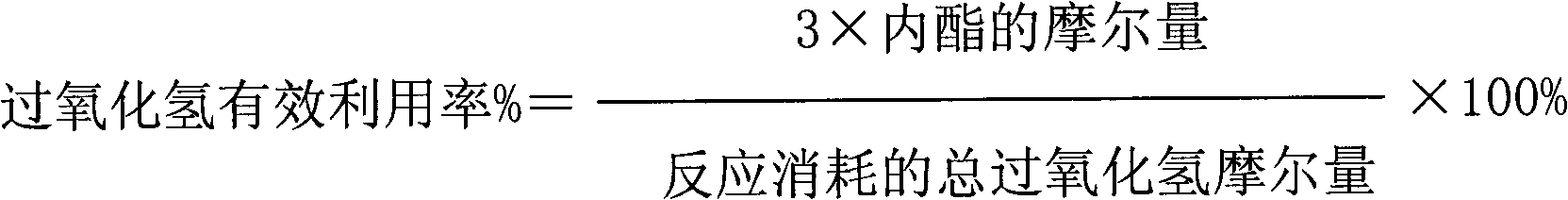Method for catalytic oxidation preparation of lactone from cycloalkane
A catalytic oxidation, naphthenic technology, applied in chemical instruments and methods, molecular sieve catalysts, physical/chemical process catalysts, etc., can solve problems such as difficulty in generating lactones, achieve high effective utilization, good activity stability, process Simplified effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Cyclohexane, hydrogen peroxide, solvent and catalyst (the mass ratio of zinc oxide and titanium silicon molecular sieve is 1, the Zn / Ti molar ratio is 5, and the Si / Ti molar ratio is 56) according to the molar ratio of cycloalkane and hydrogen peroxide 1:2, the mass ratio of solvent methanol to catalyst is 20, the mass ratio of naphthene to catalyst is 50, and the reaction is carried out at a temperature of 30° C. and a pressure of 1.5 MPa.
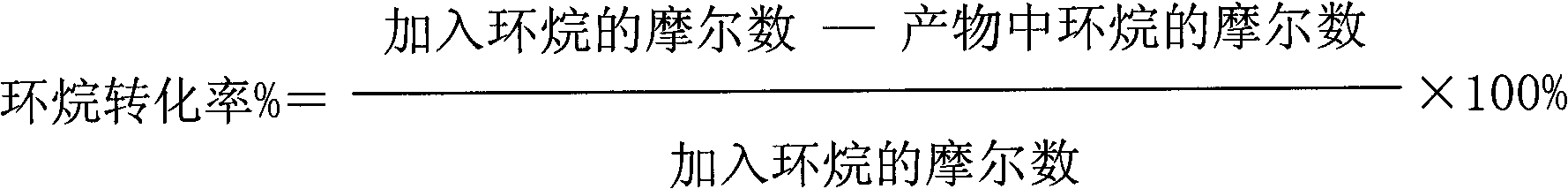
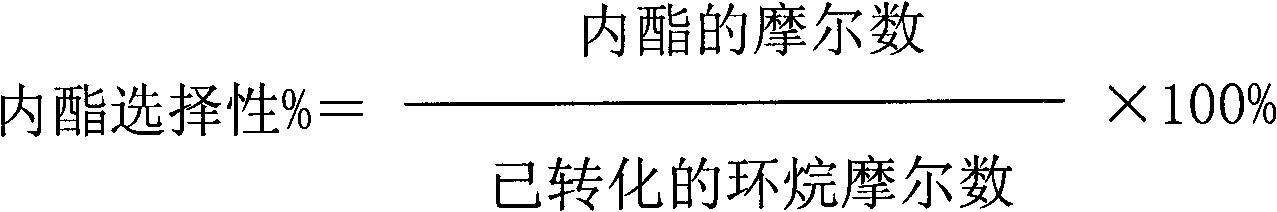
[0044] The results of the reaction for 2 hours are as follows: the conversion rate of cycloalkane is 18%; the effective utilization rate of hydrogen peroxide is 32%; the selectivity of lactone is 61%.
[0045] The results of the reaction for 12 hours are as follows: the conversion rate of cycloalkane is 17%; the effective utilization rate of hydrogen peroxide is 31%; the selectivity of lactone is 52%.
Embodiment 2
[0047] Cyclohexane, hydrogen peroxide, solvent and catalyst (mass ratio of zinc hydroxide and titanium silicon molecular sieve 0.2, Zn / Ti molar ratio is 15, Si / Ti molar ratio is 26) according to the molar ratio of cycloalkane and hydrogen peroxide The ratio is 1:5, the mass ratio of the solvent acetone to the catalyst is 50, the mass ratio of naphthene to the catalyst is 75, and the reaction is carried out at a temperature of 60° C. and a pressure of 2.5 MPa.
[0048] The results of the reaction for 2 hours are as follows: the conversion rate of naphthene is 39%; the effective utilization rate of hydrogen peroxide is 35%; the selectivity of lactone is 51%.
[0049] The results of the reaction for 12 hours are as follows: the conversion rate of cycloalkane is 37%; the effective utilization rate of hydrogen peroxide is 33%; the selectivity of lactone is 48%.
Embodiment 3
[0051] Cyclopentane, hydrogen peroxide, solvent and catalyst (the mass ratio of zinc iodide and titanium silicon molecular sieve is 5, the Zn / Ti molar ratio is 2, and the Si / Ti molar ratio is 38) according to the molar ratio of cycloalkane and hydrogen peroxide The ratio is 2:13, the mass ratio of solvent acetonitrile to catalyst is 80, the mass ratio of naphthene to catalyst is 100, and the reaction is carried out at a temperature of 50° C. and a pressure of 1.0 MPa.
[0052] The results of the reaction for 2 hours are as follows: the conversion rate of cycloalkane is 41%; the effective utilization rate of hydrogen peroxide is 40%; the selectivity of lactone is 54%.
[0053] The results of reaction for 12 hours are as follows: the conversion rate of naphthene is 38%; the effective utilization rate of hydrogen peroxide is 32%; the selectivity of lactone is 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com