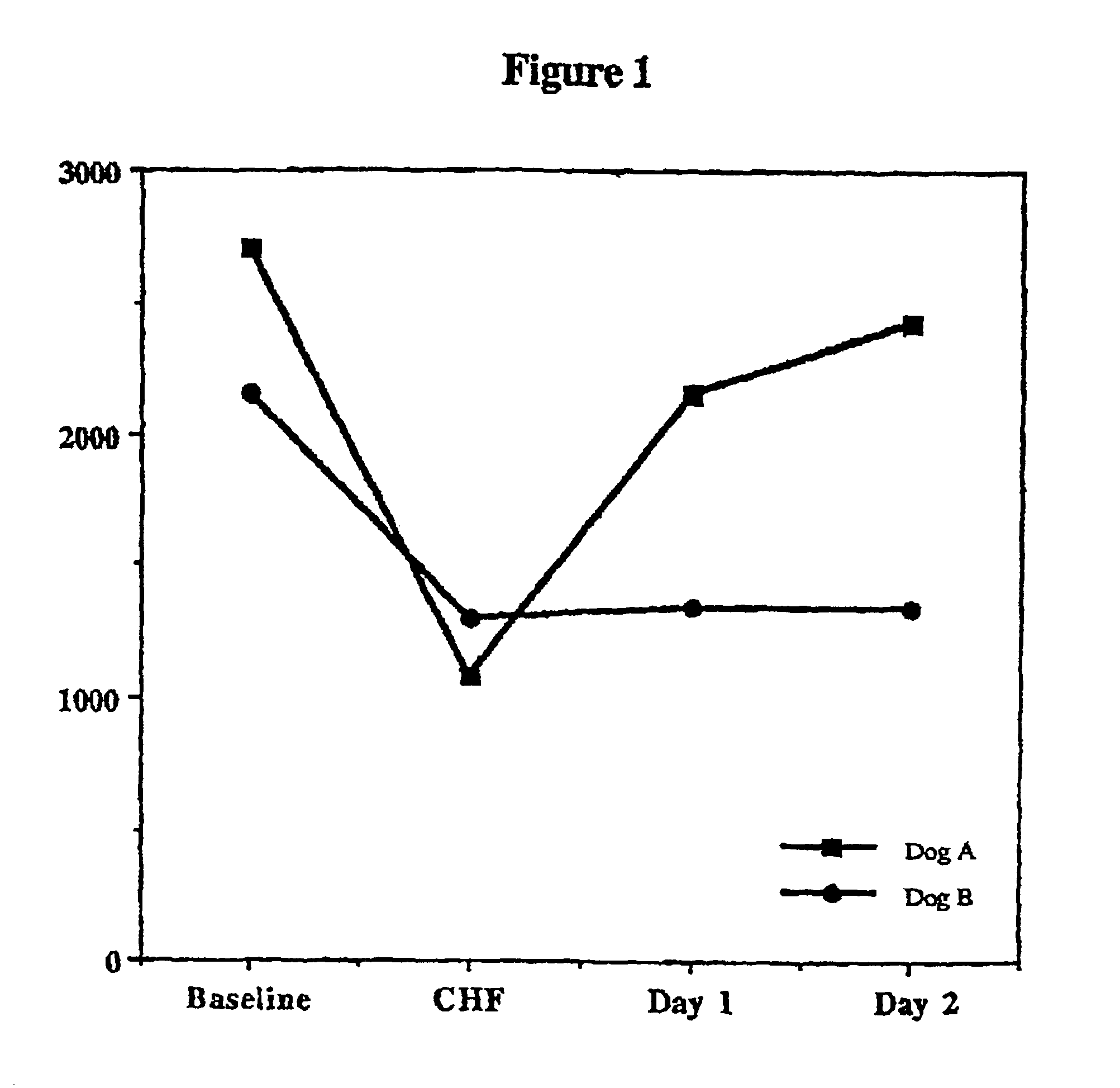
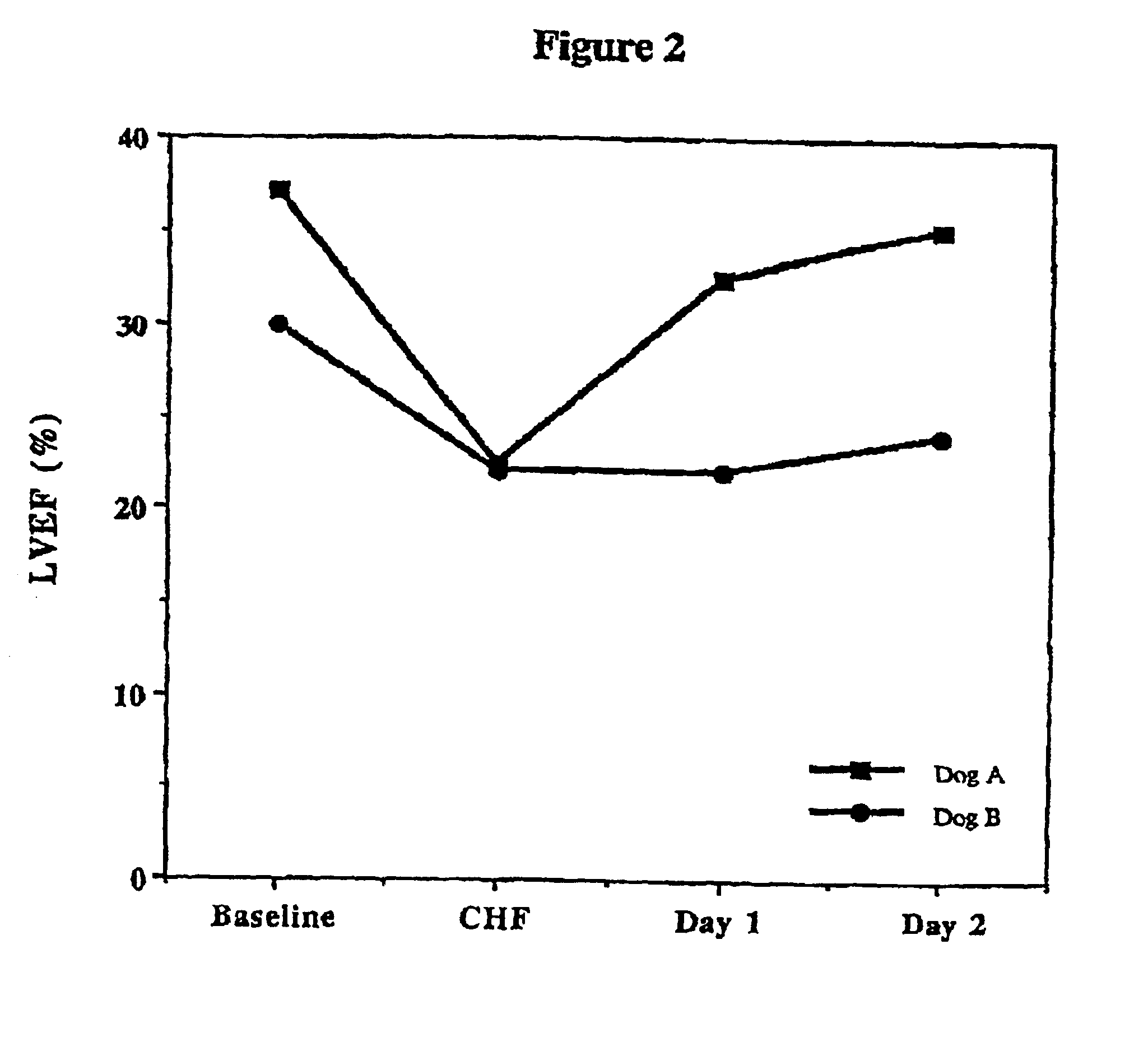
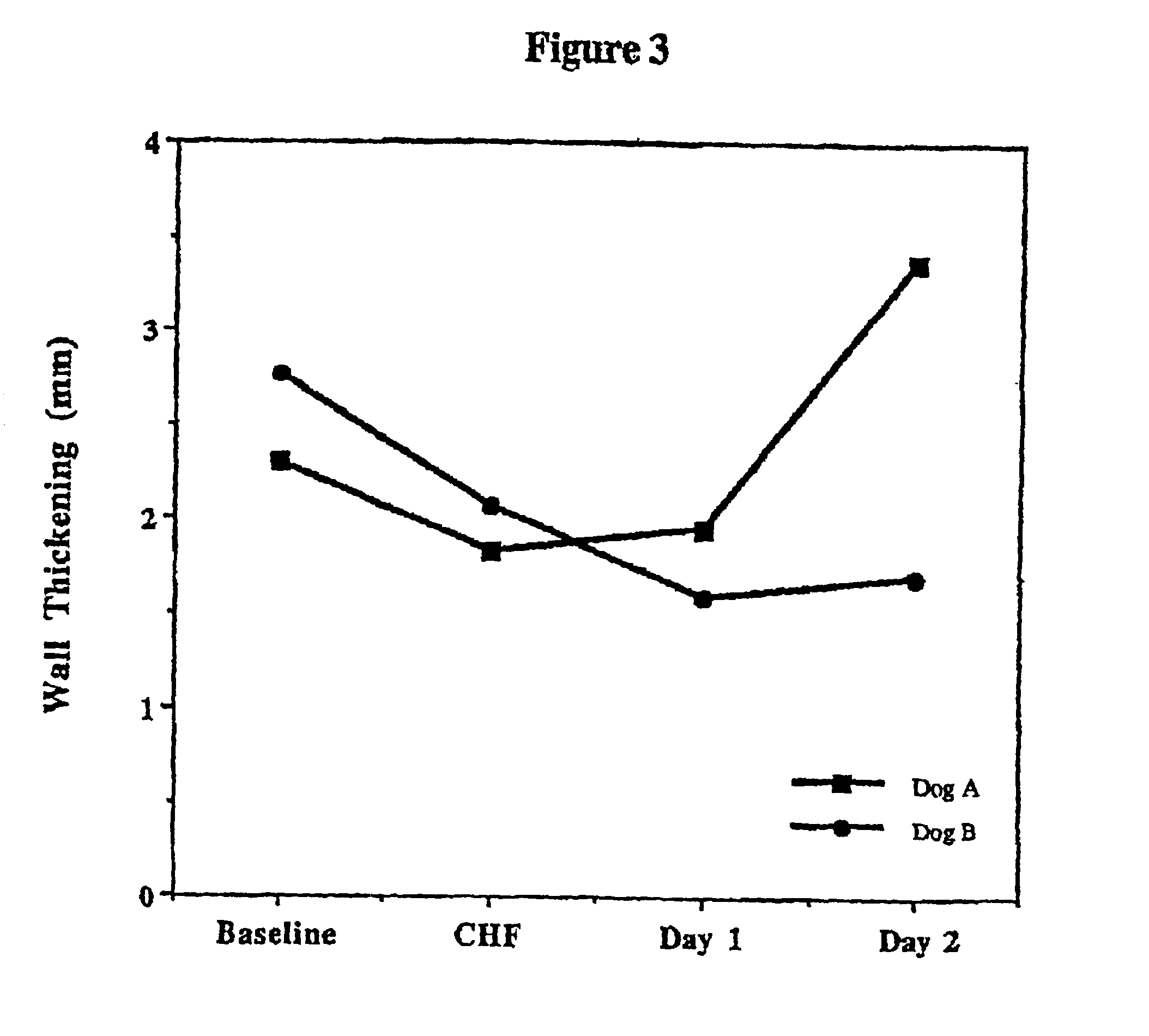
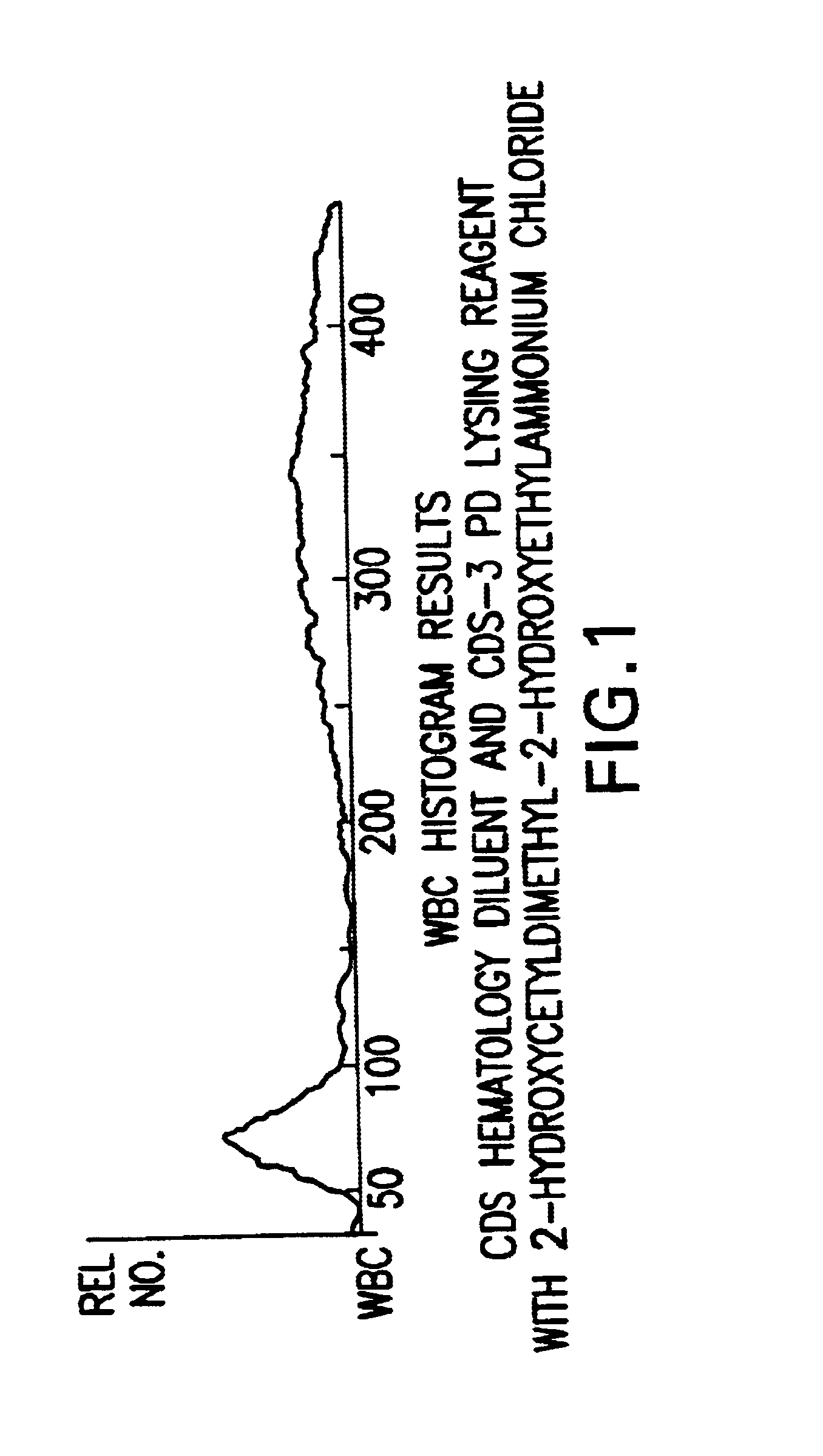
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Cell integrity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cellular Integrity. The plasma membrane (lipid bilayer) is the part of the cell that regulates the entry and exit of certain molecules. If the integrity of the membrane is lost, certain things that should not be getting in get in, and certain things that should not leave are leaking out.
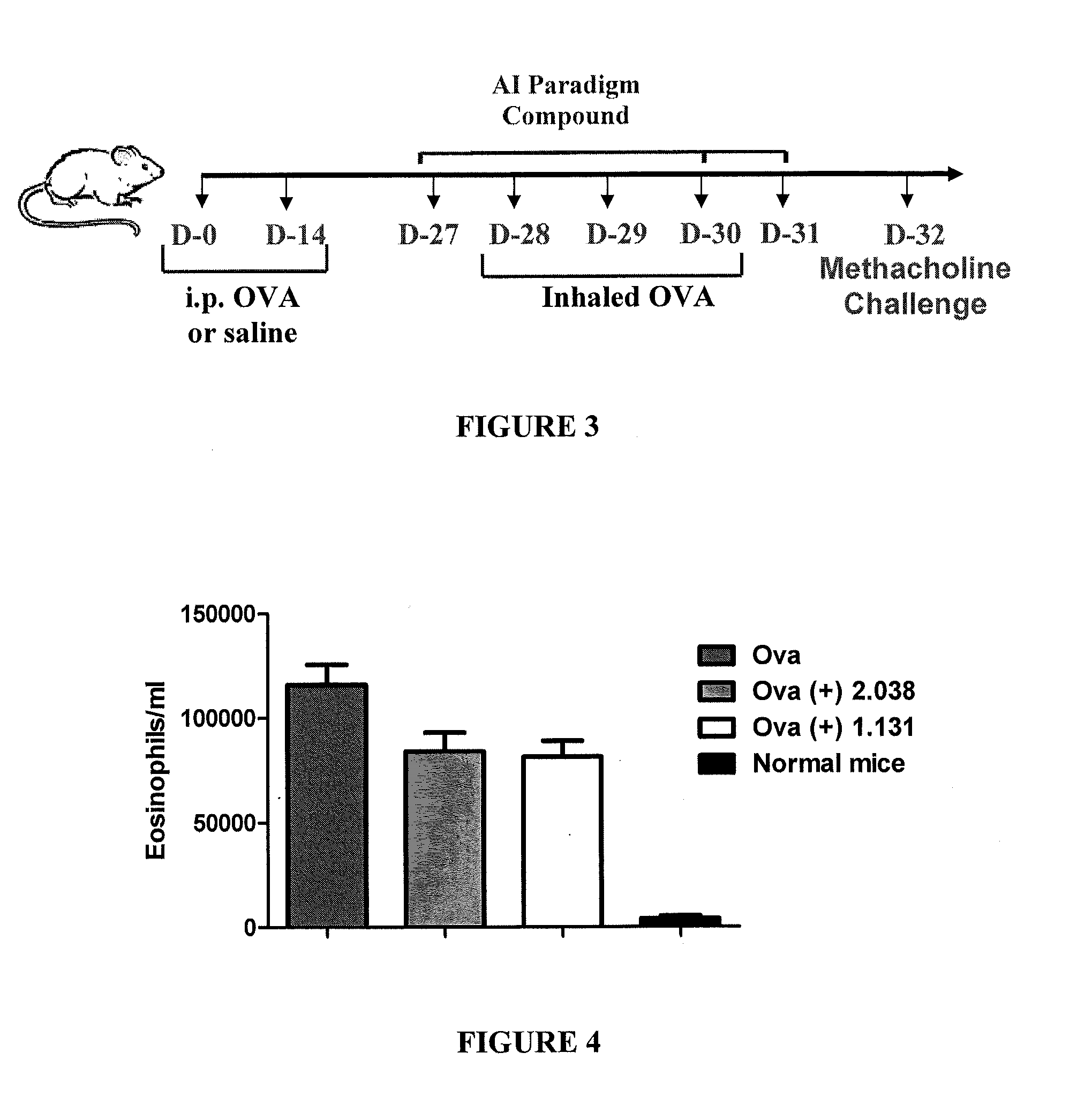
Treatment of hibernating myocardium and diabetic cardiomyopathy with a GLP-1 peptide
InactiveUS6894024B2Suppress plasma blood levelReducing norepinepherine levelPeptide/protein ingredientsMetabolism disorderHigh energyMortality rate
Hibernating myocardium is characterized by viable myocardium with impaired function due to localized reduced perfusion. Hibernating myocytes retain cellular integrity, but cannot sustain high-energy requirements of contraction. High plasma levels of catecholamines, such as norepinepherine, are believed to be predictive of mortality from hibernating myocardium. Likewise, high levels of catecholamines lead to cardiomyopathy in patients with diabetes. GLP-1 reduces plasma norepinepherine levels, and it thus is useful in a method of treating hibernating myocardium or diabetic cardiomyopathy.
Owner:ASTRAZENECA PHARMA LP
Multi-purpose reagent system and method for enumeration of red blood cells, white blood cells and thrombocytes and differential determination of white blood cells
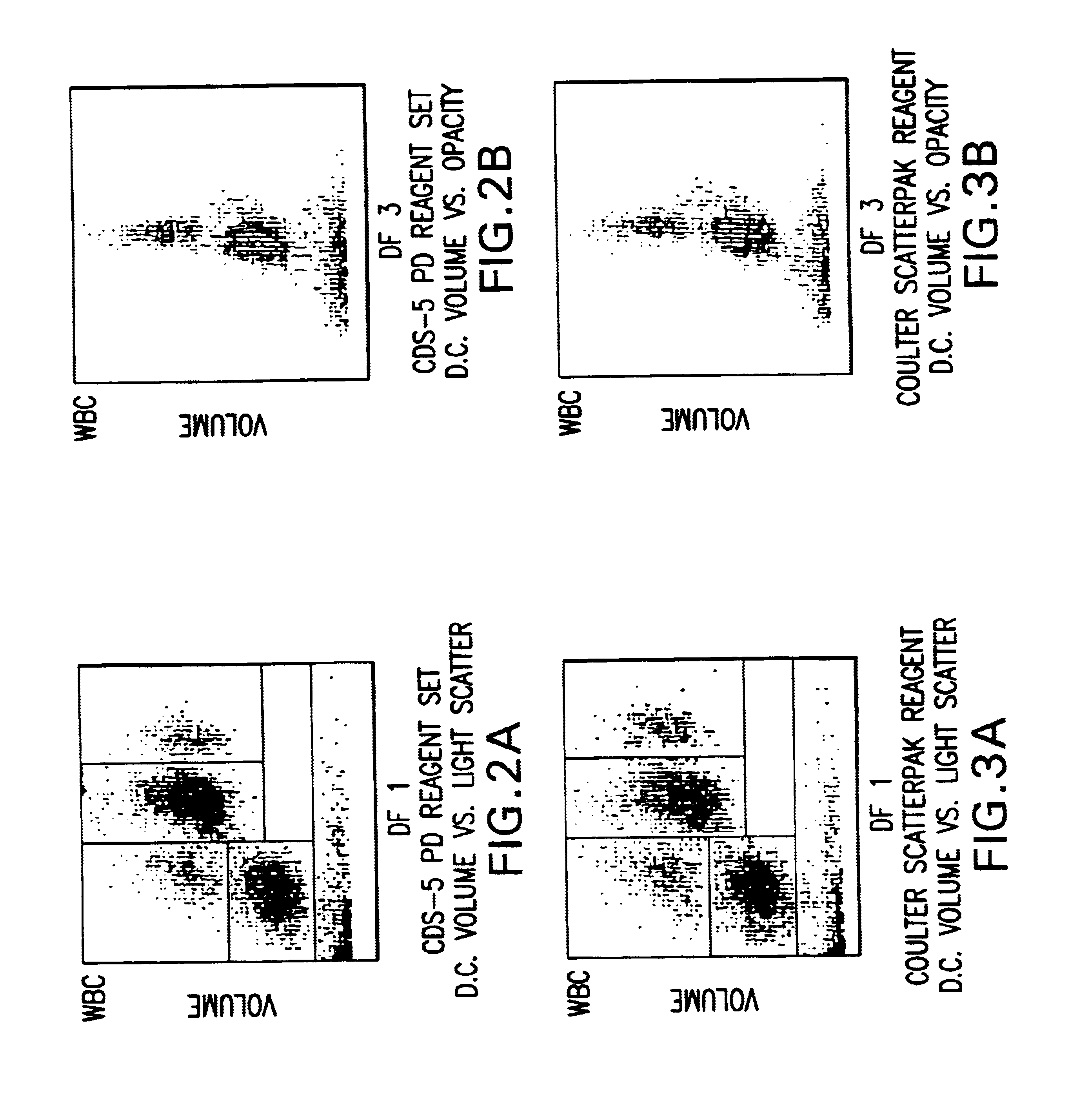
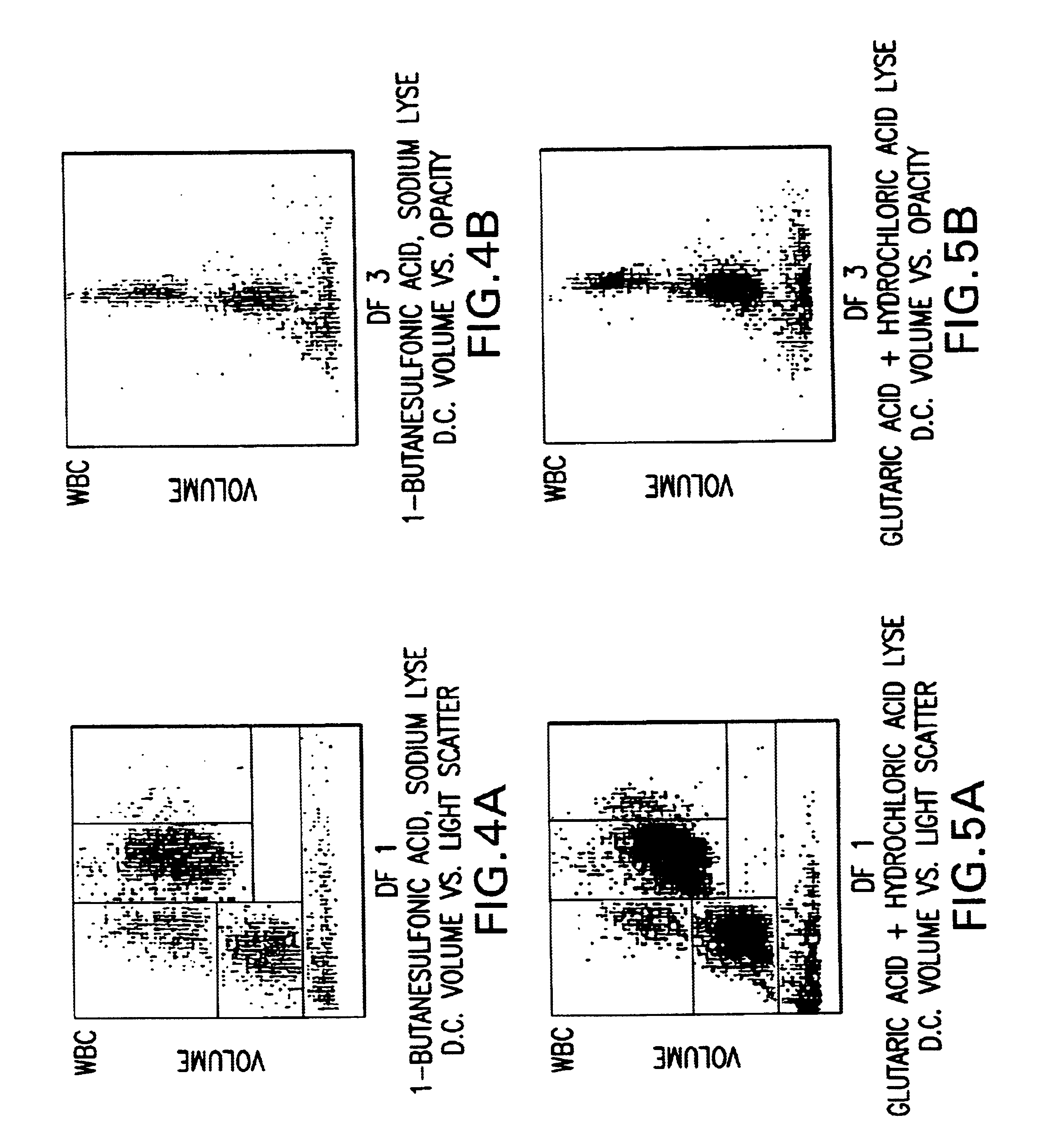
InactiveUS6632676B1Stable blood diluentWithdrawing sample devicesPreparing sample for investigationLight scatter measurementSystem requirements
A novel reagent system for use with automated and semi-automated hematology analyzers including an essentially isotonic blood diluting reagent, a blood cell lysing and hemoglobin conversion reagent, and a second lysing reagent for differentiating white blood cells into classes by size and functional characteristics. The diluent reagent enhances properties for counting and sizing blood specimens, while stabilizing cellular volume and cellular integrity for many hours. The blood cell lysing reagent removes red blood cells and enables subsequent enumeration of white blood cells and simultaneous determination of hemoglobin without use of the toxic cyanide anion. The third lysing reagent and a companion quenching differentiates blood cells into classes by size and functional characteristics, based on d.c. impedance volume, conductivity / opacity and light scatter measurements. The companion quenching reagent adjusts pH and conductivity of the final measurement solution to match the analyzer system requirements. Novel methods for use of the reagents with automated and semi-automated hematology analyzers are also provided.< / PTEXT>
Owner:CLINICAL DIAGNOSTICS SOLUTIONS
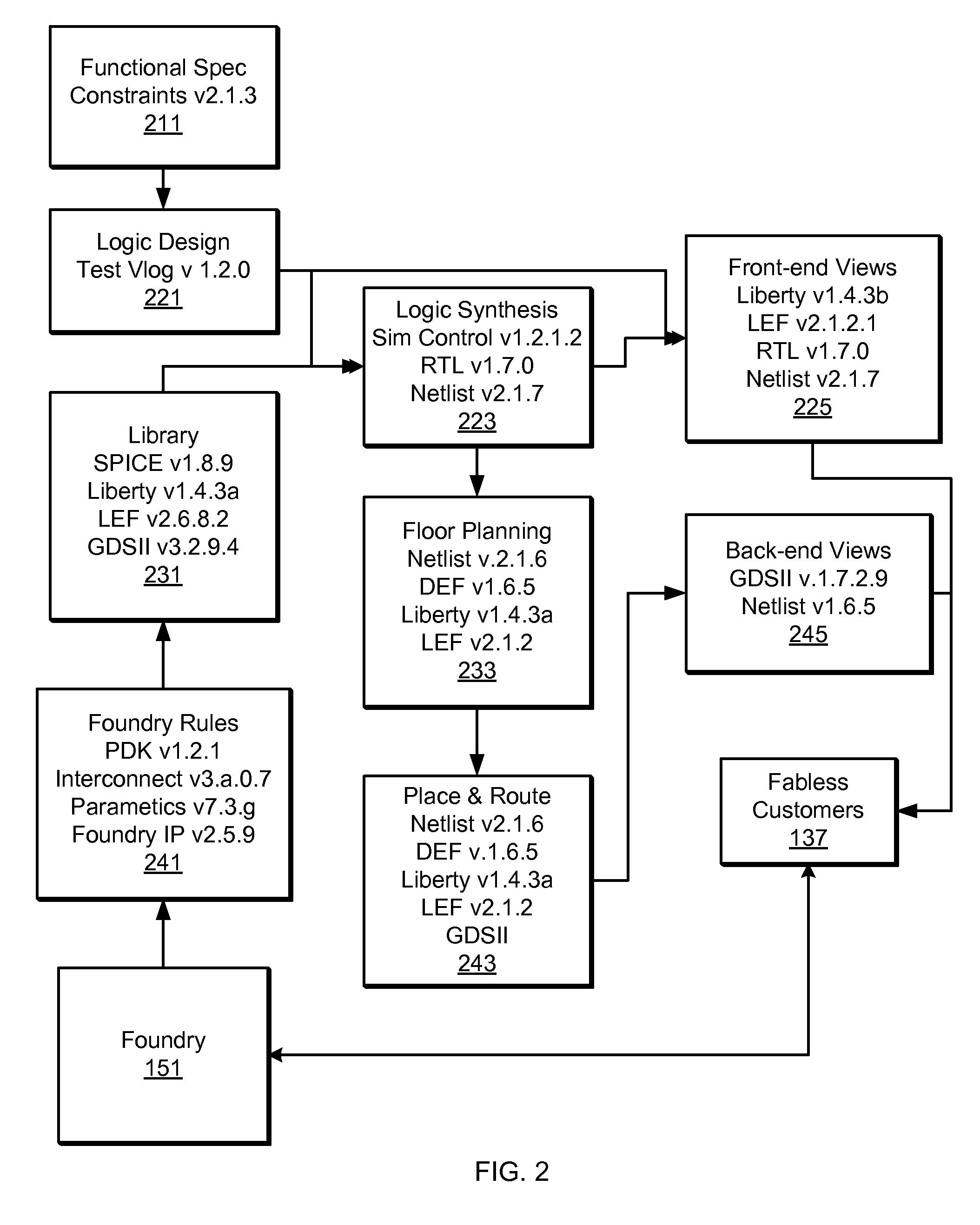
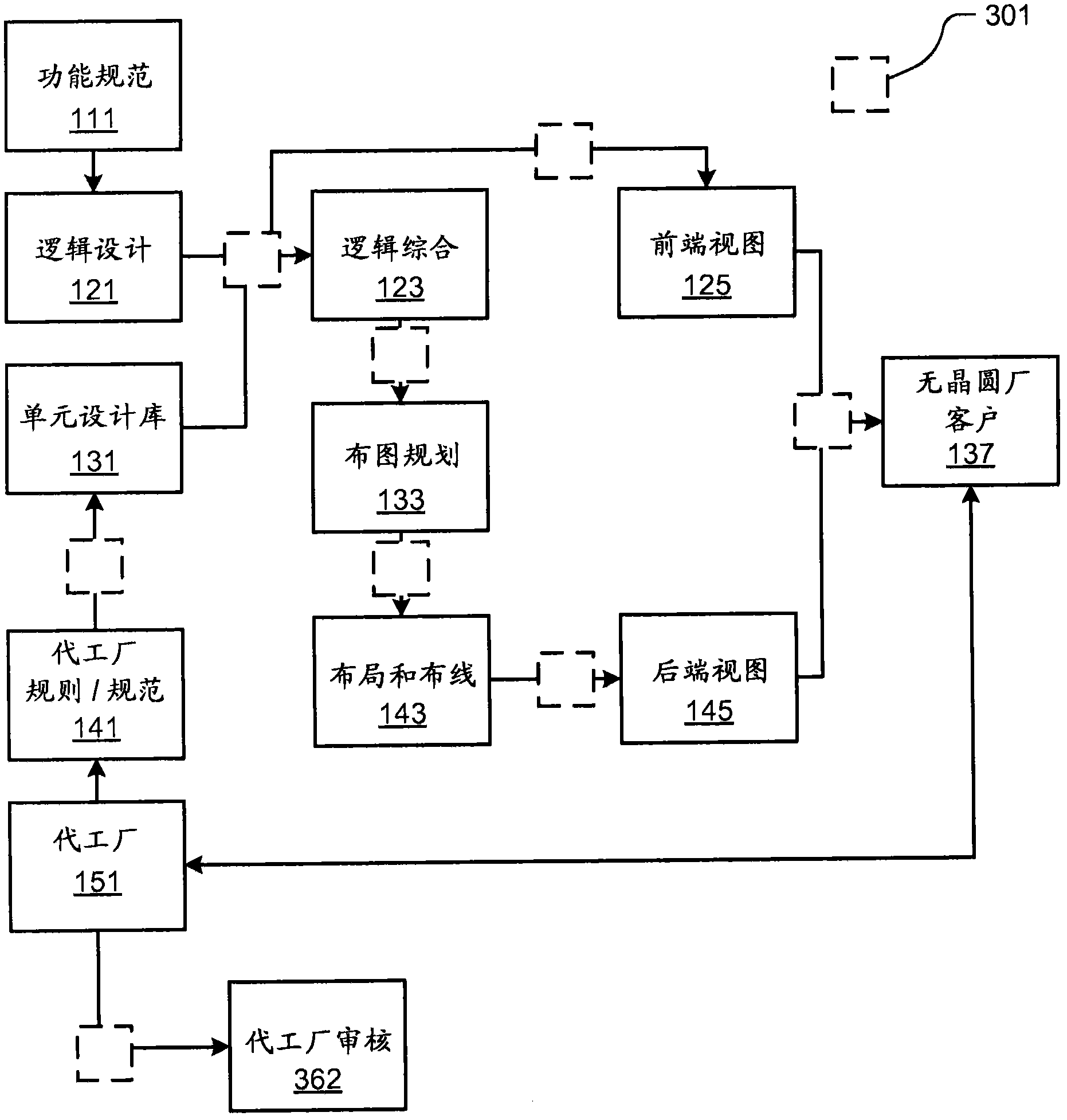
Methods and Devices for Independent Evaluation of Cell Integrity, Changes and Origin in Chip Design for Production Workflow
ActiveUS20090307640A1Reduce sensitivityDetecting faulty computer hardwareCAD circuit designCell integrityFunctional impact
The technology disclosed relates to granular analysis of design data used to prepare chip designs for manufacturing and to identification of similarities and differences among parts of design data files. In particular, it relates to parsing data and organizing into canonical forms, digesting the canonical forms, and comparing digests of design data from different sources, such as designs and libraries of design templates. Organizing the design data into canonical forms generally reduces the sensitivity of data analysis to variations in data that have no functional impact on the design. The details of the granular analysis vary among design languages used to represent aspects of a design. For various design languages, granular analysis includes partitioning design files by header / cell portions, by separate handling of comments, by functionally significant / non-significant data, by whitespace / non-whitespace, and by layer within a unit of design data. The similarities and differences of interest depend on the purpose of the granular analysis. The comparisons are useful in many ways.
Owner:OASIS TOOLING
Methods and devices for independent evaluation of cell integrity, changes and origin in chip design for production workflow
ActiveUS7685545B2Reduce sensitivityDetecting faulty computer hardwareCAD circuit designCell integrityFunctional impact
The technology disclosed relates to granular analysis of design data used to prepare chip designs for manufacturing and to identification of similarities and differences among parts of design data files. In particular, it relates to parsing data and organizing into canonical forms, digesting the canonical forms, and comparing digests of design data from different sources, such as designs and libraries of design templates. Organizing the design data into canonical forms generally reduces the sensitivity of data analysis to variations in data that have no functional impact on the design. The details of the granular analysis vary among design languages used to represent aspects of a design. For various design languages, granular analysis includes partitioning design files by header / cell portions, by separate handling of comments, by functionally significant / non-significant data, by whitespace / non-whitespace, and by layer within a unit of design data. The similarities and differences of interest depend on the purpose of the granular analysis. The comparisons are useful in many ways.
Owner:OASIS TOOLING
Method for treating diseases associated with alterations in cellular integrity using Rho kinase inhibitor compounds
This invention is directed to methods of preventing or treating diseases or conditions associated with alterations in cellular integrity including alterations in endothelial permeability, excessive cell proliferation or tissue remodeling. Particularly, this invention is directed to methods of treating diabetic nephropathy, malaria, or cancer. The method comprises identifying a subject in need of the treatment, and administering to the subject an effective amount of a novel rho kinase inhibitor compound to treat the disease.
Owner:INSPIRE PHARMA
Methods and devices for independent evaluation of cell integrity, changes and origin in chip design for production workflow
ActiveUS20090307639A1Reduce sensitivityDetecting faulty computer hardwareCAD circuit designCell integrityFunctional impact
The technology disclosed relates to granular analysis of design data used to prepare chip designs for manufacturing and to identification of similarities and differences among parts of design data files. In particular, it relates to parsing data and organizing into canonical forms, digesting the canonical forms, and comparing digests of design data from different sources, such as designs and libraries of design templates. Organizing the design data into canonical forms generally reduces the sensitivity of data analysis to variations in data that have no functional impact on the design. The details of the granular analysis vary among design languages used to represent aspects of a design. For various design languages, granular analysis includes partitioning design files by header / cell portions, by separate handling of comments, by functionally significant / non-significant data, by whitespace / non-whitespace, and by layer within a unit of design data. The similarities and differences of interest depend on the purpose of the granular analysis. The comparisons are useful in many ways.
Owner:OASIS TOOLING
Refrigeration and freezing device and freezing method thereof
InactiveCN105546905AUniform crystallizationBright colorFood freezingLighting and heating apparatusStopped workCell integrity
The invention provides a refrigeration and freezing device and a freezing method thereof. The refrigeration and freezing device comprises a liner, a refrigeration system, an interference device and an ultrasonic generation device, wherein a storage chamber for storing frozen foods is limited in the liner; the refrigeration system is configured to be controlled to supply cooling capacity to the storage chamber, so that the temperature of the storage chamber is reduced to and kept at a crystallization point temperature first preset time of the frozen foods, and then, is reduced to and kept at a preset preservation temperature of the frozen foods; the interference device is configured to be started to interfere the frozen foods after the temperature in the storage chamber is reduced to the crystallization point temperature first preset time, and works for a second preset time; and the ultrasonic generation device is configured to be started to apply ultrasonic waves to the frozen foods when the interference device is stopped working, and works for a third preset time. The refrigeration and freezing device and the freezing method thereof excellently preserve the cell integrity of the foods in the freezing process, and perfectly guarantee the freezing quality of such foods as meat loafs.
Owner:HAIER SMART HOME CO LTD
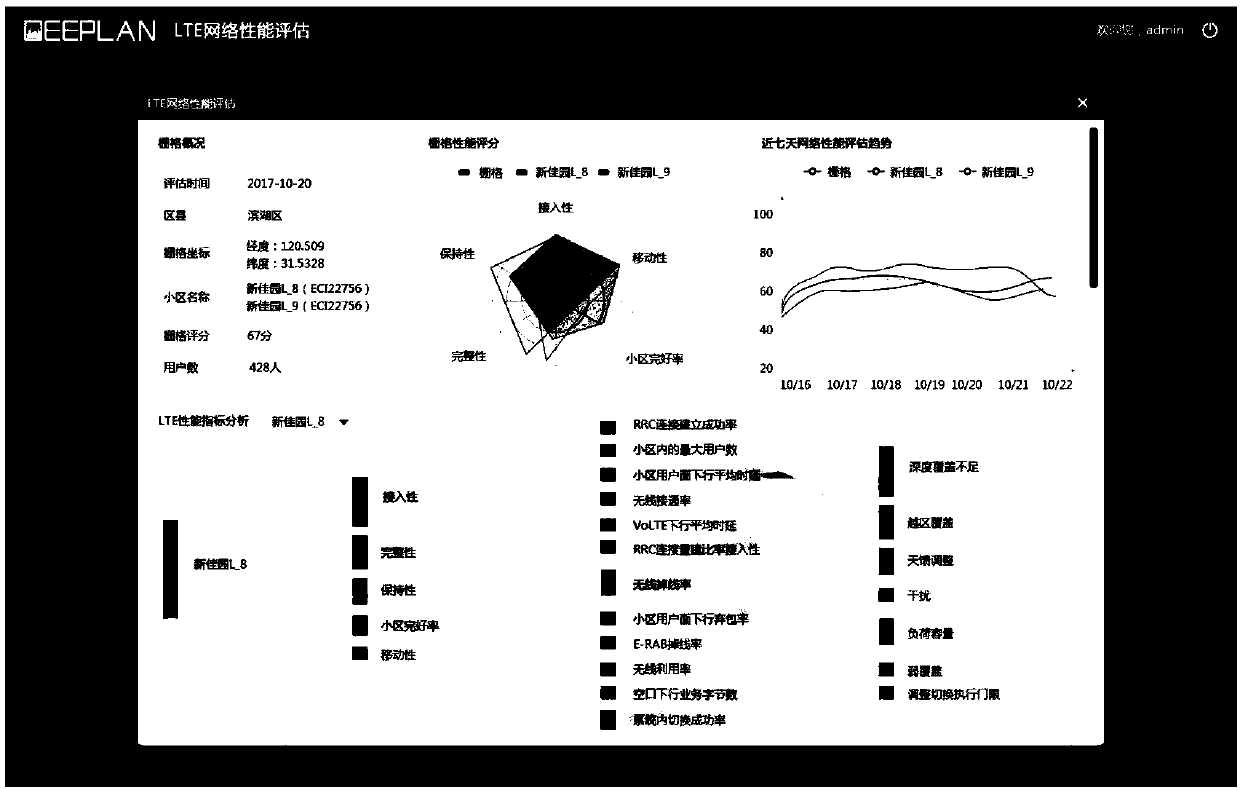
LTE network performance evaluation method based on microregions
ActiveCN107920362AAppropriate optimization needsGuide optimizationWireless communicationCell integrityData source
The invention discloses an LTE network performance evaluation method based on microregions. The method comprises the following steps: (1) data collection: collecting user level OTT information, MR data, key signaling switching data and speech data; (2) establishing a location fingerprint database; (3) data processing: integrating and associating data sources; meanwhile classifying the speech dataon five dimensions, namely, retention, access, integrity, cell integrity and mobility under two service types of LTE and VoLTE, and marking index attributes; (4) performing data calculation and analysis; (5) data analysis result: the service types are divided into an LTE (browsing service) and VoLTE service, the estimation time can be autonomously selected, and the network performance scores of grids are divided into 5 intervals of excellent, good, general, poor and serious. By means of the correlation and constraint relationship among the indicators within the dimensions, the network qualityof microregions can be evaluated objectively and objectively, and the network optimization can be effectively guided.
Owner:NANJING HOWSO TECH
Methods and devices for independent evaluation of cell integrity, changes and origin in chip design for production workflow
ActiveCN102112988AReduce sensitivityCAD circuit designSpecial data processing applicationsFunctional impactCell integrity
The technology disclosed relates to granular analysis of design data used to prepare chip designs for manufacturing and to identification of similarities and differences among parts of design data files. In particular, it relates to parsing data and organizing into canonical forms, digesting the canonical forms, and comparing digests of design data from different sources, such as designs and libraries of design templates. Organizing the design data into canonical forms generally reduces the sensitivity of data analysis to variations in data that have no functional impact on the design. The details of the granular analysis vary among design languages used to represent aspects of a design. For various design languages, granular analysis includes partitioning design files by header / cell portions, by separate handling of comments, by functionally significant / non-significant data, by whitespace / non-whitespace, and by layer within a unit of design data. The similarities and differences of interest depend on the purpose of the granular analysis. The comparisons are useful in many ways.
Owner:OASIS TOOLING
Method for preparing low-gelatinization-degree whole potato flour
InactiveCN105249346AHigh nutritional valueShorten the production cycleFood scienceNutritive valuesFluidized bed drying
A method for preparing low-gelatinization-degree whole potato flour comprises the specific steps that potatoes are cleaned, peeled and cut into granules, color protection processing is performed, and then the raw materials are sequentially subjected to drying processing, smashing and package through an airflow fluidized bed and an eddy flash evaporation drying machine to obtain a whole potato flour product. The drying processing includes two stages of fluidized bed drying and eddy flash evaporation drying. The whole potato flour prepared by means of the method is pure white in color and high in cell integrity, the heat treatment time required by the product in the preparing process is short, loss of heat-sensitive nutrient substance is reduced, nutritive value is improved, the starch curing degree is reduced, the follow-up processing performance of the product is further improved, a production period of the product is short, the efficiency is high, and the method is suitable for industrialized production.
Owner:JIANGNAN UNIV
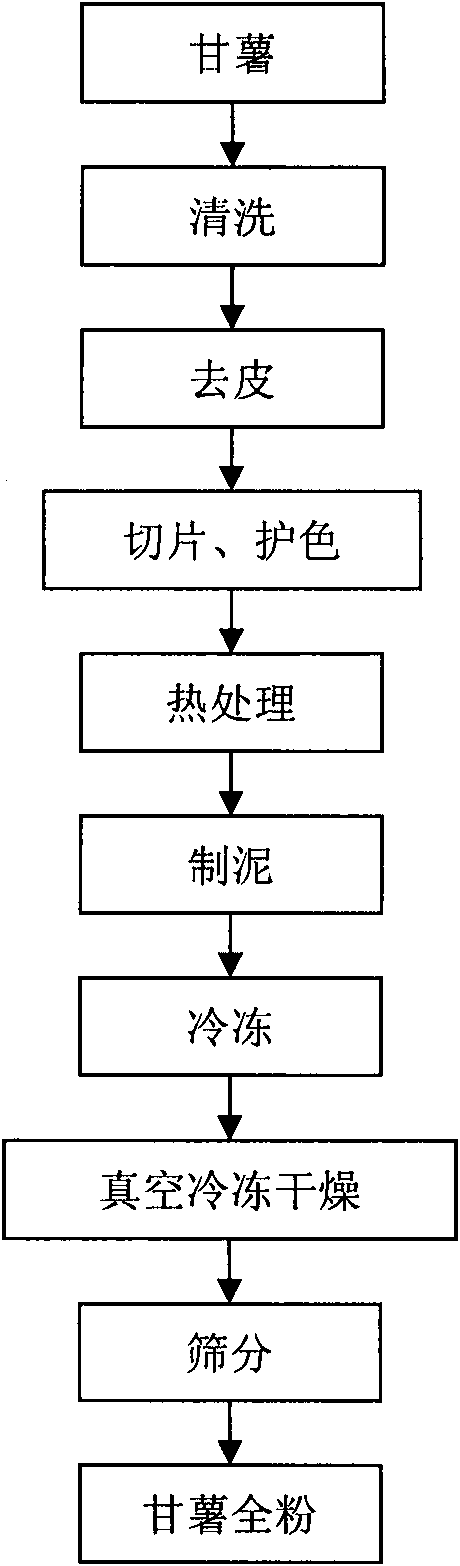
Method for preparing sweet potato whole powder
InactiveCN101810286AIncrease nutritionReduce lossesFood preparationFruits/vegetable preservation by dehydrationFreeze-dryingCell integrity
The invention discloses a method for preparing sweet potato whole powder, which comprises the following steps: 1), performing color protection treatment of peeled sweet potatoes; 2) heating the sweet potatoes treated by the step 1) for color protection; and 3), drying the sweet potatoes heated by the step 2) to obtain sweet potato whole powder. In the method, one-step heat treatment for separating cells and a vacuum freeze drying are adopted to prepare the sweet potato whole powder. The prepared sweet potato whole powder can retain high integrity of cells and adopts a vacuum freeze drying technology under vacuum in the preparation process to reduce the loss of thermosensitive substances in the sweet potatoes and improve the nutritional property of the sweet potato whole powder. Experiments show that the sweet potato whole powder prepared by the method has less than 6 percent of free starch. The method of the invention has a promising application prospect.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
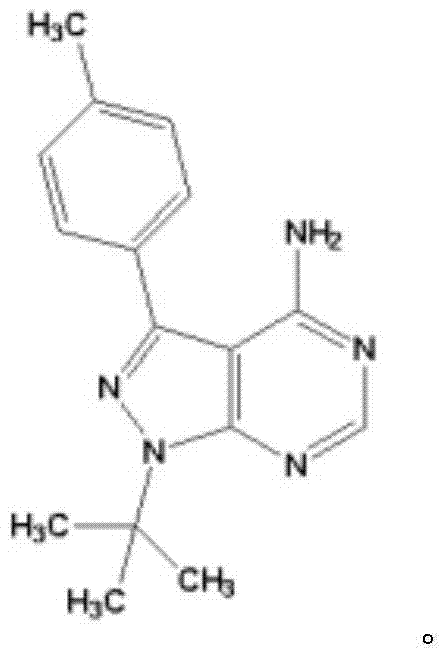
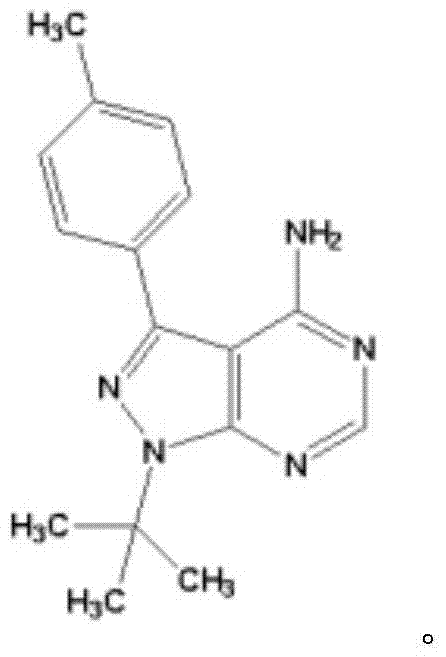
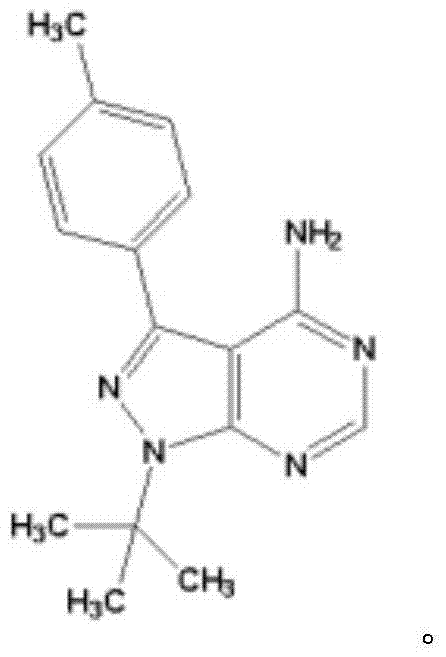
Application of thyroxine receptor analogues and salts or predrugs thereof to preparation of pulmonary disease treating and/or preventing drugs
InactiveCN107469086AEffective protectionPromote maturityOrganic active ingredientsRespiratory disorderTherapeutic effectLung tissue
The invention provides a novel application of thyroxine receptor analogues and pharmaceutically acceptable salts or predrugs thereof which are used for treating and / or preventing pulmonary diseases. The thyroxine receptor analogues and the pharmaceutically acceptable salts or predrugs thereof can effectively protect alveolar epithelial cells, adjust synthesis, storage and distribution of surface proteins of the alveolar epithelial cells, promote maturation, differentiation and integrity of the alveolar epithelial cells, are beneficial to elimination of hydrops in the lung, promote recovery of damaged mitochondria in the alveolar epithelial cells and enhance bio-synthesis of the mitochondria, so that repair of damaged lung tissue is facilitated, pulmonary fibrosis is stopped and reversed, immunoreaction is regulated, a cancerization environment is changed, pulmonary diseases are prevented and treated, pulmonary functions such as gas exchange and the like are improved, and the treatment effect is realized.
Owner:深圳市润佳通科技有限公司
Preparation method of raw whole potato powder
The invention discloses a preparation method of raw whole potato powder. The preparation method comprises selecting fresh potatoes, washing, peeling and cutting the selected potatoes, then soaking the potatoes in edible brewed white vinegar, washing the potatoes with water, carrying out ultra-high pressure treatment, carrying out freeze-drying dehydration through a freeze-drying method, and carrying out crushing and sieving to obtain raw whole potato powder. Through combination of white vinegar immersion color protection, vacuum ultra-high pressure treatment and vacuum freeze-drying, contact between a phenolic substrate and a phenol enzyme is effectively avoided and cell integrity is protected maximally. The oxygen insulation environment effectively inhibits non-enzymatic browning reactions such as a Maillard reaction and ascorbic acid oxidation so that the color of the potato blocks is well protected. A white vinegar use cost is low. The preparation method utilizes the edible brewed white vinegar so that food safety problems are avoided. The preparation method provides a novel approach for preparation of raw whole potato powder.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Human retinal pigment epithelial cell separation and cryopreservation method
PendingCN106434531AIntegrity guaranteedAvoid damageCulture processEpidermal cells/skin cellsNutrient solutionRetinal pigment epithelial cell
The invention relates to a human retinal pigment epithelial cell separation and cryopreservation method which includes the steps: firstly, cleaning and washing retinal pigment epithelial cells by nutrient solution; secondly, mixing Dispase II separase and DPBS buffer solution according to the ratio of 1:(40-50)w / v to prepare Dispase II separase solution; thirdly, mixing the Dispase II separase solution and Tryple select solution according to the volume ratio of 1:(2-3) to prepare digestive fluid for digestive separation; finally, performing cryopreservation after counting and centrifuging. The separation method is simple, the steps are easily controlled, the digestive fluid prepared by mixing the Dispase II separase solution with the Tryple select solution is used for performing digestive separation for the retinal pigment epithelial cells, adherence removing time of the retinal pigment epithelial cells can be shortened, cell integrity is kept, and cell injury is greatly reduced.
Owner:EYECURE THERAPEUTICS INC JIANGSU
Method for treating diseases associated with alterations in cellular integrity using rho kinase inhibitor compounds
This invention is directed to methods of preventing or treating diseases or conditions associated with alterations in cellular integrity including alterations in endothelial permeability, excessive cell proliferation or tissue remodeling. Particularly, this invention is directed to methods of treating diabetic nephropathy, malaria, or cancer. The method comprises identifying a subject in need of the treatment, and administering to the subject an effective amount of a novel rho kinase inhibitor compound to treat the disease.
Owner:INSPIRE PHARMA
Harvesting method for large-scale production of toxic dinoflagellate
InactiveCN101586079ANo brokenSafe brokenUnicellular algaeMicroorganism based processesHigh energyCell integrity
The present invention discloses a harvesting method for a large-scale production of a toxic dinoflagellate, employing a flocculating agent KAL(SO4)2 12H2O to precipitate the toxic dinoflagellate cells, and obtaining a toxic dinoflagellate body by a centrifugal collection. The method provides a appropriate flocculating agent dosage for different toxic dinoflagellate and is capable of avoiding the flocculating agent dosage from being oversize and preventing a cell rupture content object toxin from leaking outside by combining a centrifugal method, it is also capable of avoiding a problem that the cell will not be precipitated due to less flocculating agent dosage and avoiding a high-cost high energy consumption collection by the centrifugal method only. After precipitating the dinoflagellate cell by the flocculating agent, further the centrifugal method is selected to purify to obtain a algae mud, wherein the centrifugation is capable of improving a purifying speed of a large-scale production and purifying a good deal of precipitating liquid of the large-scale production. The method of the invention is capable of harvesting a good deal of toxic dinoflagellate safely and highly effectively without damage of the cell integrity, thereby an active secondary metabolic product of the dinoflagellate toxin is extracted.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Non-cervical exfoliated cell preserving solution with pretreatment function and application thereof
InactiveCN110352950AEasy to storeImprove integrityPreparing sample for investigationDead animal preservationLysisCervical cell
The invention relates to a non-cervical exfoliative cell preserving solution with a pretreatment function and application thereof, in particular to a non-cervical exfoliated cell preserving solution containing a cell fixative, a cleaning solution, an osmotic pressure maintenance agent, a microbial inactivating agent, and a cell lysis component.Thenon-cervical exfoliative cell preserving solution canbetter preserve non-cervical exfoliated cells comprising mucus samples, body fluid samples, puncture and brush samples to protect cell integrity and fixation. At the same time, various parts of thecells can be easily colored to be suitable for observation, long-term preservation, and analysis, and the preserved cells can also be used for cell wax blockmaking and immunohistochemical detection.
Owner:南京福怡科技发展股份有限公司
Compositions and methods for inhibiting the growth of fungi
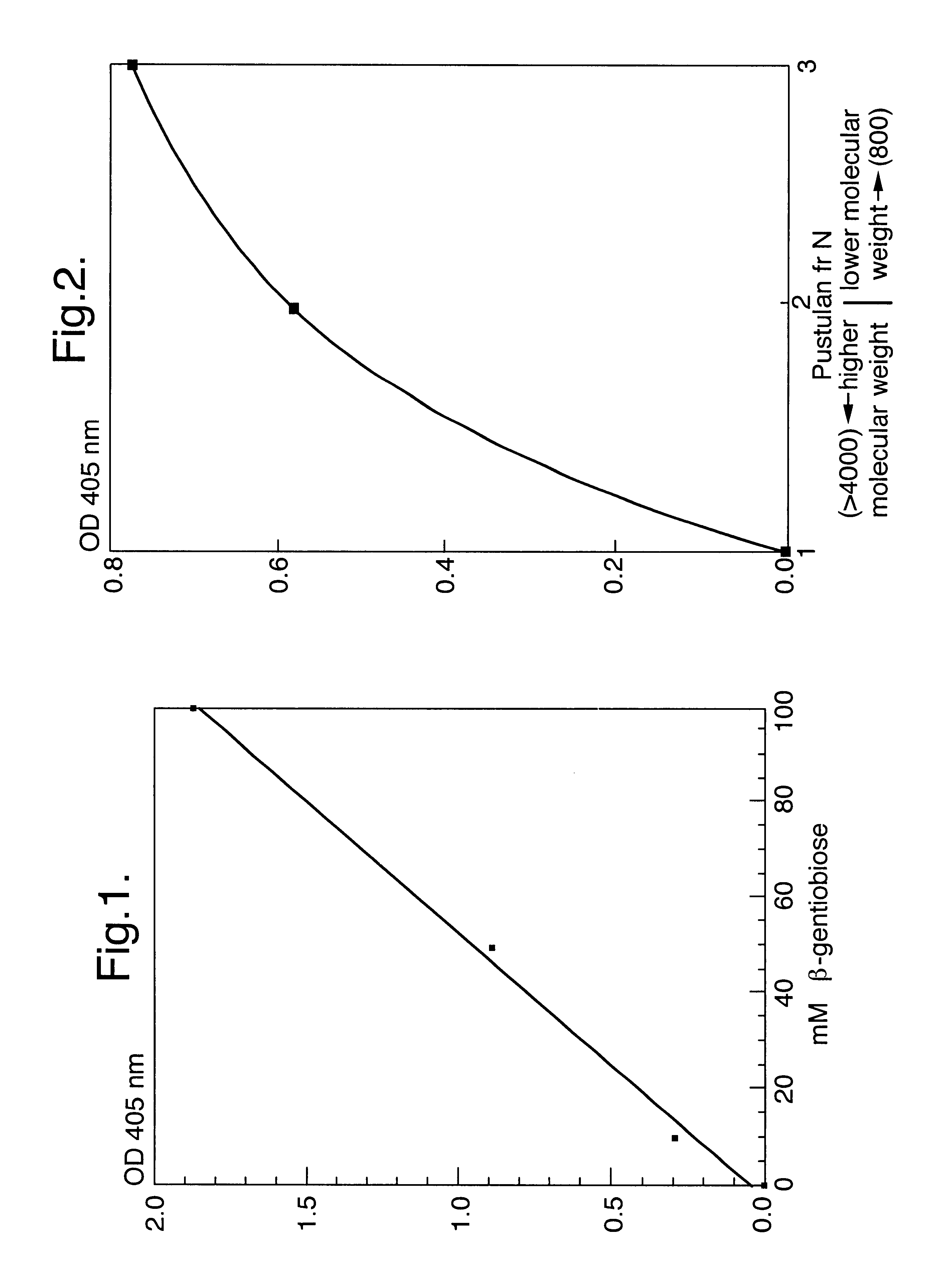
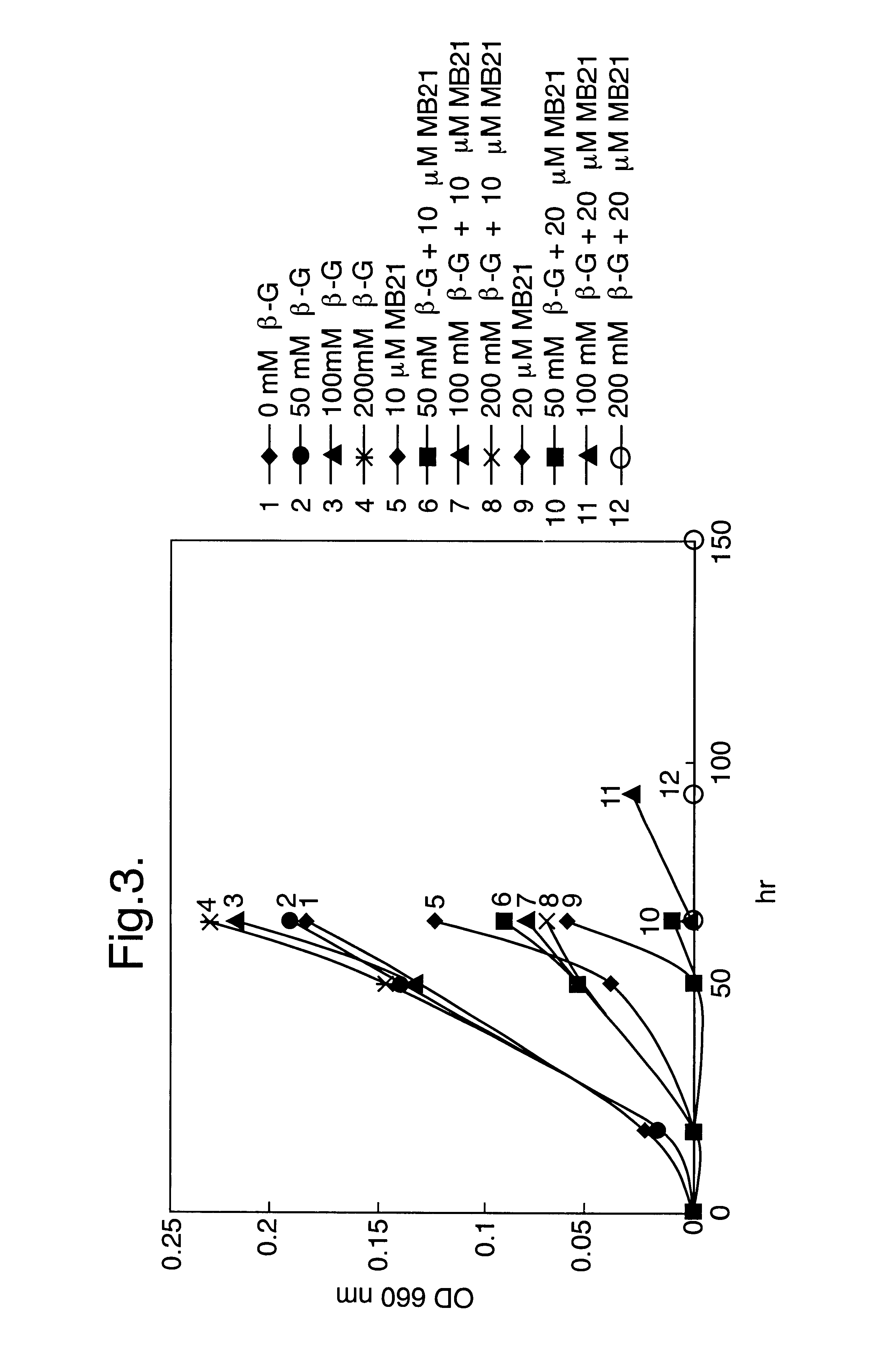
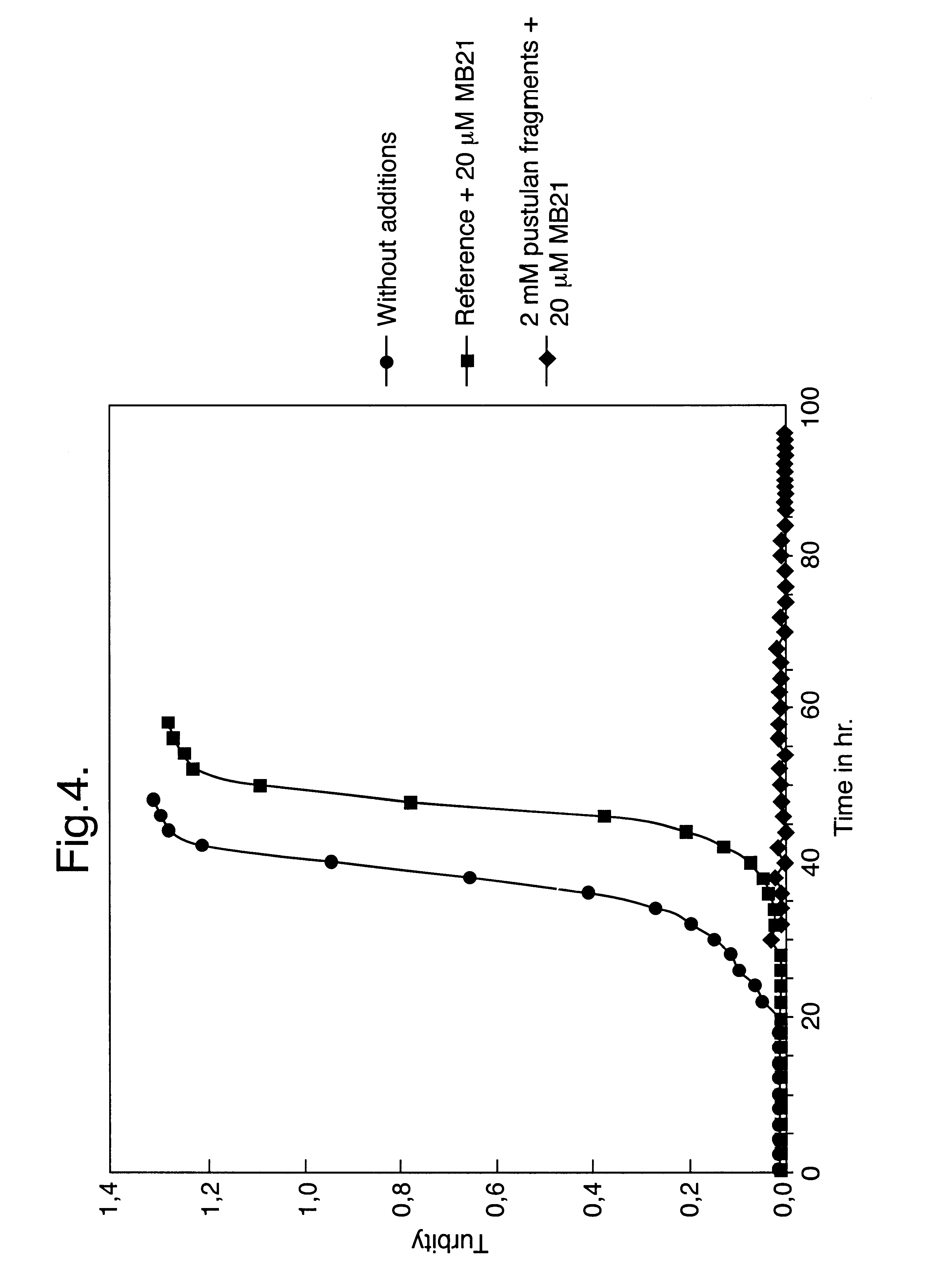
A composition suitable for inhibiting the outgrowth of fungi is provided comprising a first ingredient which inhibits the biogenesis of a normal fungal cell wall and a second ingredient which is capable of perturbing the structure of the cellular membrane of said fungi, so that either the cellular integrity is essentially lost or cell division cannot take place, or both. The first ingredient preferably inhibits the anchorage of cell wall proteins in the cell wall of the fungi and is suitably a beta-(1,6)-glucose polysaccharide, preferably beta-gentiobiose or a pustulan fragment. The second ingredient is suitably a natural microbial membrane affecting substance (MMAS), preferably MB-21. The composition is particularly suitable as a preservative for inhibiting the outgrowth of fungi in food products, such as sauces, dressings, ketchups, and soups, but is also useful for preventing or inhibiting undesired fungal growth on other products such as personal health care products, e.g. soap bars.
Owner:THOMAS J LIPTON DIV OF CONOPCO
Preparing method of carnation leaf protoplast
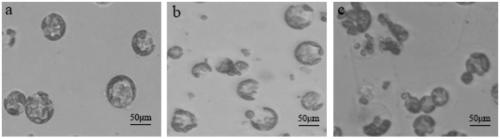
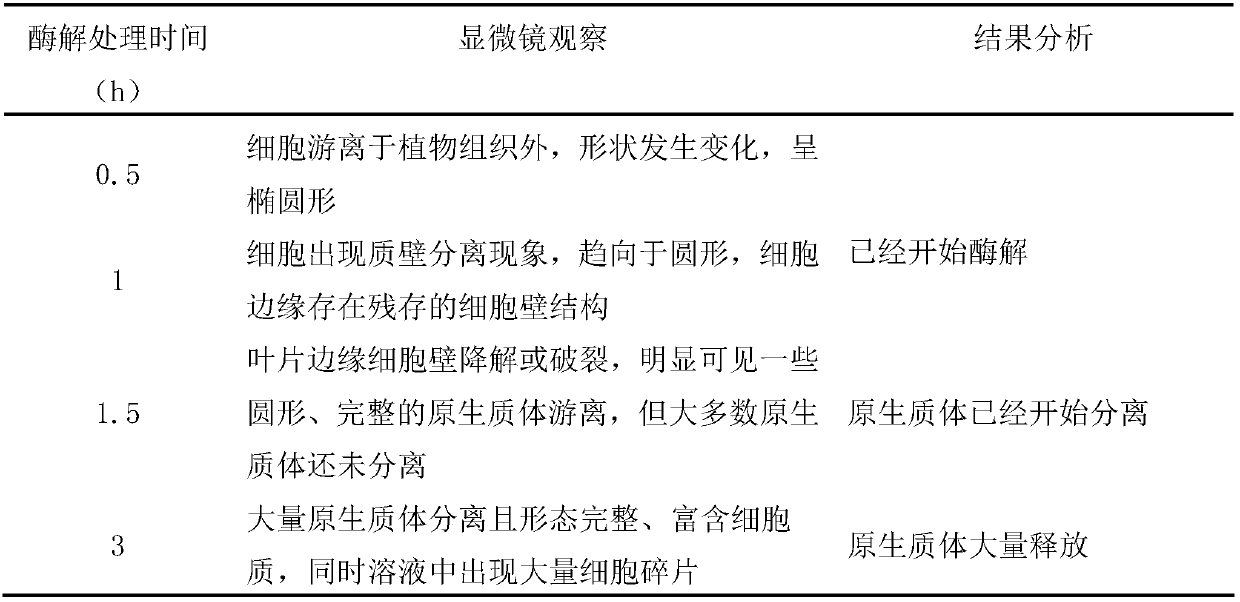
ActiveCN109628376AIncrease productionImprove completenessCell culture mediaPlant cellsPlant tissueCell integrity
The invention belongs to the technical field of plant tissue and cell culture, and particularly relates to a preparing method of carnation leaf protoplast. With a carnation tissue culture seedling asthe test material, an efficient protoplast preparing method for purification through a low-speed oscillation enzymolysis-sedimentation method is established. The method is convenient to operate and can be implemented all year round, the yield of the protoplast subjected to low-speed oscillation enzymolysis separation and density gradient purification is high and can reach 183*105 p / gFW, the cell integrity is high, and it is ensured that the activity is 75% or above. By means of the method, a foundation is laid for the research on the aspects of gene function authentication, sub-cellular localization, protein interaction and the like of a carnation leaf instantaneous converting system.
Owner:HUAZHONG AGRI UNIV
Cell freezing solution for preparing dry tumor cell quality control and application of cell freezing solution
ActiveCN109122669AResolve integritySolving activityPreparing sample for investigationDead animal preservationHuman tumorFreeze-drying
The invention discloses a cell freezing solution for preparing a dry tumor cell quality control and application of the cell freezing solution. According to a formula, the cell freezing solution is prepared by adding 5-25% of protein, 3-15% of a cell stabilizing agent A, 1-10% of a cell stabilizing agent B and 0.01-0.1% of a bacteriostatic agent into PBS (phosphate buffer solution). The cell freezing solution successfully solves the problems that a cell integrity rate after freeze-drying is very low or active ingredients of cell membrane structural proteins and cell contents cannot be reservedwell in the existing cell freeze-drying technology. A preparation method of the dry tumor cell quality control directly uses human-derived tumor cell strains containing cell membrane structures and contents to prepare the quality control; the quality control is derived from human tumor cells and has the same characteristics as those of similar tumor cells in clinical liquid biopsy detection samples; and the quality control prepared by the method is applicable to the quality control of clinical liquid biopsy and can promote extensive popularization and application of the clinical liquid biopsy.
Owner:JIANGSU CODE BIOMEDICAL TECH CO LTD
Application of lanthanum compound to bacterium growth promotion
The invention discloses application of a lanthanum compound to bacterium growth promotion. The lanthanum compound is La(NO3)3.6H2O; the addition concentration of the lanthanum compound is 5.0 to 200.0mg / L; the lanthanum compound can be used for promoting bacterial reproduction, and concretely protects bacterium cytoderm peptidoglycan structures and beta-1,4 glycosidic bonds; and the bacteria are escherichia coli. The acting target points of Penicillin and Lysozyme are used as the references; the effect that the adjacent polysaccharide chains in the cytoderm are crosslinked to protect the cytoderm peptidoglycan through the physiological effect of the La(NO3)3.6H2O capable of reversing Penicillin is discovered; the cytoderm beta-1,4 glycosidic bonds are protected through the physiological effect for reversing Lysozyme; the cell completeness is enhanced; and the bacterial reproduction is promoted.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Angiogenesis inhibition compound
InactiveCN104744474ASmall diameterOrganic chemistryAntineoplastic agentsAngiogenesis InhibitionTyrosine-kinase inhibitor
The invention relates to an angiogenesis inhibition drug. The angiogenesis inhibition drug has a structural formula shown in the following description. A result of a research on zebrafish embryonic development shows that the angiogenesis inhibition compound belongs to a Src kinase inhibitor, through VEGFR2 and MAP kinase approach inhibition, vascular endothelial cell integrity is interfered so that small blood vessel lumen diameters are gradually reduced and blood vessel endothelium is broken finally and thus the angiogenesis inhibition drug belongs to an angiogenesis inhibition compound.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Method for treating externally-autoplastic autogenous skull sheet
InactiveCN102580151AEasy to shapePrevent infiltrationProsthesisSubcutaneous tissueBiological materials
The invention relates to the field of biology materials, in particular to a method for treating an externally-autoplastic autogenous skull sheet, which includes: 1 freezing fresh skull flap for 24 hours at temperature of -10 DEG C, rewarming for 48 hours in natural environment (about 20 DEG C) so as to break integrity of cells and lead the cells to be self-dissolved fully; 2 removing soft tissue on the surface of skull manually; 3 drilling holes on the skull in mesh mode and leading areas among holes to be 1cm*1cm; 4 soaking distilled water and trypsin for 48 hours at the temperature of 37 DEG C, changing the distilled water every 24 hours so as to fully precipitine an autogenous solution; and 5 soaking for 72 hours in medical hydrogen dioxide, changing the hydrogen dioxide every 24 hours to fully remove proteins in diploe; and soaking for 12 hours in the distilled water and then draining when no adverse reactions including infection, effusion and the like appear. Aiming at the problem that ankle joints are movable so that needle heads on the foot portion can move easily, the method combines special structure of the foot portion for fixing the foot portion so as to prevent liquid medicine from permeating into subcutaneous tissues.
Owner:THE FIRST AFFILIATED HOSPITAL OF HENAN UNIV OF SCI & TECH
Method and device to aid in the inspection and certification of harvested food for human consumption
ActiveUS9952167B1Reliable and repeatable measurement of cell integrityNon-invasiveElectrical measurement instrument detailsElectrical testingContact padCell integrity
An apparatus includes an array of compression electrodes and an analysis circuit. The array of compression electrodes includes a pair of outer compression electrodes and a pair of inner compression electrodes. Each of the electrodes includes a contact pad and may be configured to automatically adjust to follow a contour of an object while maintaining contact of the contact pad with a surface of the object. The analysis circuit can be coupled to the array of compression electrodes. The analysis circuit may be configured to automatically make a bioelectrical impedance measurement using the array of compression electrodes and generate a value representing a cell integrity of said object.
Owner:SEAFOOD ANALYTICS
Applications of thyroid hormone, and pharmaceutically acceptable salts or prodrugs of thyroid hormone in preparing drugs used for treating and/or preventing pulmonary diseases
The invention provides novel applications of thyroid hormone, and pharmaceutically acceptable salts or prodrugs of thyroid hormone. According to the invention, thyroid hormone, and the pharmaceutically acceptable salts or prodrugs of thyroid hormone can be used for treating and / or preventing pulmonary diseases; thyroid hormone and the pharmaceutically acceptable salts or prodrugs of thyroid hormone can be used for protecting alveolar epithelial cells effectively, adjusting surface protein synthesis, storage, and distribution of alveolar epithelial cells, promoting maturation, differentiation, and cell integrity of alveolar epithelial cells, promoting repairing of damaged mitochondria in alveolar epithelial cells, improving mitochondria biosynthesis, preventing and reversing pulmonary fibrosis, adjusting immunity reaction, changing cancerous environment, treating and preventing pulmonary diseases, improving pulmonary functions such as gas exchange, and achieving treatment effect, and are beneficial for cleaning of pulmonary effusion and repairing of damaged lung tissue.
Owner:深圳市润佳通科技有限公司
Recovery and purity of magnetically targeted cells
InactiveUS20160168531A1Minimize CTC lossInhibit bindingBioreactor/fermenter combinationsBiological substance pretreatmentsPresent methodRare cell
Owner:BIOMAGNETIC SOLUTIONS LLC
A kind of cell cryogenic fluid and application thereof for preparing dry tumor cell quality control product
ActiveCN109122669BSolve problems that cannot be preserved intactRetain activityPreparing sample for investigationDead animal preservationHuman tumorFreeze-drying
The invention discloses a cell freezing solution for preparing a dry tumor cell quality control and application of the cell freezing solution. According to a formula, the cell freezing solution is prepared by adding 5-25% of protein, 3-15% of a cell stabilizing agent A, 1-10% of a cell stabilizing agent B and 0.01-0.1% of a bacteriostatic agent into PBS (phosphate buffer solution). The cell freezing solution successfully solves the problems that a cell integrity rate after freeze-drying is very low or active ingredients of cell membrane structural proteins and cell contents cannot be reservedwell in the existing cell freeze-drying technology. A preparation method of the dry tumor cell quality control directly uses human-derived tumor cell strains containing cell membrane structures and contents to prepare the quality control; the quality control is derived from human tumor cells and has the same characteristics as those of similar tumor cells in clinical liquid biopsy detection samples; and the quality control prepared by the method is applicable to the quality control of clinical liquid biopsy and can promote extensive popularization and application of the clinical liquid biopsy.
Owner:JIANGSU CODE BIOMEDICAL TECH CO LTD
Non-water leucorrhea specimen storing liquid and preparation method and pH value test collection tube
ActiveCN102885029AIntegrity guaranteedAvoid inactivationMaterial analysis by observing effect on chemical indicatorWithdrawing sample devicesSodium acetatePolyethylene glycol
The invention discloses a non-water leucorrhea specimen storing liquid and a preparation method and pH value test collection tube of the liquid. The non-water leucorrhea specimen storing liquid comprises the following substances by weight percent: 96.5wt% of anhydrous glycerin, 0.2wt% of polyethyleneglycol 8000 as a surface active agent, 0.25wt% of bromocresol green, 0.05wt% of methyl red, 2.0wt% of absolute ethyl alcohol and 1.0wt% of mercapto sodium acetate. The non-water leucorrhea specimen storing liquid has the advantages that visible components such as bacteriums, cells, trichomonads and sick bodies of the leucorrhea can be protected by anhydrous glycerin (glycerol) when being frozen at a temperature less than -20 DEG C; the cell completeness of the visible components such as cells, bacteriums, trichomonads and the like can still be maintained after being redissolved without changing the morphology under a microscope; the leucorrhea can be solved in water in any concentration when needing to be diluted; mercapto sodium acetate is used as a reducing agent to prevent the specimen from inactivation caused by dehydration, oxidation and autolysis during the storage or transportation; and the non-water leucorrhea specimen storing liquid does not affect the cell lysis and determination of Chlamydia trachomatis or various causative agents of the leucorrhea.
Owner:安徽信灵检验医学科技股份有限公司
Methods and devices for independent evaluation of cell integrity, changes and origin in chip design for production workflow
ActiveCN102112988BReduce sensitivityCAD circuit designSpecial data processing applicationsCell integrityFunctional impact
The technology disclosed relates to granular analysis of design data used to prepare chip designs for manufacturing and to identification of similarities and differences among parts of design data files. In particular, it relates to parsing data and organizing into canonical forms, digesting the canonical forms, and comparing digests of design data from different sources, such as designs and libraries of design templates. Organizing the design data into canonical forms generally reduces the sensitivity of data analysis to variations in data that have no functional impact on the design. The details of the granular analysis vary among design languages used to represent aspects of a design. For various design languages, granular analysis includes partitioning design files by header / cell portions, by separate handling of comments, by functionally significant / non-significant data, by whitespace / non-whitespace, and by layer within a unit of design data. The similarities and differences of interest depend on the purpose of the granular analysis. The comparisons are useful in many ways.
Owner:OASIS TOOLING
Non-water leucorrhea specimen storing liquid and preparation method and pH value test collection tube
ActiveCN102885029BIntegrity guaranteedAvoid inactivationMaterial analysis by observing effect on chemical indicatorWithdrawing sample devicesSodium acetateGlycerol
The invention discloses a non-water leucorrhea specimen storing liquid and a preparation method and pH value test collection tube of the liquid. The non-water leucorrhea specimen storing liquid comprises the following substances by weight percent: 96.5wt% of anhydrous glycerin, 0.2wt% of polyethyleneglycol 8000 as a surface active agent, 0.25wt% of bromocresol green, 0.05wt% of methyl red, 2.0wt% of absolute ethyl alcohol and 1.0wt% of mercapto sodium acetate. The non-water leucorrhea specimen storing liquid has the advantages that visible components such as bacteriums, cells, trichomonads and sick bodies of the leucorrhea can be protected by anhydrous glycerin (glycerol) when being frozen at a temperature less than -20 DEG C; the cell completeness of the visible components such as cells, bacteriums, trichomonads and the like can still be maintained after being redissolved without changing the morphology under a microscope; the leucorrhea can be solved in water in any concentration when needing to be diluted; mercapto sodium acetate is used as a reducing agent to prevent the specimen from inactivation caused by dehydration, oxidation and autolysis during the storage or transportation; and the non-water leucorrhea specimen storing liquid does not affect the cell lysis and determination of Chlamydia trachomatis or various causative agents of the leucorrhea.
Owner:安徽信灵检验医学科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com