Recombined hepatitis E hepatitis virus protein, preparation method and usage thereof
A hepatitis E virus and polynucleotide technology, applied in the fields of molecular biology and medicine, can solve the problems of unsuccessful development and achieve good antigenicity and immunogenicity, strong pertinence and practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] This implementation describes the construction method of recombinant bacteria BL21(DE3)-pET-28a(+)-179:
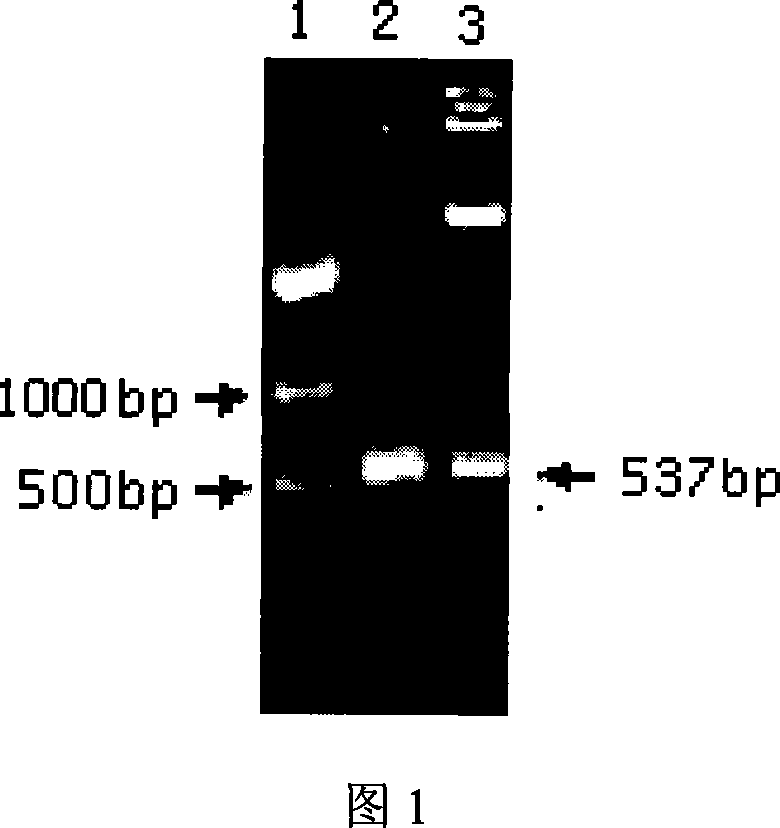
[0051] (a) Obtaining the target gene: Using the sequence of the Chinese strain of HEV genotype 4 as a template, use primer 1 (5'-CCCCCCATGGTTATCCAGGACTATGATAATC-3') and primer 2 (5'-CCCCTCGAGTCAAGGGTAATCAACAGTGTCCTCCA-3') to amplify the ORF2 encoded polypeptide 453 -631 (p179) gene fragment. The PCR conditions are: 94°C-45 seconds, 52°C-45 seconds, 72°C-50 seconds, 35 cycles. Among them, primer 1 and primer 2 respectively contain NcoI and XhoI enzyme cutting sites. PCR products were recovered and purified by agarose gel.
[0052] The above amino acid sequence from position 453 to position 631 (p179), the amino acid sequence described in it is as follows:
[0053] VIQDYDNQHEQDRPTPSPAPSRPFSVLRANDVLWLSLTAAEYDQTTYGSSTNPM
[0054] YVSDTVTFVNVATGAQGVRSRSLDWSKVTLDGRPLTTIQQYSKTFYVLPLRGKLS
[0055] FWEAGTTKAGYPYNYNTTASDQILIENAAGHRVCISTYTTNLGSGPVSISAVGVL
[0056] APHSAL...
Embodiment 2
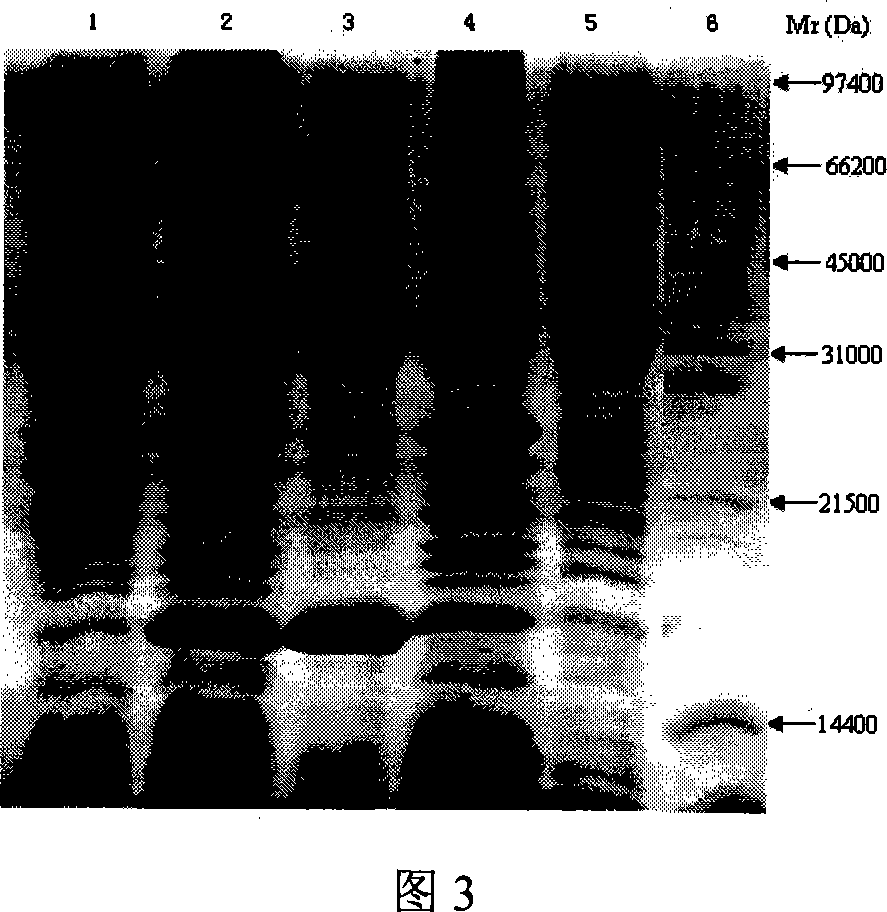
[0073] This implementation describes the purification method of recombinant p179 protein and its electron microscope analysis results (Fig. 6-Fig. 8):
[0074] Purification of the recombinant p179 protein was carried out by the following method: the recombinant strain BL21(DE3)-pET-28a(+)-179 expressed the target protein under the induction of IPTG at 37°C. The bacteria were collected by centrifugation at 5000g at 4°C for 15 minutes, and the bacteria were resuspended in buffer A (20mM Tris-HCl, pH 7.4), and the bacteria were lysed by ultrasonic method. Then, the precipitate was removed by centrifugation at 20,000 g at 4°C for 30 minutes. It should be noted that HEV p179 is a non-fusion protein and mostly exists in the supernatant of the lysate in a soluble form. HEV p179 was purified using Biologic LP System (Bio-Red, England). First, add the supernatant obtained from the lysis to an anion exchange chromatography column (SOURCE 30Q, 10ml bed volume) that has been pre-balanced...
Embodiment 3
[0077] This implementation describes the evaluation of recombinant p179 protein as an antigen for the indirect ELISA detection of HEV IgG antibody, wherein the coating dose range of recombinant p179 protein is 20-80ng / well, and the preferred concentration is 50ng / well.
[0078] (a) Detection steps:
[0079] Coat polystyrene microwell strips (50ng / well) with recombinant p179 protein as an antigen in advance, overnight at 4°C; wash the plate 3 times with 1×PBST washing solution the next day, and then dry it; seal the pre-coated microwell plate with blocking solution , 37°C for 1h; washed twice with PBST, button-dried; sealed, and stored at 4°C for later use.
[0080] Adding samples: add 95 μl sample diluent to each well, add 5 μl serum samples in turn, set up blank control wells, negative control serum, positive control serum and samples to be tested, and pat to mix;
[0081] Incubation: incubate at 37°C for 30 minutes;
[0082] Washing: Discard the liquid in the well, wash 5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com