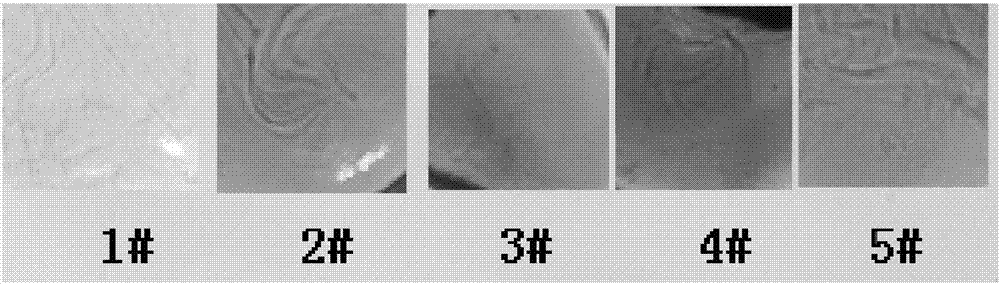
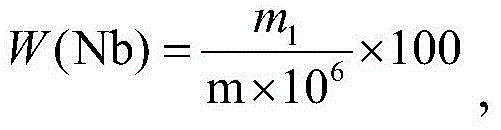Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Beryllium hydroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Beryllium hydroxide, Be(OH)₂, is an amphoteric hydroxide, dissolving in both acids and alkalis. Industrially, it is produced as a by-product in the extraction of beryllium metal from the ores beryl and bertrandite. The natural pure beryllium hydroxide is rare (in form of the mineral behoite, orthorhombic) or very rare (clinobehoite, monoclinic). When alkali is added to beryllium salt solutions the α-form (a gel) is formed. If this left to stand or boiled, the rhombic β-form precipitates. This has the same structure as zinc hydroxide, Zn(OH)₂, with tetrahedral beryllium centers.
Preparation method of high-purity ammonium fluoroberyllate and application thereof
The invention provides a preparation method of high-purity ammonium fluoroberyllate and application thereof, and in particular relates to a method of preparing high-purity ammonium fluoroberyllate; purity of the high-purity ammonium fluoroberyllate is greater than 99.95%, and the method comprises the following steps: (i), providing a mixture a of beryllium hydroxide and water; (ii), adding hydrofluoric acid into the mixture a to form a transparent mixture b; (iii), adding ammonia water into the transparent mixture b to form a mixture c containing precipitates; and (iv), carrying out recrystallization for at least twice onto the precipitates to obtain a recrystallization product, i.e., the high-purity ammonium fluoroberyllate. According to the preparation method disclosed by the invention, material requirements are low, preparation needs can be satisfied by only needing industrial beryllium hydroxide; and moreover, the obtained product contains extremely few impurities, and therefore, the preparation method can be conveniently applied to preparing the high-purity ammonium fluoroberyllate.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
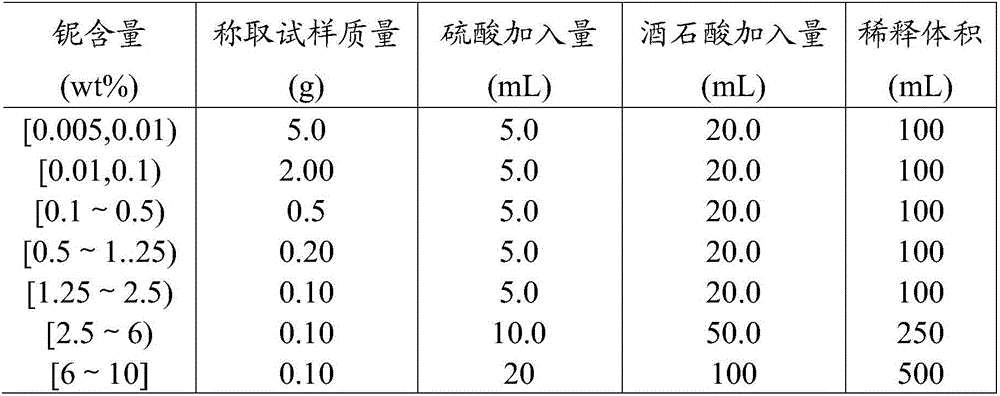
Method for measuring niobium content in iron steel through beryllium hydroxide separating sulfochlorophenol S spectrophotometric method
ActiveCN104101573AHigh precisionImprove stabilityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsBeryllium sulfateNiobium
The invention discloses a method for measuring niobium content in iron steel through a beryllium hydroxide separating sulfochlorophenol S spectrophotometric method. The method comprises the following steps: (1) weighing a sample, and dissolving into a first container with acid; (2) adding perchloric acid; (3) adding an ethylenediamine tetraacetic acid tetramine solution and / or an ethylenediamine tetraacetic acid disodium diamine solution; (4), adding ammonia water, a beryllium sulfate solution and oxalic acid; (5) filtering; (6) dissolving precipitation into a second container through acid; (7) after the precipitation is dissolved, adding tartaric acid so as to obtain a to-be-measured solution; (8) moving quantitative to-be-measured solution into a second container, and adding a sulfochlorophenol S solution, an ethylenediamine tetraacetic acid disodium diamine solution and hydrochloric acid so as to obtain a color developing liquid; (9) adding the color developing liquid into an absorption vessel, preparing a reference liquid, adding the reference liquid into the absorption vessel, and measuring the absorbency of the color developing liquid by comparison with the reference liquid at the position with the wavelength of 652 or 654; (10) calculating the niobium content in the sample according to a niobium working curve. According to the method, the measuring precision of the niobium content in the measured iron steel can be obviously improved.
Owner:PANGANG GROUP JIANGYOU CHANGCHENG SPECIAL STEEL
Method for preparing high-purity beryllium hydroxide from low-purity beryllium hydroxide
InactiveCN105585034AHigh purityReduce Si contentBeryllium oxides/hydroxidesSolubilityHigh concentration
The invention discloses a method for preparing high-purity beryllium hydroxide from low-purity beryllium hydroxide. According to the method, low-purity beryllium hydroxide is leached by high-concentration sulfuric acid from high-grade beryllium ore or beryllium concentrate, a leaching solution is subjected to impurity removal and precipitation by ammonium hydroxide, and a product is obtained; the low-purity beryllium hydroxide product contains impurities, and main components of the impurities include 1.0%-10.0% of SO4<2->, 0.05%-0.50% of Si, 0.02%-0.20% of Al and 0.05%-0.5% of Fe. Beryllium hydroxide is amphoteric hydroxide, can react with strong acid and strong base, but has low water solubility, the water solubility is 2*10<-3>g / L at the temperature of 25 DEG C, beryllium hydroxide can be sufficiently dissolved under the heating condition when reacting with strong base-sodium hydroxide, the impurities in beryllium hydroxide after dissolving are dissolved in a solution and are removed through hydrolysis, filtering and washing, and high-purity beryllium hydroxide is prepared accordingly.
Owner:NANHUA UNIV
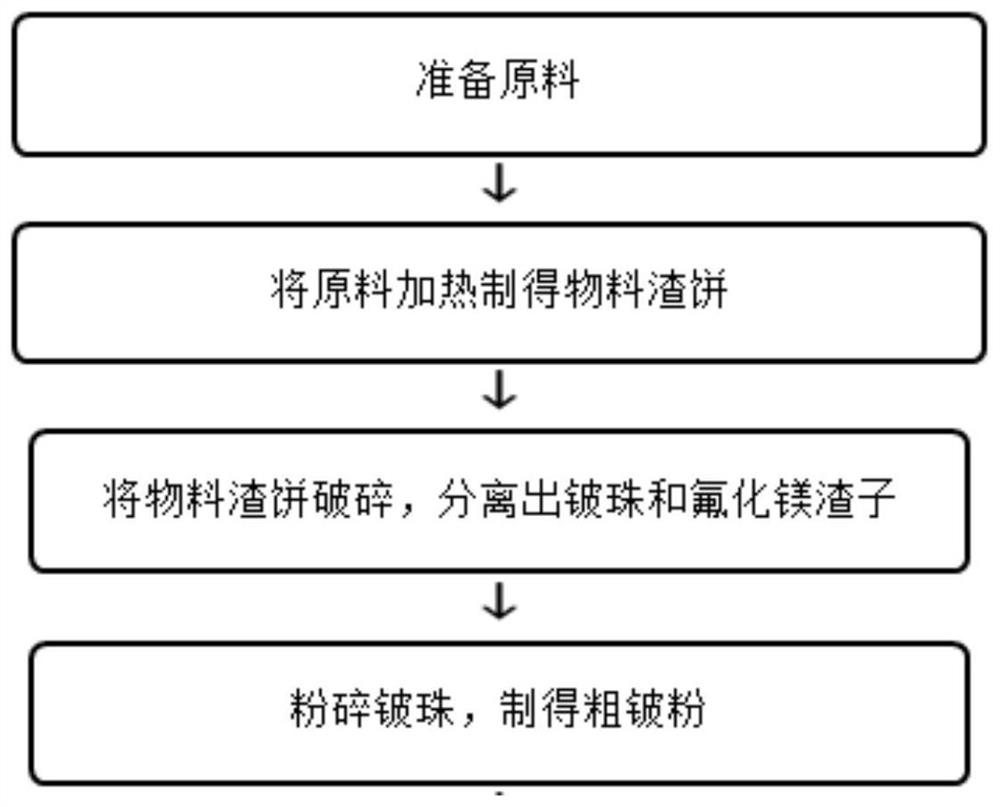
Method for preparing beryllium hydroxide by extracting from beryllium-containing ore
InactiveCN107641712AEfficient removalSimple production processProcess efficiency improvementBeryllium sulfateBeryllium hydroxide
The invention discloses a method for a method for preparing beryllium hydroxide by extracting from beryllium-containing ore. The method comprises the following steps: carrying out ball milling on rawore, adding quick lime and roasting; then pouring a mixture into water for freezing, and drying and breaking an obtained material to obtain a fine particle material; adding sulfate or aluminate into the obtained fine particle material, adding sulfuric acid and water, and curing to obtain a cured material; adding block rubber and water into the cured material, and leaching and filtering to obtain aberyllium sulfate solution, wherein the beryllium sulfate solution contains ferric sulfate impurity and aluminum sulfate impurity; adding ammonium sulfate into the beryllium sulfate solution for performing evaporation concentration; adding calcium carbonate, water and hydrogen peroxide and removing the ferric sulfate impurity; then filtering and introducing ammonia or ammonia water into the solution to obtain the beryllium hydroxide. The method disclosed by the invention has the characteristics of short flow process, simple production conditions, wide application range, high recovery rate, low production cost and the like; the difficult problem of extracting beryllium from low-grade raw ore is solved.
Owner:LONGZHOU WANHE TRADING CO LTD
Magnesium oxide material and preparing method thereof
InactiveCN105692661AImprove high temperature stabilityGood effectMagnesiaBeryllium hydroxideRefractory
The invention relates to a magnesium oxide material and a preparing method thereof. According to the technical scheme, magnesium oxide fine powder and mixed solution are blended according to the mass ratio of 1:2 and placed in a mixing machine to be mixed for 0.5-8 h, so that mixed pulp is obtained; the mixed pulp is filtered and dried for 24 h at 110 DEG C, so that a mixture block is obtained; heat insulation is conducted on the mixture block for 1-6 h at 1000-1300 DEG C, cooling is conducted, and ball milling is conducted to 1-200 microns, so that the magnesium oxide material is obtained. The mixed solution is prepared from 5-30 wt% of beryllium hydroxide and 70-95 wt% of oxalic acid solution. The prepared magnesium oxide material has the advantages of being high in hydration resistance, stable in high temperature performance, high in high-temperature strength and excellent in erosion resistance, and is a novel raw material for preparing a high-temperature industrial high-grade refractory material.
Owner:WUHAN UNIV OF SCI & TECH
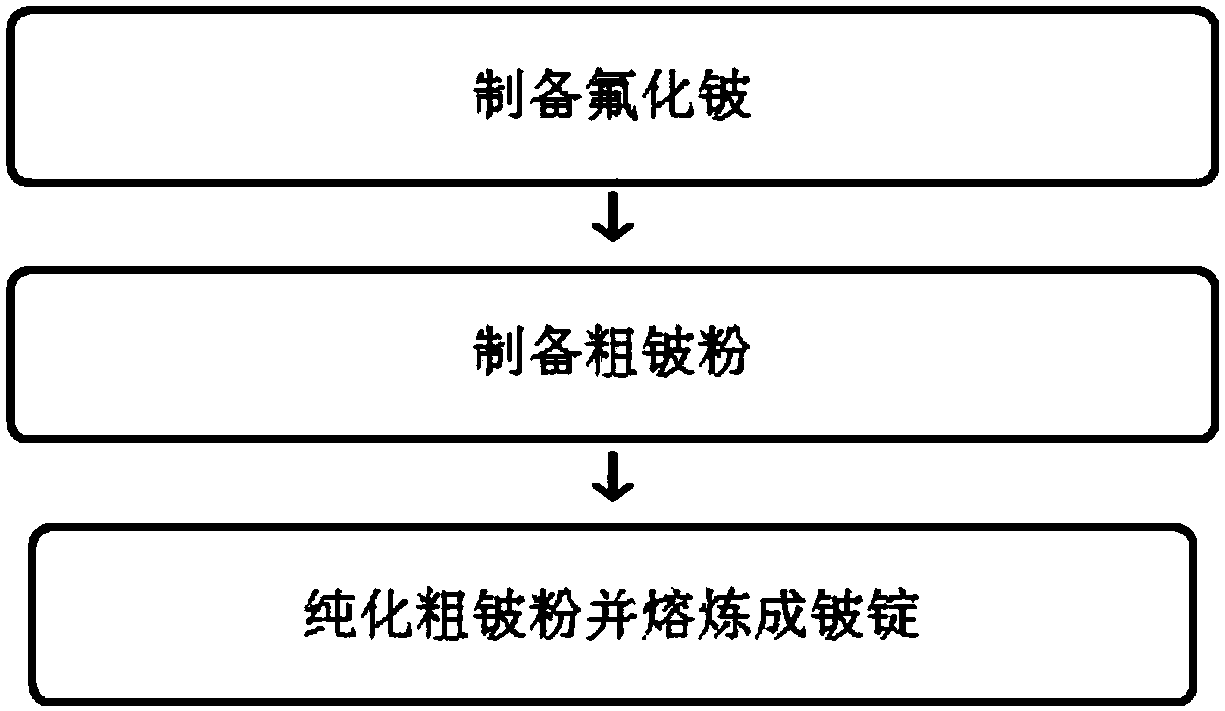
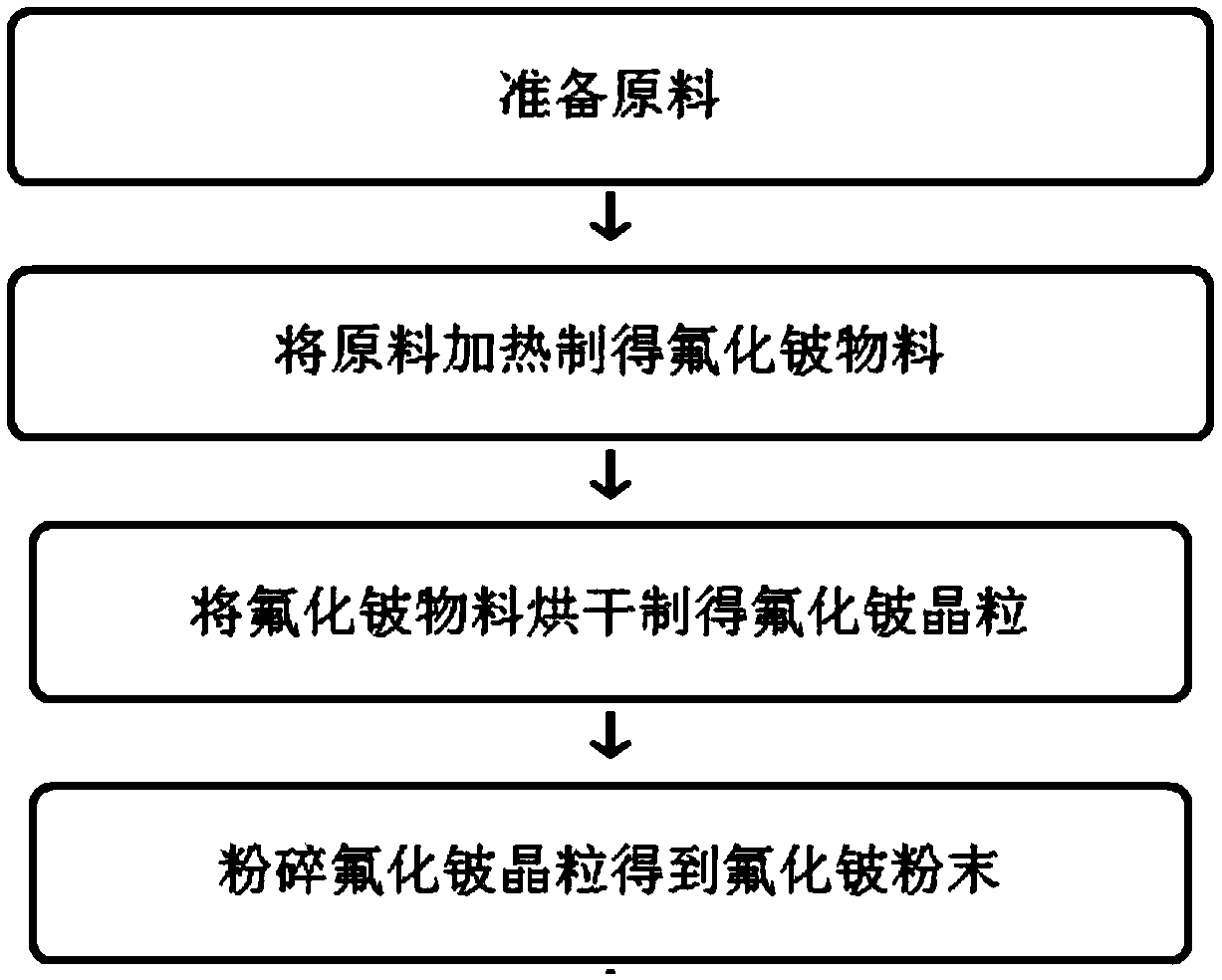
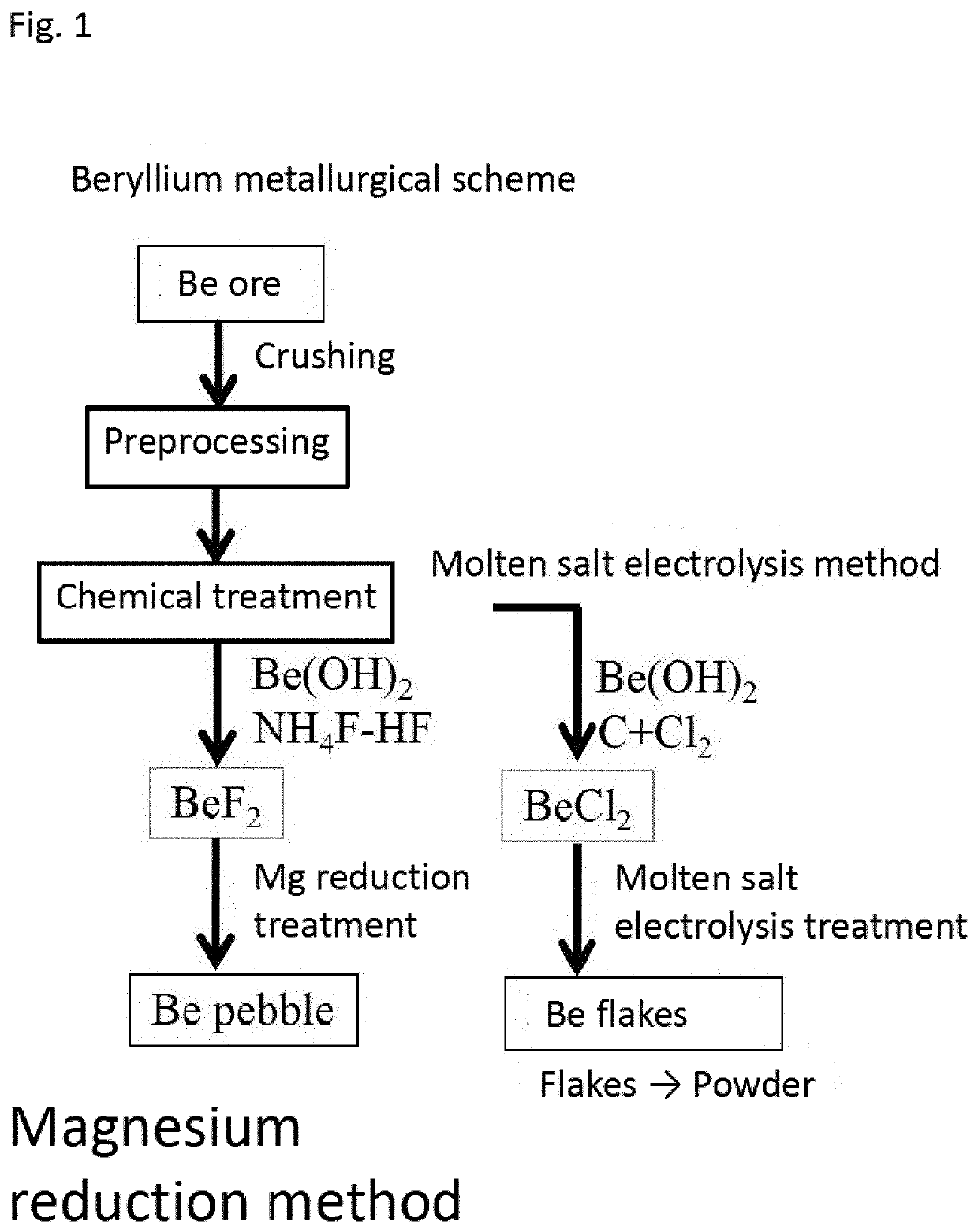
Preparation method of beryllium fluoride and preparation mode of ultrahigh-purity metal beryllium
ActiveCN109110789ASimple processIncrease productivityBeryllium fluorides/double-fluoridesHydrofluoric acidBeryllium hydroxide
The invention relates to a preparation method of beryllium fluoride and a preparation mode of ultrahigh-purity metal beryllium. The beryllium fluoride is prepared by mixing and heating beryllium hydroxide and hydrofluoric acid with the following steps: pouring the beryllium hydroxide and the hydrofluoric acid into a reactor, and sealing; energizing, stirring, and powering on for heating; putting aprepared beryllium fluoride material into a drying box for drying, so as to obtain beryllium fluoride crystal grains; pouring into a stainless steel pulverizer, and pulverizing, so as to obtain beryllium fluoride powder, and storing; preparing the beryllium fluoride powder into coarse beryllium powder, purifying, and smelting into a beryllium ingot. The preparation method of the beryllium fluoride and the preparation mode of ultrahigh-purity metal beryllium have the advantages that the technology is simple, and introduced impurities are fewer; compared with the traditional technology, the production efficiency is higher; the purity of the obtained product is high, energy is saved, the treatment cost is low, and the purity of the prepared beryllium is high.
Owner:陆世强
Preparation process of beryllium oxide
PendingCN108950181AHigh purityHigh recovery rateProcess efficiency improvementBeryllium oxides/hydroxidesHydrogenBeryllium hydroxide
The invention discloses a preparation process of beryllium oxide. The preparation process of the beryllium oxide comprises the steps of (1) raw material pretreatment, (2) block calcining, (3) block regeneration, (4) hydrogen and oxygen treatment, (5) acid treatment of powder and (6) preparation of beryllium oxide. The beryllium recovery rate of beryllium in ores can be increased above 93%; the constant-temperature calcining, not the high-temperature smelting is adopted, so that the problems of high energy consumption and high pollution can be overcome; and the purity of beryllium hydroxide canbe improved through repeated washing, so that the preparation of beryllium oxide is facilitated.
Owner:峨眉山市中山新材料科技有限公司
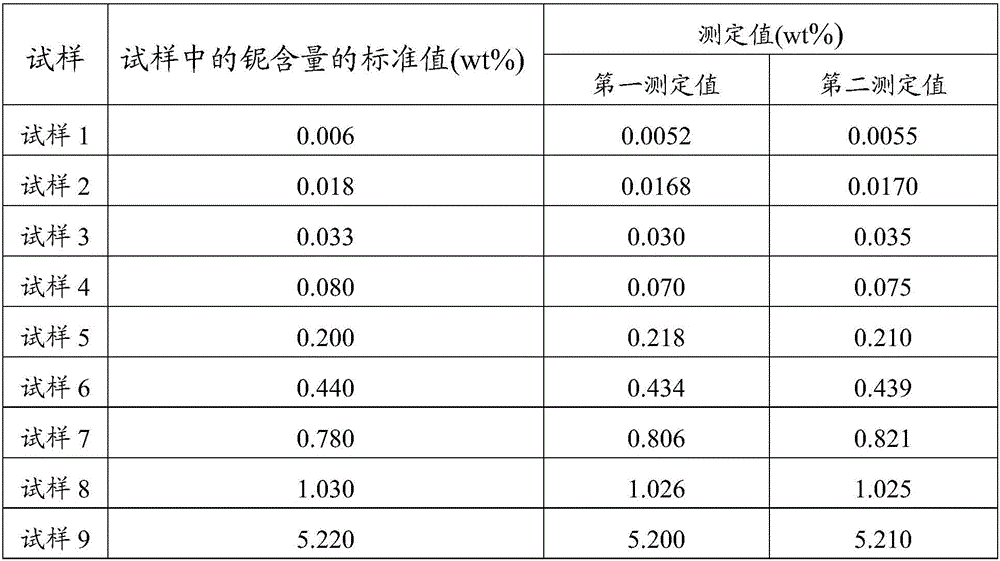
Method for determining content of beryllium in sludge
A method for determining the content of beryllium in sludge comprises the following steps: absorbing a beryllium oxide standard solution, adding an EDTA solution, stirring, heating for agglomerating a beryllium hydroxide precipitate, filtering through adding a small amount of paper pulp by using a medium speed filter paper, washing the precipitate by using hot ammonia water, purging with hot hydrochloric acid through using a washing bottle several times until the precipitate is dissolved in an original beaker, washing by using the hot hydrochloric acid, washing by using hot water to obtain a solution with the volume of about 100mL, adding the EDTA solution, and stirring. No national standard methods of determination of beryllium in soil exist. The method has the advantages of satisfactory determination result, simple operation, small dosage of the acid, and reduction of the work strength of analyzers.
Owner:大连大公检验检测有限公司
Heat absorbing temperature control devices that include hydroxide
InactiveUS7566484B2WallsSemiconductor/solid-state device detailsTemperature controlCalcium hydroxide
The increase of temperature of heat sensitive devices during heat generating conditions is prevented through the absorption of heat, by providing a hydroxide, such as Lithium Hydroxide, Sodium Hydroxide, Potassium Hydroxide, Magnesium Hydroxide, Calcium Hydroxide, Beryllium Hydroxide, Aluminum Hydroxide, Ammonium Hydroxide and mixtures thereof, in an amount sufficient to effect the required heat absorption. Where the heat generating conditions are generated by a heat generator, separate and distinct from the heat sensitive device, the hydroxide may be supported in a position between the heat sensitive device and the heat generator. Where the heat sensitive device is itself the heat generator, the hydroxide is in contact with the heat sensitive device, either directly or indirectly.
Owner:HAYES & ASSOCS
Orangered glass and production method and application thereof
InactiveCN107285623AHigh light transmittanceReasonable formulaGlass shaping apparatusBeryllium hydroxideRoom temperature
The invention relates to orangered glass and a production method and application thereof; the orangered glass is made from, by weight, 5-13.0% of Na2O, 1-4.5% of Rb2O, 6.4-10.0% of CaO, 2.0-3.0% of BeO, 60-65% of SiO2, 1% of Na2SO4, 4.5% of C, and 5.6% of Se; the above components are introduced in the forms of sodium carbonate, rubidium chloride, limestone, beryllium hydroxide, silica sand, sodium sulfate, carbon powder, and selenium powder respectively. The above materials are mixed well, the mixture is melted at 1485+ / -5 DEG C, the temperature is held for 90 min, the glass melt is poured into a mold that is preheated to 580 DEG C, the mold is placed a resistance furnace held at 580 DEG C for the purpose of annealing, and the temperature is held for 60 min before natural cooling to room temperature. Carbon and selenium are mixed for coloring; as carbon and selenium are used together under controlled usage, the orangered glass can be produced which has bright appearance and full color, never fades and is a novel material for making ornaments.
Owner:LUOYANG INST OF SCI & TECH
Preparation method of nanometer beryllium oxide material
PendingCN114455615ASolve the problem of environmental pollutionLow firing temperatureNanotechnologyChemical/physical/physico-chemical stationary reactorsBeryllium sulfateBeryllium hydroxide
The invention discloses a preparation method of a nanometer beryllium oxide material, relates to the field of preparation of nanometer beryllium oxide materials, and solves the problems that an existing preparation method of the nanometer beryllium oxide material is tedious in step, needs to consume a large amount of heat energy and easily causes environmental pollution. Beryllium hydroxide gel is obtained through the steps of aging, filtering, washing and the like; polyvinylpyrrolidone and ammonium persulfate are introduced to form a polymer chain with a three-dimensional network structure in a water phase, and beryllium crystal grains are further refined through ultrasonic treatment; the beryllium-containing mixed solution is subjected to hydro-thermal treatment under certain conditions, insoluble substances are filtered, washed, dried and subjected to final high-temperature roasting to obtain the nanometer beryllium oxide material, the preparation method of the nanometer beryllium oxide material can reduce the roasting temperature, the particle size of nanometer particles is uniform, the particle size is about 10 nm, and the nanometer beryllium oxide material is environmentally friendly and has the advantage of being suitable for industrial large-scale production.
Owner:上海太洋科技有限公司
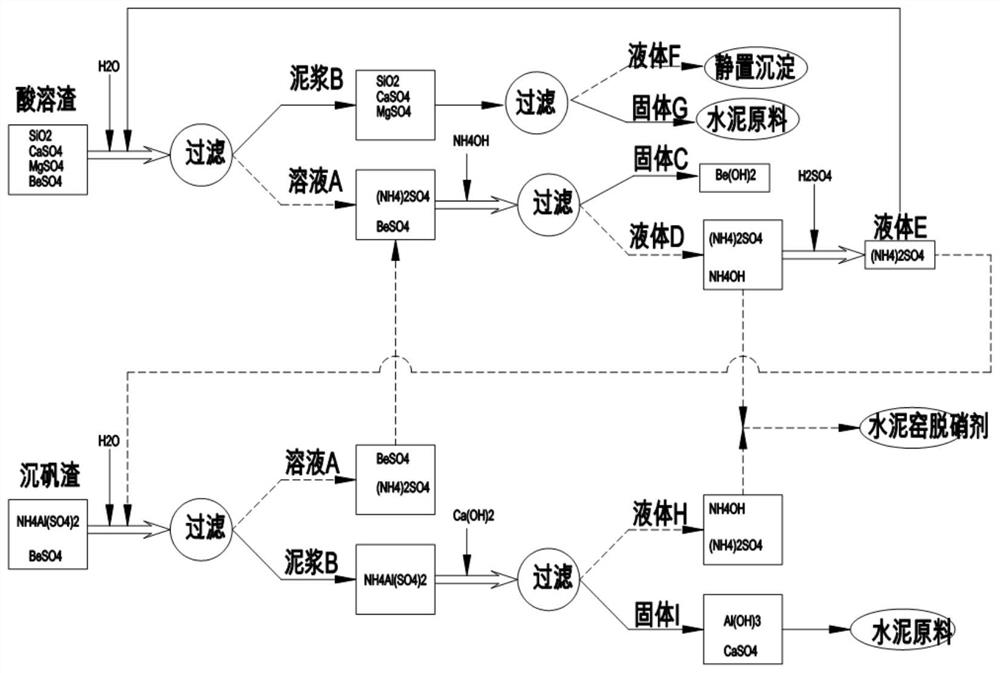
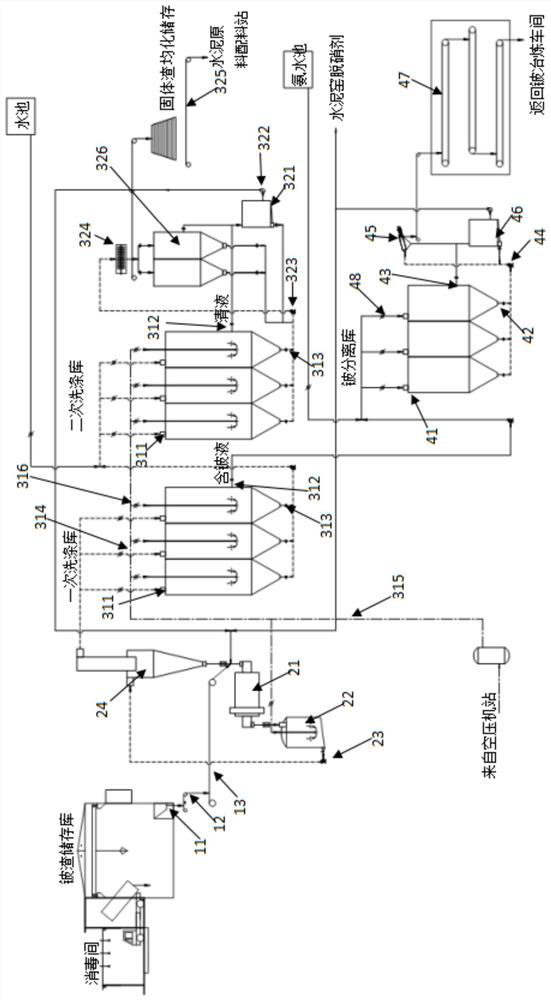
Beryllium slag detoxification and cement kiln collaborative resource treatment system and process
ActiveCN109761514BRealize harmless disposalMeet the protection requirementsDispersed particle separationCement productionBeryllium sulfateBeryllium hydroxide
The invention discloses a beryllium slag detoxification and cement kiln collaborative resource treatment system and process. The beryllium slag can be washed with water under acidic conditions to form a beryllium sulfate solution and solid slag, wherein the beryllium sulfate solution reacts with ammonia water to generate hydrogen Beryllium oxide precipitation, the precipitation is separated and used to recover beryllium hydroxide; and the solid slag is sent to the system cement kiln for high-temperature calcination after treatment, and the beryllium contained in the solid slag is solid-fused in the cement clinker lattice to form iron beryllium acid Minerals such as calcium, aluminum beryllium and calcium beryllium, the content of beryllium in cement reaches a trace amount, and the leaching solubility is less than 0.1μg / l, which meets the requirements of cement raw materials. The ammonia nitrogen compound produced during the reaction is converted into ammonia water or ammonium salt, which can be used as The cement kiln denitrification agent or returned to the beryllium smelter as an auxiliary agent can completely eliminate the residue, completely eliminate the toxic characteristics, and realize the recycling and harmless treatment of beryllium slag.
Owner:长沙中硅环保科技有限公司
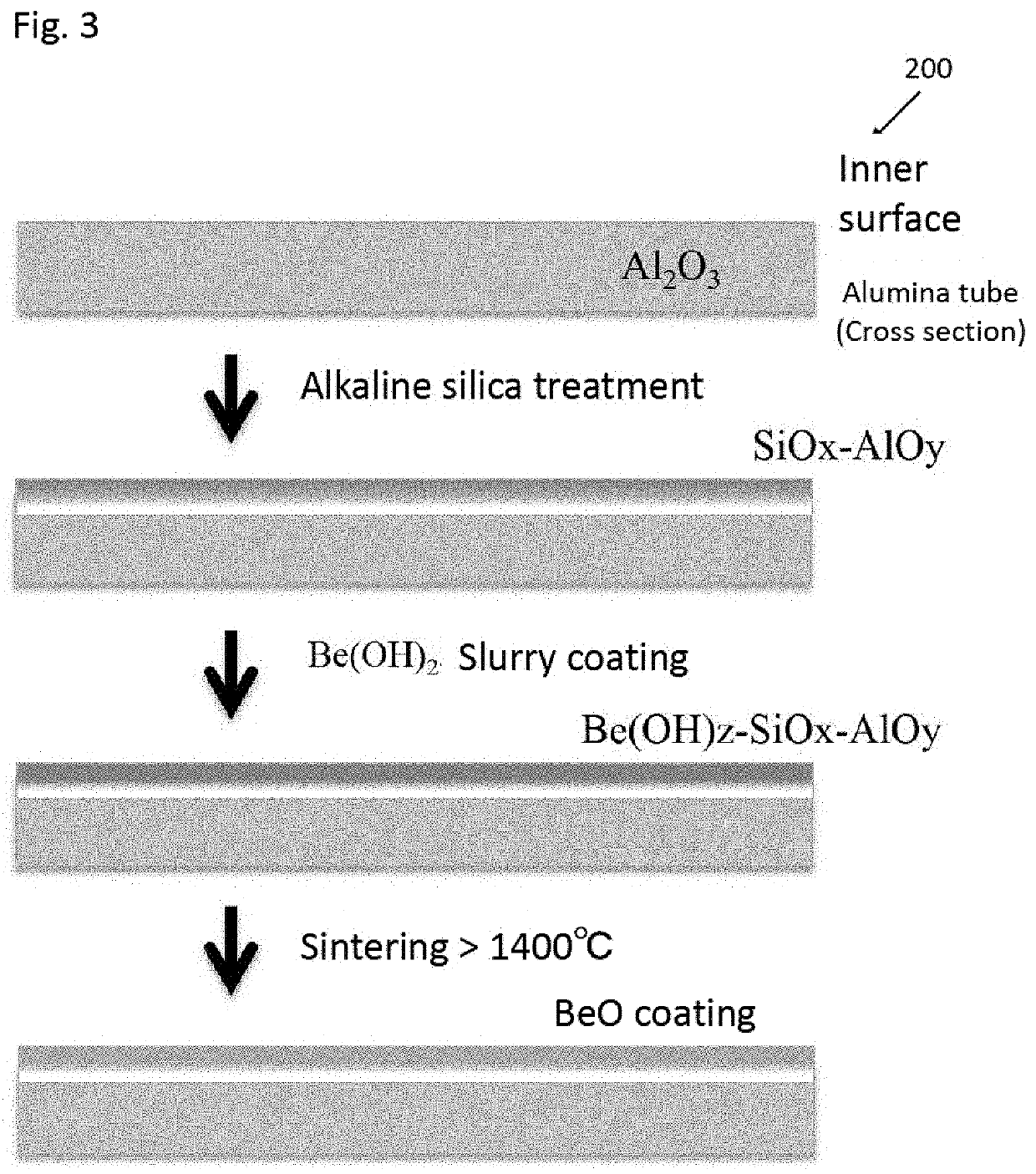
Continuous Producing Method of Beryllium Metal Sphere
ActiveUS20210114103A1Efficient mass productionLow costNuclear energy generationTransportation and packagingBeryllium hydroxideSlurry coating
To produce metallic beryllium spheres with high sphericity in a large quantity efficiently at a low cost by a simple method. The continuously producing method of metal beryllium spheres, comprising the steps of: collecting granulated beryllium spheres produced b by charging beryllium powder into a rotary kiln; classifying the collected beryllium spheres by particle size with an automatic sieve; and crushing particles of beryllium spheres of non-target diameters and mixing them with the raw material beryllium powder for reuse. The rotary kiln has a core tube the inner surface of which is coated with beryllium oxide by sintering the slurry coating of beryllium hydroxide applied after alkaline silica treatment.
Owner:SK KAKEN CO LTD
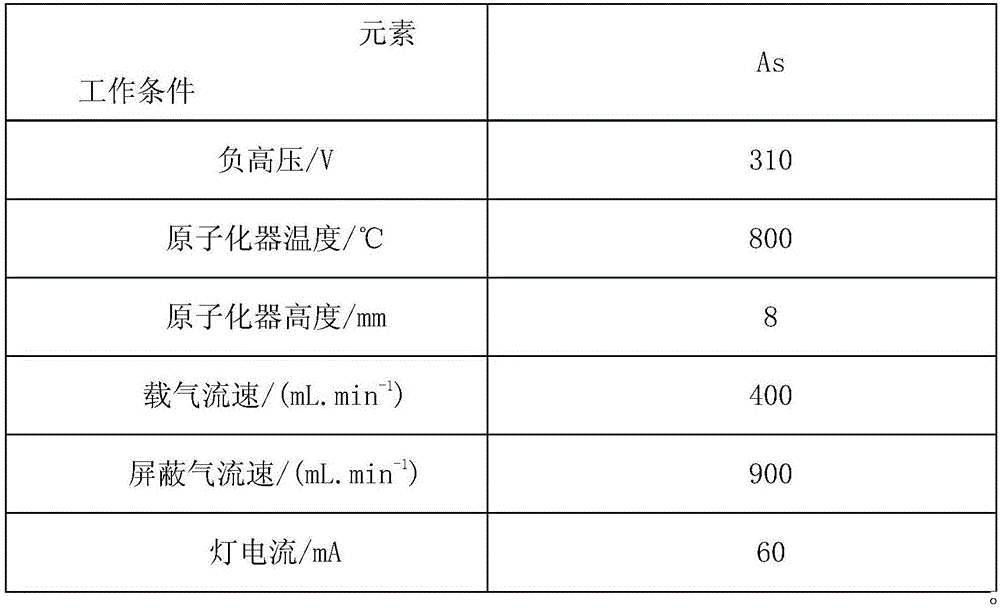
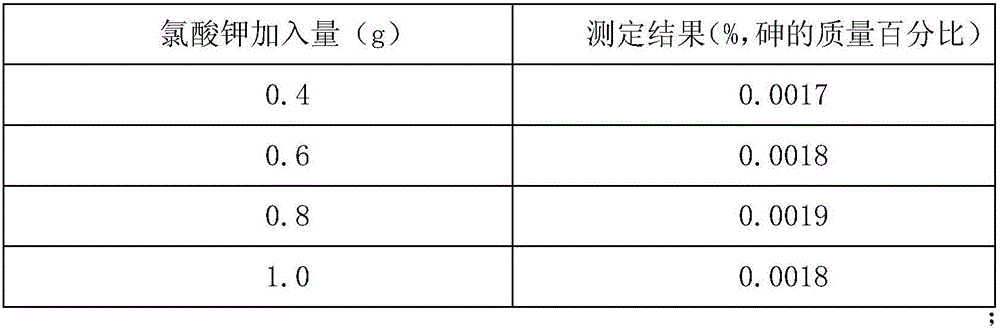
Method for measuring arsenic in APT through atomic fluorescence spectrometer
ActiveCN105891179AEasy to separateEliminate distractionsFluorescence/phosphorescenceFluorescenceBeryllium hydroxide
The invention relates to a method for measuring arsenic in APT through an atomic fluorescence spectrometer. Specifically, a 100 g / L potassium hydroxide solution is adopted for dissolving an APT sample; in an ammoniacal solution, beryllium hydroxide is used for coprecipitation, and arsenic and base tungsten are separated; hydrochloric acid with the volume ratio of 8% is used for dissolving sediment, arsenic enters the solution, and finally the atomic fluorescence spectrometer is used for measuring arsenic. Through the method, tungsten and arsenic can be completely separated, disturbance caused by tungsten to arsenic measurement is completely eliminated, the accuracy of measurement is greatly improved, the requirement for measurement of trace arsenic in APT can be completely met, and the method is economical, simple, convenient, rapid and easy to operate.
Owner:GANZHOU HUAXING TUNGSTEN PRODS
Ceramic plate
InactiveCN109851317AImprove heat resistanceImprove visual effectsClaywaresBeryllium hydroxidePolyvinyl alcohol
The invention provides a ceramic plate, and belongs to the field of ceramics. The ceramic plate comprises a plate and a glaze; the plate comprises the following components, in parts by weight: 15-25 parts of kaolin, 5-15 parts of talc powder, 0.5-1.5 parts of polyvinyl alcohol, 0.2-0.5 part of citric acid, 25-35 parts of cordierite, 2-4 parts of beryllium oxide, 3-5 parts of beryllium hydroxide, and 5-10 parts of strontium carbonate; and the glaze comprises the following components, in parts by weight: 65-75 parts of petalite, 5-15 parts of potassium feldspar, 4-6 parts of zinc oxide, 4-6 parts of kaolin, 6-8 parts of burnt talc, 2-4 parts of lithium carbonate, 2-4 parts of pyrochlore, 2-5 parts of spinel, and 0.5-1.5 parts of bismuth iron molybdenum oxide. The ceramic plate provided by the invention has good heat resistance and high strength and is not easy to deform.
Owner:HEYUAN DONGYUAN EAGLE CERAMICS CO LTD
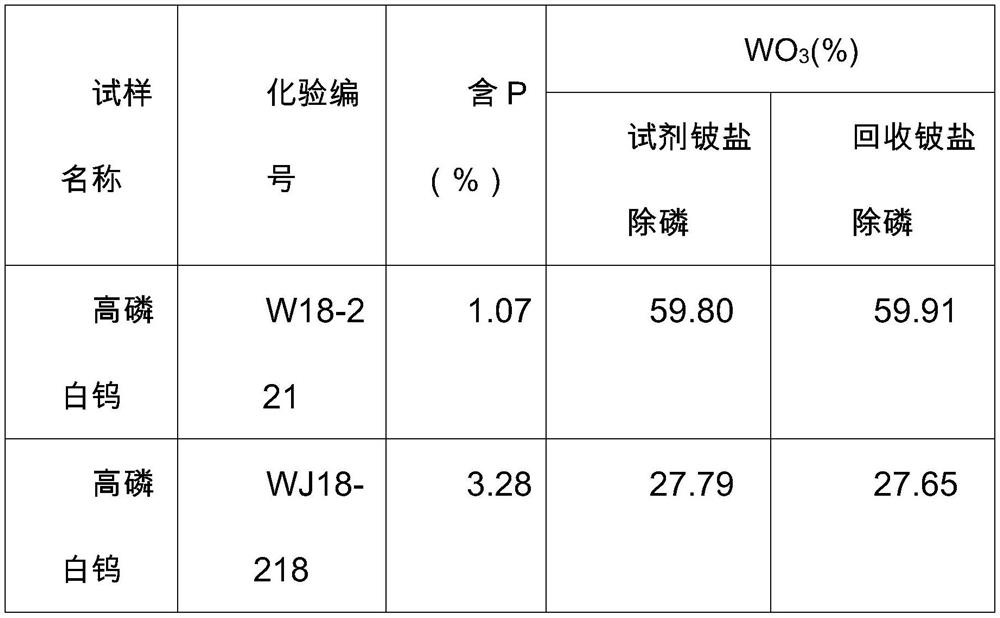
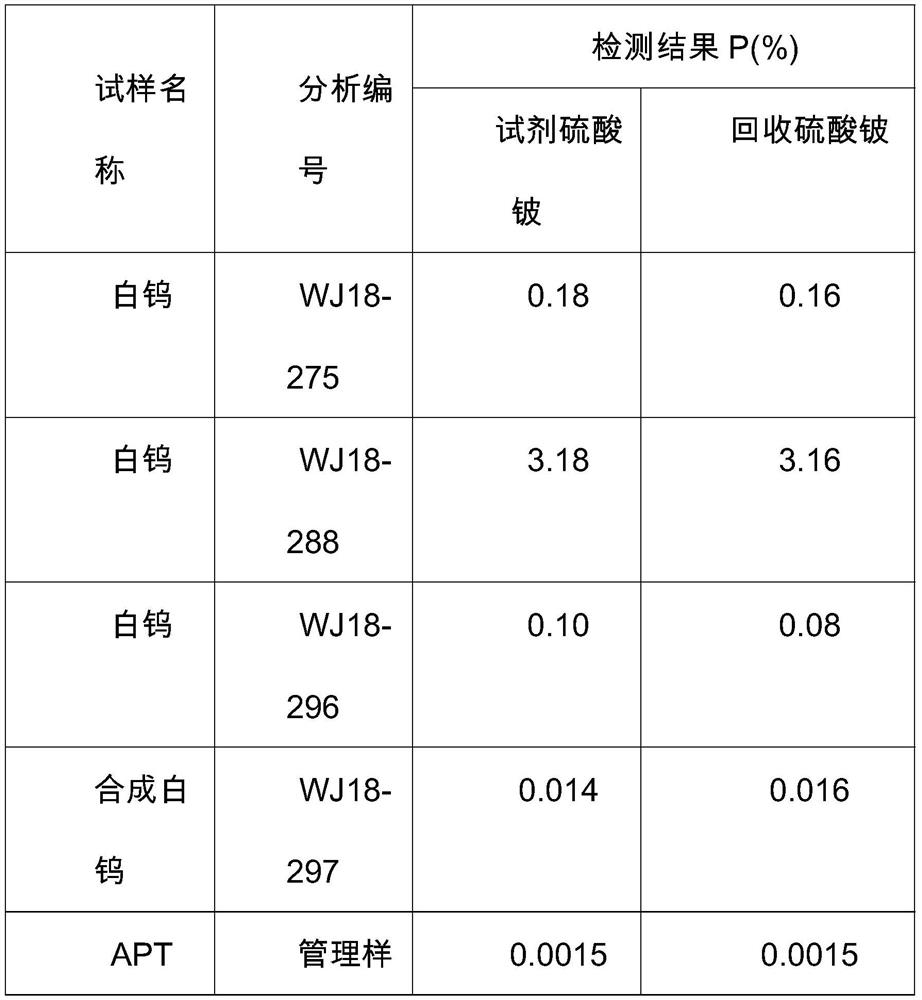
A kind of recycling method of beryllium in beryllium waste liquid measured by beryllium loading method
ActiveCN109319973BOvercome the disadvantages of polluting the environmentReduce pollutionTreatment involving filtrationMultistage water/sewage treatmentBüchner funnelBeryllium hydroxide
The invention discloses a method for recovering and reusing beryllium in the waste liquid of P measured by the beryllium loading method. The method comprises the following steps: placing the waste liquid containing beryllium after measuring P by the beryllium loading method in a 3000mL beaker, heating and concentrating on an electric furnace Volume, neutralized with alkali, then add ammonia water until beryllium hydroxide precipitates completely; filter with Buchner funnel, wash the precipitate with ammonia water-ammonium nitrate solution or ammonia water-ammonium sulfate solution for 10 times, transfer the precipitate into a beaker, add nitric acid or sulfuric acid Dissolve the precipitate; add ammonia water until the beryllium hydroxide precipitates completely, filter again with a Buchner funnel, wash the precipitate with ammonia water-ammonium nitrate solution or ammonia water-ammonium sulfate solution 10 times, transfer the precipitate into a beaker, add nitric acid or sulfuric acid to dissolve the precipitate. The invention is simple and easy to implement and low in cost. The beryllium salt is recovered and then used for the determination of P, or used for the phosphorus removal step of the determination of tungsten trioxide in high-phosphorus tungsten ore. The beryllium waste liquid is discharged outside, causing the disadvantage of beryllium polluting the environment, and greatly saving the cost of reagents.
Owner:江钨世泰科钨品有限公司
Method for Determination of Niobium Content in Iron and Steel by Separation of Chlorosulfophenol S by Beryllium Hydroxide
ActiveCN104101573BHigh precisionImprove stabilityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsBeryllium sulfateBeryllium hydroxide
Owner:PANGANG GROUP JIANGYOU CHANGCHENG SPECIAL STEEL
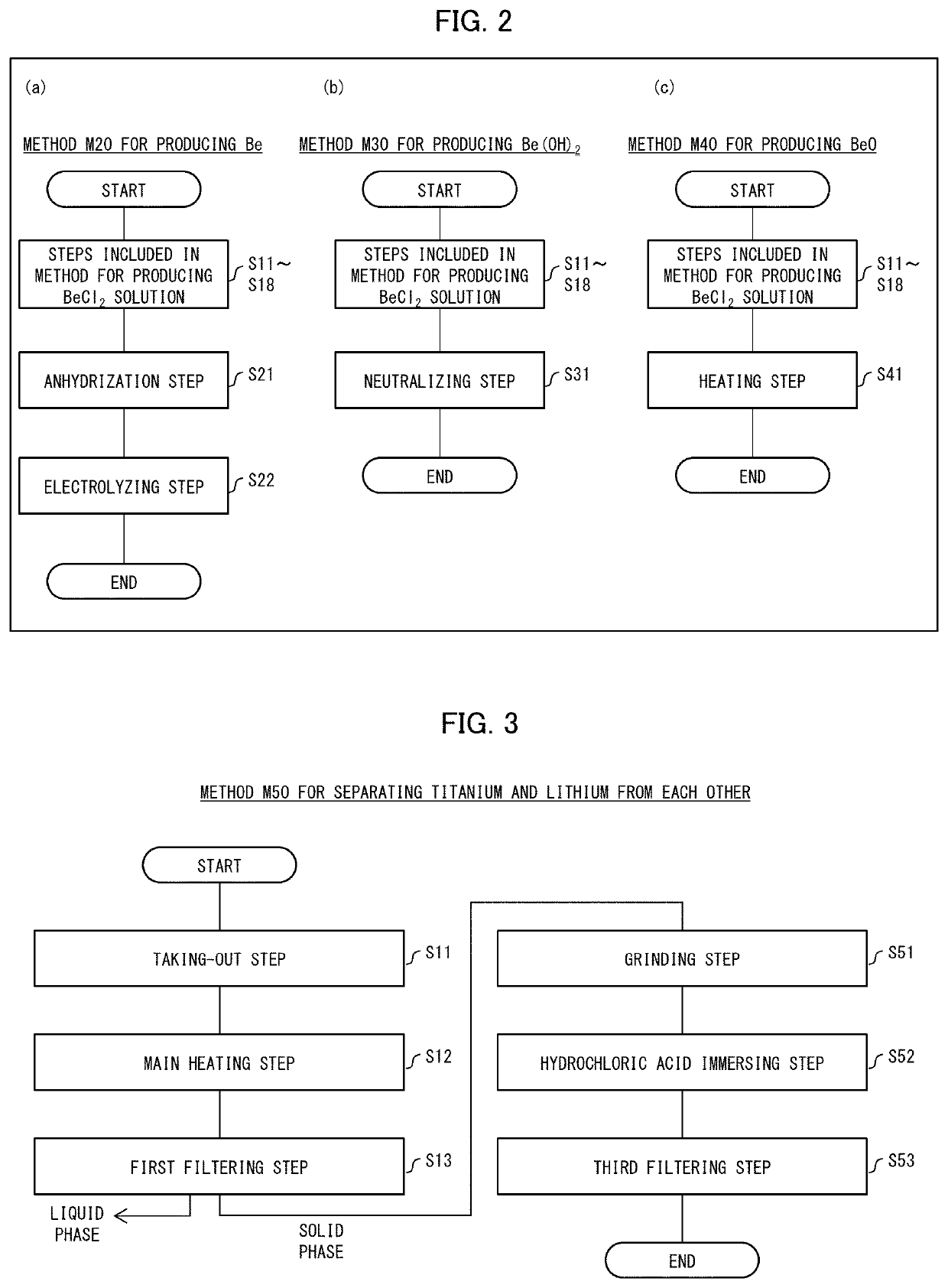
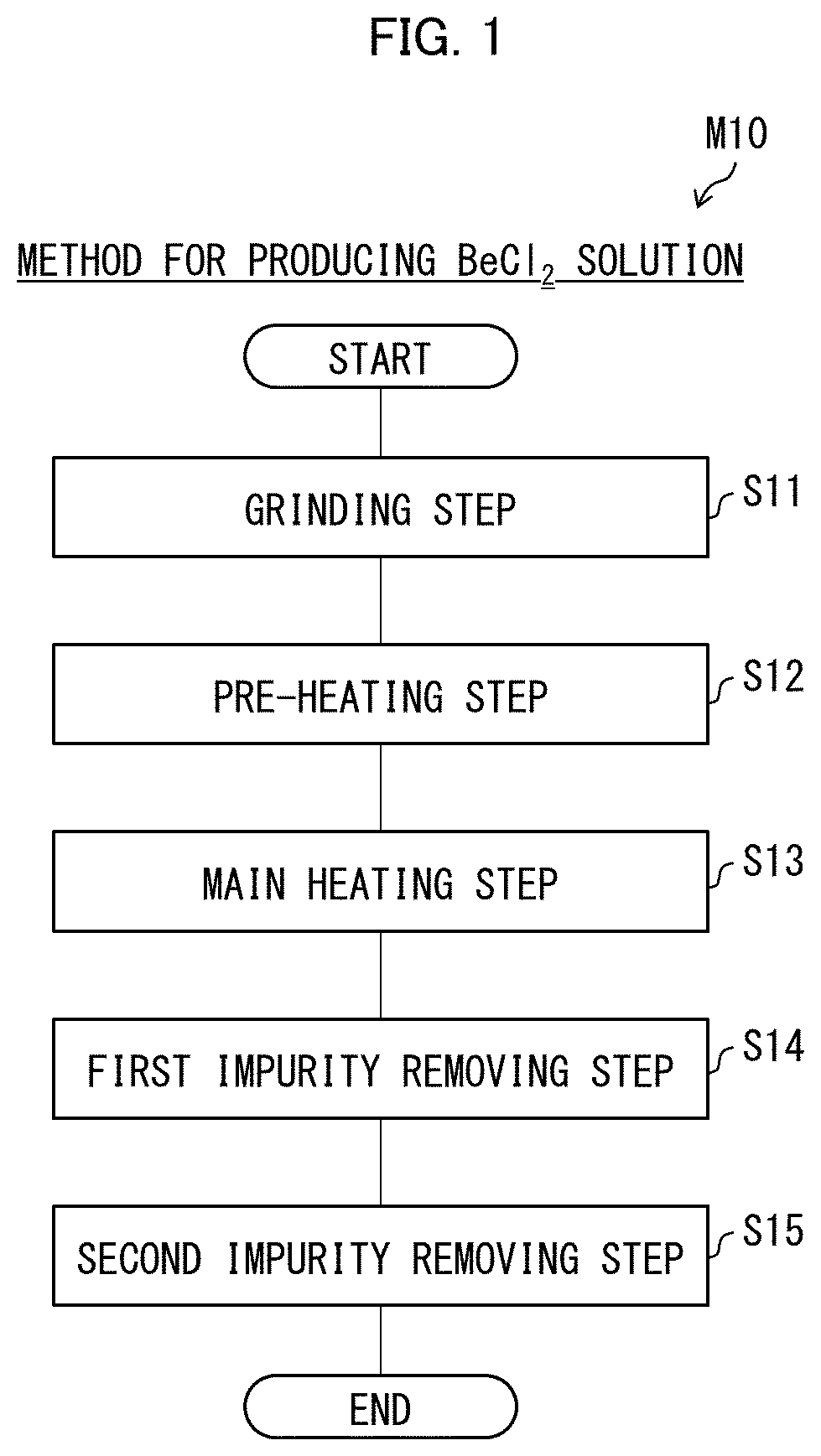
Beryllium solution production method, beryllium production method, beryllium hydroxide production method, beryllium oxide production method, solution production device, beryllium production system, and beryllium
PendingUS20220315438A1Improve energy efficiencyRadioactive decontaminationBeryllium oxides/hydroxidesBeryllium hydroxideHigh energy
This invention has an object to provide a method for producing a beryllium solution, the method being novel and having high energy efficiency. The method (M10) for producing a beryllium solution includes a main heating step (S13) of dielectrically heating an acidic solution containing a starting material so as to generate a beryllium solution, the starting material being beryllium or a substance containing beryllium.
Owner:NAT INST FOR QUANTUM SCI & TECH
A kind of orange-red glass and its preparation method and application
InactiveCN107285623BHigh light transmittanceReasonable formulaGlass shaping apparatusBeryllium hydroxideRoom temperature
The invention relates to orangered glass and a production method and application thereof; the orangered glass is made from, by weight, 5-13.0% of Na2O, 1-4.5% of Rb2O, 6.4-10.0% of CaO, 2.0-3.0% of BeO, 60-65% of SiO2, 1% of Na2SO4, 4.5% of C, and 5.6% of Se; the above components are introduced in the forms of sodium carbonate, rubidium chloride, limestone, beryllium hydroxide, silica sand, sodium sulfate, carbon powder, and selenium powder respectively. The above materials are mixed well, the mixture is melted at 1485+ / -5 DEG C, the temperature is held for 90 min, the glass melt is poured into a mold that is preheated to 580 DEG C, the mold is placed a resistance furnace held at 580 DEG C for the purpose of annealing, and the temperature is held for 60 min before natural cooling to room temperature. Carbon and selenium are mixed for coloring; as carbon and selenium are used together under controlled usage, the orangered glass can be produced which has bright appearance and full color, never fades and is a novel material for making ornaments.
Owner:LUOYANG INST OF SCI & TECH
Method for determining arsenic in apt by atomic fluorescence spectrometer
ActiveCN105891179BEasy to separateEliminate distractionsFluorescence/phosphorescenceBeryllium hydroxideAtomic fluorescence spectrometry
The invention relates to a method for measuring arsenic in APT through an atomic fluorescence spectrometer. Specifically, a 100 g / L potassium hydroxide solution is adopted for dissolving an APT sample; in an ammoniacal solution, beryllium hydroxide is used for coprecipitation, and arsenic and base tungsten are separated; hydrochloric acid with the volume ratio of 8% is used for dissolving sediment, arsenic enters the solution, and finally the atomic fluorescence spectrometer is used for measuring arsenic. Through the method, tungsten and arsenic can be completely separated, disturbance caused by tungsten to arsenic measurement is completely eliminated, the accuracy of measurement is greatly improved, the requirement for measurement of trace arsenic in APT can be completely met, and the method is economical, simple, convenient, rapid and easy to operate.
Owner:GANZHOU HUAXING TUNGSTEN PRODS
A method for preparing beryllium fluoride and a method for preparing ultra-high purity metal beryllium
ActiveCN109110789BPrecise flow controlReasonable structureBeryllium fluorides/double-fluoridesBeryllium hydroxidePhysical chemistry
The invention relates to a preparation method of beryllium fluoride and a preparation mode of ultrahigh-purity metal beryllium. The beryllium fluoride is prepared by mixing and heating beryllium hydroxide and hydrofluoric acid with the following steps: pouring the beryllium hydroxide and the hydrofluoric acid into a reactor, and sealing; energizing, stirring, and powering on for heating; putting aprepared beryllium fluoride material into a drying box for drying, so as to obtain beryllium fluoride crystal grains; pouring into a stainless steel pulverizer, and pulverizing, so as to obtain beryllium fluoride powder, and storing; preparing the beryllium fluoride powder into coarse beryllium powder, purifying, and smelting into a beryllium ingot. The preparation method of the beryllium fluoride and the preparation mode of ultrahigh-purity metal beryllium have the advantages that the technology is simple, and introduced impurities are fewer; compared with the traditional technology, the production efficiency is higher; the purity of the obtained product is high, energy is saved, the treatment cost is low, and the purity of the prepared beryllium is high.
Owner:陆世强
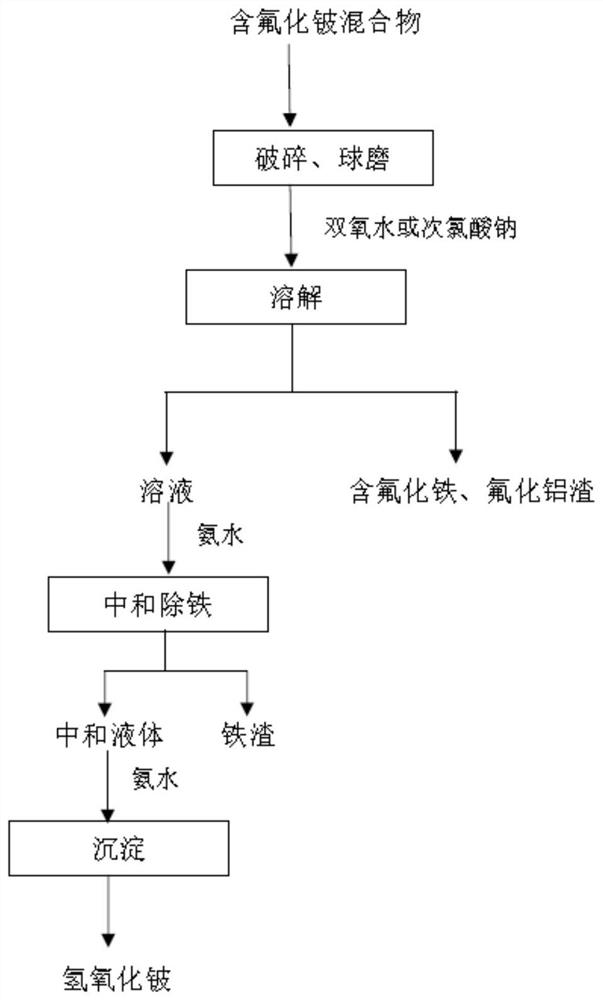
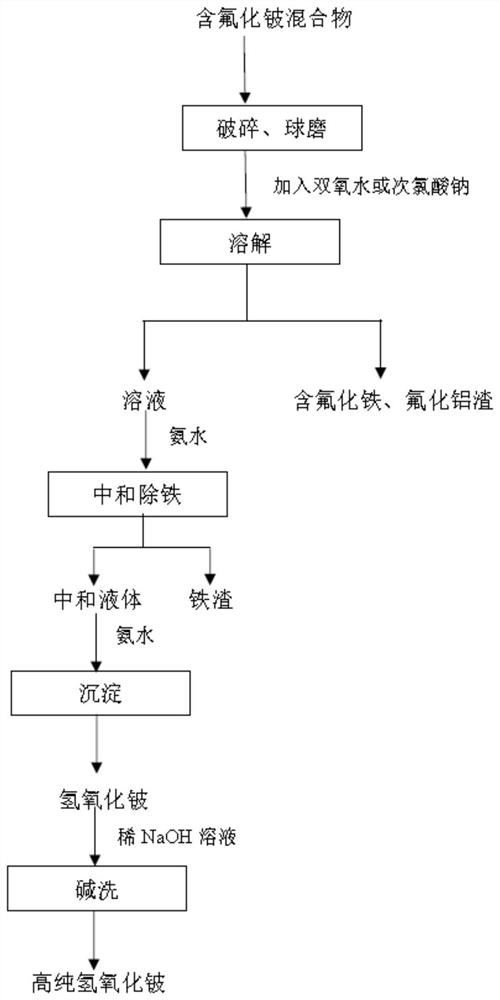
Method for purifying and preparing beryllium hydroxide from beryllium fluoride-containing mixture
ActiveCN113003591AImprove processing efficiencySimple processProcess efficiency improvementBeryllium oxides/hydroxidesBeryllium hydroxidePhysical chemistry
The invention relates to a method for purifying and preparing beryllium hydroxide from a beryllium fluoride-containing mixture, belongs to the technical field of metal beryllium metallurgy, and solves the problems that an existing beryllium hydroxide preparation technology is tedious in process and high in equipment requirement, and the purity of the prepared beryllium hydroxide is low. The method for purifying and preparing beryllium hydroxide from the beryllium fluoride-containing mixture comprises the steps: crushing and ball-milling the beryllium fluoride-containing mixture into beryllium fluoride-containing mixture powder; dissolving the beryllium fluoride-containing mixture powder with water to obtain a beryllium fluoride-containing mixture suspension; carrying out solid-liquid separation on the beryllium fluoride-containing mixture suspension to obtain a first separation solution and residues; adding ammonia water into the first separation solution to adjust the pH value, carrying out primary precipitation, and removing the primary precipitate to obtain a second separation solution; and adding ammonia water into the second separation solution to adjust the pH value, carrying out secondary precipitation, carrying out solid-liquid separation, and drying the obtained solid to obtain beryllium hydroxide. The beryllium hydroxide is prepared by smelting an intermediate product beryllium fluoride-containing mixture from beryllium ore.
Owner:钢研晟华科技股份有限公司 +1
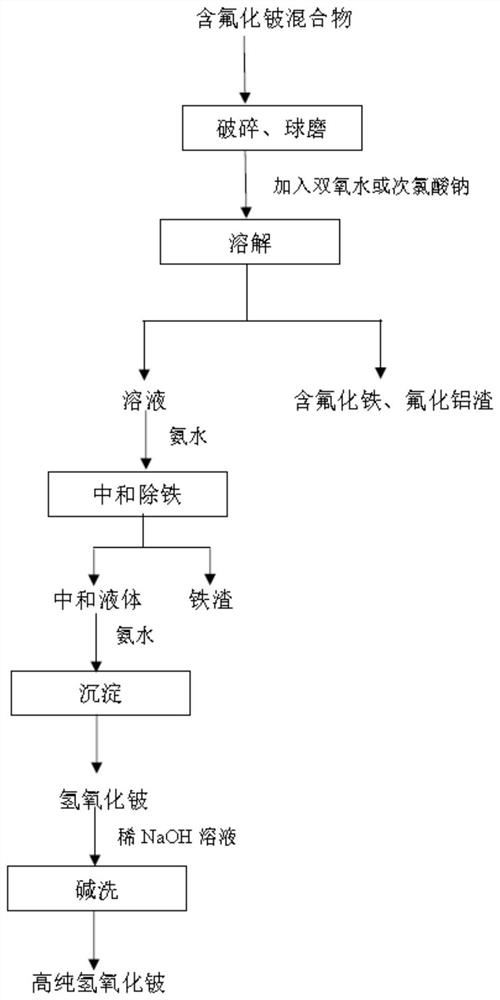
A kind of method for purifying and preparing beryllium hydroxide from mixture containing beryllium fluoride
ActiveCN113003591BImprove processing efficiencySimple processProcess efficiency improvementBeryllium oxides/hydroxidesBeryllium hydroxidePhysical chemistry
The invention relates to a method for purifying and preparing beryllium hydroxide from a mixture containing beryllium fluoride, belonging to the technical field of metal beryllium metallurgy, and solves the complicated process and high equipment requirements in the existing technology for preparing beryllium hydroxide; The problem of low purity of beryllium. The method for purifying and preparing beryllium hydroxide from a beryllium fluoride-containing mixture of the present invention includes: crushing and ball-milling the beryllium fluoride-containing mixture into powder of the beryllium fluoride-containing mixture; dissolving the beryllium fluoride-containing mixture powder with water to obtain a beryllium fluoride-containing mixture The suspension liquid of the beryllium fluoride mixture; the suspension liquid containing the beryllium fluoride mixture is subjected to solid-liquid separation to obtain the first separation solution and the residue; the first separation solution is added with ammonia water to adjust the pH value to carry out a precipitation, and the removal is carried out once The precipitate obtains the second separation solution; the second separation solution is added with ammonia water to adjust the pH value to carry out secondary precipitation, solid-liquid separation, and drying of the obtained solid to obtain beryllium hydroxide. The beryllium hydroxide is prepared by using the beryllium ore smelting intermediate product containing beryllium fluoride mixture.
Owner:钢研晟华科技股份有限公司 +1
A kind of magnesium oxide material and preparation method thereof
InactiveCN105692661BImprove high temperature stabilityGood effectMagnesiaOXALIC ACID DIHYDRATEBeryllium hydroxide
The invention relates to a magnesium oxide material and a preparing method thereof. According to the technical scheme, magnesium oxide fine powder and mixed solution are blended according to the mass ratio of 1:2 and placed in a mixing machine to be mixed for 0.5-8 h, so that mixed pulp is obtained; the mixed pulp is filtered and dried for 24 h at 110 DEG C, so that a mixture block is obtained; heat insulation is conducted on the mixture block for 1-6 h at 1000-1300 DEG C, cooling is conducted, and ball milling is conducted to 1-200 microns, so that the magnesium oxide material is obtained. The mixed solution is prepared from 5-30 wt% of beryllium hydroxide and 70-95 wt% of oxalic acid solution. The prepared magnesium oxide material has the advantages of being high in hydration resistance, stable in high temperature performance, high in high-temperature strength and excellent in erosion resistance, and is a novel raw material for preparing a high-temperature industrial high-grade refractory material.
Owner:WUHAN UNIV OF SCI & TECH
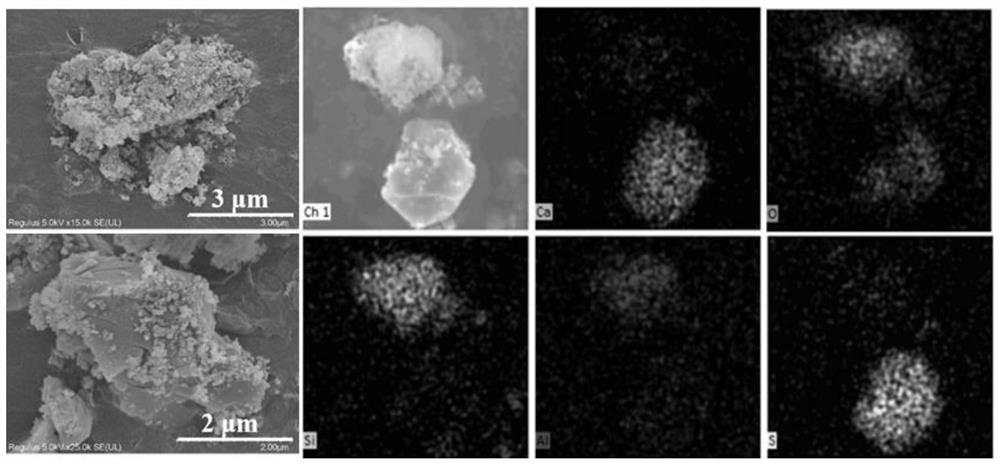
A method for separating beryllium from beryllium-containing sludge based on mineral phase reconstruction
ActiveCN113817923BHigh reactivityEffective dissolutionSludge treatment by de-watering/drying/thickeningCement productionBeryllium hydroxideSludge
Owner:CENT SOUTH UNIV
A kind of preparation method and application of high-purity ammonium fluoroberyllate
The invention provides a preparation method of high-purity ammonium fluoroberyllate and application thereof, and in particular relates to a method of preparing high-purity ammonium fluoroberyllate; purity of the high-purity ammonium fluoroberyllate is greater than 99.95%, and the method comprises the following steps: (i), providing a mixture a of beryllium hydroxide and water; (ii), adding hydrofluoric acid into the mixture a to form a transparent mixture b; (iii), adding ammonia water into the transparent mixture b to form a mixture c containing precipitates; and (iv), carrying out recrystallization for at least twice onto the precipitates to obtain a recrystallization product, i.e., the high-purity ammonium fluoroberyllate. According to the preparation method disclosed by the invention, material requirements are low, preparation needs can be satisfied by only needing industrial beryllium hydroxide; and moreover, the obtained product contains extremely few impurities, and therefore, the preparation method can be conveniently applied to preparing the high-purity ammonium fluoroberyllate.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Alumina material and preparation method thereof
InactiveCN105837188AImprove high temperature stabilityReduce solubilityBeryllium hydroxideMass ratio
The invention relates to an alumina material and a preparation method thereof. According to a technical scheme in the invention, the preparation method comprises the following steps: carrying out batching according to a mass ratio of fine alumina powder to a mixed solution of 1: 2 and placing the fine alumina powder and the mixed solution in a mixer for blending for 0.5 to 8 h so as to obtain mixed slurry; draining the mixed slurry through filtering and then carrying out drying at 110 DEG C for 24 h so as to obtain a mixture block; and maintaining the mixture block at 1000 to 1300 DEG C for 1 to 6 h, then cooling the mixture block and carrying out ball milling until a particle size of 1 to 200 [mu]m is obtained so as to obtain the alumina material. The mixed solution is composed of 5 to 30% of beryllium hydroxide and 70 to 95% of an oxalic acid solution. The alumina material prepared in the invention has the characteristics of stable high temperature performance, great high-temperature strength, a small thermal expansion coefficient and high thermal shock stability, and is a novel raw material for preparation of a refractory material used in high-temperature industry.
Owner:WUHAN UNIV OF SCI & TECH
A kind of aluminum oxide material and preparation method thereof
The invention relates to an alumina material and a preparation method thereof. According to a technical scheme in the invention, the preparation method comprises the following steps: carrying out batching according to a mass ratio of fine alumina powder to a mixed solution of 1: 2 and placing the fine alumina powder and the mixed solution in a mixer for blending for 0.5 to 8 h so as to obtain mixed slurry; draining the mixed slurry through filtering and then carrying out drying at 110 DEG C for 24 h so as to obtain a mixture block; and maintaining the mixture block at 1000 to 1300 DEG C for 1 to 6 h, then cooling the mixture block and carrying out ball milling until a particle size of 1 to 200 [mu]m is obtained so as to obtain the alumina material. The mixed solution is composed of 5 to 30% of beryllium hydroxide and 70 to 95% of an oxalic acid solution. The alumina material prepared in the invention has the characteristics of stable high temperature performance, great high-temperature strength, a small thermal expansion coefficient and high thermal shock stability, and is a novel raw material for preparation of a refractory material used in high-temperature industry.
Owner:WUHAN UNIV OF SCI & TECH
A combined dressing and metallurgy method for extracting beryllium oxide from chrysoberyl beryllium ore
InactiveCN105671341BPlay a fluxing effectAct as a bondProcess efficiency improvementWet separationKeroseneDecomposition
Owner:广东省资源综合利用研究所 +1
Method for producing beryllium solution, method for producing beryllium, method for producing beryllium hydroxide, method for producing beryllium oxide, and beryllium oxide
PendingUS20220298021A1Improve energy efficiencyBeryllium oxides/hydroxidesBeryllium hydroxideHigh energy
This invention has an object to provide a method for producing a beryllium solution by dissolving beryllium oxide, the method being novel and having high energy efficiency. A production method (M10) for producing a beryllium solution includes a main heating step (S13) of dielectrically heating an acidic solution containing beryllium oxide to generate a beryllium solution.
Owner:NAT INST FOR QUANTUM SCI & TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com