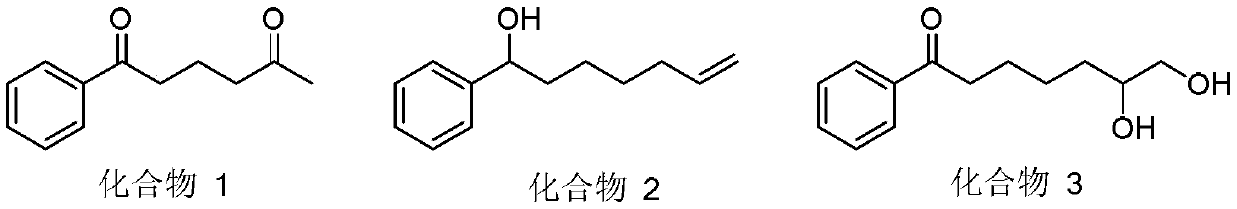
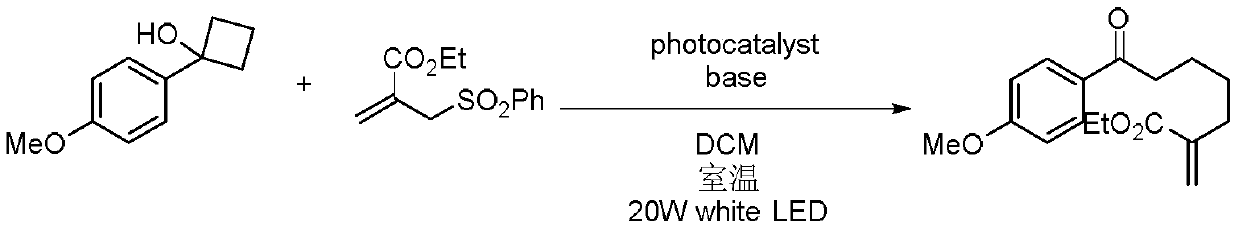
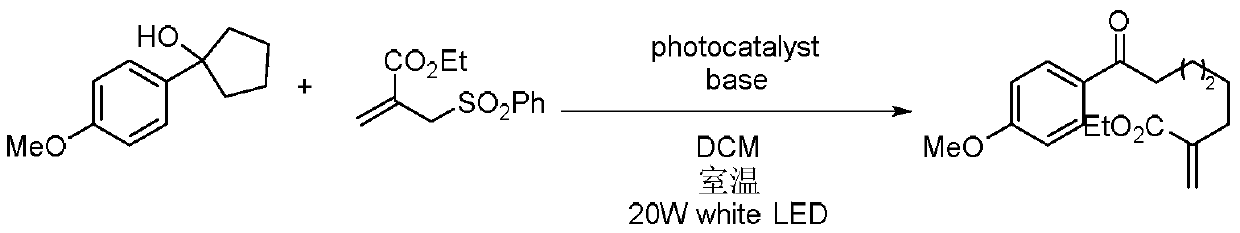
A kind of preparation method of multi-substituted distal allyl ketone derivatives
A technology for allyl ketone and derivatives, which is applied in the field of preparation of multi-substituted distal allyl ketone derivatives, can solve the problems of large equipment requirements, harsh conditions, and low yield, and achieve low cost and mild reaction conditions , the effect of high research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0013] Specific embodiment 1: In this embodiment, a method for preparing a multi-substituted distal allyl ketone derivative comprises the following steps:
[0014] Dissolve tertiary cyclic alcohol derivatives, unsaturated ethylenic compounds, bases and photocatalysts in an organic solvent in a nitrogen atmosphere at room temperature, mix them evenly, and then place them under a blue LED light for photoreaction. Separation and purification by column chromatography, the resulting product is a multi-substituted distal allyl ketone derivative; the molar ratio of unsaturated ethylenic compound, tertiary cyclic alcohol derivative, base and photocatalyst is 2:1:2.5:0.5;
[0015] Wherein the chemical structural formula of tertiary cyclic alcohol derivative is:
[0016] The chemical structural formula of unsaturated ethylenic compounds is:
[0017] The chemical structural formula of the photocatalyst is:
[0018] Among them, R 1 , R 2 and R 3 is alkyl, alkoxy or halogen, and...
specific Embodiment approach 2
[0023] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that the unsaturated ethylenic compound is α, β unsaturated ester substituted by methyl, α, β unsaturated ester substituted by ethyl, α, β substituted by nitro Unsaturated esters or cyano-substituted α, β unsaturated esters. Others are the same as the first embodiment.
specific Embodiment approach 3
[0024] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that the three-level cyclic alcohol derivatives are three-membered cyclic alcohols, four-membered cyclic alcohols, five-membered cyclic alcohols, twelve-membered cyclic alcohols, ortho polycyclic alcohols Alkyl substituted cyclic alcohols or ortho aryl substituted cyclic alcohols. Others are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com