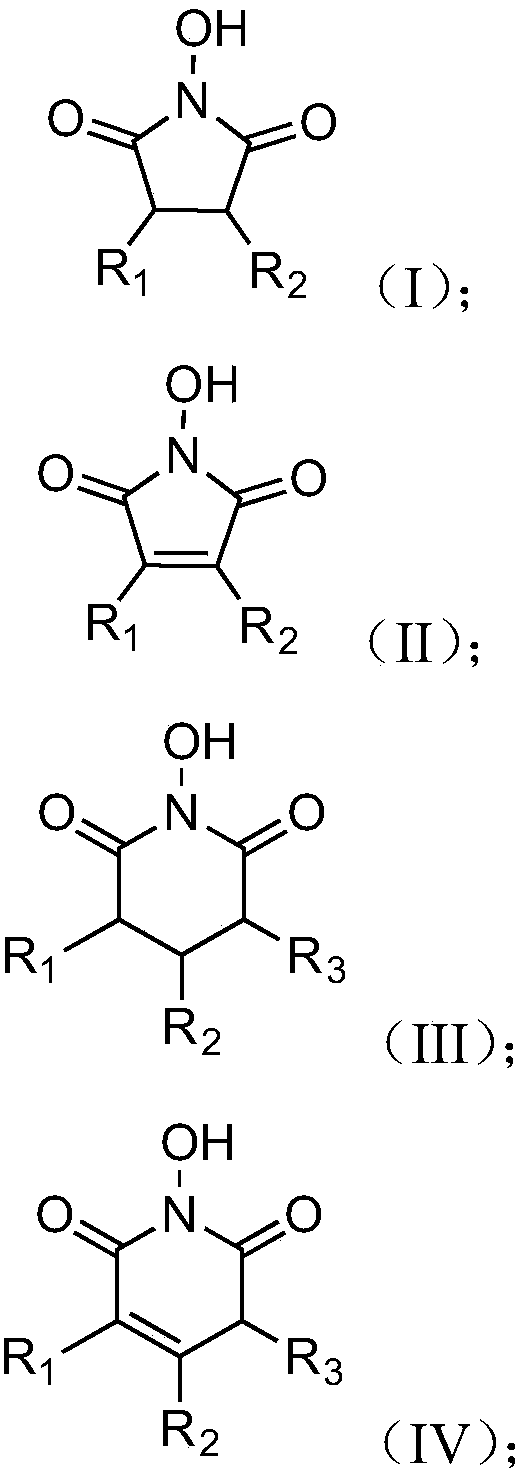
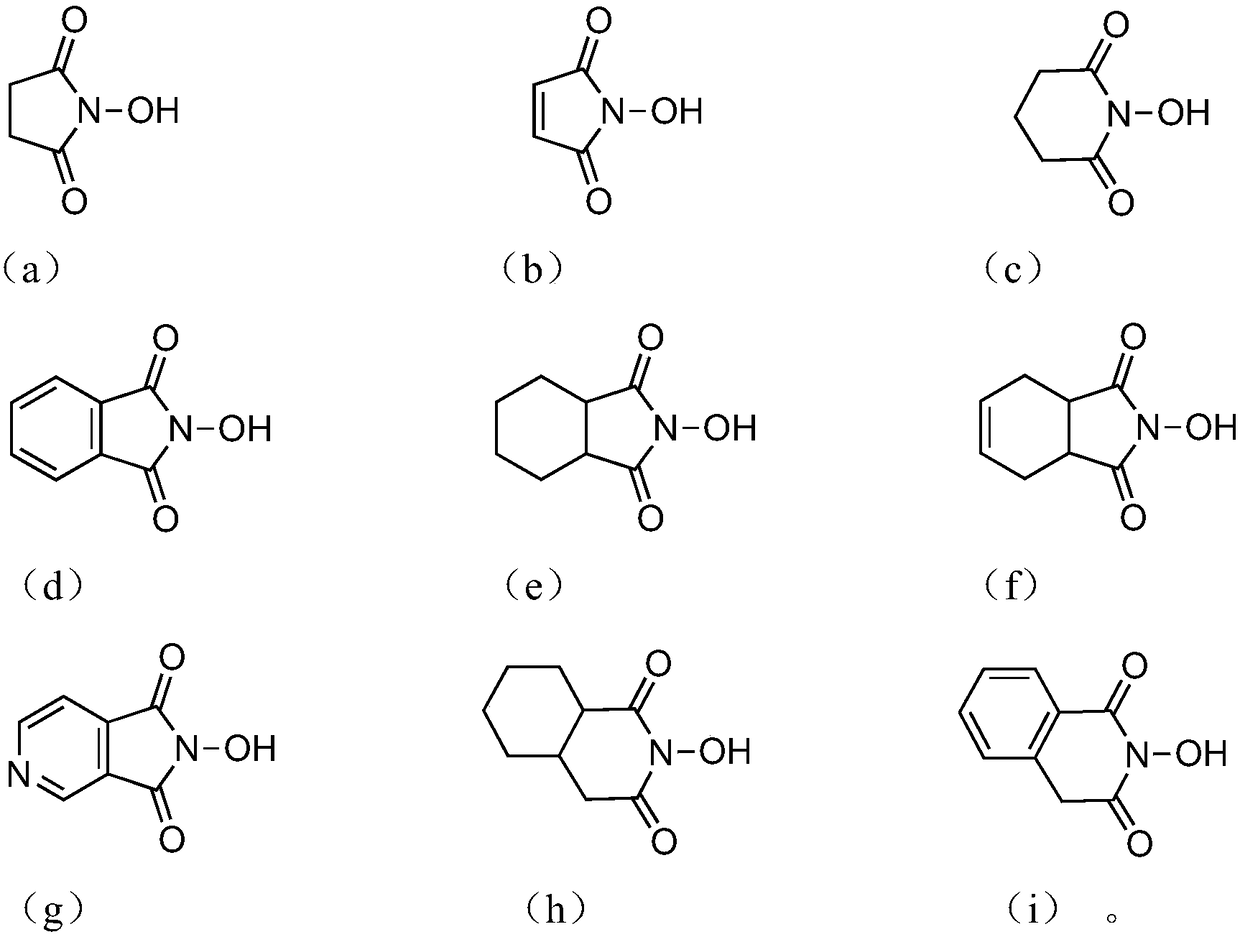
Oxidizing method of cycloalkane compound
A technology of naphthenes and compounds, which is applied in the field of oxidation of naphthenes compounds, can solve the problems of many intermediate steps, insufficient steps, fouling of reactors, etc., avoiding operation and safety problems, good industrial application prospects, and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
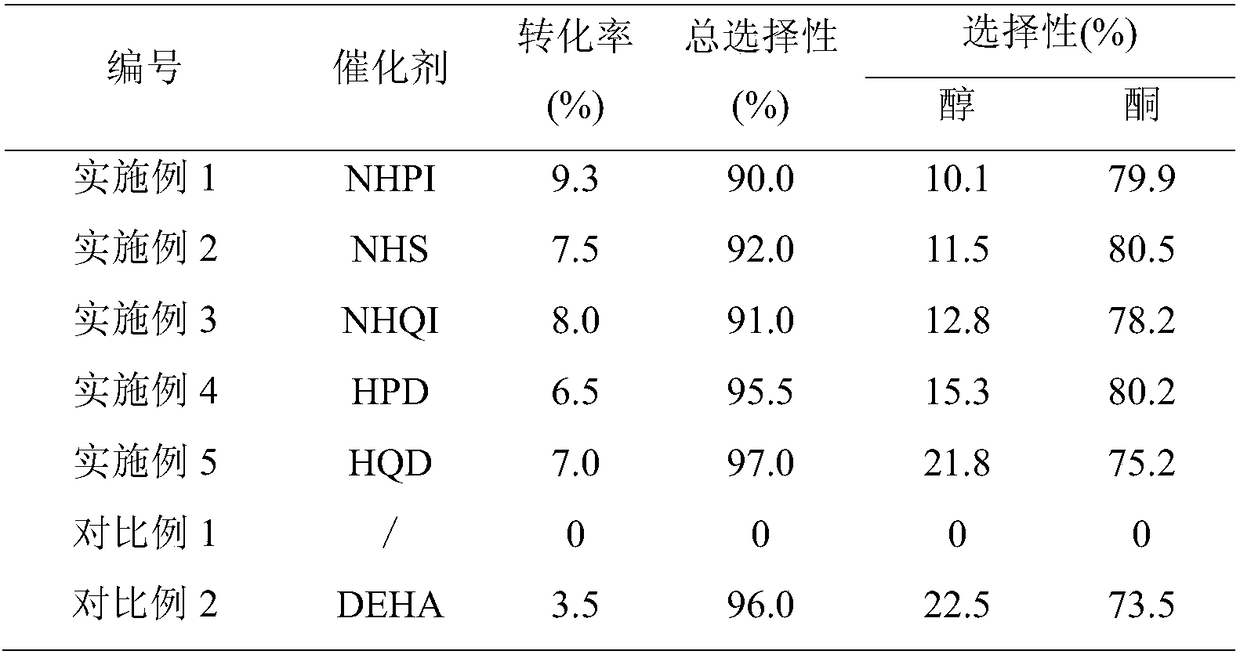
[0079] Add 0.0652g (0.4mmol) of NHPI, 0.219g (0.4mmol) of ammonium cerium nitrate (abbreviation: CAN), 0.1683g (2mmol) of cyclohexane, 1,2 - Dichloroethane (DCE) 20 mL. Under normal pressure, after stirring at 45°C for 24 hours at a constant temperature, cool to room temperature, and detect and analyze by gas-phase internal standard method, the conversion rate of cyclohexane is 9.3%, and the total selectivity is 90.0%, wherein the selectivity of cyclohexanol is 10.1% %, the selectivity of cyclohexanone is 79.9%.
Embodiment 2
[0081] Add 0.0460g (0.4mmol) of NHS, 0.219g (0.4mmol) of ammonium cerium nitrate, 0.1683g (2mmol) of cyclohexane, and 1,2-dichloroethane into a three-necked flask connected with a condenser tube, a thermometer, and an oxygen bag. (DCE) 20mL. Under normal pressure, after stirring at 45°C for 24 hours at a constant temperature, cool to room temperature, and detect and analyze by gas-phase internal standard method, the conversion rate of cyclohexane is 7.5%, the total selectivity is 92.0%, and the selectivity of cyclohexanol is 11.5%. %, the selectivity of cyclohexanone is 80.5%.
Embodiment 3
[0083] Add NHQI 0.0656g (0.4mmol), cerium ammonium nitrate 0.219g (0.4mmol), cyclohexane 0.1683g (2mmol), 1,2-dichloroethane in the three-necked flask connected to the condenser tube, thermometer and oxygen bag (DCE) 20mL. Under normal pressure, after 24 hours of constant temperature stirring at 45°C, it was cooled to room temperature and detected and analyzed by the gas-phase internal standard method. The conversion rate of cyclohexane was 8.0%, and the total selectivity was 91.0%. %, the selectivity of cyclohexanone is 78.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com