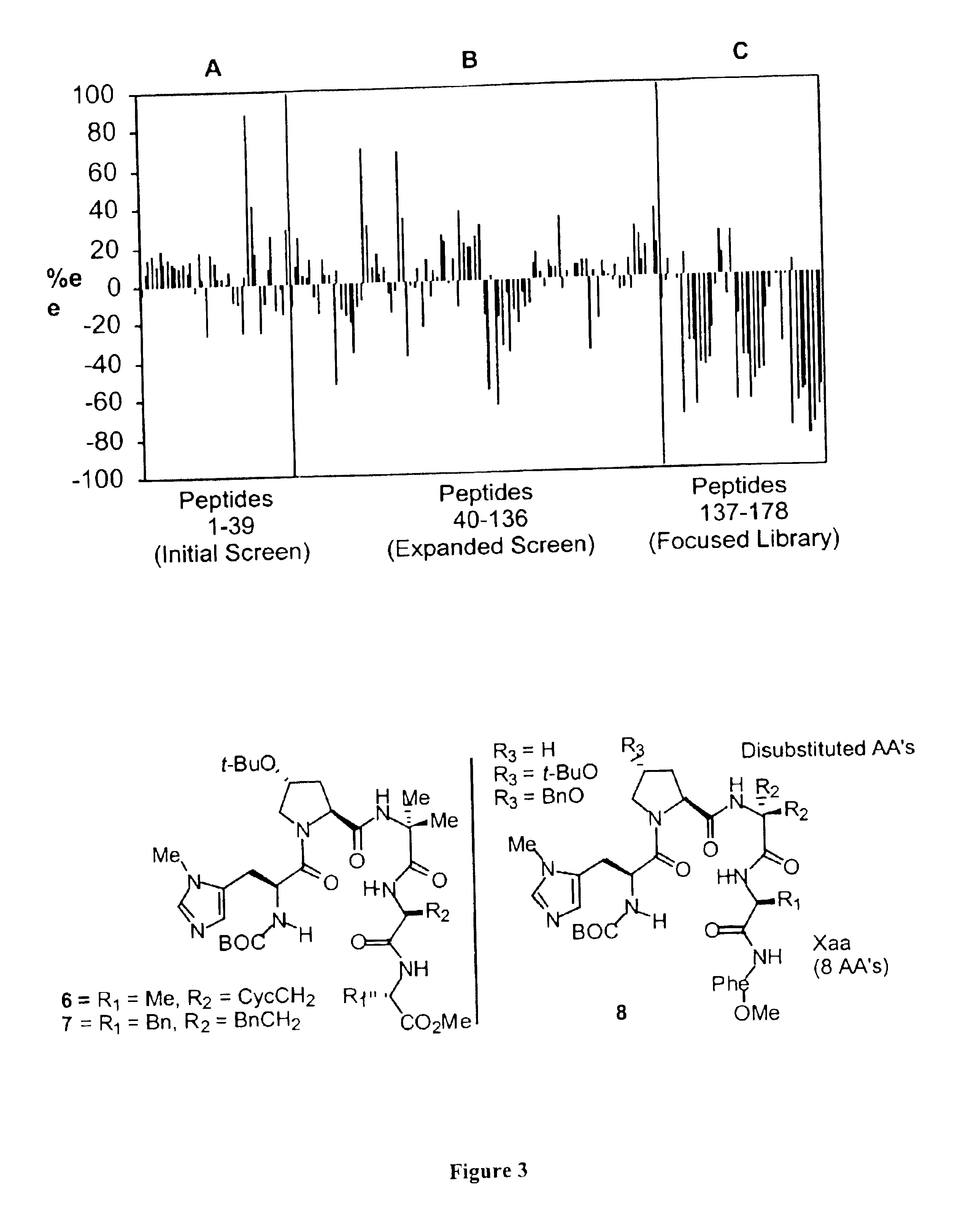
The present invention provides peptide-based phosphorylation catalysts (PBPC's) for the asymmetric monophosphorylation of cyclitols, particularly myo-inositols. The PBPC's of the invention effect a regio and enantioselective phosphorylation of a myo-inositol in a manner analogous to enzymatic kinases, thereby functioning as effective “kinase mimics.” Although orders of magnitude less complex in terms of structure than macromolecular proteins, the PBPC's of the invention control product formation with high enantioselectivity (>98% ee). The synthetic (+)-myo-inositol-1-phosphate is optically and spectroscopically equivalent to naturally occuring compound. The ability of the low molecular weight PBPC's of the present invention to mimic stereoselective enzymes represents a powerful approach toward catalytic asymmetric synthesis of biologically important molecules, and for mechanistic modeling of biochemical transformations to enable their use in drug applications.
Owner:TRUSTEES OF BOSTON COLLEGE THE
Highly efficient enzymatic process to produce (r)-3-quinuclidinol
PendingUS20200270656A1Delayed reaction timeIncrease substrate loadingOrganic chemistryOxidoreductasesRhodotorulaEnzymatic process
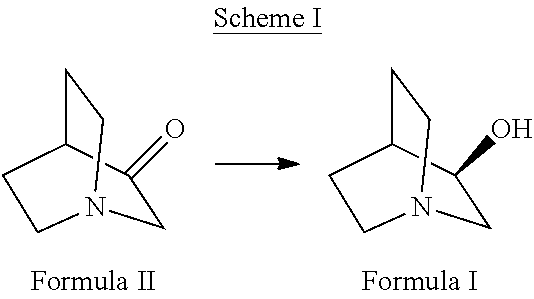
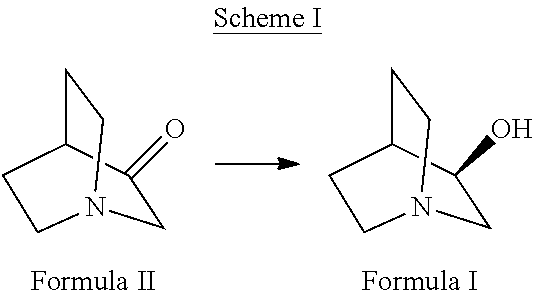
The present invention relates to enzymatic reduction of 3-quinuclidinone to (R)-3-quinuclidinol (Scheme I), by reacting 3-quinuclidinone with a variant of ketoreductase enzyme derived from Rhodotorula rubra. The invention also relates to enzymatically produced (R)-3-quinuclidinol wherein the substrate loading capacity of the enzyme is not less than 100 g / L.
Owner:UNICHEM LAB LTD
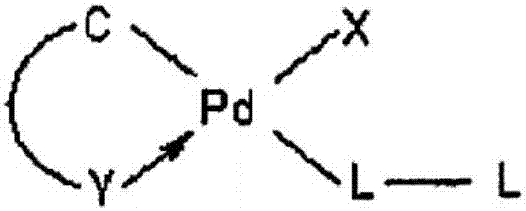
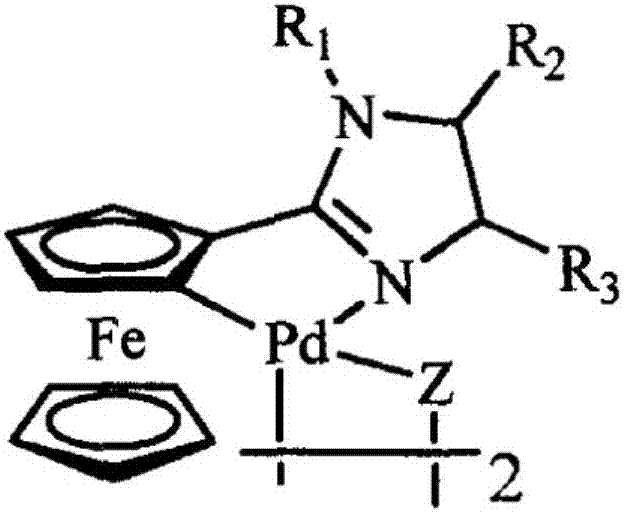
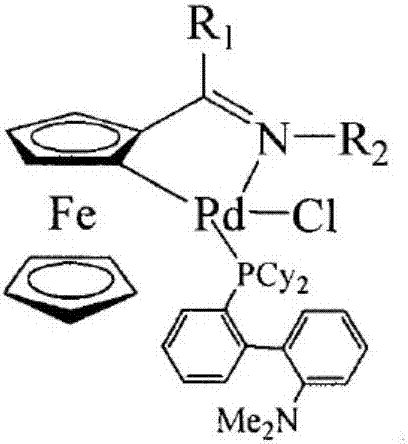
Aromatic heterocycte cyclitol palladium metal catalyst and application thereof
ActiveCN107262151AReduce dosageUniversalOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenMetal catalyst
The invention provides an aromatic heterocycte cyclitol palladium metal catalyst. The catalyst is a compound or a corresponding compound salt of a structure shown by a formula (1) or a formula (2). In the formula, n is 1 or 2, R1, R2 and R3 are independent hydrogen (H), methyl (CH3), propyl (C3H7), n-butyl (n-C4H9), n-hexyl (n-C6H13), cyclohexyl, adamantyl, camphoryl, Ph, p-OCH3-Ph, p-F-Ph or fluorenyl. The invention further provides application of the aromatic heterocycte cyclitol palladium metal catalyst in aromatic ring cyanation reaction, aromatic heterocycte C-H activation and arylation reaction, aromatic heterocycte oxidization and dehydrogenation Heck reaction and Suzuki coupling reaction. The formulas are shown in the specification.
Owner:ZHENGZHOU UNIV
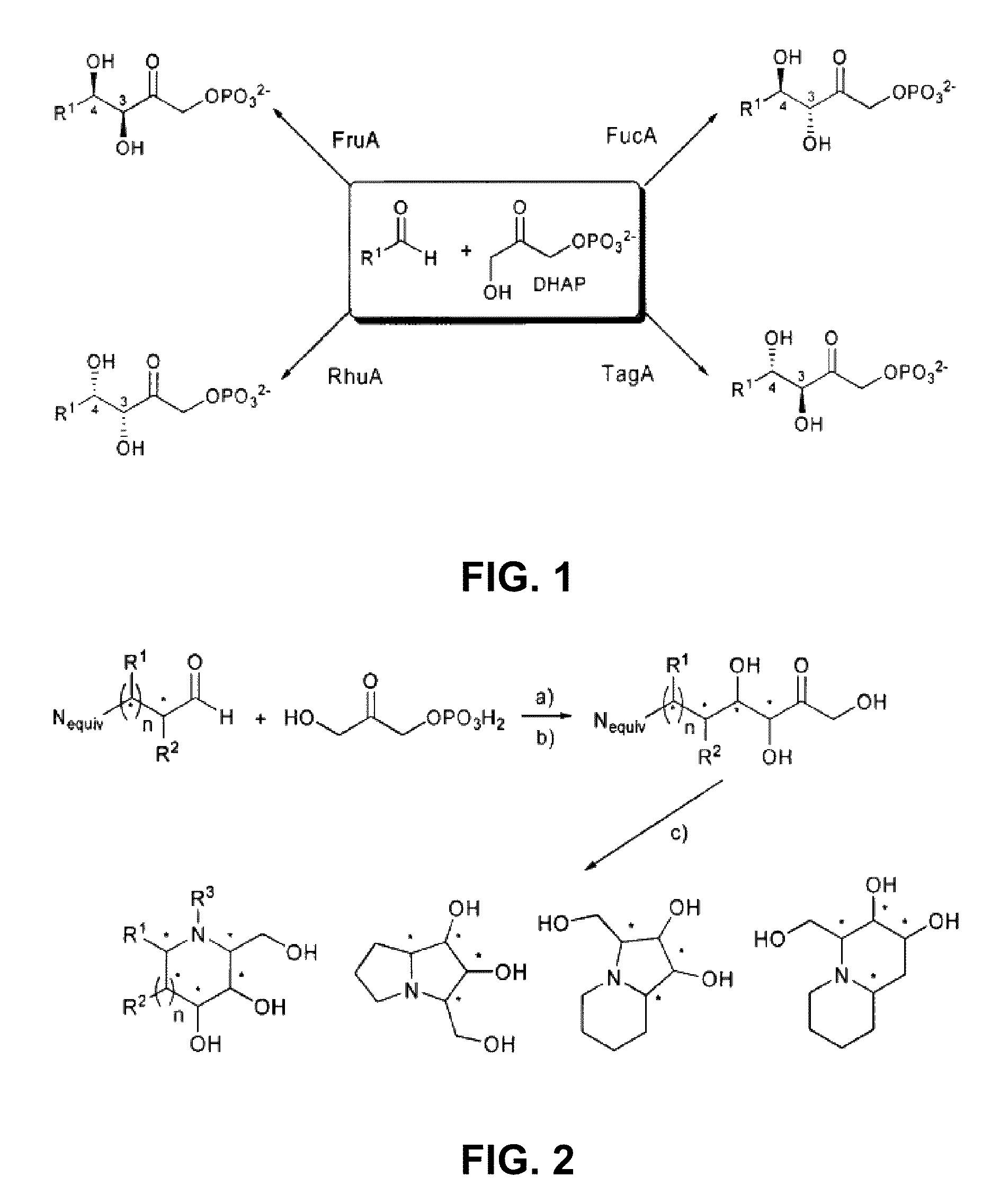
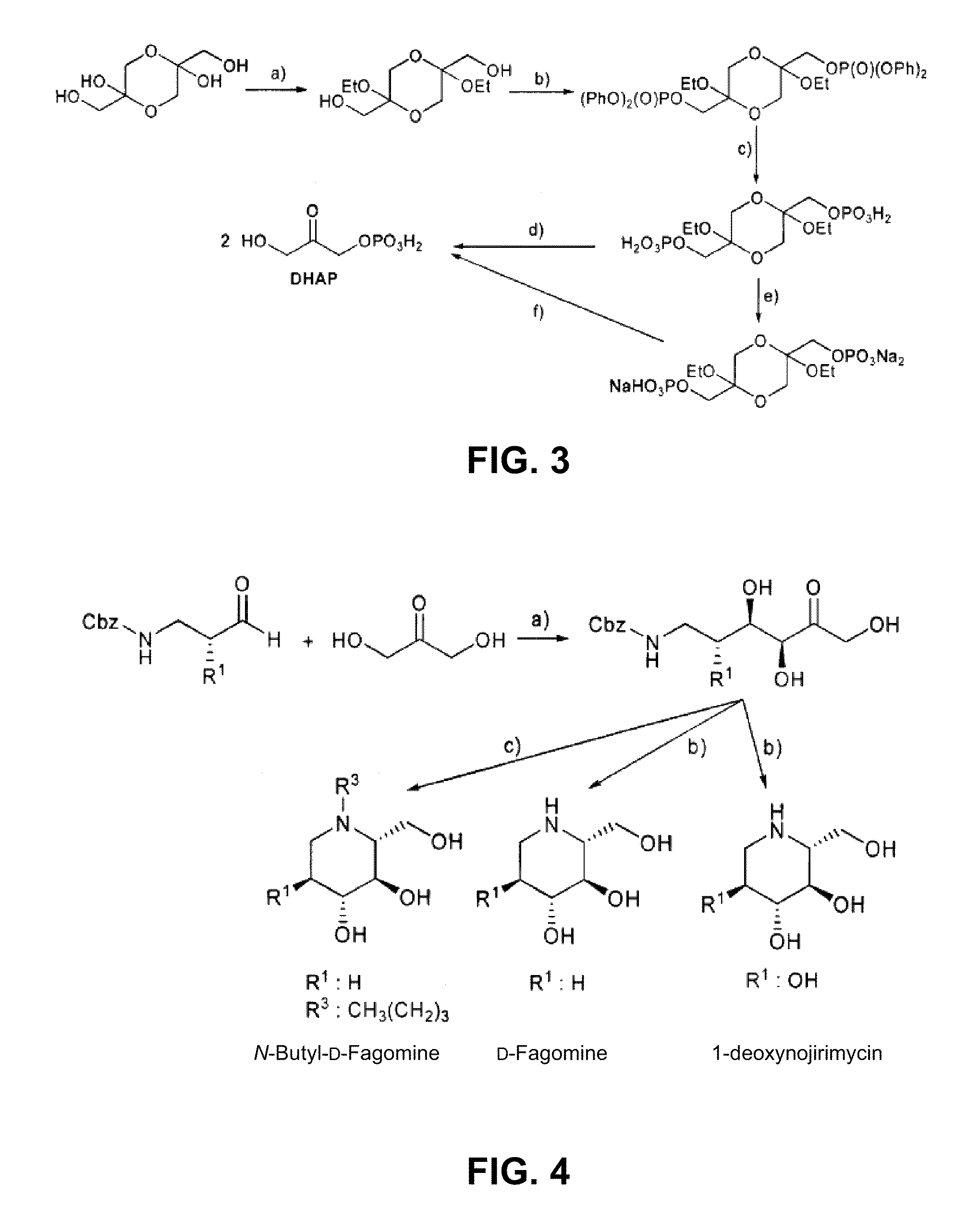
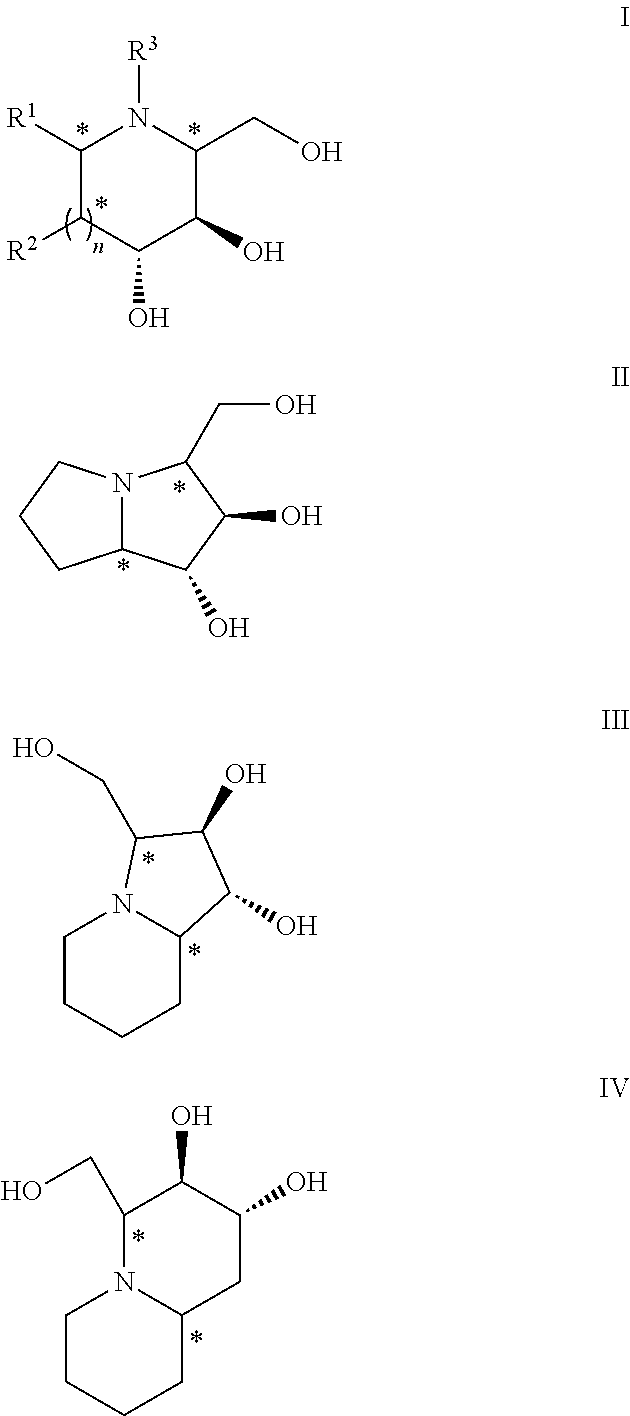
Chemoenzymatic process for the preparation of iminocyclitols
InactiveUS8288132B2Different propertyAldol addition is carried out efficientlyFermentationLyasesDodecanePtru catalyst
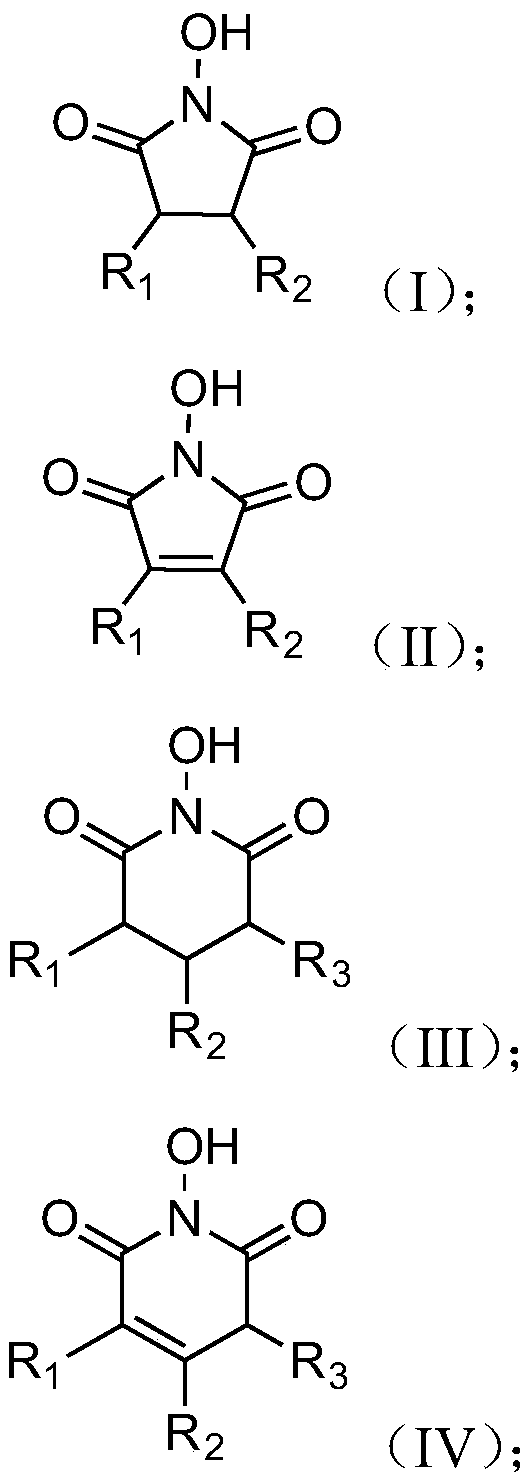
The present invention discloses a chemoenzymatic process for the preparation of an iminocyclitol corresponding to formula (I), (II), (III) or (IV), wherein: R1 and R2 are the same or different, and independently selected from the group consisting of: H, OH, hydroxymethyl, methyl, ethyl, butyl, pentyl, hexyl, octyl, isopropyl, isobutyl, 2-methylbutyl, and benzyl; R3 is selected from the group consisting of: H, hydroxymethyl, hydroxyethyl, ethyl, butyl, pentyl, hexyl, octyl, dodecyl, isobutyl, isopropyl, isopentyl, 2-methylbutyl, benzyl, and phenylethyl; n: 0 or 1; the process comprising: (i) an aldol addition catalyzed by a D-fructose-6-phosphate aldolase enzyme (FSA) and an acceptor aminoaldehyde; and (ii) an intramolecular reductive amination of the addition adduct obtained in step (i) with H2, in the presence of a metallic catalyst, optionally being carried out said step (ii) in the presence of an aldehyde of formula R3—CHO, wherein R3 is as defined above.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Linked cyclitols and their polysulfated derivatives
InactiveUS7173019B2Suitable pH and isotonicity and stabilityBiocideOrganic chemistryArylLymphatic Spread
The invention relates to compounds of the following formula (I). In these compounds, R1, R2, R3 and R4 are each independently a substituted or unsubstituted cyclitol with a ring comprising six carbon atoms, or hydrogen, substituted or unsubstituted alkyl, cycloalkyl, aryl, acyl, alkyloxycarbonyl, or alkylaminocarbonyl. At least two of R1, R2, R3 and R4 comprise the substituted or unsubstituted cyclitol. The linker can be any one of the following: —(CH2)w—, —(CH2)x—C6H4—(CH2)x—, —(CH2)y—NR5—(CH2)y—, and —(CH2)z—HCR6—(CH2)z—; wherein: w, x, y and z are independently an integer having a value of 0–10; R5 is a substituted or unsubstituted cyclitol with a ring comprising six carbon atoms; and, R6 is —OH, —OSO3Na, —OSO3Na substituted with alkyl, cycloalkyl or aryl, or substituted or unsubstituted alkyl, cycloalkyl or aryl. The compounds can also include substituted or unsubstituted cyclitol carbamides with the linker bond at the carbamide nitrogen. In other embodiments, the invention provides pharmaceutical compositions which include the compounds, and use of the compounds in the prevention or treatment in a mammalian subject of a disorder resulting from angiogenesis, metastasis, inflammation and / or coagulation / thrombosis
Owner:AUSTRALIEN NAT UNIV
A kind of preparation method of multi-substituted distal allyl ketone derivatives
ActiveCN107973715BVarious conversion pathwaysHigh research valueOrganic compound preparationCarboxylic acid esters separation/purificationChromatographic separationOrganic synthesis
The invention relates to a method for preparing a polysubstituted distal-end allyl ketone derivative. An object of the invention is to provide the method for preparing the polysubstituted distal-end allyl ketone derivative. The method comprises the steps of dissolving a third-grade cyclitol derivative, an unsaturated olefin compound and a photocatalyst in an organic solvent in a nitrogen gas atmosphere at room temperature, carrying out uniform mixing, carrying out a lighting reaction under a blue LED (light emitting diode) lamp, carrying out rotary evaporation to remove the solvent, then, carrying out silica-gel column chromatographic separation and purification, thereby obtaining a product, i.e., the polysubstituted distal-end allyl ketone derivative. According to the method provided by the invention, the reaction can be carried out at normal temperature under normal pressure the reaction conditions are mild, and the yield can reach 80%; and the method has the advantages of simplicityin operation, no pollution, safety, environment-friendliness and low cost. The invention is applied to the field of organic synthesis.
Owner:HARBIN INST OF TECH
Aromatic heterocyclic alcohol ring palladium metal catalyst and its application
ActiveCN107262151BReduce dosageUniversalOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystAlcohol
The invention provides an aromatic heterocycte cyclitol palladium metal catalyst. The catalyst is a compound or a corresponding compound salt of a structure shown by a formula (1) or a formula (2). In the formula, n is 1 or 2, R1, R2 and R3 are independent hydrogen (H), methyl (CH3), propyl (C3H7), n-butyl (n-C4H9), n-hexyl (n-C6H13), cyclohexyl, adamantyl, camphoryl, Ph, p-OCH3-Ph, p-F-Ph or fluorenyl. The invention further provides application of the aromatic heterocycte cyclitol palladium metal catalyst in aromatic ring cyanation reaction, aromatic heterocycte C-H activation and arylation reaction, aromatic heterocycte oxidization and dehydrogenation Heck reaction and Suzuki coupling reaction. The formulas are shown in the specification.
Owner:ZHENGZHOU UNIV
Purification of 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy)quinuclidine and preparation method of penehyclidine hydrochloride
Owner:北京鑫开元医药科技有限公司
A kind of catalytic hydrogenation saturation method of rich phenolic bio-oil fraction
ActiveCN110205205BPromote generationImprove conversion abilityFatty acid hydrogenationLiquid carbonaceous fuelsLiquid productPtru catalyst
The invention discloses a catalytic hydrogenation saturation method for phenol-rich biological oil fractions. The method comprises the steps that the biological oil fractions and fatty alcohols are mixed to obtain a reaction raw material, the reaction raw material is atomized through preheating, and then passes through a stationary bed reactor filled with a SiO2-loaded Pt-Ni bimetallic catalyst, H2 is also introduced into the reaction raw material, a hydrogenation reaction is carried out, the reaction temperature is 240-330 DEG C, the reaction pressure is 1-6 MPa, and a liquid product is prepared. The overall conversion rate of phenol catalytic hydrogenation saturation can reach 92%, the yield of the liquid product is close to 90.0 wt%, and the overall selectivity of cyclitol and cycloalkane products in the liquid product can reach 97%. The catalytic hydrogenation saturation method for the phenol-rich biological oil fractions solves the problem that phenols are difficult to convert orhave strict conversion conditions in a traditional hydrogenation process through the prepared bimetallic catalyst by taking more difficultly converted phenol-rich biological oil as an object, and improves the activity and stability of subsequent cracking of the biological oil.
Owner:ZHEJIANG UNIV
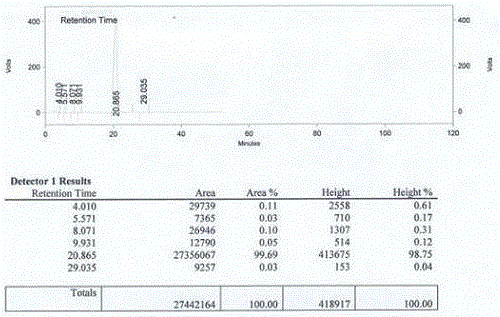
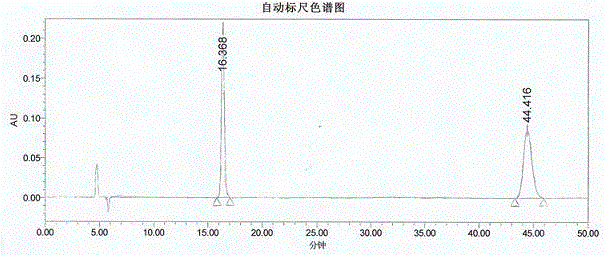
A kind of assay method of r-3-quinuclidinol optical purity
The invention belongs to the technical field of pharmaceutical chemical engineering, and in particular relates to a method for measuring the optical purity of (R)-3-quinuclidinol. The method for measuring the optical purity of (R)-3-quinuclidinol comprises the steps of (a) preparing a sample; (b) preparing a test solution; (c) preparing a chromatographic condition, and performing detection. According to the method, a precolumn derivatization chiral chromatography method is adopted to measure the optical purity of (R)-3-quinuclidinol; specifically, the precolumn derivatization method is used for enabling (RS)-3-quinuclidinol racemate and paratoluensulfonyl chloride to react to generate (RS)-3-quinuclidinol paratoluensulfonyl chloride derivative, namely quinuclidinol 3-paratoluene sulfonate; then the chiral chromatography method is used for measuring the optical purity; therefore, the technical difficulty in measurement of the optical purity of (R)-3-quinuclidinol is creatively solved.
Owner:JINAN ASIA PHARMA TECH
Denatured carob flour (DCF) with a low content of soluble tannins and sugars, meant for human consumption and process to obtain it
The present invention provides an improved denatured carob flour, which includes 2 to 15% sugars, 0.2 to 1.5% cyclitols (pinitol), 2 to 10% lignins, 10 to 30% celluloses, 3 to 20% hemicelluloses, 1 to 6% pectins, 25 to 55% condensed tannins, 3 to 9% protein and less than 8% water. The invention further provides a process to obtain a denatured carob flour that includes the following steps: a. cleaning the whole fruit; b. crushing the carob fruits; c. separating the carob seeds and kibbled carob pulp, d. toasting between 130 to 200° C.; e. an extracting process; f. separating; g. milling 90% of particles below 250 pm; h. separating; i. drying below 8%; and j. classifying (sieving).
Owner:INVESTIGACION Y NUTRICION +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com