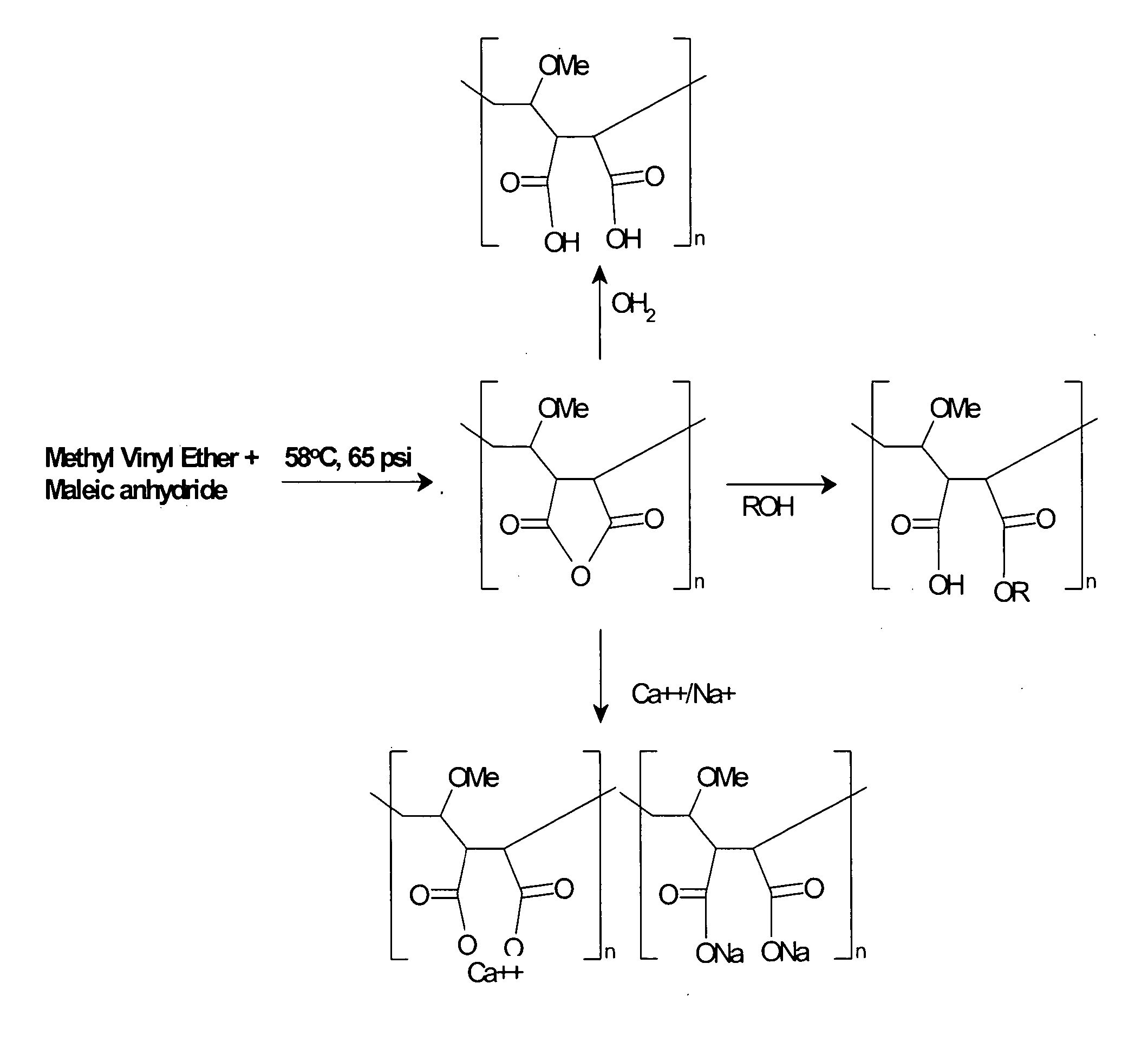
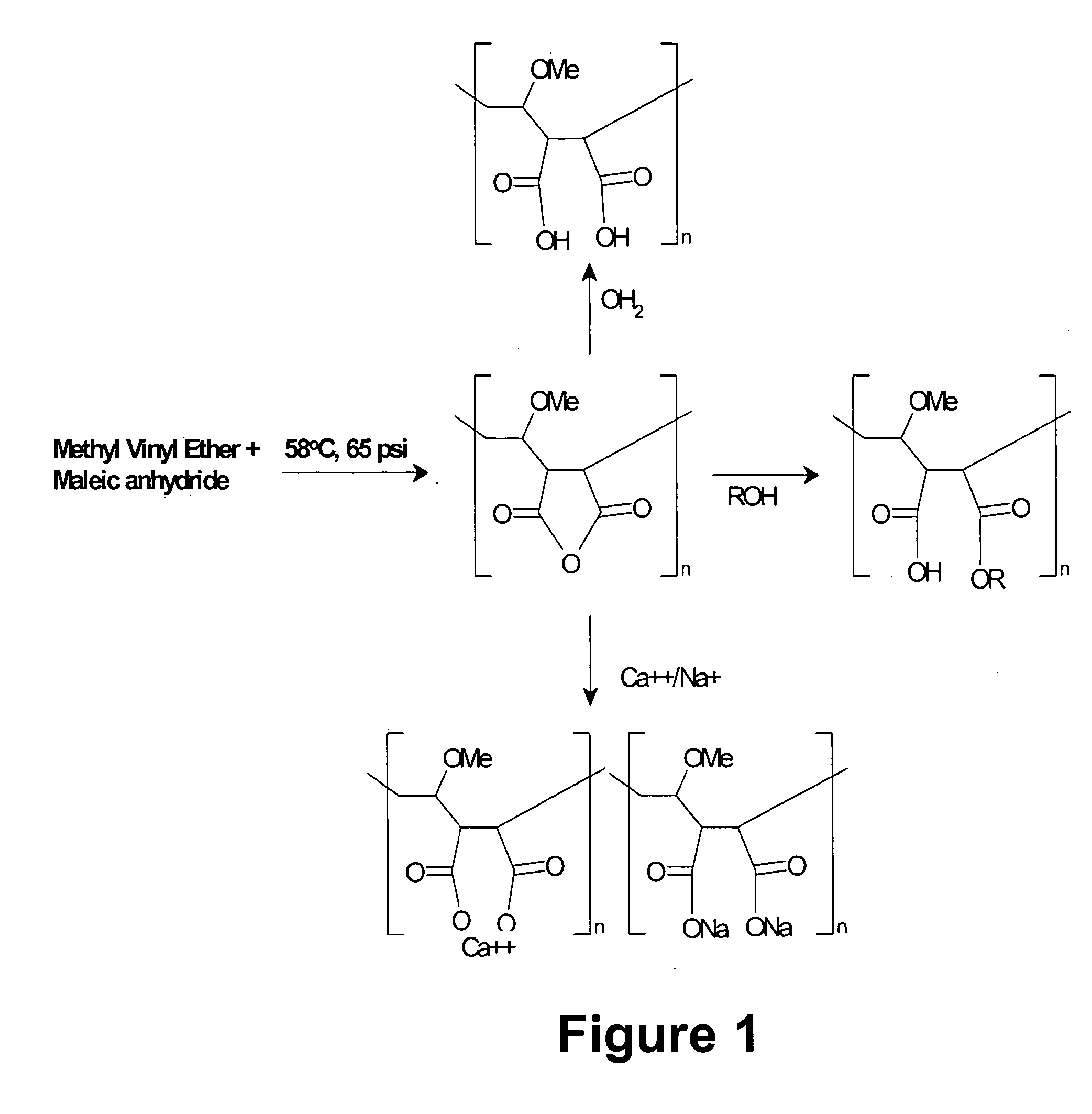
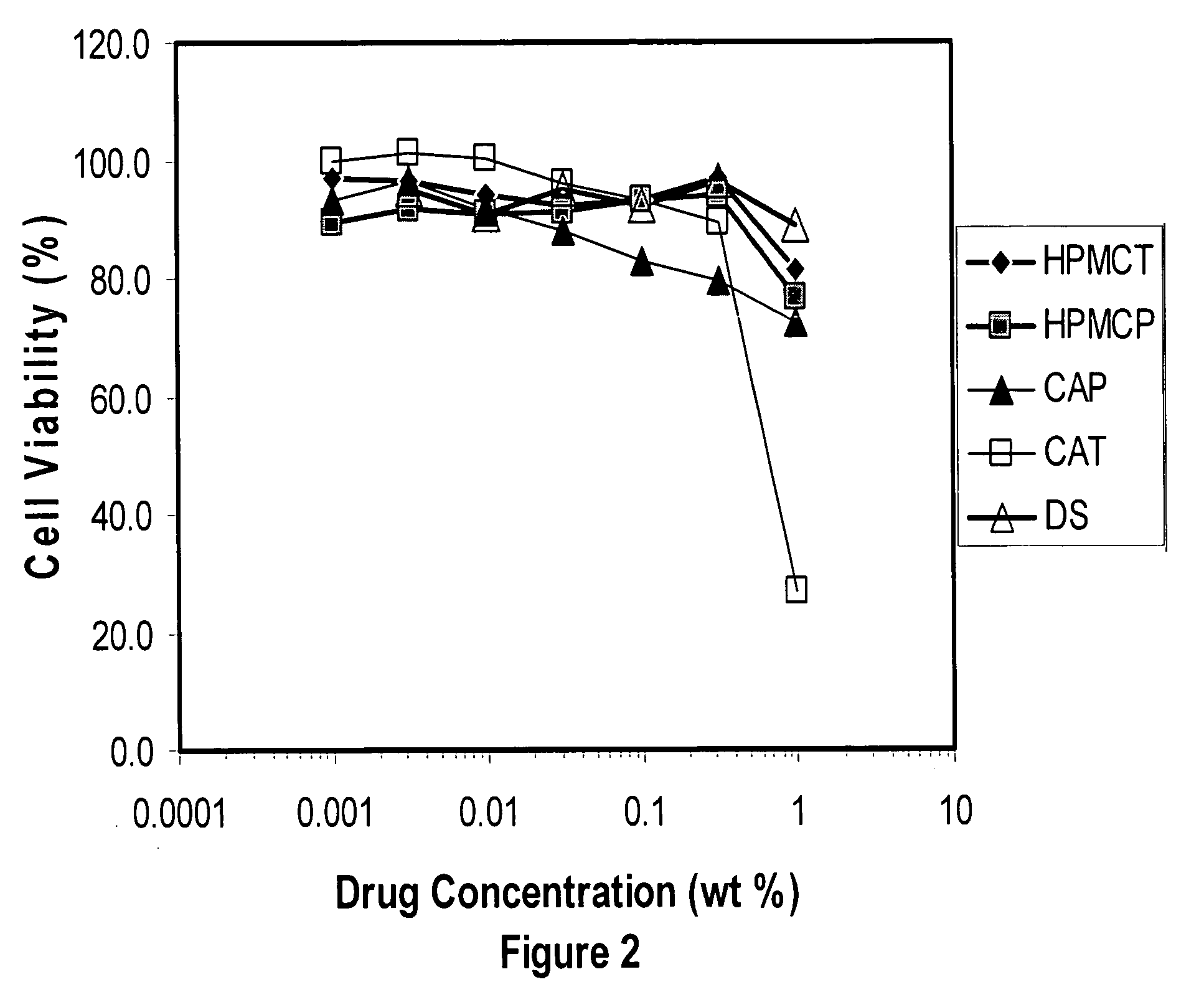
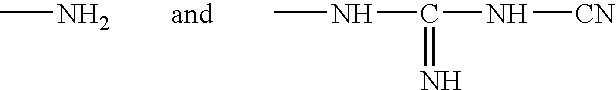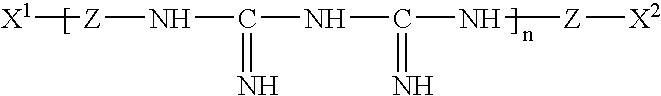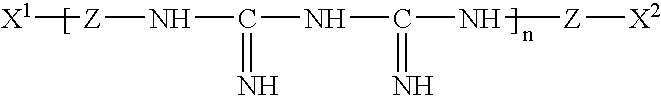Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
73 results about "Contact lens solutions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
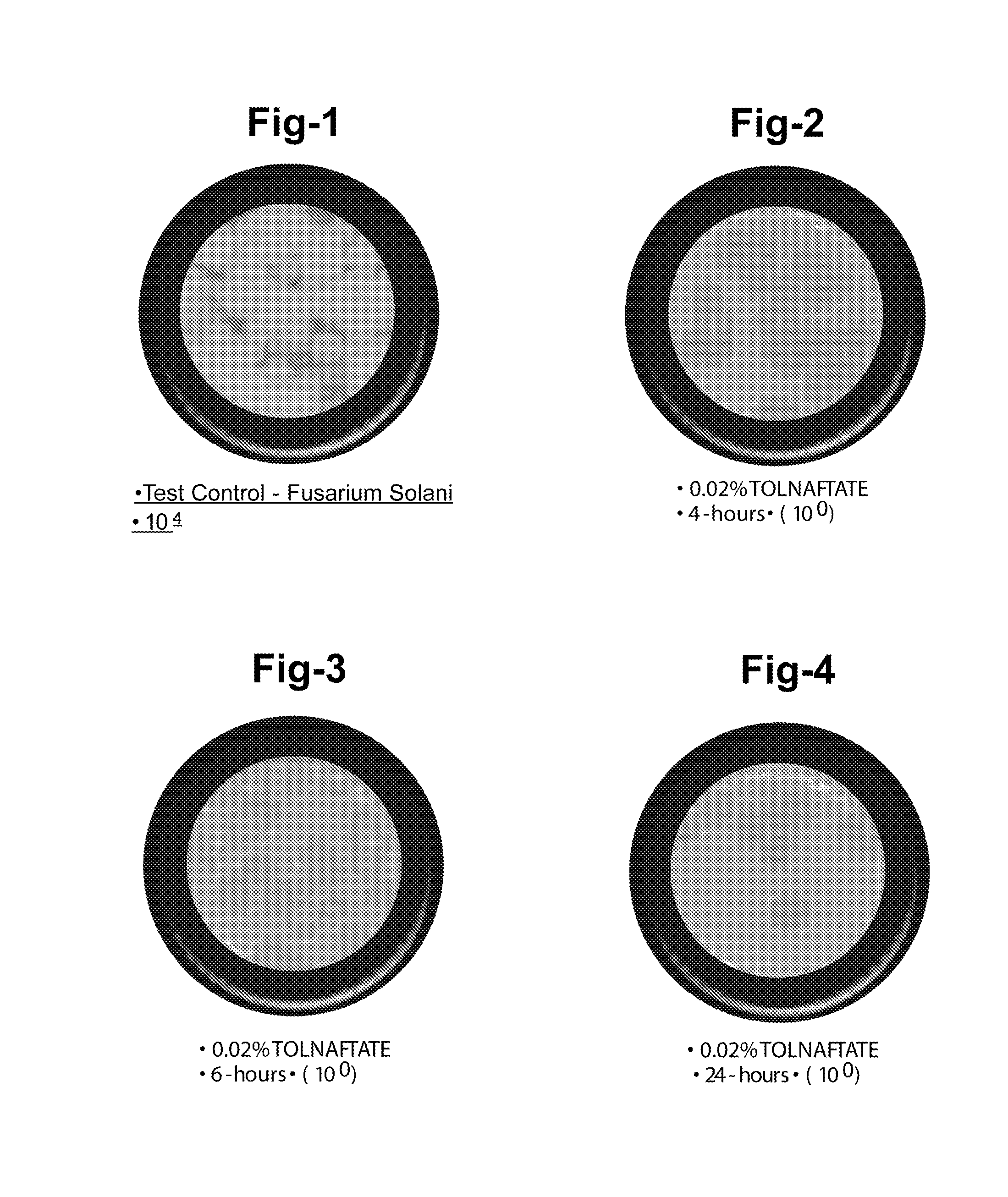
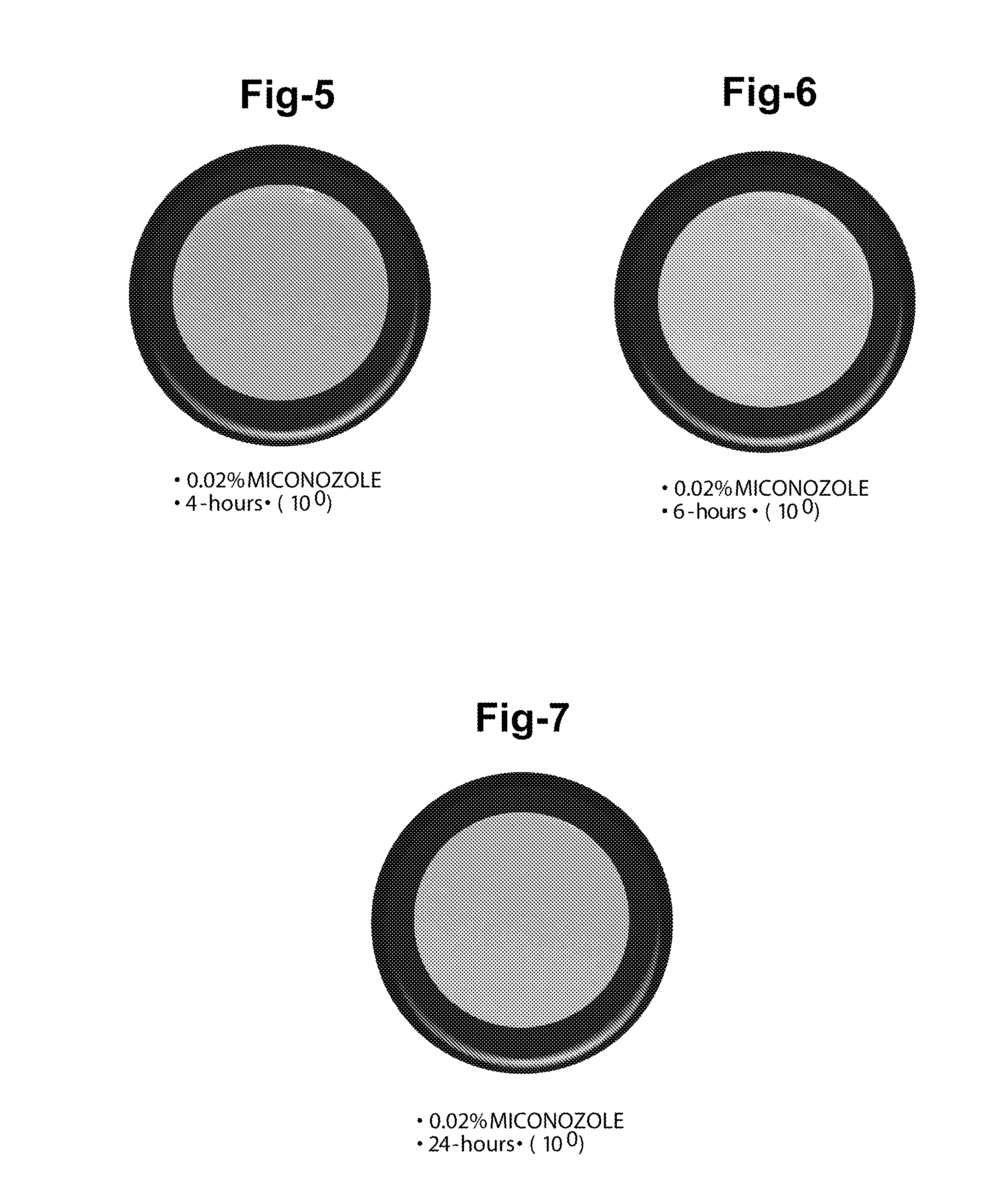
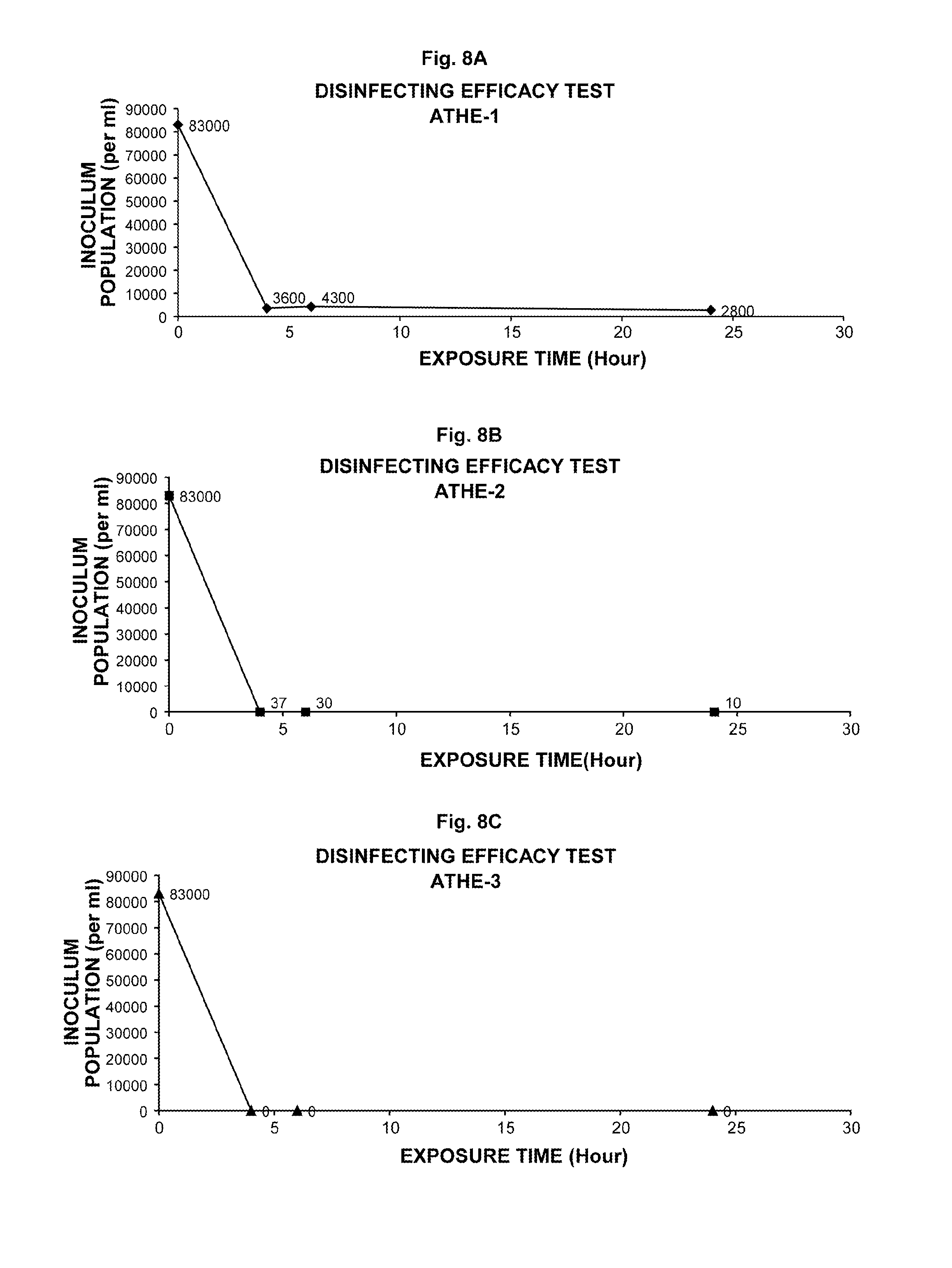
A: Most multipurpose contact lens solutions contain three classes of ingredients: surfactants, disinfectants and enzymatic cleaners. The disinfectant is designed to kill bacteria, while the surfactant removes any debris that might be stuck to the lens.
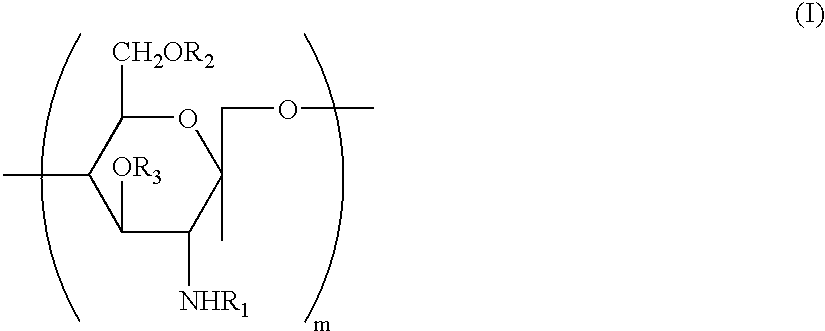
Contact lens solution
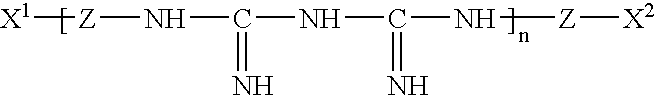
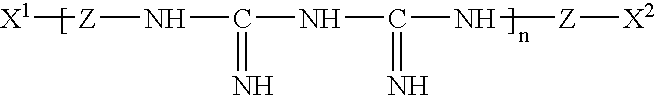
InactiveUS20050074467A1Safe for eyeSuppresses the adhesion of a large amount of polylysineSpectales/gogglesOrganic detergent compounding agentsPhosphoric acidNitrogen
A liquid formulation for contact lenses which comprises polylysine, polyphosphoric acid and / or salt thereof as a substance for suppressing the adhesion of polylysine to a contact lens, nitrogen-containing organic anti-microbial agent excluding polylysine and water. Contact lenses can be cleaned, disinfected and stored simply by soaking them in the liquid formulation without cleaning by digital rubbing or without rinsing before they are worn in eyes.
Owner:OPHTECS CORP
Ocular solutions
InactiveUS7083803B2Reduce inflammationReduce bacterial growthBiocideSenses disorderDiseaseEverolimus
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease.
Owner:PEYMAN GHOLAM A DR
Ophthalmic and related aqueous solutions containing antifungal agents, uses therefor and methods for preparing them
The invention relates generally to concentrates and aqueous solutions for topical application comprising antifungal additives or agents as well as to preparation and use of such concentrates and solutions. More specifically, the invention relates to preparation and use of solutions that come in contact with the eye lids and / or eyes, such as but not limited to contact lens solutions, aqueous ophthalmic rinse solutions, and aqueous surgical scrubs for ophthalmic use.
Owner:SAWAYA ASSAD S
Ocular solutions
InactiveUS20050063996A1Reduce turbidityReduce inflammationAntibacterial agentsBiocideEverolimusCell migration
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
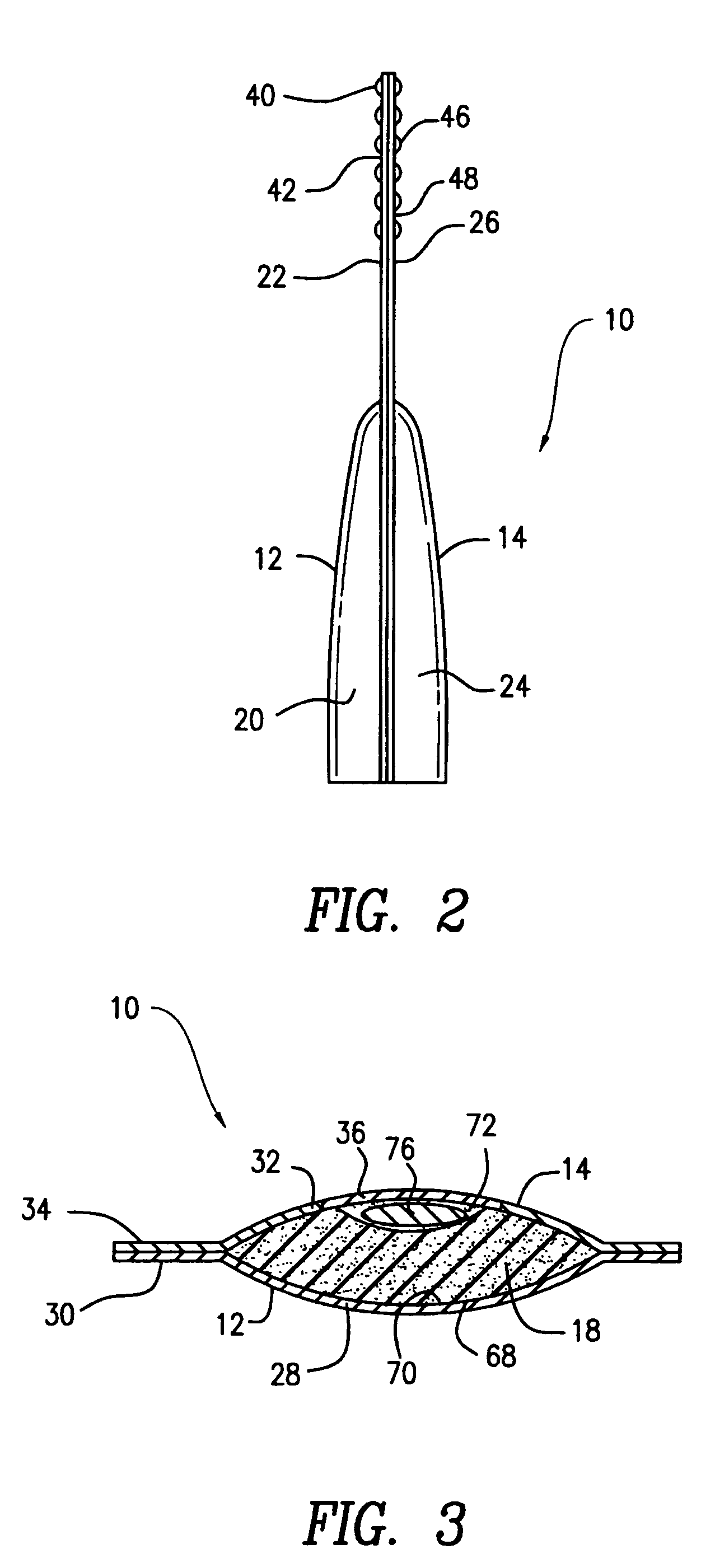
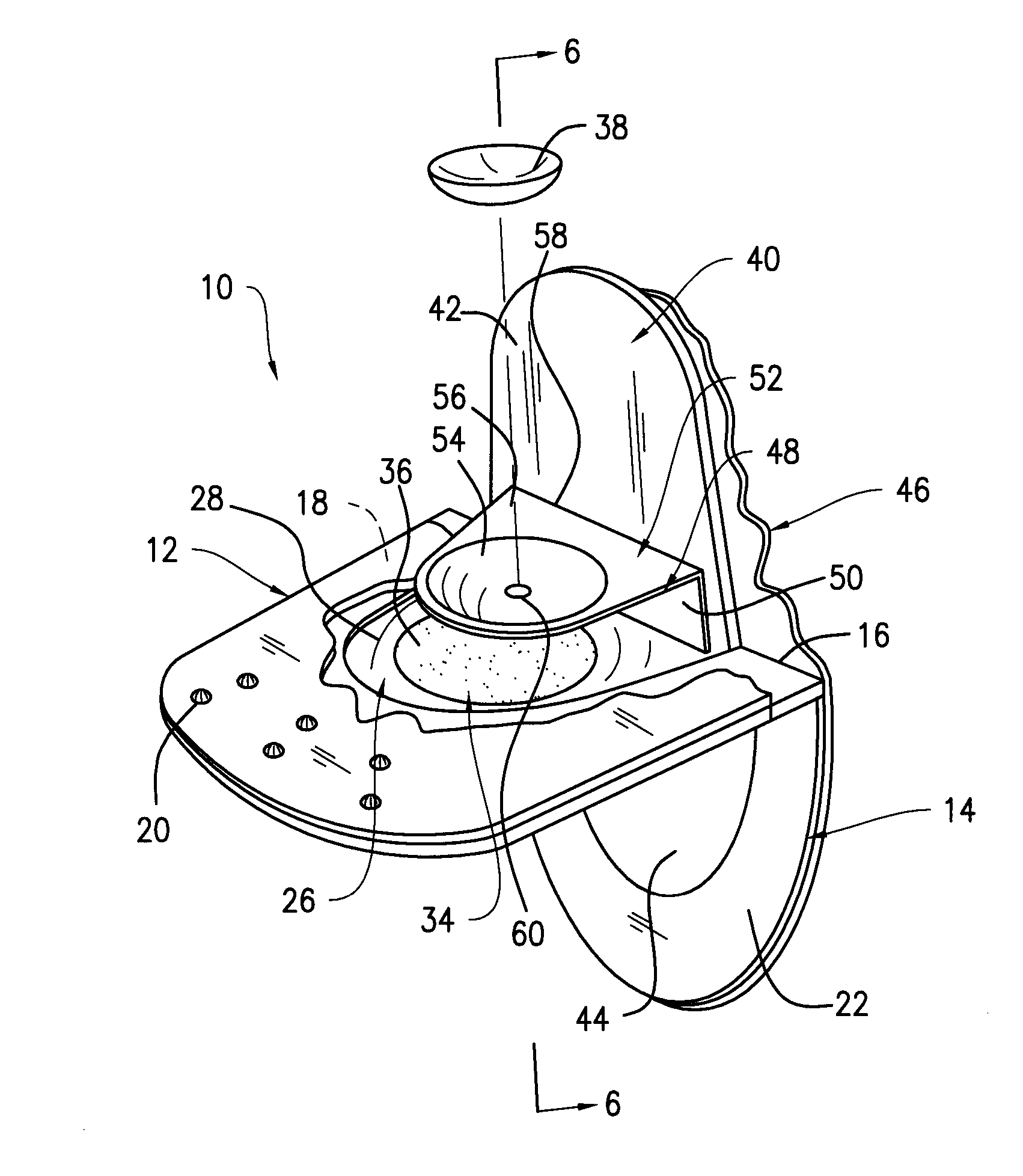
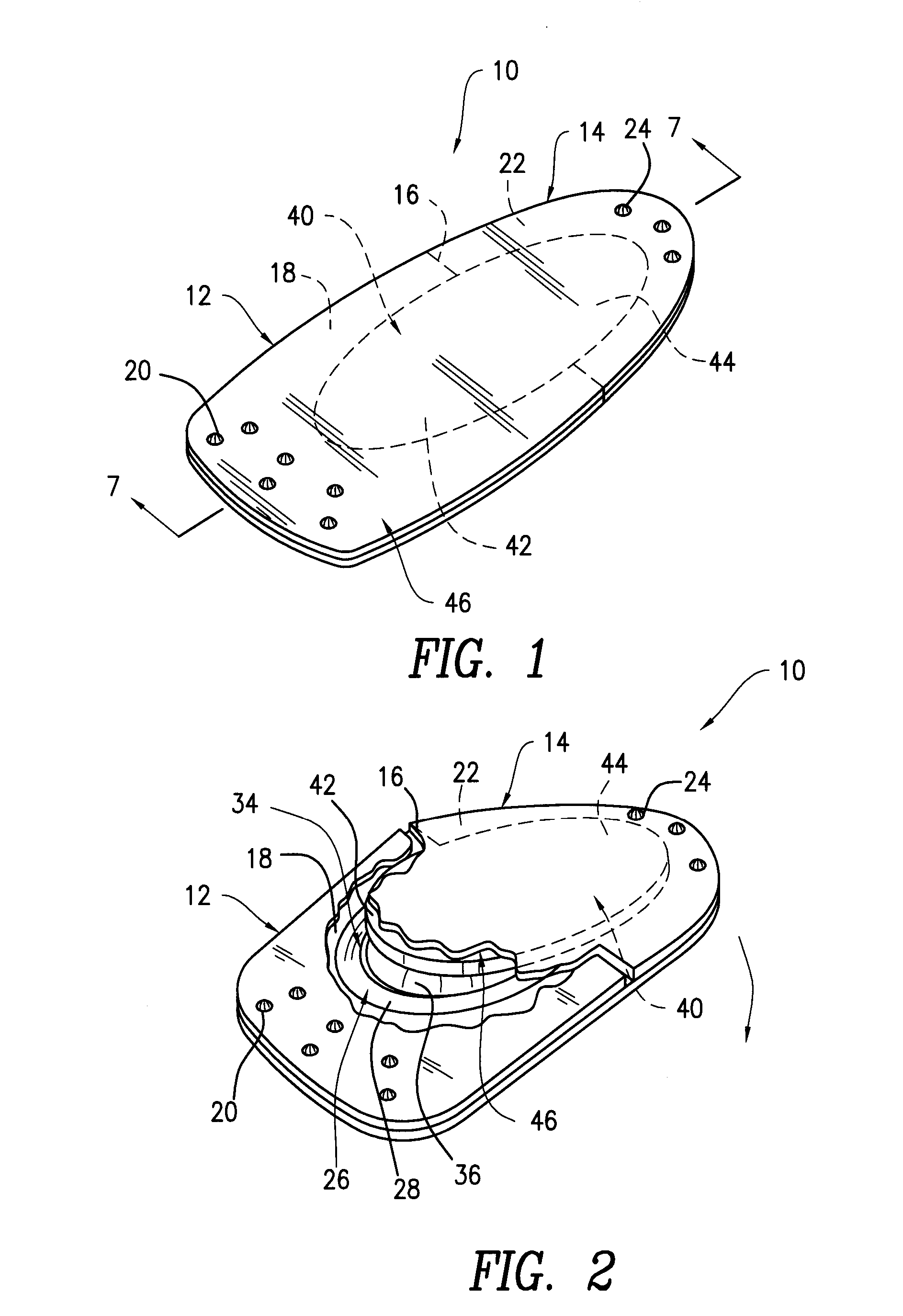
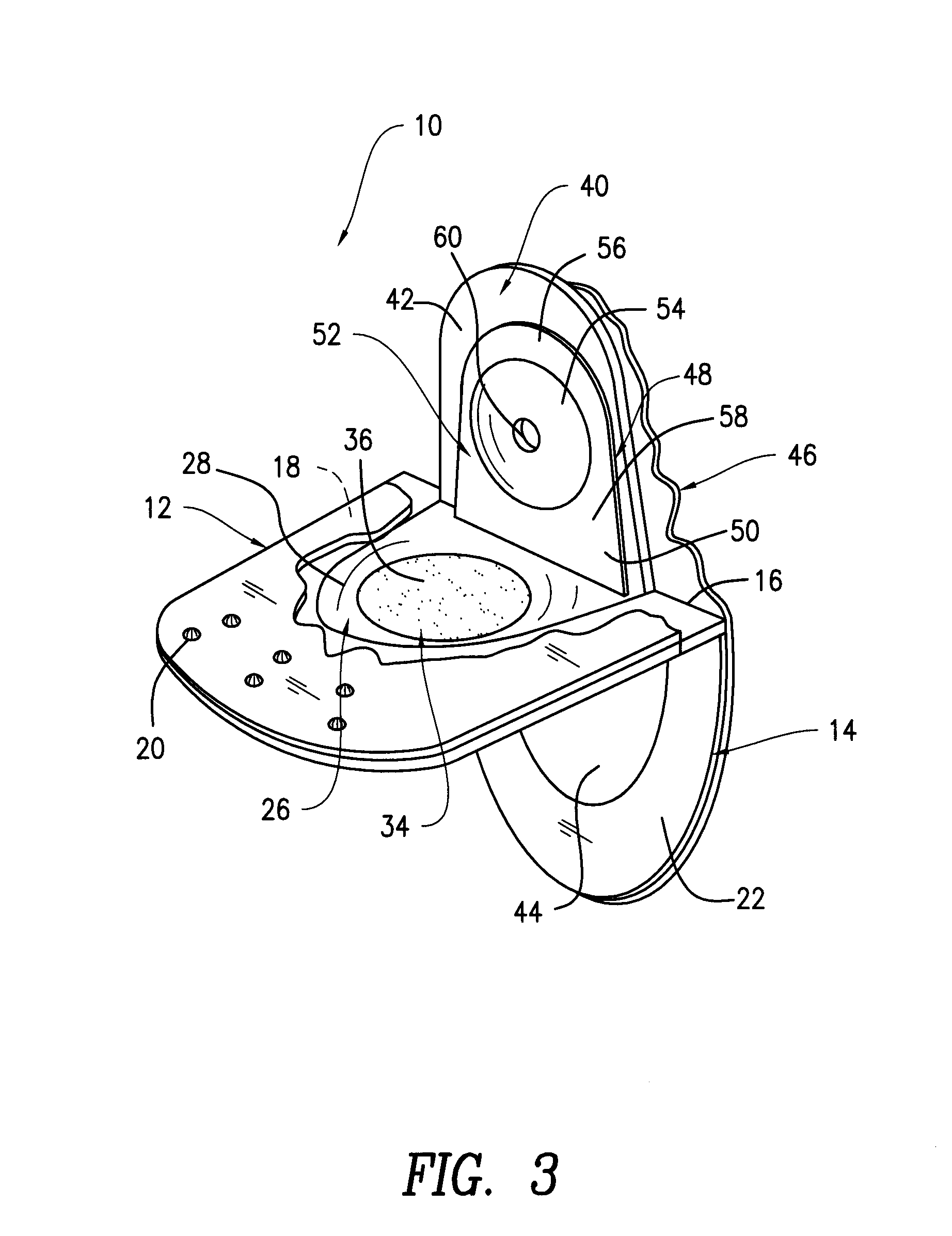
Contact lens package
InactiveUS20060054514A1Other accessoriesContainer/bottle contructionEngineeringMechanical engineering
A contact lens package includes a pair of shell members that are removably attached to each other. An sponge member positioned between the shell members contains a recess for housing a contact lens and contact lens solution. One end of each of the shell members contains a tab, the tabs being juxtaposed with one another when the shell members are attached to each other. The package is opened by pulling apart the juxtaposed tabs and separating the shell members, thereby enabling access to the contact lens. The tabs have aligned apertures for hanging the package from one end thereof. An opposite end of the package is provided with a gusset that is sized and shaped for positioning the package in a free standing position. One of the shell members contains a window for allowing visual exposure of the contact lens.
Owner:TOKARSKI MICHAEL +6
Ocular solutions
InactiveUS20060228394A1Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:MINU
Ocular solutions
InactiveUS7087237B2Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusMacrolide resistance
Containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
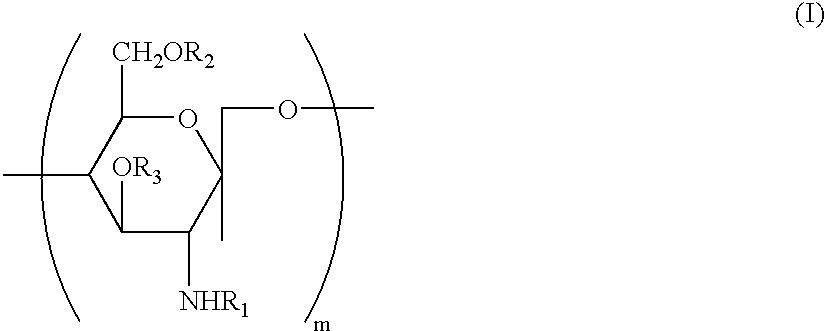
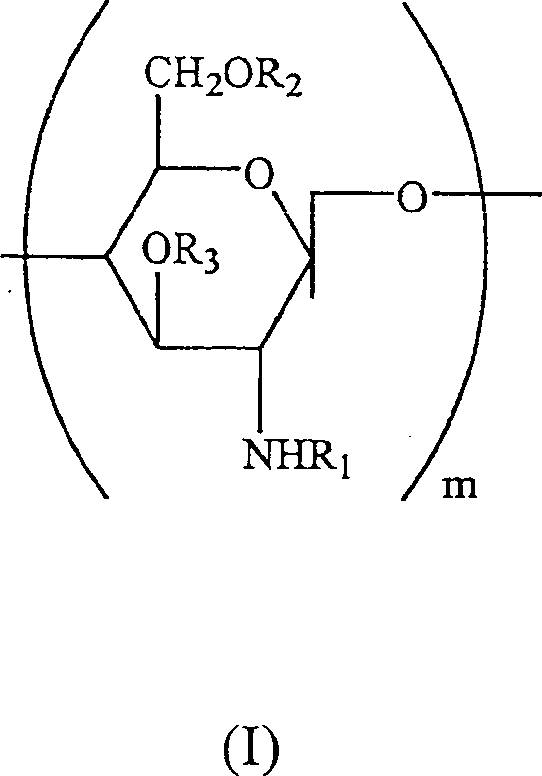
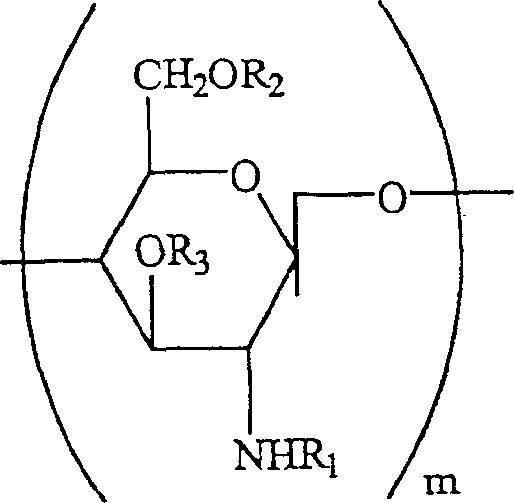
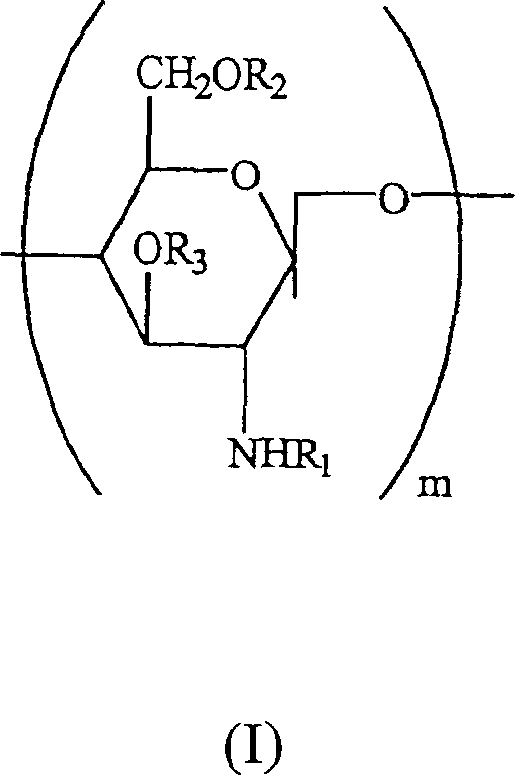
Preserving compositions containing chitosan and processes for making water soluble O-acetylated chitosan and chitosan
InactiveUS20020018732A1Minimal preserving activityAlleviate challengeBiocidePharmaceutical delivery mechanismPolymer sciencePharmaceutical drug
The present invention is directed to a pharmaceutical preserving composition comprising: (a) at least one chitosan or chitosan derivative and (b) at least one buffer solution, as well as methods of preserving contact lens solutions and disinfecting contact lens using such composition. The present invention is further directed to a method of preparing O-acetylated chitosan or chitosan derivatives comprising the steps of dissolving the chitosan or chitosan derivative into an aqueous acidic solution and reacting the chitosan or chitosan derivative with an acetylating agent in the presence of a phase transfer reagent.
Owner:TECH RESOURCE INT
Ophthalmic and contact lens solutions containing simple saccharides as preservative enhancers
InactiveUS20070098818A1Effective preservationDegree of reductionBiocideHydroxy compound active ingredientsTagatoseSucrose
The present invention relates to an ophthalmic solution comprising 0.00001 to 10.0 weight percent of a simple saccharide, at least 0.00001 weight percent of a preservative, and not more than about 0.2 percent by weight chloride. The simple saccharide is chosen from the group consisting of: inositol; mannitol; sorbitol; sucrose; dextrose; glycerin; propylene glycol; ribose; triose; tetrose; erythrose; threose; pentose; arabinose; ribulose; xylose; xylulose; lyxose; hexose; allose; altrose; fructose; galactose; glucose; gulose; idose; mannose; sorbose; talose; tagatose; adlose; ketose; heptose; sedoheptulose; monosaccharides; disaccharides; sugar alcohols; xylitol; and polyol.
Owner:FXS VENTURES LLC
Use of lipid conjugates in the treatment of diseases or disorders of the eye
In one embodiment, the invention provides a method of treating, reducing the incidence, reducing the severity or pathogenesis of an eye disease or disorder in a subject, including, inter alia, retinal detachment, macular degeneration, glaucoma or retinopathy, comprising the step of administering an effective amount of a lipid or phospholipid moiety bound optionally via a spacer to a physiologically acceptable monomer, dimer, oligomer, or polymer via an ester or amide bond, and / or a pharmaceutically acceptable salt or a pharmaceutical product thereof. This invention also provides a contact lens solution comprising a lipid or phospholipid moiety bound optionally via a spacer to a physiologically acceptable monomer, dimer, oligomer, or polymer via an ester or amide bond, and / or a pharmaceutically acceptable salt or a pharmaceutical product thereof.
Owner:YEDGAR SAUL +1
Methods, compositions, formulations, and uses of cellulose and acrylic-based polymers
InactiveUS20050244365A1Easy to chargeLow pKaAntibacterial agentsCosmetic preparationsDisinfectantReverse transcriptase
Compositions, formulations, and methods for the treatment or prevention, or decreasing the frequency of transmission of a virus (such as human immunodeficiency virus type 1 (HIV-1), Herpes Simplex virus type 1 (HSV1), or Herpes Simplex Virus Type 2 (HSV2), or other virus), or a bacterial infection (such as Trichomonas vaginalis, Neisseris gonorrhoeae Haemopholus ducreyl, or Chlamydia trachomatis, or other bacterial species), or a fungal infection, using an anionic cellulose- or acrylic-based oligomer, polymer, or copolymer. The present invention also includes administering a therapeutically effective amount of said oligomer, polymer, or copolymer, or a pharmaceutically acceptable salt thereof, or with a pharmaceutically acceptable carrier or diluent, thereof. The invention relies on the unique biochemical substitution of the cellulose or acrylic backbone such that the resultant molecule can remain molecularly dispersed in solution (or gel or other formulation) and mostly dissociated over a wide range of physiological microenvironments, such as the low pH found within the vaginal lumen, preferably from a pH of 14 to below 3.5. These specific substitutions also impart on the resultant molecule potent antiviral, anti-bacterial, and anti-fungal properties. In addition, these compositions can be used as general disinfectants for human use such as in contact lens solutions, mouthwashes, toothpastes, suppositories, or as more generalized disinfectants found in soaps, household cleaning products, paints, water treatments modalities, or can be incorporated into cosmetic, and can be used as vehicles for drug delivery, an adjuvant in a therapeutic formulation, or as a preservative. These compounds can be delivered in a liquid or solid dosage form and can be incorporated into barrier devices such as condoms, diaphragms, or cervical caps, to help prevent the transmission of STDs. The compounds of this invention can also be used in combination therapies with other classes of antiviral, antibacterial, or antifungal agent having similar or differing mechanisms of action including, but not limited to, anionic or cationic polymers, copolymers, or oligomers, surfactants, protease inhibitors, DNA or RNA polymerase inhibitors (including reverse transcriptase inhibitors), fusion inhibitors, cell wall biosynthesis inhibitors, integrase inhibitors, or virus or bacterial attachment inhibitors.
Owner:NOVAFLUX INC +1
Ocular solutions
InactiveUS20050063997A1Reduce turbidityReduce inflammationBiocideSenses disorderEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:PEYMAN GHOLAM A DR
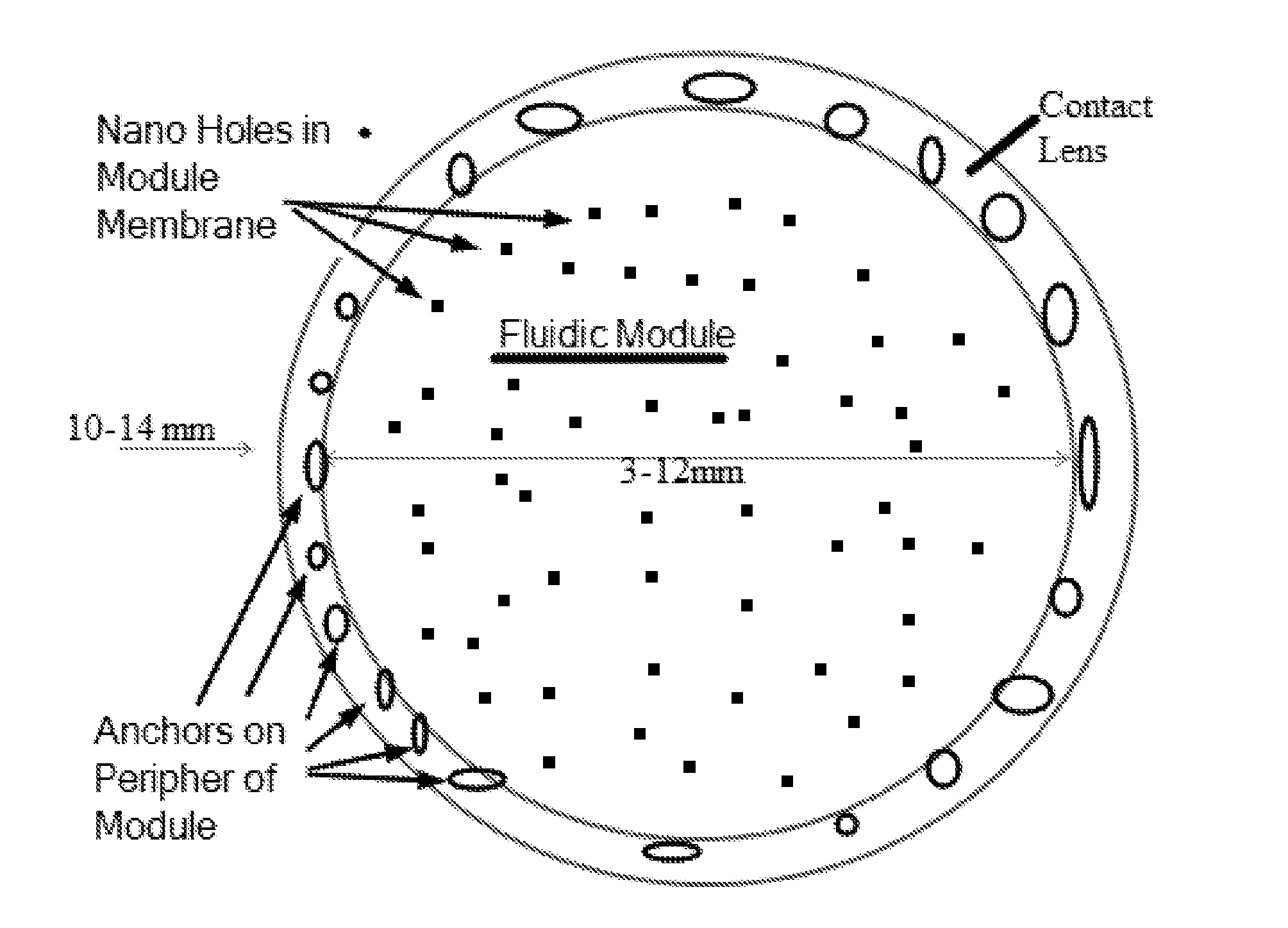
Drug delivery from contact lenses with a fluidic module
InactiveUS20160018671A1Inhibited DiffusionReduce diffuseOrganic active ingredientsSenses disorderAqueous solutionOptometry
A soft contact lens comprises a module embedded in a soft contact lens material. The module comprises a hydrophobic material having channels formed therein, such that a surface tension of the aqueous solution within the channels inhibits release of therapeutic agent, such as a drug, through the one or more channels. The surface tension of the aqueous solution within the channel can inhibit diffusion of the therapeutic agent through the channel. The channels may comprise a cross-sectional area and optionally a length, such that therapeutic agent is released through the channels when pressure of the eyelid increases. In many embodiments, the contact lens is configured to inhibit release of the therapeutic agent when the contact lens comprises a free floating configuration, for example when stored in a contact lens solution, such that the storage time of the contact lens can be increased substantially.
Owner:ONEFOCUS TECH
Composition for contact lens solution containing peppermint and preparation method thereof
ActiveCN101574537AReduce drynessRelieve sorenessSpectales/gogglesOrganic detergent compounding agentsFiltrationPeppermints
The invention discloses a composition a contact lens solution containing peppermint, which is characterized in that the composition consists of the following components in percentage by weight: 0.001 to 0.3 percent of peppermint, 0.01 to 4 percent of isotonic regulator, 0.001 to 6 percent of lubricant-humectant, 0.001 to 5 percent of surfactant, 0.001 to 3 percent of chelating agent, 0.001 to 10 percent of buffering agent, 0.00001 to 2 percent of biocide, and 80 to 99.7 percent of pure water. A preparation method comprises the steps of: orderly placing the components into the pure water to dissolve, using sodium hydroxide and hydrochloric acid to adjust the pH value to be between 6.5 and 7.8, ensuring that the osmotic pressure is between 280 and 320 mo sm / kg.H2O, and performing aseptic filtration (0.22 mu m) on a solution. In the composition, the peppermint has special fragrance, sense of pungency and cool sense, stops pain and itching, and is effective to relieve the dryness and discomfort as well as the swelling of eyes. The contact lens solution is used for treating, disinfecting, cleaning, soaking, lubricating and storing contact lenses.
Owner:HAICHANG CONTACT LENSES
Ophthalmic and contact lens solutions containing forms of vitamin b
InactiveUS20070104744A1Discomfort to userDegree of reductionOrganic detergent compounding agentsLens cleaning compositionsOphthalmic solutionsBiology
The present invention relates to improved ophthalmic solutions that employ select B vitamins; pyridoxine and its salts; and thiamine and its salts in order to more effectively preserve solutions and to reduce the degree to which cationic preservatives will deposit on contact lenses. Ophthalmic solutions are here understood to include contact lens treatment solutions, such as cleaners, soaking solutions, conditioning solutions and lens storage solutions, as well as wetting solutions and in-eye solutions for treatment of eye conditions.
Owner:FXS VENTURES LLC
Snap and lift package for contact lens
InactiveUS20080047848A1Overcome disadvantagesOther accessoriesContainer/bottle contructionEngineeringMechanical engineering
A contact lens package includes a first housing member and a second housing member pivotably connected to each other. The package also includes a holding mechanism for holding a contact lens and a receiving mechanism for receiving contact lens solution. When the housing members are in a substantially co-planar orientation, the holding mechanism is positioned within the receiving mechanism. When the housing members are in a non-planar orientation, the holding mechanism is removed from the receiving mechanism such that the holding mechanism is pivotable from a closed position, in which the contact lens held in the holding mechanism is inaccessible to a user, toward an open position, in which the contact lens held in the holding mechanism is accessible to the user.
Owner:TOKARSKI MICHAEL +6
Water soluble, randomly substituted partial N-partial O-acetylated chitosan, preserving compositions containing chitosan, and processes for making thereof
InactiveUS20020177577A1Minimal preserving activityAlleviate challengeOrganic active ingredientsBiocideWater solubleAqueous solution
The present invention is directed to a water soluble, randomly substituted partial N-, partial O-acetylated chitosans or chitosan derivatives and methods of preparing water soluble, randomly substituted partial N-, partial O-acetylated chitosans or chitosan derivatives comprising the steps of dissolving the chitosan or chitosan derivative into an aqueous acidic solution and reacting the chitosan or chitosan derivative with an acetylating agent in the presence of a phase transfer reagent. The present invention is further directed to a pharmaceutical preserving composition comprising: (a) at least one chitosan or chitosan derivative and (b) at least one buffer solution, as well as methods of preserving contact lens solutions and disinfecting contact lens using such composition.
Owner:ADJUVANT PHARMA
Opthalmic and contact lens solutions with a peroxide source and a cationic polymeric preservative
An ophthalmic solution including 0.01 to 0.0001 percent by weight of a peroxide producing agent and 0.1 to 500 parts per million of a cationic, polymeric preservative.
Owner:BIO CONCEPT LAB
Ophthalmic and contact lens solutions containing simple saccharides as preservative enhancers
InactiveUS20060142169A1Effective preservationDegree of reductionBiocideSenses disorderMANNITOL/SORBITOLSucrose
A contact lens solution comprising 0.001 to 10 weight percent or a preservative enhancer chosen from the group consisting of: inositol; mannitol; sorbitol; sucrose; dextrose; glycerin and propylene glycol; and at least 0.0001 weight percent of a cationic polymeric preservative, and where the concentration of chloride in said solution is less than 0.2 percent by weight.
Owner:FXS VENTURES LLC
Ophthalmic and contact lens solutions containing forms of vitamin b
InactiveUS20060148665A1Degree of reductionEffectively preserve solutionsCosmetic preparationsOrganic detergent compounding agentsDiseasePreservative
The present invention relates to improved ophthalmic solutions that employ select B vitamins; pyridoxine and its salts; and thiamine and its salts in order to more effectively preserve solutions and to reduce the degree to which cationic preservatives will deposit on contact lenses. Ophthalmic solutions are here understood to include contact lens treatment solutions, such as cleaners, soaking solutions, conditioning solutions and lens storage solutions, as well as wetting solutions and in-eye solutions for treatment of eye conditions.
Owner:FXS VENTURES LLC
Novel ophthalmic compositions containing human recombinant lysozyme and use thereof for treating eye conditions and as contact lens solutions
InactiveUS20080213188A1Ultrasonic/sonic/infrasonic diagnosticsSenses disorderSubject matterEyes inflammation
An ophthalmic solution comprising: a) a human recombinant lysozyme; b) one or more natural lacrophyl substances; c) water; and d) optionally one or more therapeutic substances. The ophthalmic solution is useful to treat dry eye conditions and eye inflammation and also to condition and / or cleanse contact lenses. It is emphasized that this abstract is provided to comply with the rules requiring an abstract which will allow a searcher or other reader quickly to ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the appended issued claims.
Owner:SAINT SIMEON
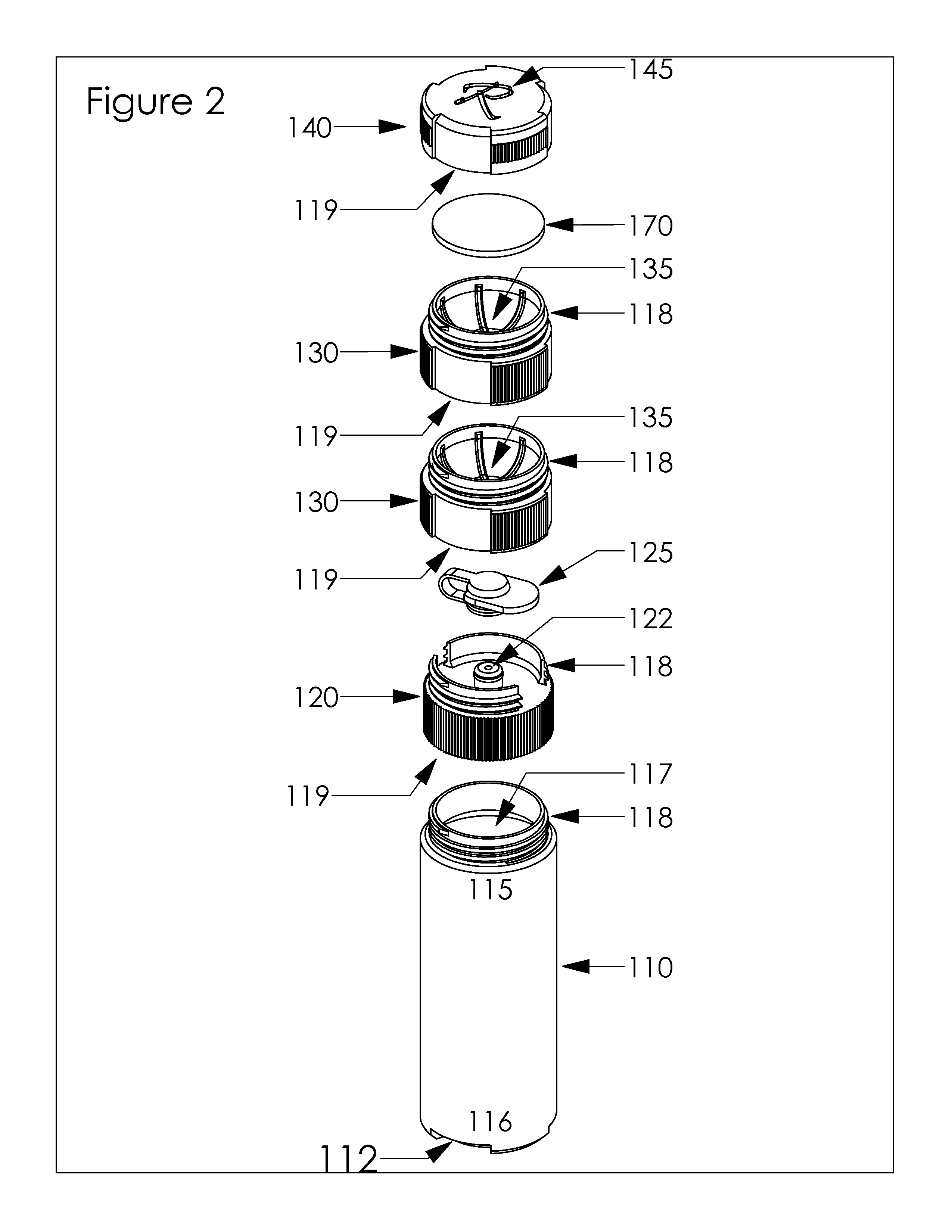
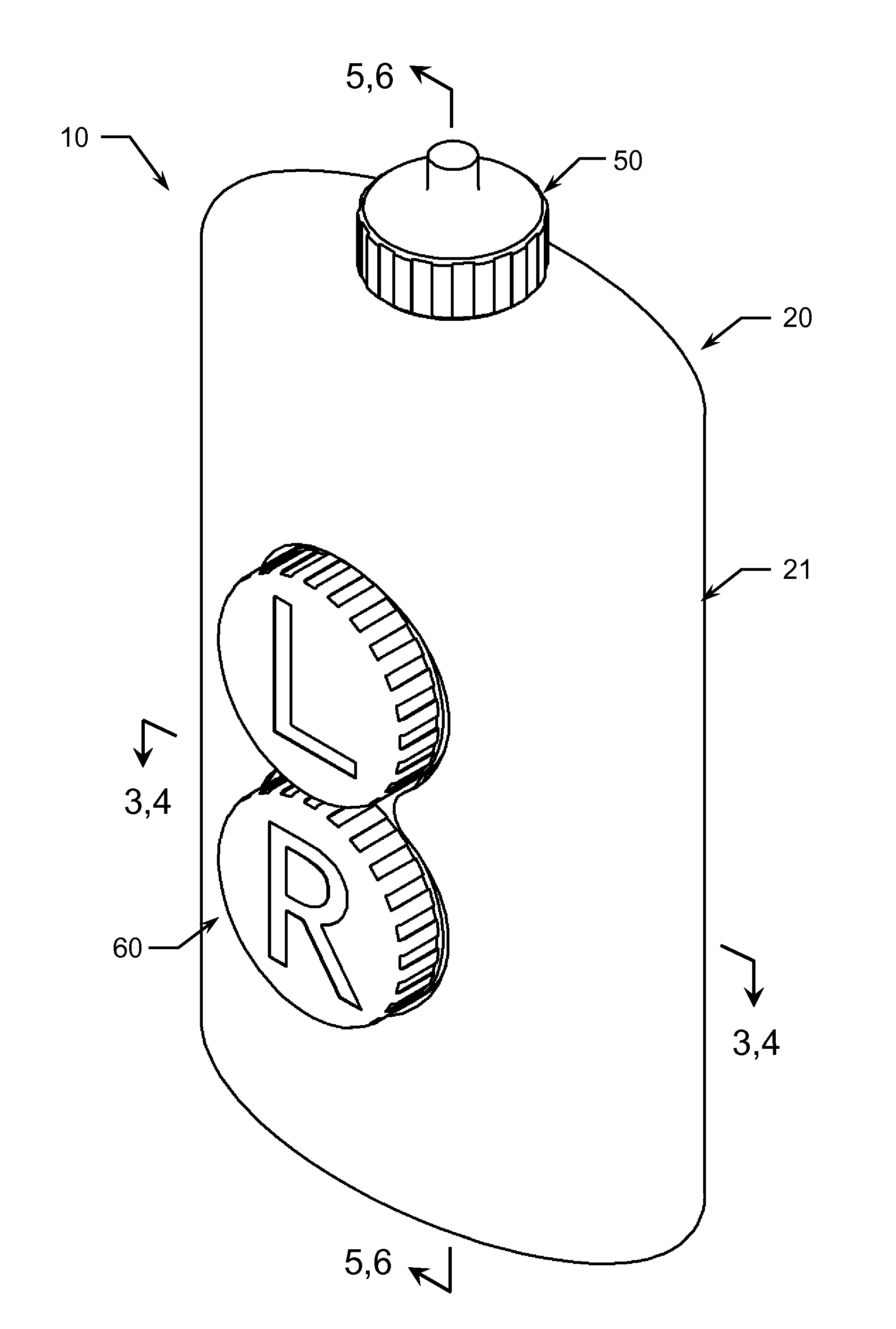
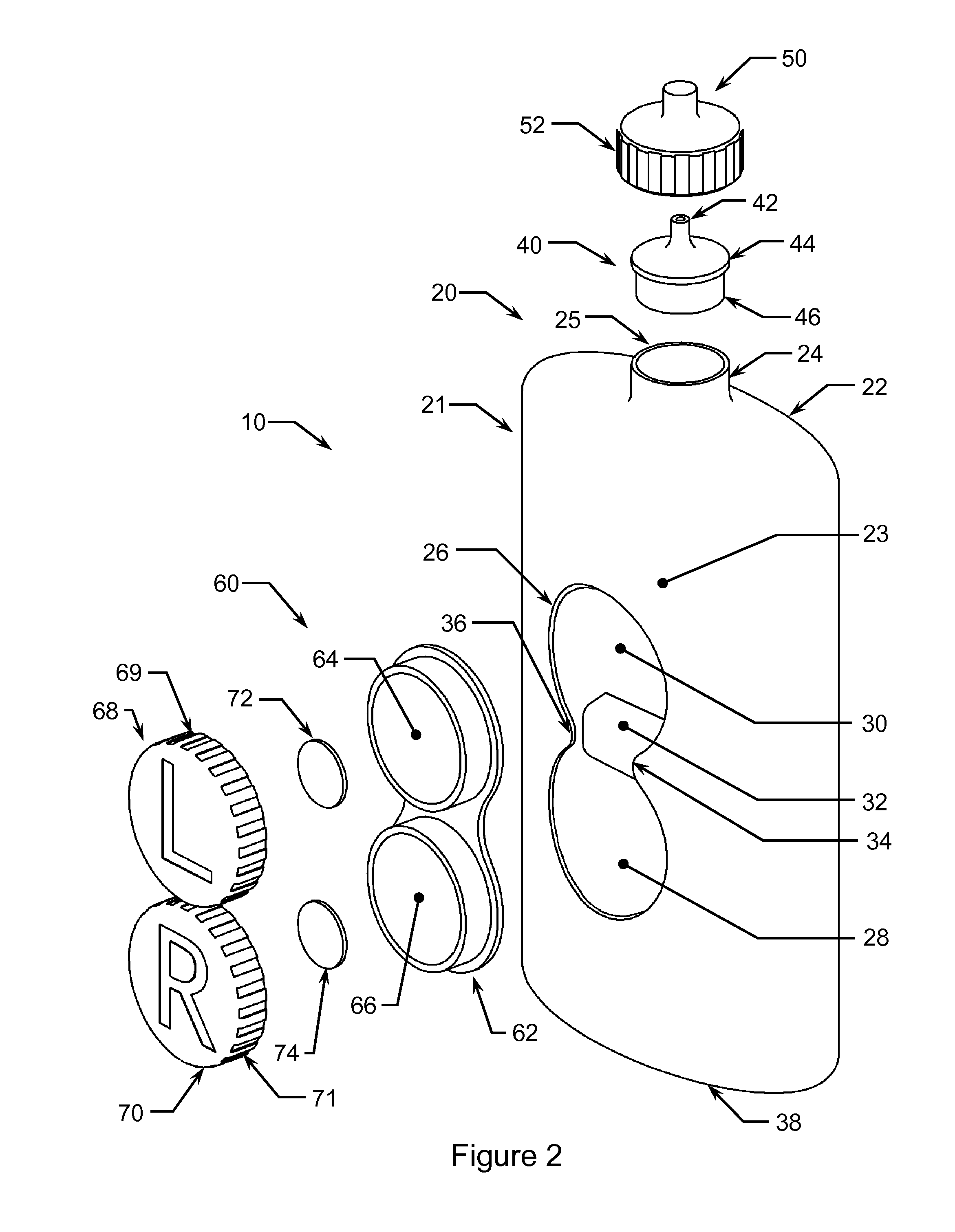
Integrated Bottle, Case and Mirror
A compact, stackable, integrated, and re-useable contact lens case and contact lens solution bottle comprising at least one bottle for storage of at least one solution, at least one bottle cap comprising a nozzle with a closing cap, at least one contact lens case, and at least one cover. Embodiments of the device further comprise at least one mirror. The components of the device are securely and removably stackable upon each other. Preferably, when stacked together, the integrated device is one unit that is easily stored and transported.
Owner:FRANKLIN DAMON L
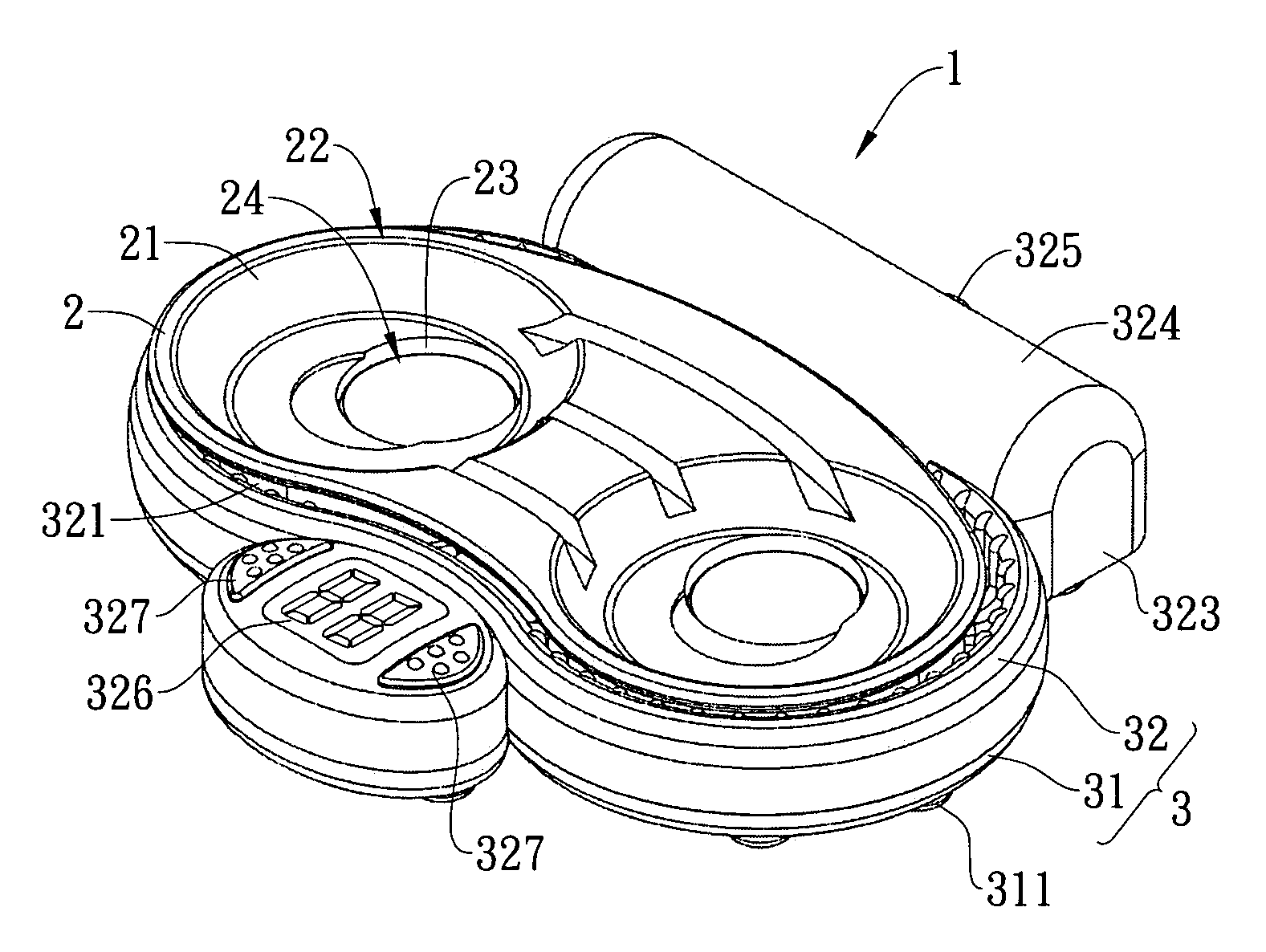
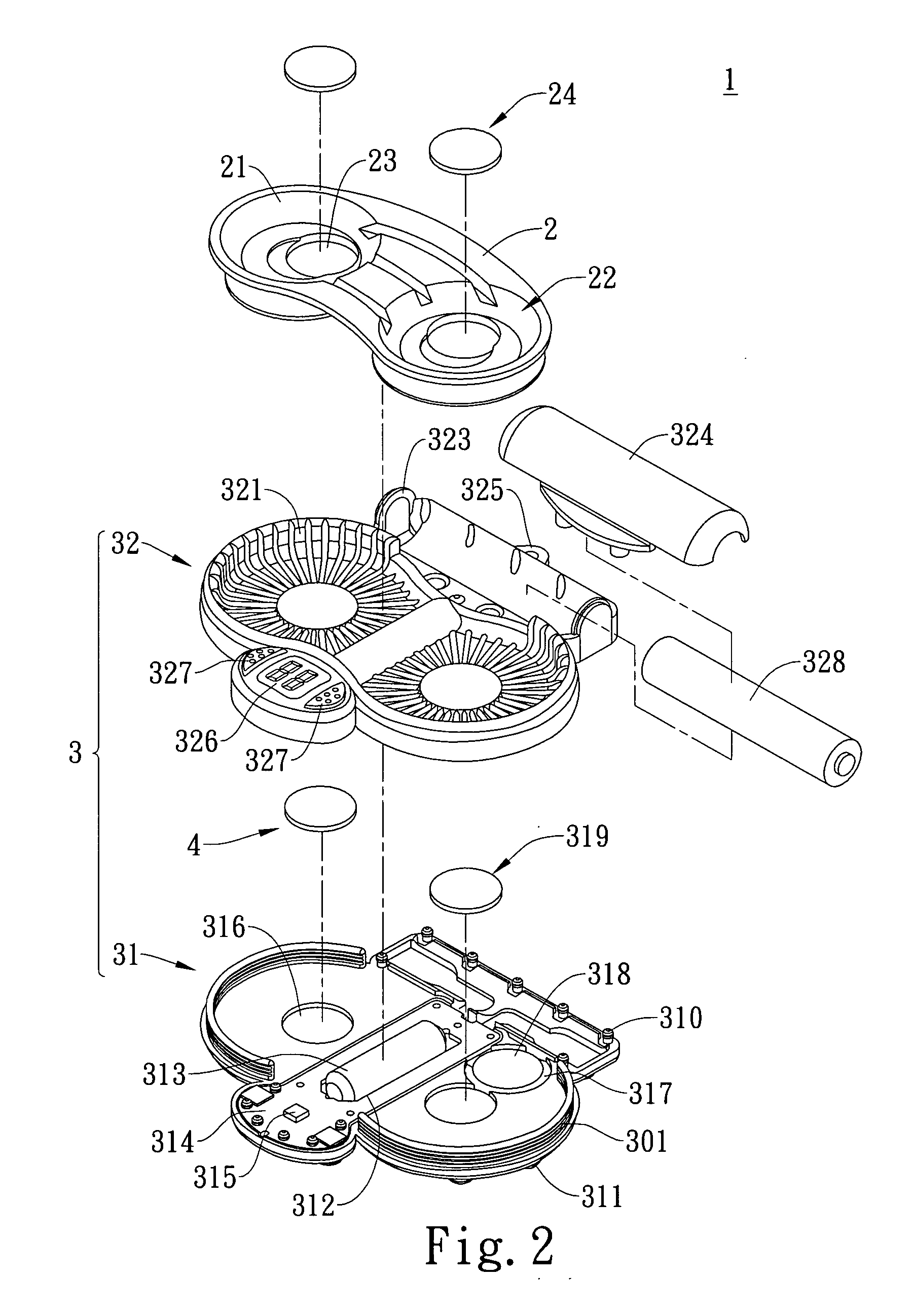
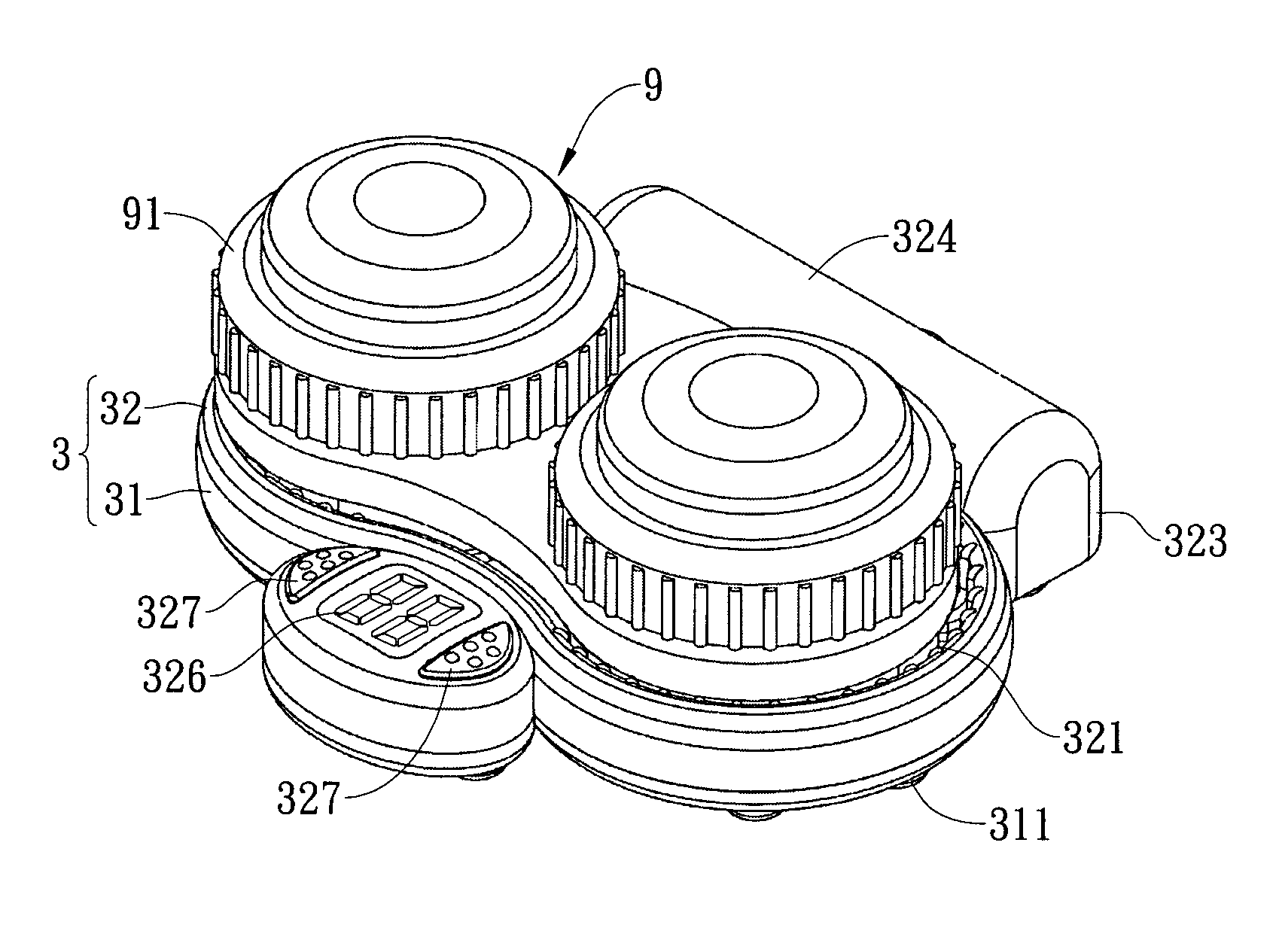
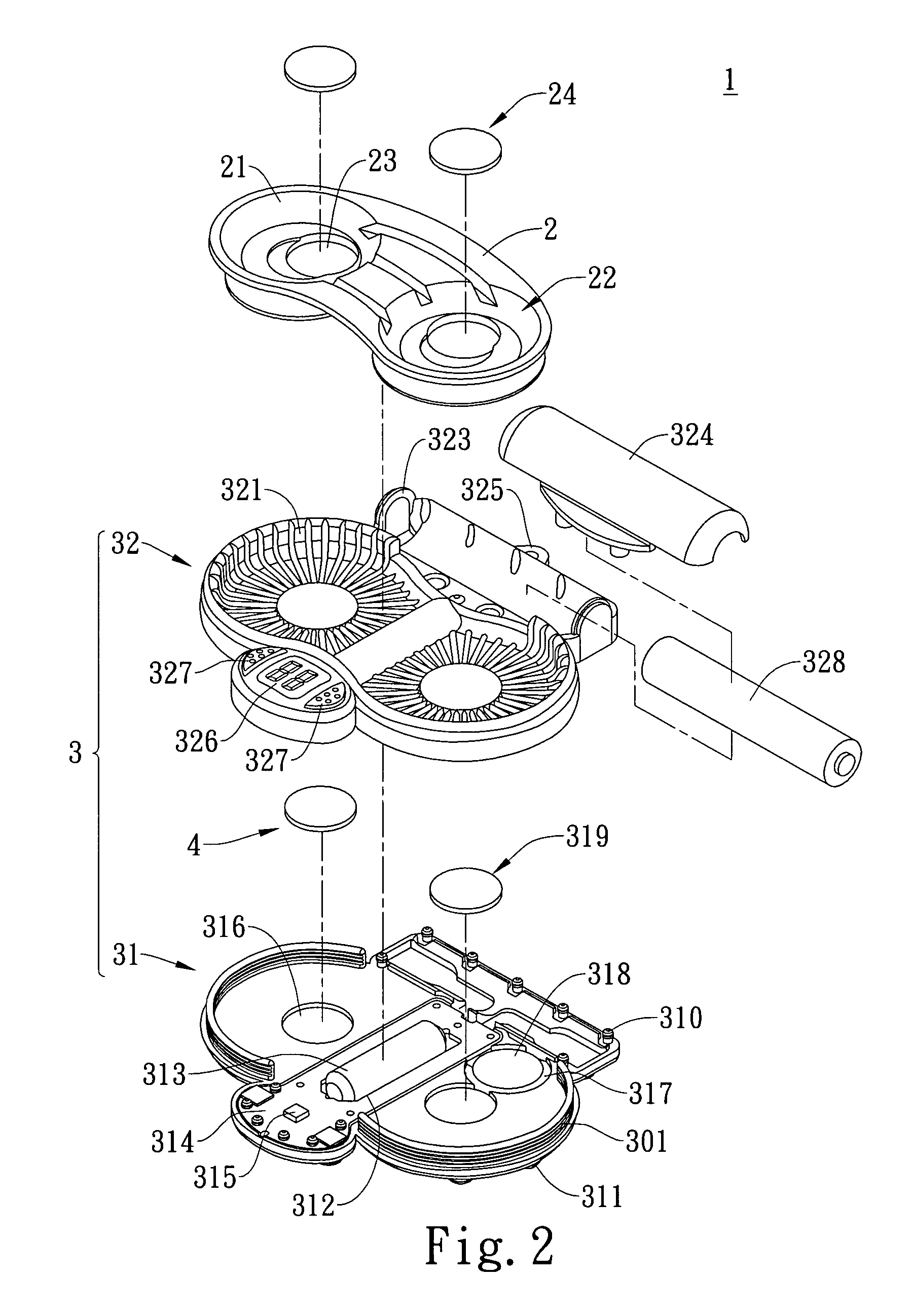
Vibration-type cleaning device for contact lenses
ActiveUS20090283118A1Low costReduce noiseElectrostatic cleaningOther accessoriesEngineeringContact Lens Case
A vibration-type cleaning device for contact lenses comprises an adaptor that is provided with a support part thereon for supporting a contact lens case and a base that is provided under the bottom of the adaptor. The base is connected with the adapter via a positioning element so that the base can be attached to or detached from the adaptor. The base is provided with a vibrator therein and the vibrator is connected with a power source. Consequently, the vibrator can be driven under the control of a user to vibrate the contact lens solution and the contact lenses in the contact lens case and the cleaning effect of the contact lenses in the contact lens solution can be enhanced through vibration.
Owner:ZAKUTIN DAVID MICHAEL
Water soluble, randomly substituted partial N-partial O-acetylated chitosan, preserving compositions containing chitosan, and processes for making thereof
InactiveUS6716970B2Synergistic effectAlleviate challengeBiocideSugar derivativesWater solubleAqueous solution
The present invention is directed to a water soluble, randomly substituted partial N-, partial O-acetylated chitosans or chitosan derivatives and methods of preparing water soluble, randomly substituted partial N-, partial O-acetylated chitosans or chitosan derivatives comprising the steps of dissolving the chitosan or chitosan derivative into an aqueous acidic solution and reacting the chitosan or chitosan derivative with an acetylating agent in the presence of a phase transfer reagent. The present invention is further directed to a pharmaceutical preserving composition comprising: (a) at least one chitosan or chitosan derivative and (b) at least one buffer solution, as well as methods of preserving contact lens solutions and disinfecting contact lens using such composition.
Owner:ADJUVANT PHARMA
Vibration-type cleaning device for contact lenses
InactiveUS8015987B2Low costReduce noiseElectrostatic cleaningOther accessoriesEngineeringContact Lens Case
A vibration-type cleaning device for contact lenses comprises an adaptor that is provided with a support part thereon for supporting a contact lens case and a base that is provided under the bottom of the adaptor. The base is connected with the adapter via a positioning element so that the base can be attached to or detached from the adaptor. The base is provided with a vibrator therein and the vibrator is connected with a power source. Consequently, the vibrator can be driven under the control of a user to vibrate the contact lens solution and the contact lenses in the contact lens case and the cleaning effect of the contact lenses in the contact lens solution can be enhanced through vibration.
Owner:ZAKUTIN DAVID MICHAEL
Water soluble, randomly substituted partial N-, partial O-acetylated chitosan, preserving compositions containing chitosan, and processes for making thereof
InactiveCN1507458APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPolymer scienceAcetylation
Owner:ADJUVANT PHARMA
Ophthalmic and contact lens solutions with a peroxide source and a preservative
InactiveUS20070098813A1Increase capacityEffective capacityBiocideLens cleaning compositionsPreservativePeroxide
The present invention relates to an ophthalmic solution including 0.00001 to about 3.0 percent by weight of a peroxide producing agent and 0.1 to 500 parts per million of a preservative.
Owner:FXS VENTURES LLC
Contact lens solution
A contact lens solution containing polylysine, polyphosphoric acid and / or its salt preventing polylysine from adsorption by contact lenses, a nitrogen-containing organic antibacterial agent other than polylysine and water. Contact lenses can be washed, disinfected and preserved merely by soaking in the above solution without rubbing or rinsing before putting into eyes.
Owner:OPHTECS CORP
Contact Lens Case and Solution container Travel Apparatus
InactiveUS20100252082A1Small sizeSatisfy regulationOther accessoriesCleaning using liquidsOff the shelfEngineering
The combined contact lens case and contact lens solution container apparatus is an invention that allows a user to conveniently carry both an off-the-shelf contact lens case and a container of contact lens solution in one compact package. Because the apparatus includes both the case and container, the apparatus avoids the problem of having contact lenses on-hand when traveling but not having contact lens solution. The contact lens case easily snaps into and out of the side of the contact lens solution container requiring minimal manual dexterity. The solution container, having a solution capacity of no more than three ounces, complies with air travel carry-on requirements, allowing the user to conveniently travel with apparatus whether by commercial air travel or by other modes of travel.
Owner:LIM MOO YOUNG
Ophthalmic contact lens solutions containing forms of vitamin B
InactiveUS9308264B2Degree of reductionEffectively preserve solutionsBiocideOrganic detergent compounding agentsPreservativeB Vitamin Family
The present invention relates to improved ophthalmic solutions that employ select B vitamins; pyridoxine and its salts; and thiamine and its salts in order to more effectively preserve solutions and to reduce the degree to which cationic preservatives will deposit on contact lenses. Ophthalmic solutions are here understood to include contact lens treatment solutions, such as cleaners, soaking solutions, conditioning solutions and lens storage solutions, as well as wetting solutions and in-eye solutions for treatment of eye conditions.
Owner:FXS VENTURES LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com