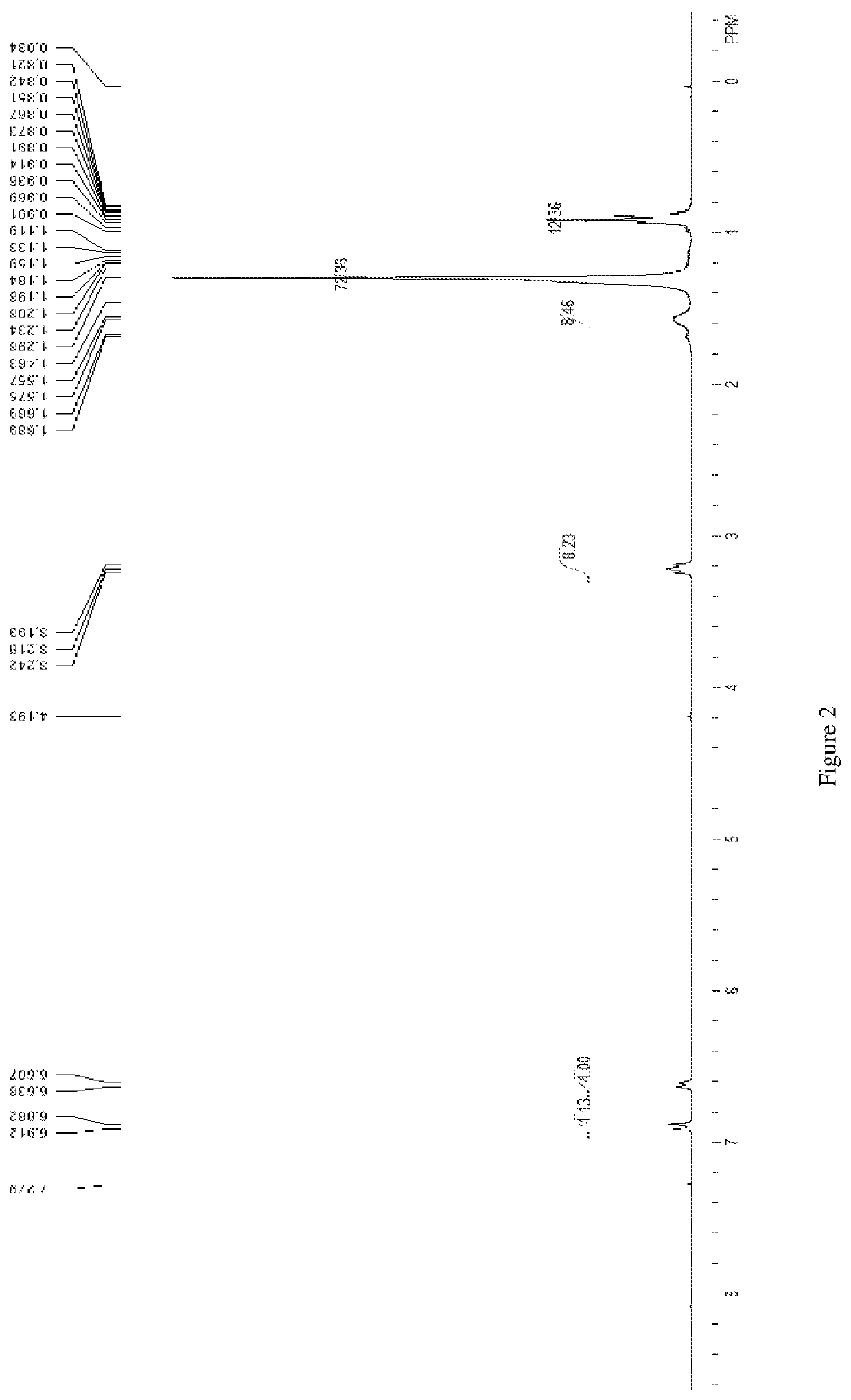
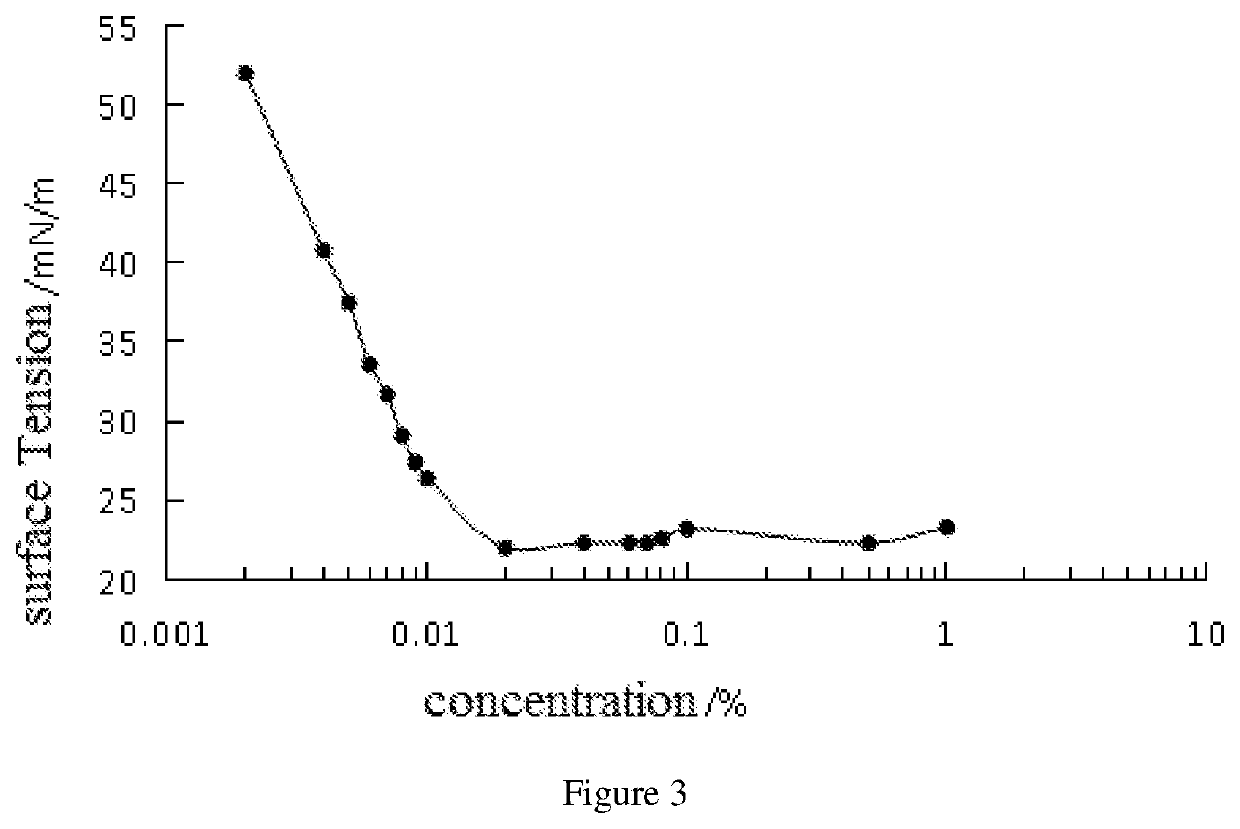
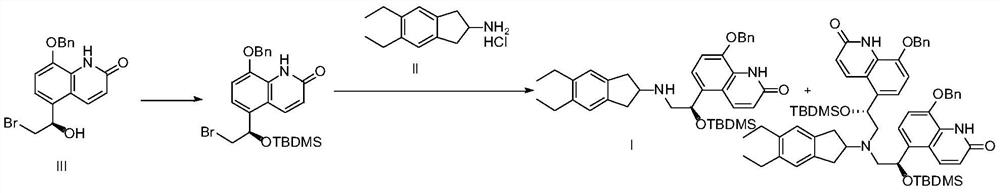
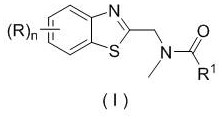
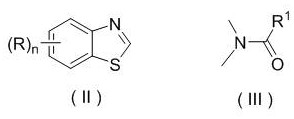
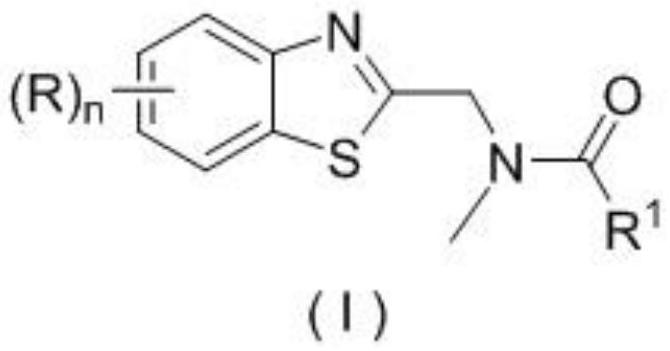
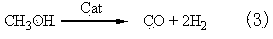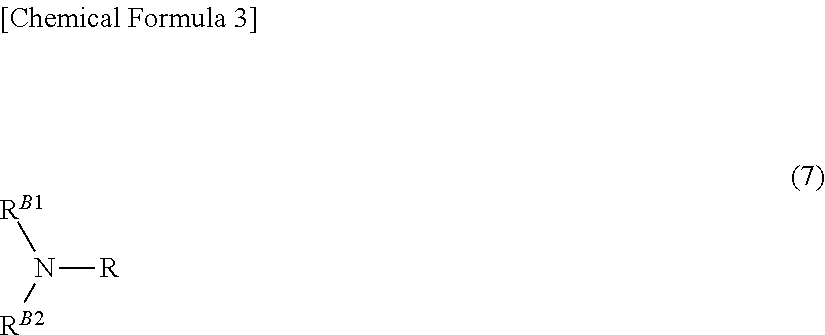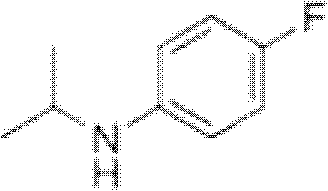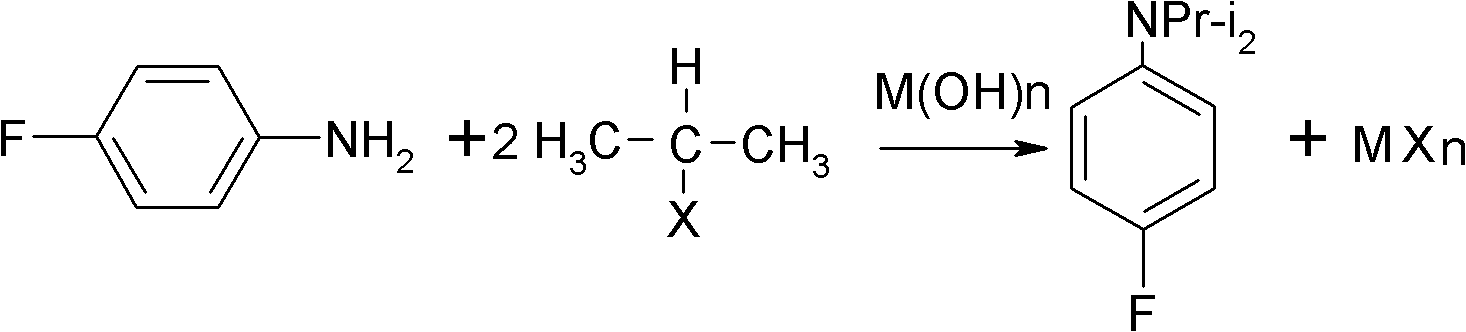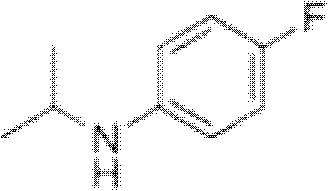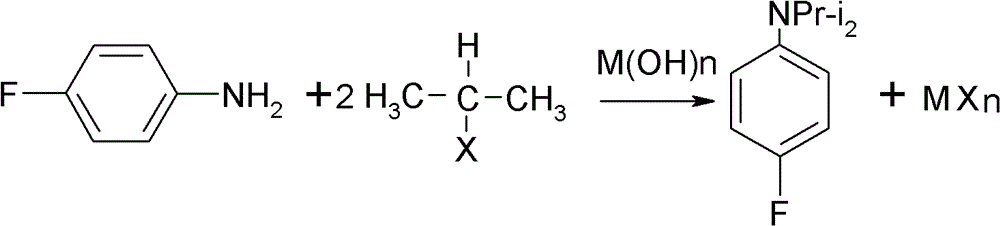Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Amine alkylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amine alkylation (amino-de-halogenation) is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution (of the halide), and the reaction product is a higher substituted amine. The method is widely used in the laboratory, but less so industrially, where alcohols are often preferred alkylating agents.
Method for producing ethylbenzene and styrene through side chain alkylation of toluene and methanol
ActiveCN103664485AImprove the utilization rate of alkylationDecomposition facilitates controlHydrocarbon by hydrocarbon and non-hydrocarbon condensationAlkyl transferAmine alkylation
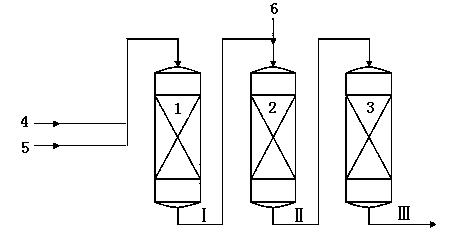
The invention mainly relates to a method for producing ethylbenzene and styrene through side chain alkylation of toluene and methanol, which is mainly used for solving the problems that methanol is easily decomposed and consequently the utilization rate of methanol is low in the prior art. The method comprises the steps that a) toluene and the first part of methanol enter a first reaction area and contact with a catalyst to generate a material flow I; b) the material flow I and the second part of methanol enter at least one second reaction area and contact with a catalyst to generate a material flow II; c) the material flow II enters at least one third reaction area and contacts with a catalyst to generate a material flow III containing ethylbenzene and styrene. By adopting the technical scheme, the problems are better solved, and the method can be applied to the industrial production of preparing ethylbenzene and styrene through side chain alkylation of toluene and methanol.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing ethylbenzene and styrene through toluene and methanol side chain alkylation
ActiveCN104926580AIncrease the degree of exchangeImprove stabilityMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationAmine alkylationRubidium
The invention relates to a method for preparing ethylbenzene and styrene through toluene and methanol side chain alkylation, and is used for mainly solving the problems that the toluene conversion rate is low, the ethylbenzene and styrene selectivity is low and a catalyst is unstable in the prior art. The problems are solved by adopting the technical scheme that under conditions of toluene and methanol side chain alkylation, raw materials and a catalyst make contact to generate ethylbenzene and styrene; and the catalyst comprises the following components by the weight percentage: a) 0.1-99% of an alkali metal ion exchanged X molecular sieve, b) 0.1-99% of an alkali metal ion exchanged ZSM-5 molecular sieve, and c) 0-5% of a rare earth metal, wherein alkali metals are at least two selected from potassium, rubidium or cesium. The method can be used for industrial production of preparation of ethylbenzene and styrene through toluene and methanol side chain alkylation reaction.
Owner:CHINA PETROLEUM & CHEM CORP +1
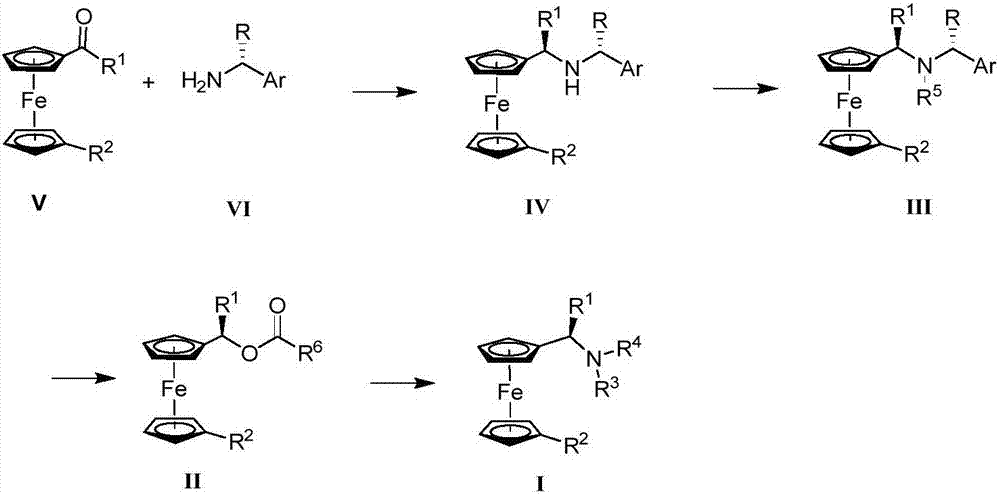
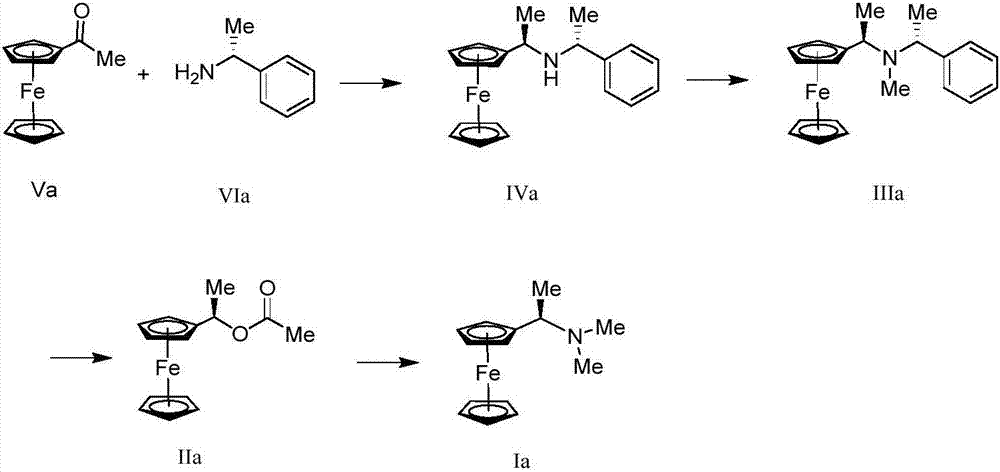
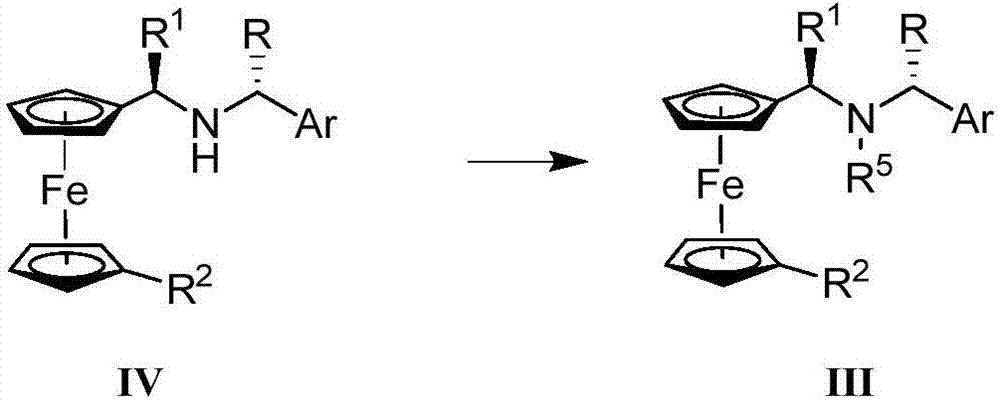
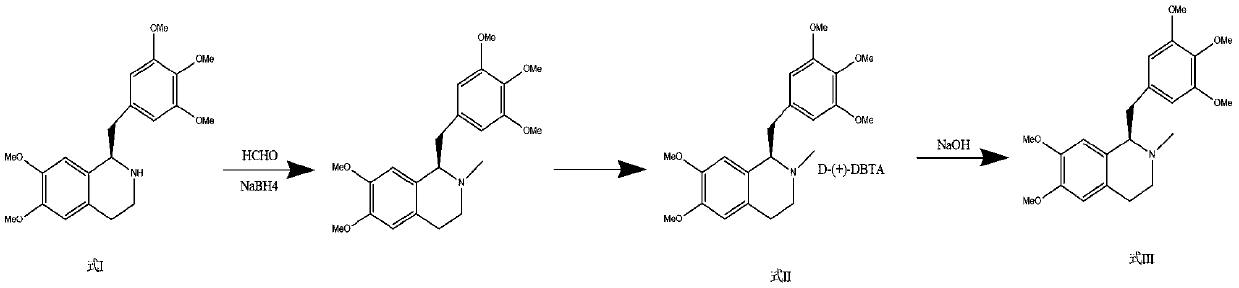
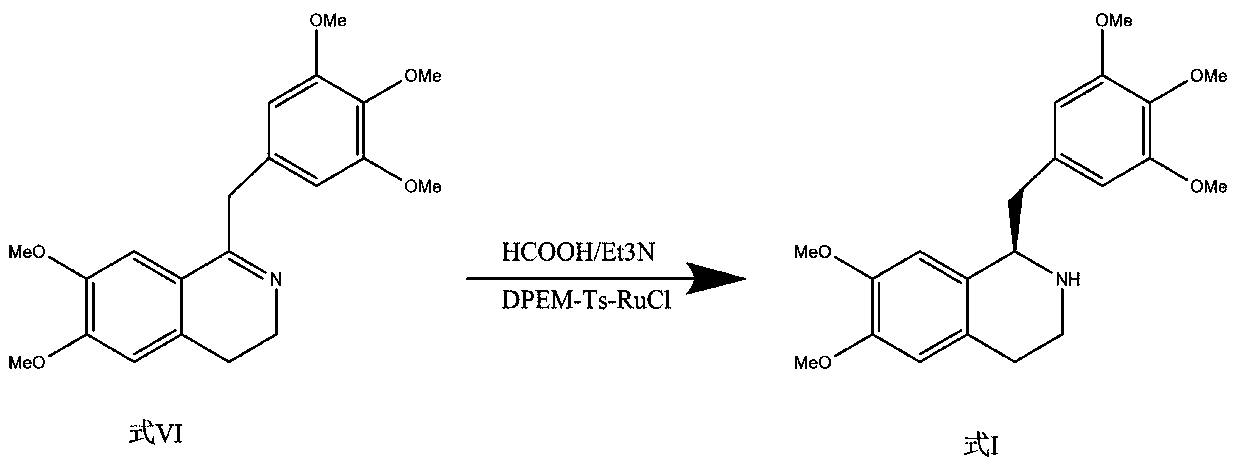
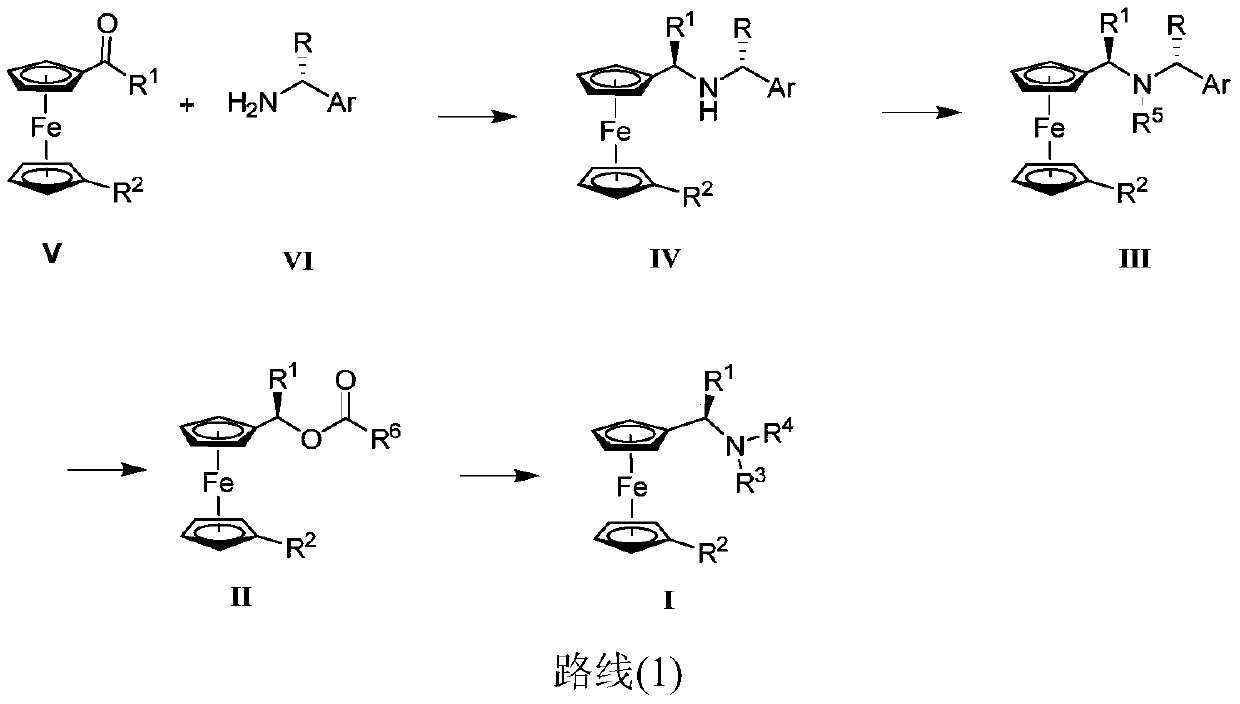
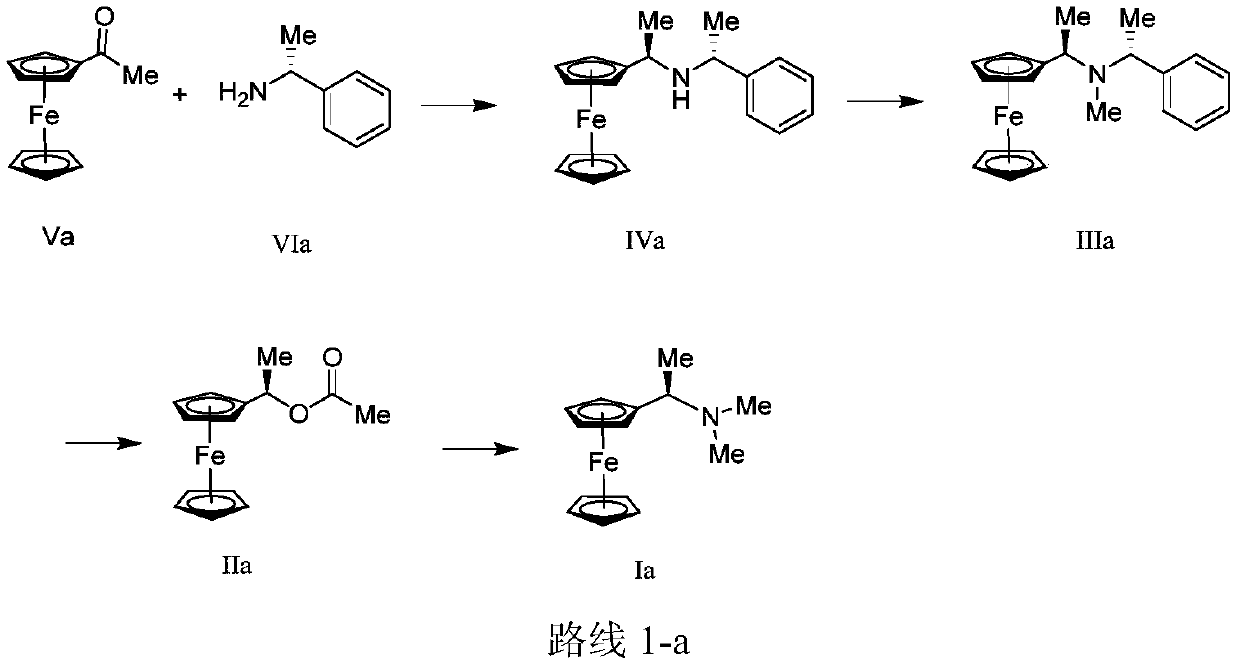
Synthesis method and application of chiral Ugi's amine as well as derivative and optical isomer thereof
The invention discloses a synthesis method of Ugi's amine as shown in formula (I) as well as a derivative and an optical isomer thereof. Chiral amine as shown in formula (VI) and a ferrocene compound as shown in formula (V) are used as starting raw materials, and then are subjected to reductive amination, reductive amination or alkylation of amido, substitution reaction and amine substitution reaction, thus obtaining the chiral Ugi's amine as shown in formula (I) as well as the derivative and the optical isomer thereof, or then are subjected to reductive amination, hydrogenation debenzylation, reductive amination or amino-alkylation reaction, thus obtaining the chiral Ugi's amine as shown in formula (I) as well as the derivative and the optical isomer thereof. The chiral Ugi's amine as shown in formula (I) as well as the derivative and the optical isomer thereof can be used for synthesizing a Josiphos type ferrocene diphosphine ligand which is used as a chiral ligand for various metal complex catalysts and is an important chiral catalyst ligand for preparing a medicine intermediate and an agricultural chemical; and the ligand is widely applied to asymmetric reaction of metal catalysis, and is applicable to industrial large-scale production.
Owner:SHANGHAI MAOSHENG KAIHUI TECH CO LTD
Method for alkylation of amines
ActiveUS20160009632A1Easy to prepareImprove stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAmine alkylationAlcohol
The present invention provides a simple, efficient, and industrially advantageous method for the alkylation of amines. The present invention relates to a production method for N-alkylamines whereby an amine is reacted with an alcohol in the presence of a ruthenium complex represented by general formula (1): RuXY(CO)(L) (wherein X and Y can be the same or different and represent a monovalent anionic ligand, and L represents a tridentate aminodiphosphine ligand).
Owner:TAKASAGO INTERNATIONAL CORPORATION
N,N,N',N'-dodecyl-tetrasubstituted diphenyl ether sulfonate anionic gemini surfactant and synthesis method thereof
ActiveCN109851530AEasy to separateEasy to purifySurface-active detergent compositionsTransportation and packagingDiphenyl etherAmine alkylation
The invention discloses an N,N,N',N'-dodecyl-tetrasubstituted diphenyl ether sulfonate anionic gemini surfactant and a synthesis method thereof. The structural formula of the anionic gemini surfactantis shown in the description. The anionic gemini surfactant is prepared by a two-step reaction. The synthesis method comprises the following steps: S1, carrying out amine alkylation on 4,4'-diaminodiphenyl ether and bromododecane to obtain N,N,N',N'-dodecyl-tetrasubstituted diphenyl ether; and S2, carrying out a sulfuration reaction on N,N,N',N'-dodecyl-tetrasubstituted diphenyl ether and concentrated sulfuric acid to obtain the target product N,N,N',N'-dodecyl-tetrasubstituted diphenyl ether sulfonate. The surfactant of the invention has a high surface activity, and the synthesis method has the advantages of simplicity, mild reaction conditions, and easiness in separation and purification. The surfactant of the invention is expected to be applied to alkali / surfactant combination flooding,polymer / surfactant binary combination flooding, alkali / surfactant / polymer ternary combination flooding, micro-emulsion emulsifiers in tertiary oil recovery, and also can be compounded with common surfactants to reduce the use cost and create conditions for large-scale application.
Owner:PETROCHINA CO LTD
Method for synthesizing 1,2-dissubstituted olefin compounds by alkyne alkylation
ActiveCN110563535AReduce consumptionUniversalCarboxylic acid nitrile preparationOrganic compound preparationAmine alkylationSolvent
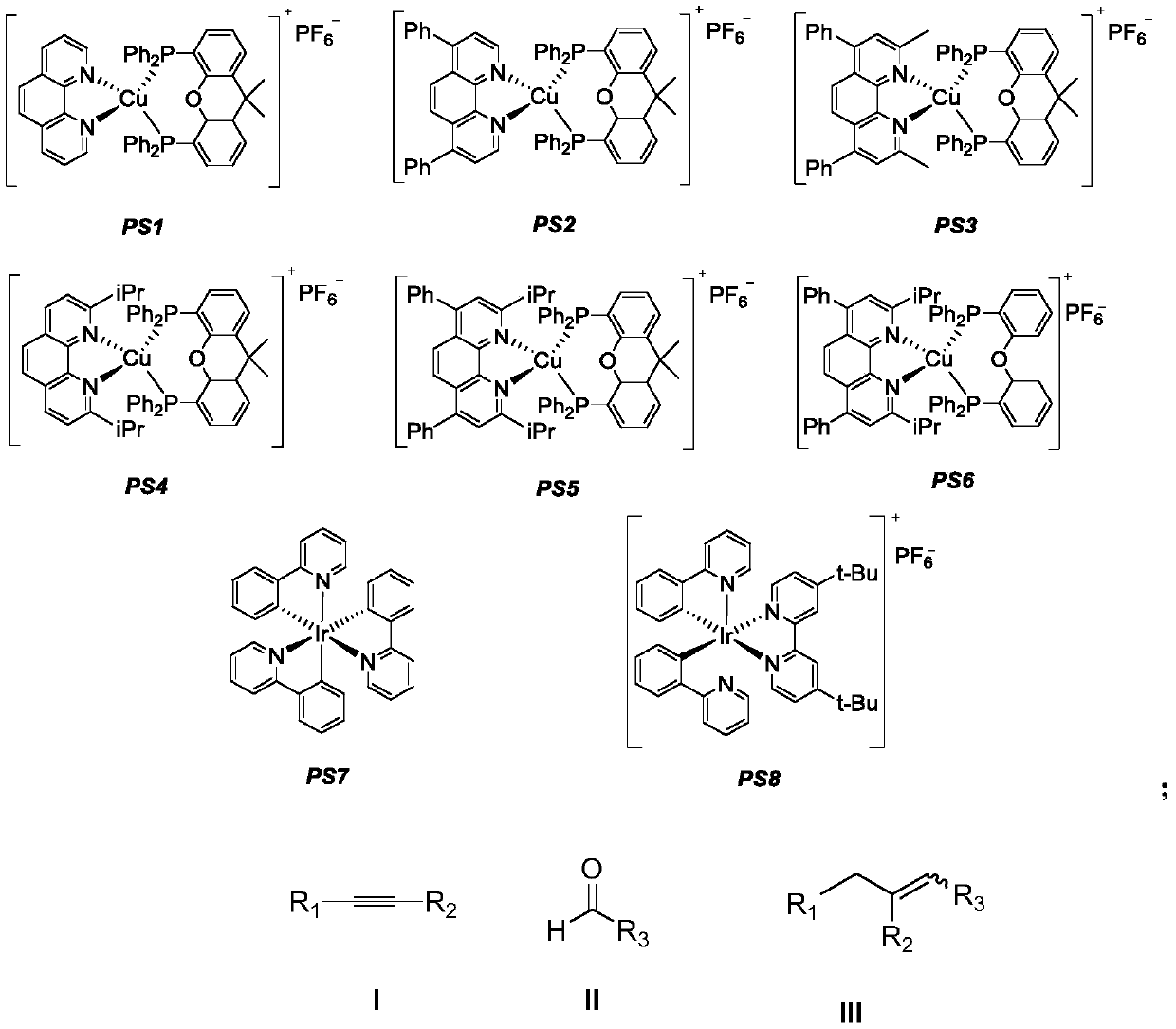
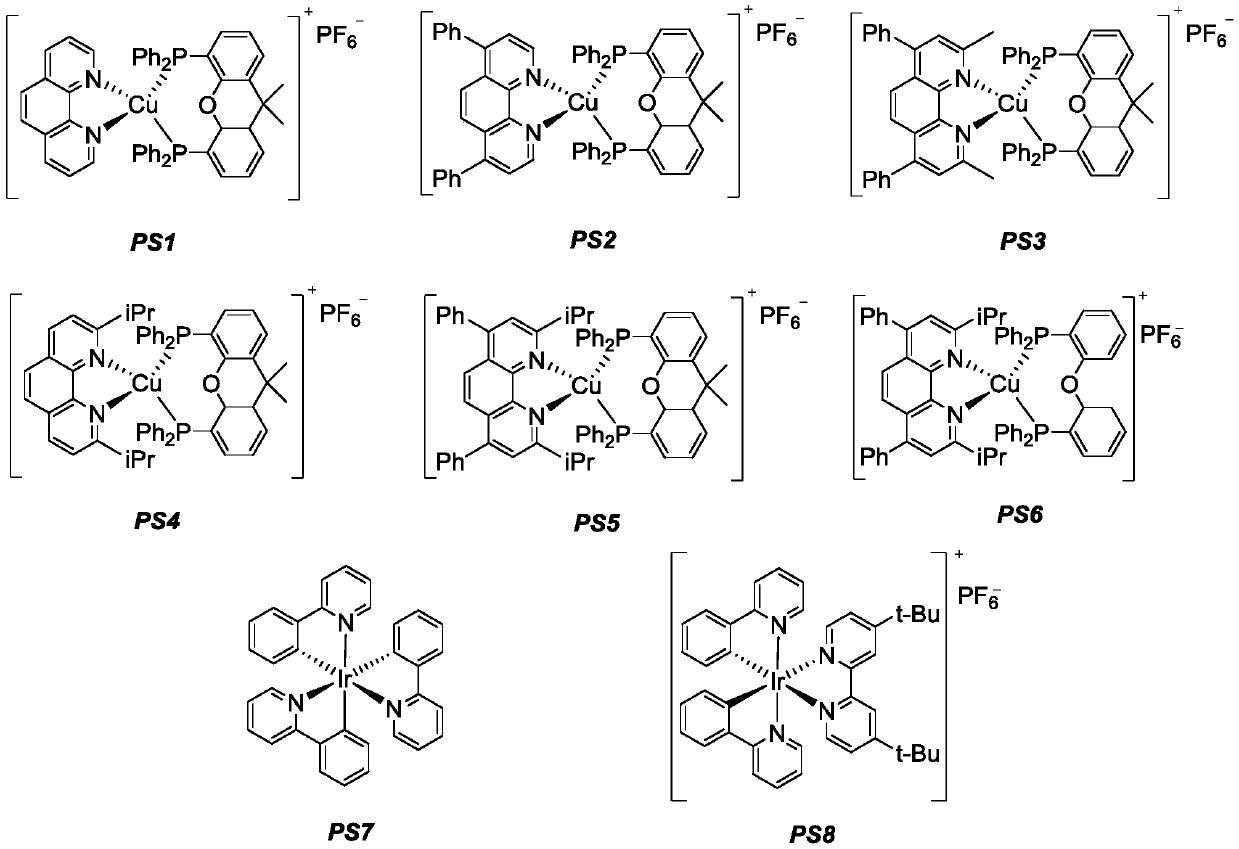
The invention discloses a method for synthesizing 1,2-dissubstituted olefin compounds by high-selectivity alkyne alkylation. The method comprises the following specific steps: a photosensitizer, an alkyne compound represented as the formula I and hantzsch ester are added to a Schlenk reaction tube, aldehyde represented as the formula II and secondary amine are dissolved in an organic solvent or amixed solvent of 1,4-dioxane and water under protective gas atmosphere, a mixed solution is obtained, the mixed solution is added to the Schlenk reaction tube under the condition of protective gas andis stirred for a reaction at 25 DEG C for 6-14 h, preferably 12 h under the irradiation of a light source, and the 1,2-dissubstituted olefin compounds represented as the formula III are obtained after the obtained reaction solution is subjected to aftertreatment. Polysubstituted alkenes which are difficult to prepare with the conventional method can be synthesized with the method, and the methodhas the advantages of being high in reaction stereoselectivity, relatively mild in reaction condition, high in yield, high in substrate universality and simple and convenient to operate, a catalyst ischeap and available and has lower toxicity, and energy consumption is reduced.
Owner:ZHEJIANG UNIV OF TECH
Method for alkylating amine and amino acid
InactiveCN101851143AAvoid it happening againOrganic compound preparationCarboxylic acid amides preparationHalohydrocarbonAmine alkylation
Owner:XIAMEN UNIV
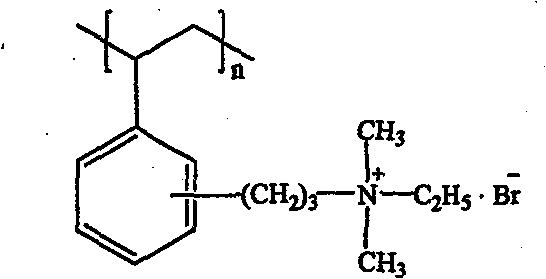
Method for preparing strong alkali anion exchange resin with long spacer arm
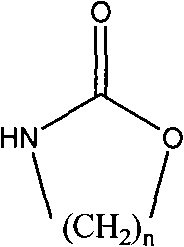
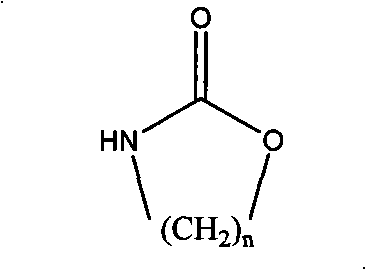
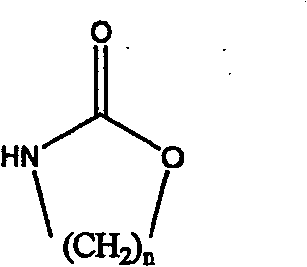
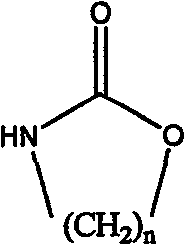
The invention relates to a method for preparing a strong anion exchange resin with long spacer arms, belonging to the field of the synthesis of ion exchange resins. The method enables macropore or hydrogel polystyrene resin swollen by inert solvent such as nitrobenzene to have amine-alkylation reaction by taking cyclic carbamate as alkylation reagent and Lewis acid or ionic liquid as catalyzer, thus producing polystyrene resin with long spacer arms primary amines; the polystyrene resin with long spacer-arm primary amines reacts with formic acid and formaldehyde to get tert-aminated polystyrene resin which then reacts with ethyl bromide to get strong anion exchange resin with long spacer arms. Directly introducing primary amine groups into white beads through amine-alkylation, the method disclosed by the invention simplifies process, saves cost and is simple in process; therefore, the method is of wide application prospect.
Owner:CHANGZHOU UNIV
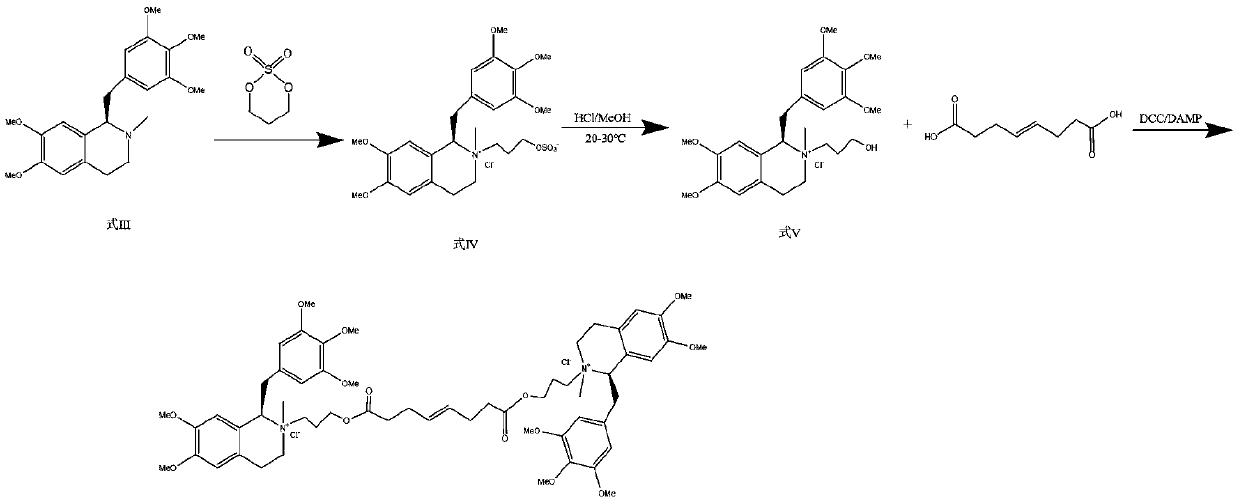
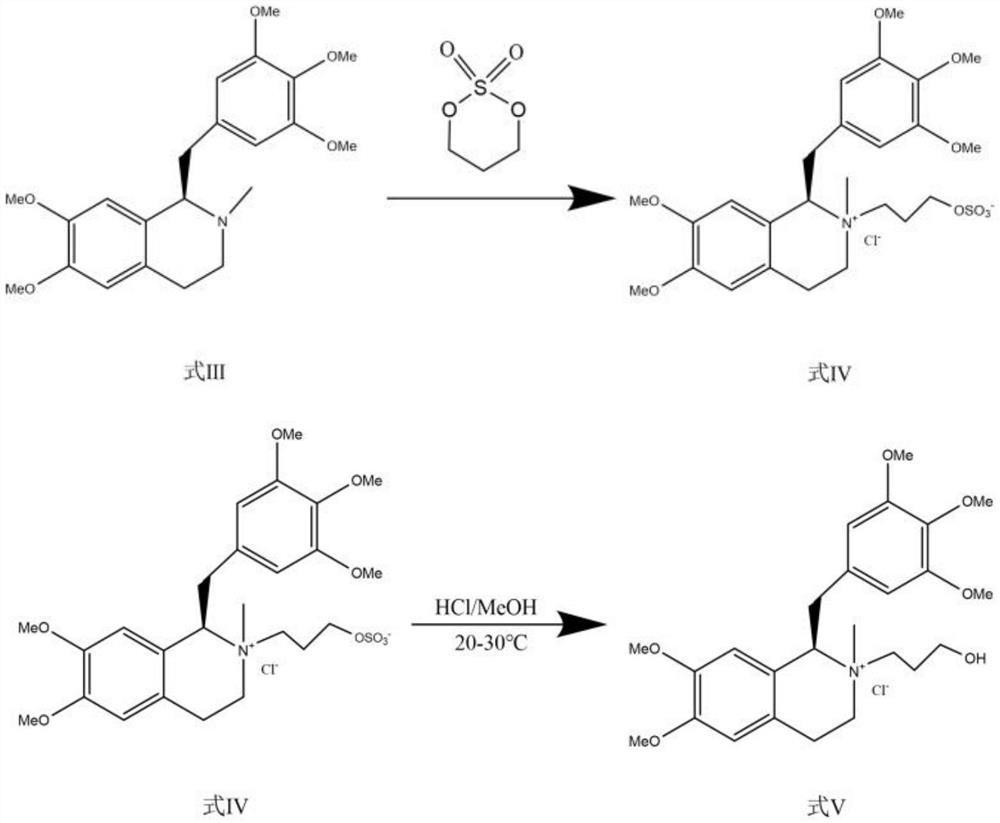
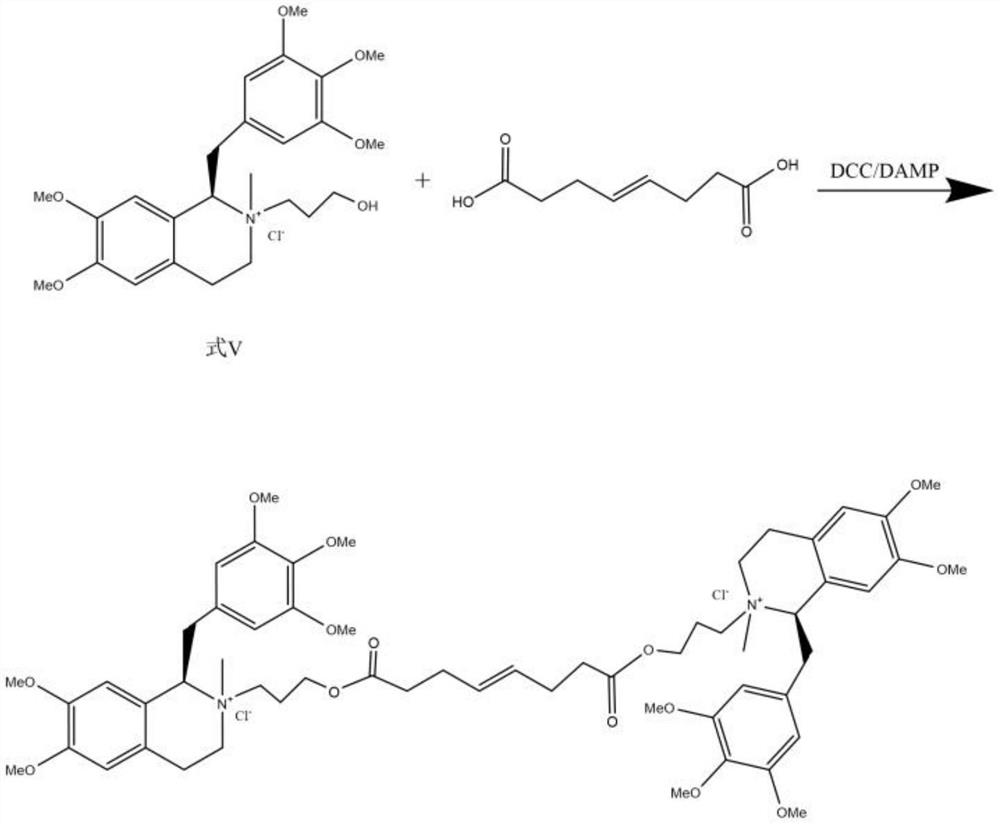
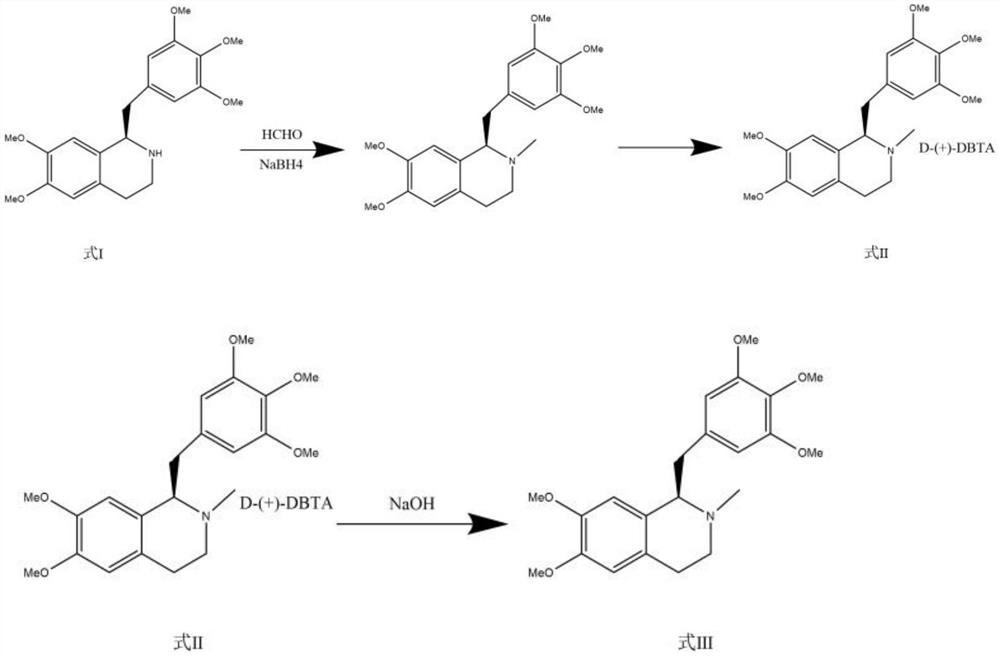
Preparation method of mivacurium chloride and injection thereof
ActiveCN111471013AImprove stabilityHas a pH buffering effectOrganic active ingredientsOrganic chemistryTrans esterificationAmine alkylation
The invention belongs to the technical field of biological medicine, and particularly relates to mivacurium chloride and a preparation method of an injection thereof. The mivacurium chloride is prepared from R-5'-methoxy laudanosine and trans-octenedioic acid as raw materials through amine alkylation, ester exchange and other reactions, and finally high-purity mivacurium chloride is obtained. Themivacurium chloride is less in impurity generation, the product is easy to separate and purify, meanwhile, formation and discharge of a large amount of (S)-5-methoxy laudanosine are avoided, and the injection prepared from the raw materials is less in impurity and stabler.
Owner:朗天药业(湖北)有限公司
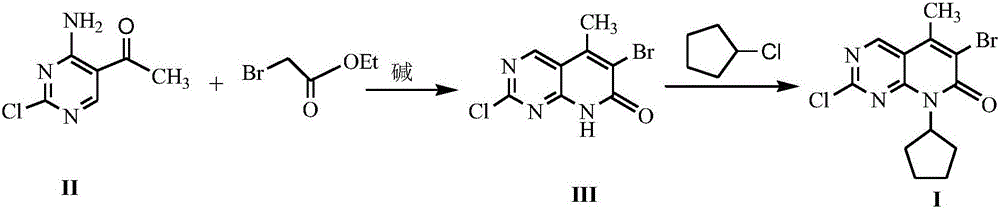
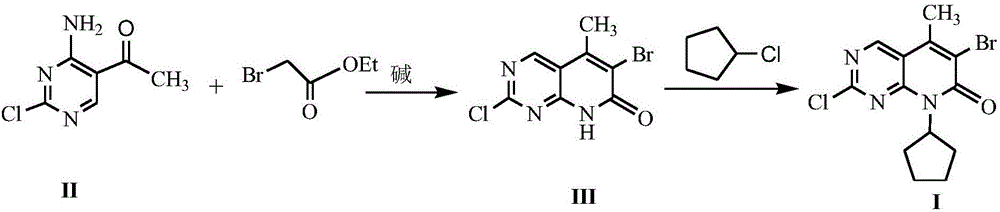
Method for synthesizing palbociclib intermediate
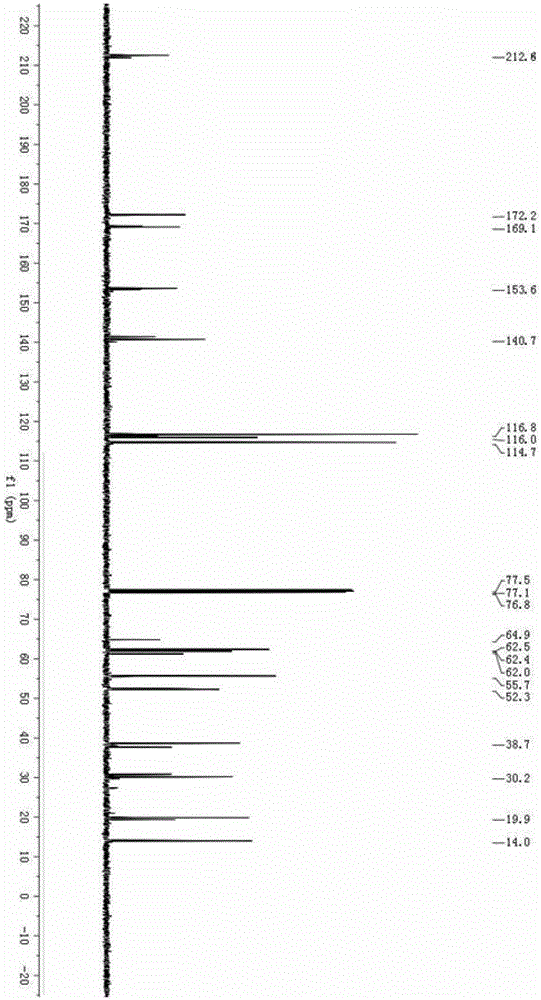
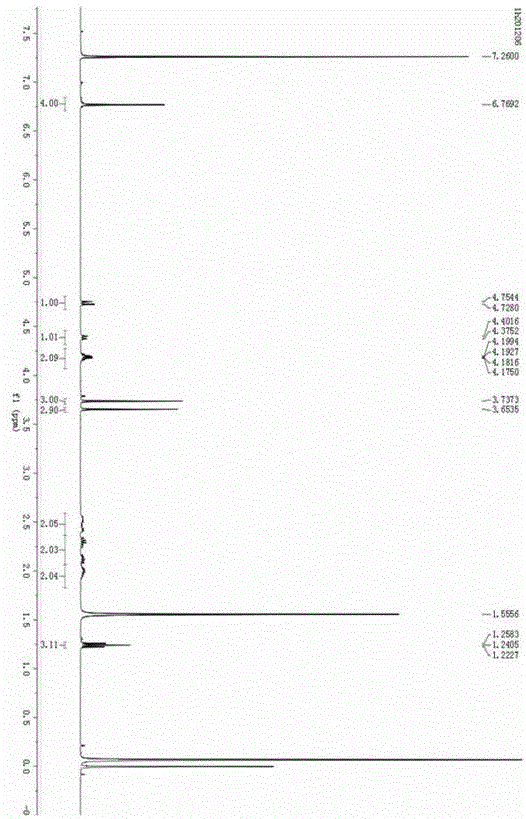
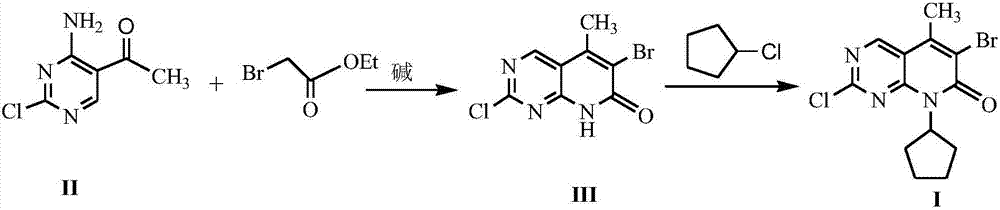
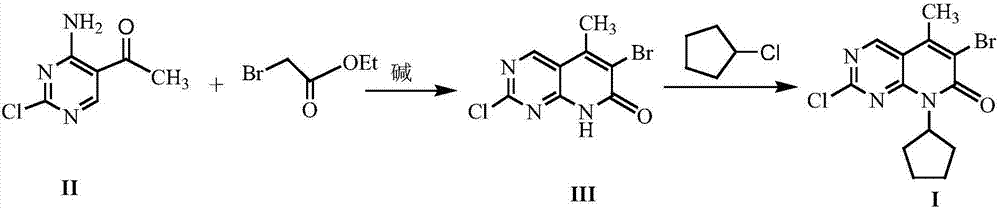
The invention discloses a method for synthesizing a palbociclib intermediate 2-chloro-6-bromo-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one. The method comprises the following steps of 1) preparing 2-chloro-6-bromo-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one by means of performing cyclization on 4-amino-2-chloro-5-pyrimidine ethanone and ethyl bromoacetate under the effect of alkali; 2) preparing a target product by means of performing amine alkylation reaction on the 2-chloro-6-bromo-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one and chlorinated cyclopentane. The method for synthesizing an important palbociclib intermediate 2-chloro-6-bromo-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one provided by the invention has the advantages that the steps are few, the reaction condition is mild, the operation is easy and convenient, the synthesis efficiency is high, impurities are few, the method is suitable for industrialized production, and a new approach is provided for the preparation of palbociclib and the palbociclib intermediate. The formula is shown in the description.
Owner:HUAIHAI INST OF TECH
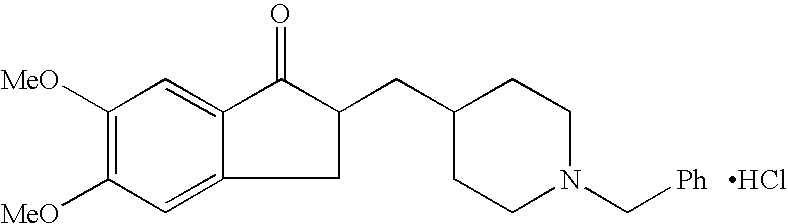
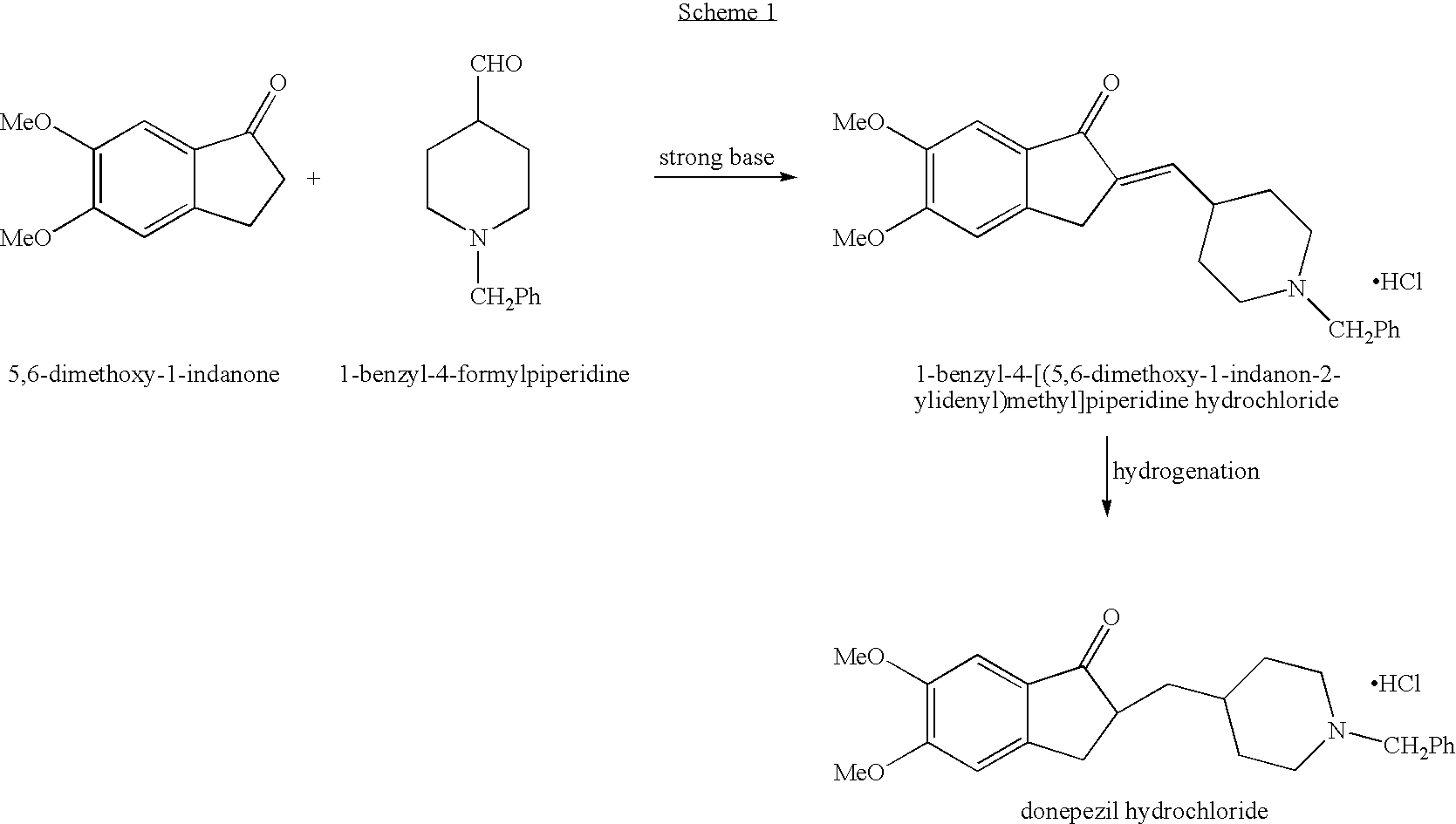
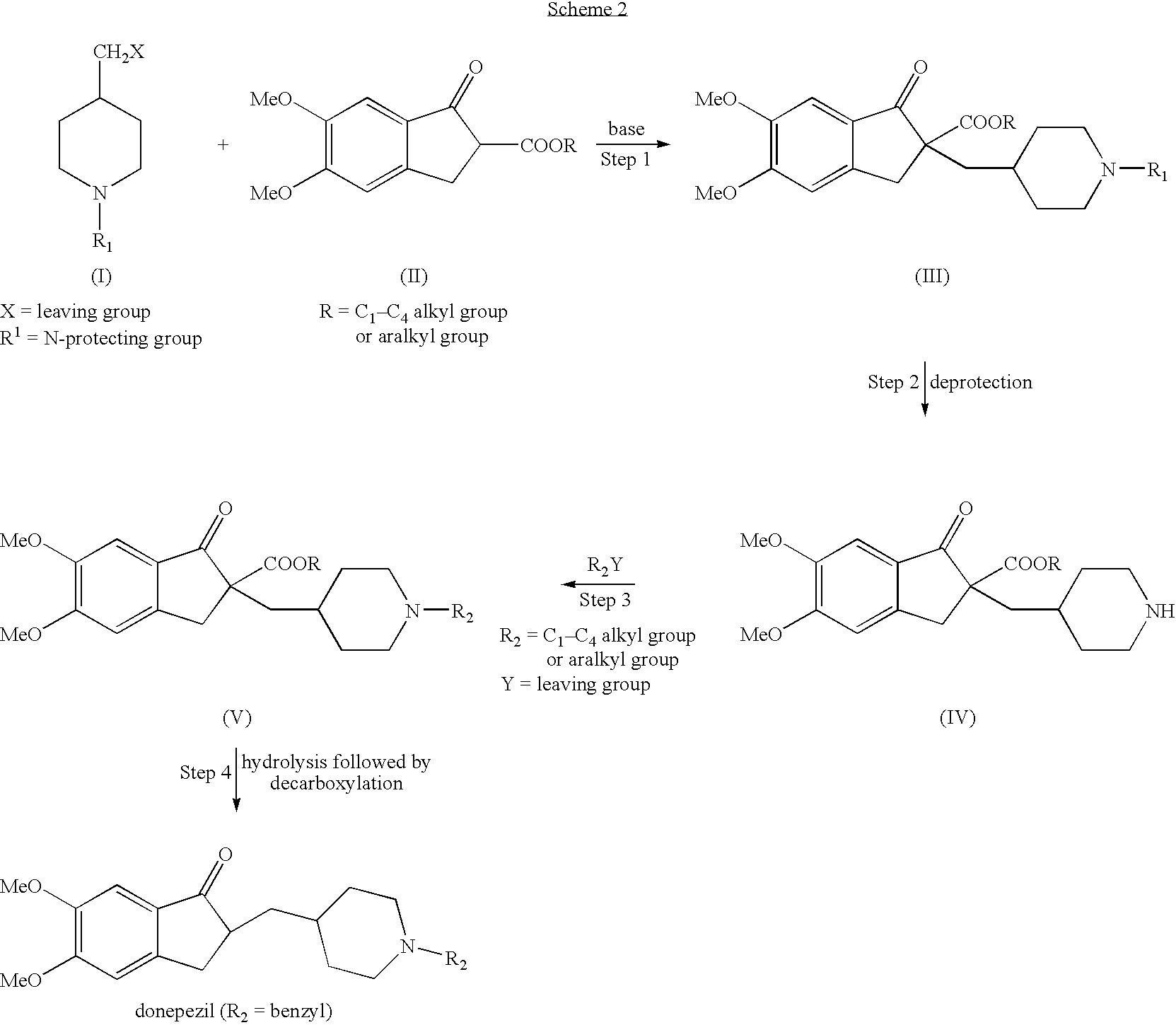
Process for alkylating secondary amines and the use in donepezil preparation thereof
The present invention relates to an improved process of alkylating secondary amines, more particularly of alkylating compounds having amino piperidinic group, which are useful as donepezil intermediates, wherein an alcohol serves as reaction facilitator thus enabling to obtain donepezil and salts thereof in high quality and yield. The present invention also relates to the prevention of unwanted alkylation of donepezil precursors, having amino piperidinic group, by using suitable reaction conditions.
Owner:CHEMAGIS
N,n,n',n'-tetradodecyl-substituted diphenyl ether sulfonate anionic gemini surfactant and synthesis method thereof
ActiveUS20200208045A1Improve surface activityEasy to purifySurface-active detergent compositionsTransportation and packagingDiaminodiphenyl etherAmine alkylation
The present invention discloses a N,N,N′,N′-tetradodecyl-substituted diphenyl ether sulfonate anionic Gemini surfactant and the synthesis method thereof. It has a structural formula of:and is prepared in a two-step reaction comprising: S1. subjecting 4,4′-diaminodiphenyl ether and bromododecane to an amine alkylation reaction to obtain N,N,N′,N′-tetradodecyl-substituted diphenyl ether; and S2. sulfonating the N,N,N′,N′-tetradodecyl-substituted diphenyl ether with concentrated sulfuric acid to produce the target product, N,N,N′,N′-tetradodecyl-substituted diphenyl ether sulfonate. The surfactant of the present invention has a high surface activity and can be synthesized with a simple procedure under mild reaction conditions, and can be easily separated and purified. The surfactant of the present invention is promising in applications for alkaline / surfactant in tertiary oil recovery, for polymer / surfactant binary compound flooding, alkaline / surfactant / polymer tertiary compound flooding, microemulsion emulsifier, and the like, and may also be compounded with common surfactants to lower the cost, thereby enabling its application in a large scale.
Owner:PETROCHINA CO LTD
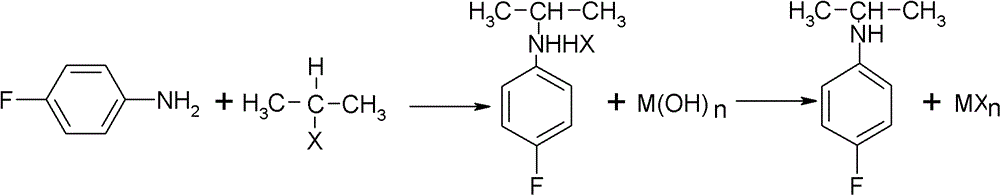
Preparation method of oriented single alkylation of 4-fluorine-N-isopropyl aniline
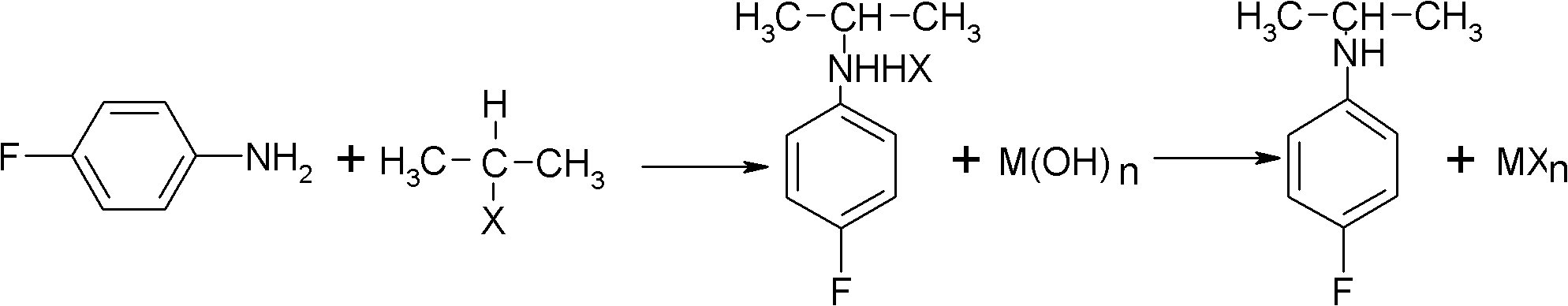
ActiveCN102993027AImprove conversion rateHigh reaction yieldOrganic compound preparationAmino compound preparationAlkyl transferAmine alkylation
The invention discloses a preparation method of 4-fluorine-N-isopropyl aniline by oriented single substituted N-alkylation reaction, which takes 4-fluoroaniline as a substrate, halogenated iso-propane as an alkylation reagent under the effects of a phase-transfer catalyst, a cocatalyst and an acid binding agent. The method has the advantages of mild reaction condition, simple operation, high raw material conversion rate and products selectivity, convenient products separation and the like. The prepared 4-fluorine-N-isopropyl aniline is an important pesticide intermediate, and can synthesize an oxyacetamide herbicide flufenacet and the like.
Owner:SINOCHEM LANTIAN +1
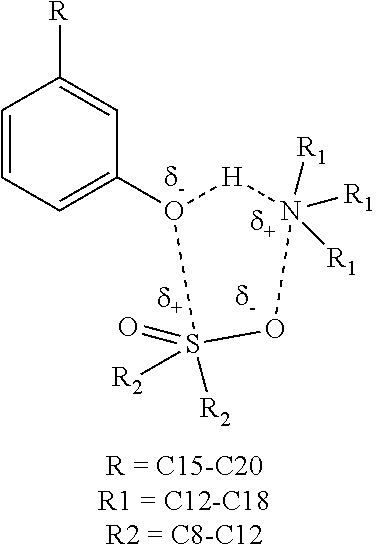
Lubricant composition
Disclosed herein is a process for lubricating an internal combustion engine with a lubricant composition that may be substantially ash free. The ashless lubricant composition described herein may comprise primary antioxidants, such as aminic diphenyl amines, alkylated phenyl-naphthyl amines, and phenolic antioxidants. The ashless lubricant composition described herein may also comprise sulfur containing products that may work as secondary antioxidants. Other components such as ashless antiwear components, dispersants, pour point depressants, friction modifiers, and metal deactivators may also be used to formulate an ashless lubricant composition according to an embodiment.
Owner:BASF AG
A synthetic method for α-position alkylation of secondary amines catalyzed by visible light
ActiveCN104230734BSynthetic reaction conditions are mildEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAir atmosphereAmine alkylation
The invention discloses a synthesis method for α-position alkylation of a secondary amine catalyzed by visible light, comprising the following steps: 1) adding an inorganic metal salt and N-aryl glycine ester into an organic solvent to obtain a solution A; 2 ) adding β-keto ester to solution A to obtain solution B; 3) irradiating solution B with visible light in an air atmosphere to obtain an alkylated product of N-aryl glycinate. In the invention, the compound formed by in-situ coordination of cheap inorganic metal salt and secondary amine is used not only as a photosensitizer, but also as a catalyst to realize the α-position alkylation reaction of the secondary amine. The system of the present invention uses LED blue light or green light as a visible light source, does not need to add additional photosensitizers, does not need to add additional electron acceptors, uses oxygen in the air as the terminal oxidant, and has mild synthesis reaction conditions, simple operation, and atom economy. .
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
A kind of method of synthesizing palbociclib intermediate
The invention discloses a method for synthesizing a palbociclib intermediate 2-chloro-6-bromo-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one. The method comprises the following steps of 1) preparing 2-chloro-6-bromo-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one by means of performing cyclization on 4-amino-2-chloro-5-pyrimidine ethanone and ethyl bromoacetate under the effect of alkali; 2) preparing a target product by means of performing amine alkylation reaction on the 2-chloro-6-bromo-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one and chlorinated cyclopentane. The method for synthesizing an important palbociclib intermediate 2-chloro-6-bromo-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one provided by the invention has the advantages that the steps are few, the reaction condition is mild, the operation is easy and convenient, the synthesis efficiency is high, impurities are few, the method is suitable for industrialized production, and a new approach is provided for the preparation of palbociclib and the palbociclib intermediate. The formula is shown in the description.
Owner:HUAIHAI INST OF TECH
Preparation method and application of chiral ugi's amine and its derivatives and optical isomers
The invention discloses a synthesis method of Ugi's amine as shown in formula (I) as well as a derivative and an optical isomer thereof. Chiral amine as shown in formula (VI) and a ferrocene compound as shown in formula (V) are used as starting raw materials, and then are subjected to reductive amination, reductive amination or alkylation of amido, substitution reaction and amine substitution reaction, thus obtaining the chiral Ugi's amine as shown in formula (I) as well as the derivative and the optical isomer thereof, or then are subjected to reductive amination, hydrogenation debenzylation, reductive amination or amino-alkylation reaction, thus obtaining the chiral Ugi's amine as shown in formula (I) as well as the derivative and the optical isomer thereof. The chiral Ugi's amine as shown in formula (I) as well as the derivative and the optical isomer thereof can be used for synthesizing a Josiphos type ferrocene diphosphine ligand which is used as a chiral ligand for various metal complex catalysts and is an important chiral catalyst ligand for preparing a medicine intermediate and an agricultural chemical; and the ligand is widely applied to asymmetric reaction of metal catalysis, and is applicable to industrial large-scale production.
Owner:SHANGHAI MAOSHENG KAIHUI TECH CO LTD
A kind of preparation method of mivacurium chloride and injection thereof
ActiveCN111471013BImprove stabilityLittle fluctuation in contentOrganic active ingredientsOrganic chemistryTrans esterificationAmine alkylation
The invention belongs to the technical field of biomedicine, and specifically relates to a preparation method of mivacurium chloride and an injection thereof, wherein the mivacurium chloride uses R-5'-methoxylabdansin and fumaric acid as raw materials , through amine alkylation, transesterification and other reactions, finally get high-purity mivacurium chloride. The impurity of mivacurium chloride of the present invention is less, the product is easy to separate and purify, avoids the formation and discharge of a large amount of (S)-5-methoxylaudansu simultaneously, the injection liquid impurity that this raw material is made is smaller ,more stable.
Owner:朗天药业(湖北)有限公司
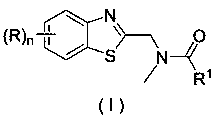
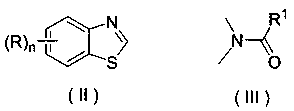
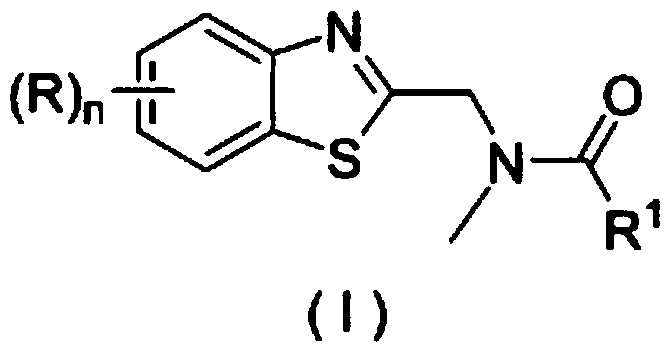
Application of a substituted benzothiazole C2 amide alkylation derivative as a fungicide
ActiveCN110432282AMild reaction conditionsGet rid of base catalysisBiocideFungicidesAlkyl transferFungicide
The invention discloses application of a substituted benzothiazole C2 amide alkylation derivative as a fungicide. The structure of the derivative is shown as a formula (I). In the formula (I), a substitute R1 is hydrogen or C1-C5 alkyl; H on a benzothiazole ring is monosubstituted, polysubstituted or unsubstituted by a substituent R, with the C2 site of the benzothiazole ring is not substituted bythe R; n is an integer of 0 to 4, and represents the number of the substituents R on the benzothiazole ring; when the n is 0, indicating that H on the benzothiazole ring is not substituted; when then is 1, indicating that the H on the benzothiazole ring is monosubstituted by the substituent R; when the n is 2 to 4, indicating that H on the benzothiazole ring is polysubstituted by the substituents R, with the substituents R at different substation positions being same or different; the substituent R is hydrogen, C1-C5 alkyl, C1-C2 alkoxyaryl, or halogen. The derivative has good inhibitory effects on fungi causing wheat scab, corn southern leaf blight, cucumber anthracnose and rice sheath blight disease.
Owner:ZHEJIANG UNIV OF TECH
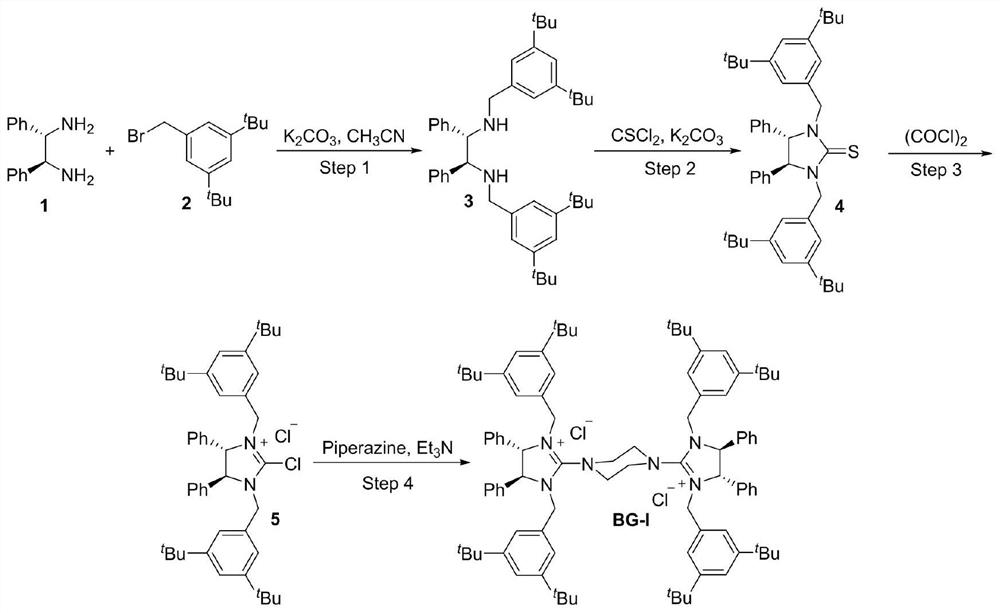
Kilogram-level amplification-scale production chiral biguanide catalyst synthesis method
PendingCN113185461AHigh purityAvoid repeated contactOrganic compound preparationOrganic chemistry methodsBenzoyl bromideAmine alkylation
The invention discloses a kilogram-level amplification-scale production chiral biguanide catalyst synthesis method, which comprises: S1, carrying out an amine alkylation reaction by using chiral ethylenediamine and benzyl bromide as raw materials to obtain chiral bis-secondary amine; s2, adding a solvent and thiophosgene into the same kettle, carrying out a thiocarbonylation reaction to obtain chiral thiourea, and carrying out pulping purification treatment on the chiral thiourea; s3, adding oxalyl chloride into the same kettle, and performing reflux reaction in a solvent to obtain chiral 2-chloroimidazoline onium salt; and s4, evaporating out the solvent in the step S3 from the original kettle, then adding piperazine into the same kettle, and carrying out amination coupling reaction to obtain a chiral biguanide salt ion pair catalyst BG-I; and according to the synthesis method, one-kettle multi-step same-kettle feeding is adopted, the production stability and safety are improved, the synthesis route is shortened, meanwhile, the traditional tedious pulping purification process is simplified, the synthesis efficiency is improved, the synthesis cost is reduced, and the method is stable in process and suitable for industrial large-scale production.
Owner:CHINA-SINGAPORE INT JOINT RES INST
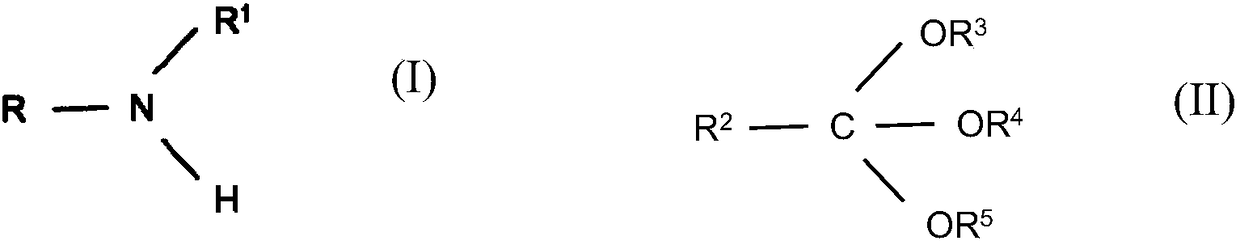
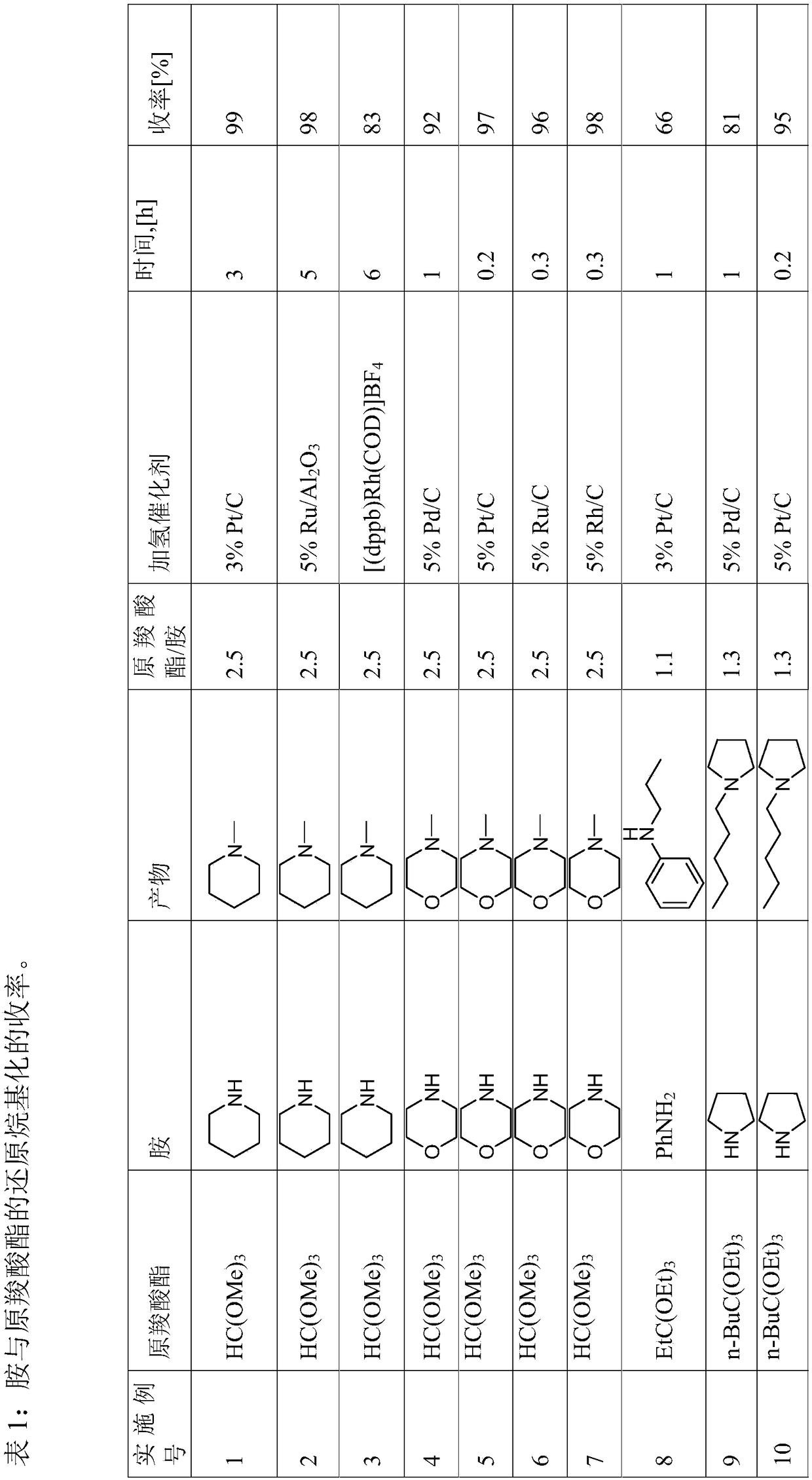
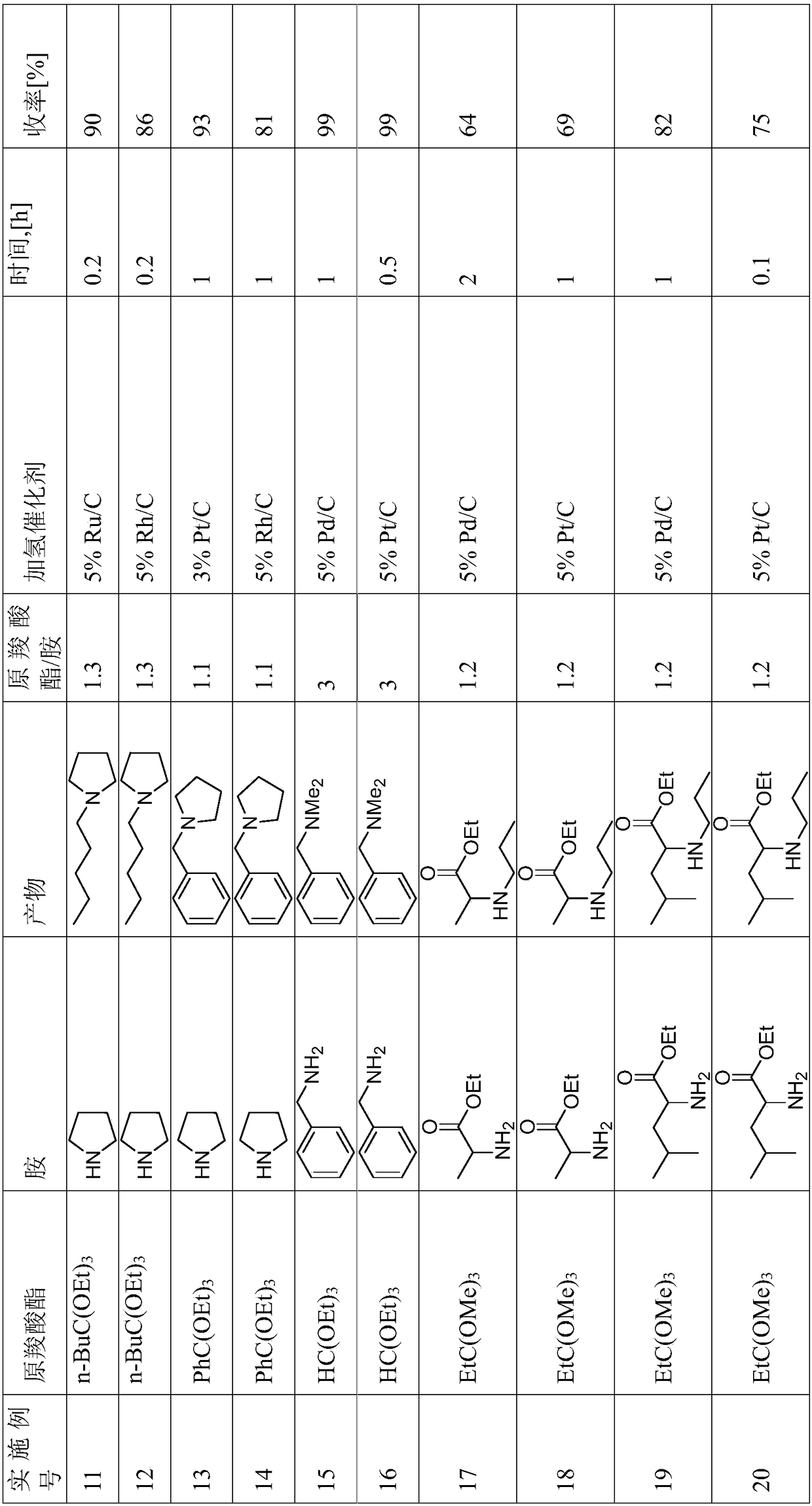
Reductive alkylation of amines with orthocarboxylic acid esters
ActiveCN108602753AOrganic compound preparationAmino-carboxyl compound preparationAlkyl transferHydrogen
The present invention relates to a process for the N-alkylation of amines by reacting an amine with an orthocarboxylic acid ester and with hydrogen in the presence of a hydrogenation catalyst.
Owner:EVONIK OPERATIONS GMBH
Preparation method of ambroxol hydrochloride impurity
InactiveCN112961113ATo meet preparation needsEasy to storeGroup 4/14 element organic compoundsBulk chemical productionAmine alkylationCarbonyl reduction
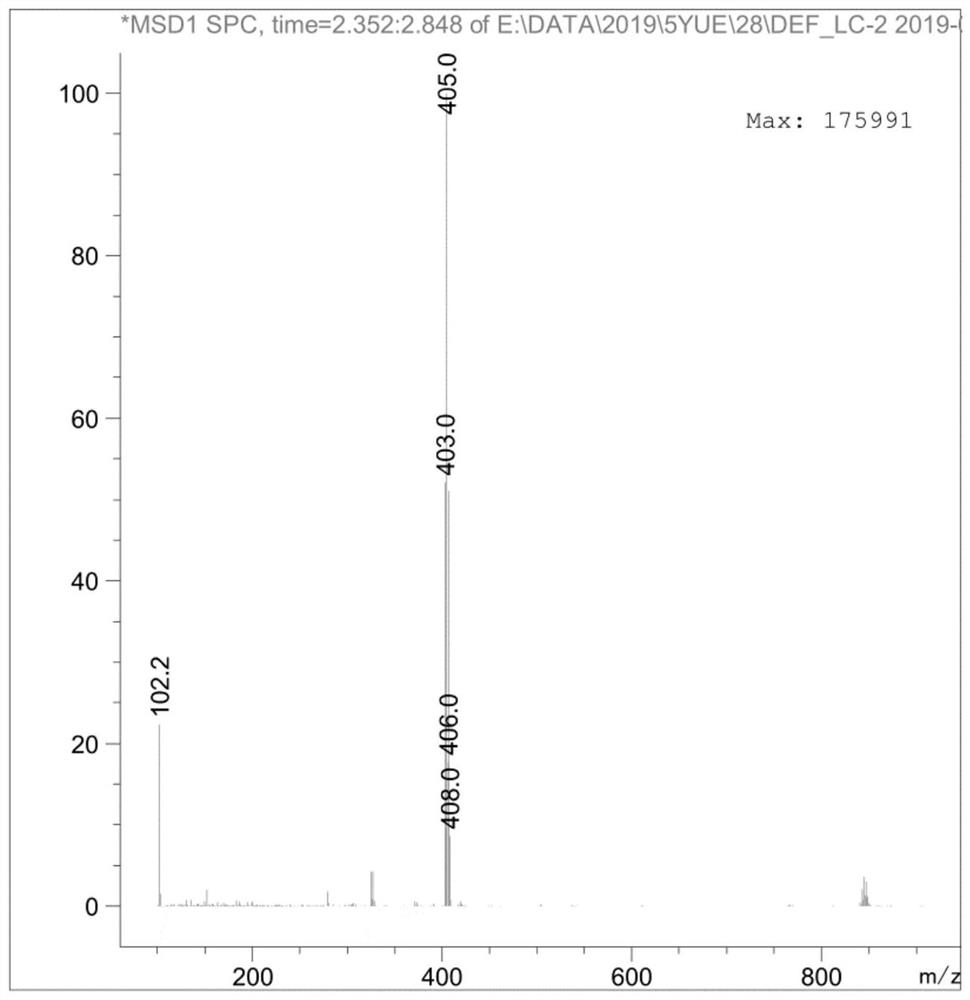
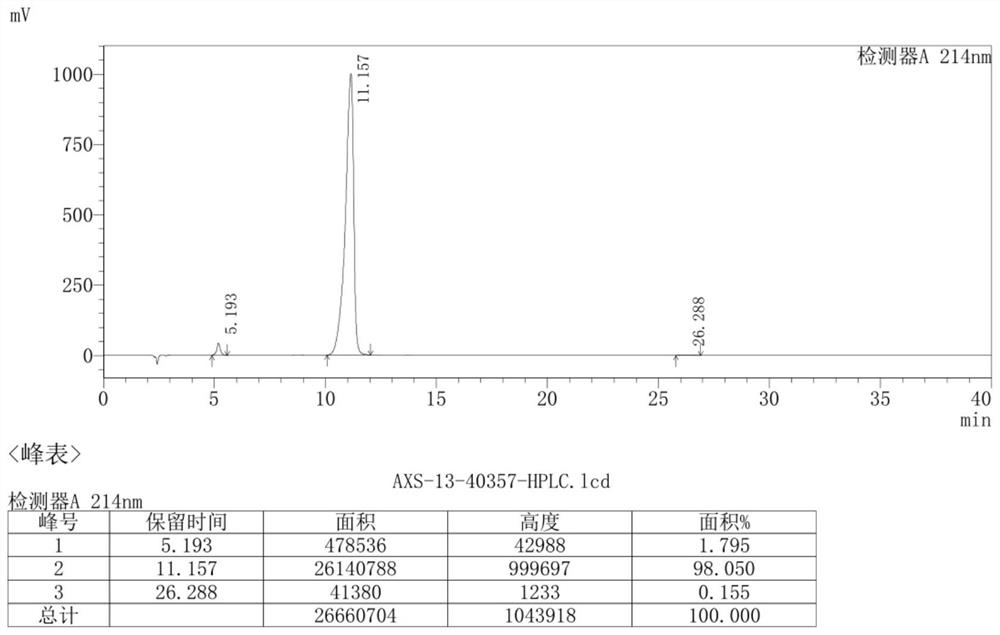
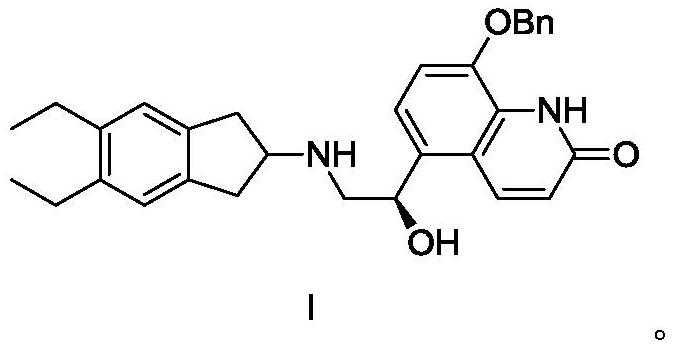
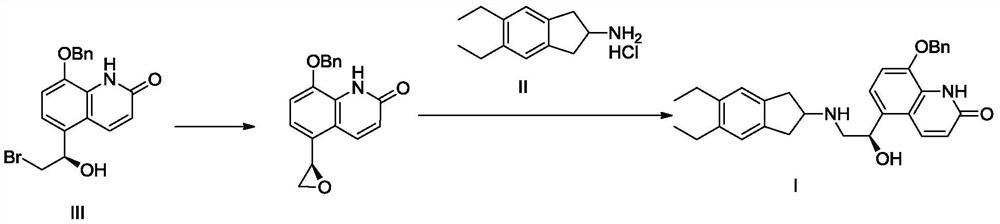
The invention discloses a preparation method of an ambroxol hydrochloride impurity reference substance. With ambroxol hydrochloride as a raw material, the ambroxol impurity AXS-13 is obtained through hydroxyl protection, amine alkylation, cyclization, carbonyl reduction, deprotection and other reactions. A basis is provided for research of ambroxol hydrochloride related substances. The method is simple, raw materials are easy to obtain, the product is easy to purify, the yield and the purity are high, and the product can be used as a reference substance in impurity detection.
Owner:北京新康哌森医药科技有限公司
A kind of synthetic method and synthetic intermediate of indacaterol and its salt derivatives
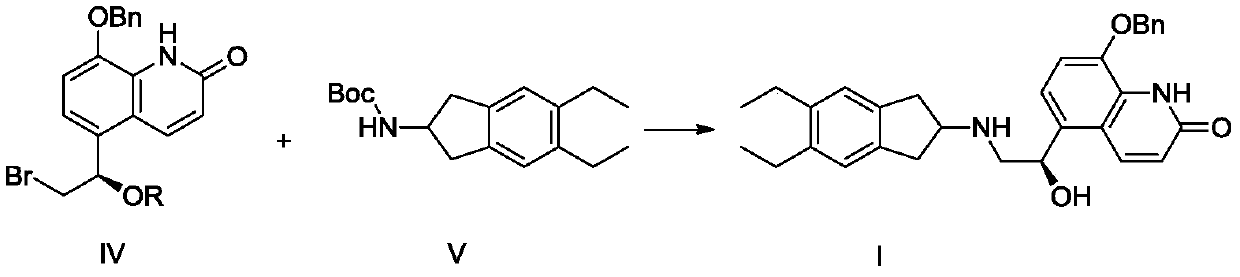
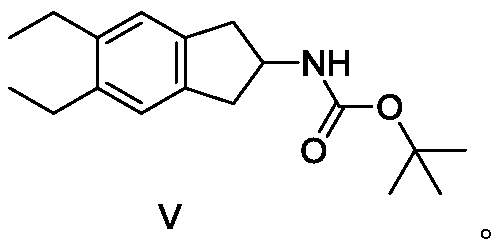
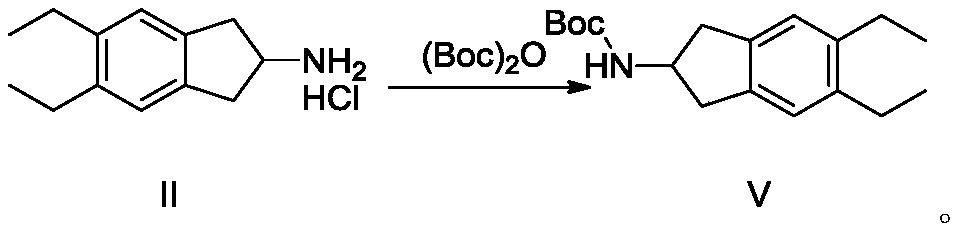
ActiveCN110128339BAvoid polyalkylationAvoid it happening againCarbamic acid derivatives preparationOrganic compound preparationAmine alkylationChemical compound
The invention belongs to the technical field of medicine synthesis, and in particular relates to a synthesis method of indacaterol and its salt derivatives and an intermediate for synthesis. The synthesis method of indacaterol, which uses the compound of general formula IV and compound of formula V as raw materials to obtain the compound of formula I through amine alkylation and deprotection one-pot reaction, the reaction equation is as follows: wherein, R is a silyl protecting group . The technical solution provided by the present invention cleverly avoids polyalkylation on the amino group through Boc protection of the amino group. Under the condition that both the hydroxyl group and the amino group are properly protected, the alkylation reaction effectively avoids the generation of by-products, and After the alkylation reaction, the deprotection of hydroxyl group and amino group can be carried out directly, and the multi-step reaction can be completed in one reactor, which saves the process of intermediate purification and separation, and also improves the reaction yield to the greatest extent and reduces the production cost.
Owner:SUZHOU ZHIYU BIOTECH CO LTD
Application of a substituted benzothiazole C2 amide alkylated derivative as a fungicide
ActiveCN110432282BMild reaction conditionsHigh antibacterial activityBiocideFungicidesFungicideAmine alkylation
Owner:ZHEJIANG UNIV OF TECH
Synthesis method of indacaterol and salt derivatives thereof and intermediate for the synthesis
ActiveCN110128339AAvoid polyalkylationAvoid it happening againCarbamic acid derivatives preparationOrganic compound preparationIndacaterolAlkyl transfer
The invention belongs to the technical field of drug synthesis, and particularly relates to a synthesis method of indacaterol and salt derivatives thereof and intermediate for the synthesis. The synthesis method of indacaterol comprises the following steps: carrying out amine alkylation and deprotection reaction, in a one-pot manner, on a compound shown as a general formula IV and a compound shownas a formula V serving as raw materials to obtain a compound shown as a formula I, wherein the reaction equation is shown as the specification; wherein R is a silane protecting group. According to the technical scheme provided by the invention, by carrying out Boc protection on the amino group, polyalkylation on the amino group is ingeniously avoided, so that under the condition that both the hydroxyl group and the amino group have proper protection, the alkylation reaction effectively avoids the generation of by-products; after the alkylation reaction, hydroxyl and amino deprotection can bedirectly carried out, so that the multi-step reaction is completed in one reactor; according to the method, the intermediate purification and separation process is omitted, the reaction yield is increased to the maximum extent, and the production cost is reduced.
Owner:SUZHOU ZHIYU BIOTECH CO LTD
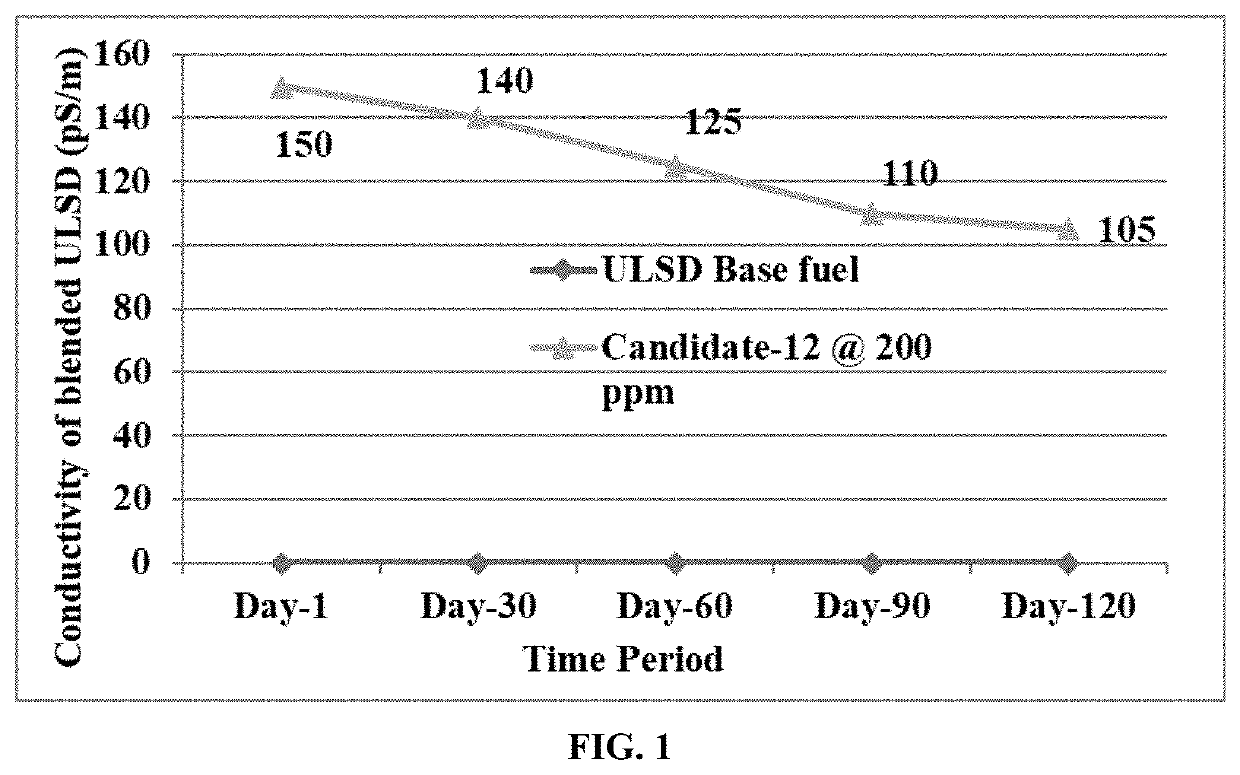
Lubricity and conductivity improver additive for ultra low sulfur diesel fuels
ActiveUS20210189270A1Improve the lubrication effectIncrease conductivity propertiesLiquid carbonaceous fuelsFuel additivesAmine alkylationCarboxylic acid
The present invention discloses a single package additive for improving lubricity and conductivity properties of ultra-low sulfur diesel fuels. The single package additive is a reaction product of a fatty acid composition, a glycerol tricarboxylates, a polysulfone, a polyamine, an alkylated benzene sulfonic acid and a phenol derivative. More specifically, the present invention discloses a reaction product of: a fatty acid composition in the range of 60-95% wt / wt; a glycerol tricarboxylate in the range of 0.1-10.0% wt / wt; a polysulfone in the range of 0.1-5.0% wt / wt; a polyamine in the range of 0.1-5.0% wt / wt; an alkylated benzene sulfonic acid in the range of 0.1-5.0% wt / wt; and a phenol derivative in the range of 0.1-10.0% wt / wt. The present invention also discloses a single-pot process for the preparation of said reaction product.
Owner:INDIAN OIL CORPORATION
Aromatic ring containing amine compound and preparation method and application thereof
InactiveCN105254602AEnhanced inhibitory effectHigh activityOrganic compound preparationAmino-carboxyl compound preparationProstate cancer cellLymphatic Spread
The invention discloses an aromatic ring containing amine compound and salt acceptable pharmaceutically. The aromatic ring containing amine compound has very good tumorigenesis and tumor metastasis inhibiting activity, especially has obvious inhibiting effect on prostate cancer cells and breast cancer cells, is an excellent antitumor lead compound, can be further developed into an antitumor drug, is used for treating tumor metastasis, advanced tumors, non-entity tumors and other cancers and has potential and wide application prospect the field of tumor treatment. The aromatic ring containing amine compound is prepared by adopting a halogenated part and a primary amine part to perform amine alkylation reaction in a DMSO solvent, is simple and is suitable for industrial production.
Owner:KUNMING UNIV
Preparation method of oriented single alkylation of 4-fluorine-N-isopropyl aniline
ActiveCN102993027BHigh selectivityEasy to separateOrganic compound preparationAmino compound preparationAlkyl transferAmine alkylation
The invention discloses a preparation method of 4-fluorine-N-isopropyl aniline by oriented single substituted N-alkylation reaction, which takes 4-fluoroaniline as a substrate, halogenated iso-propane as an alkylation reagent under the effects of a phase-transfer catalyst, a cocatalyst and an acid binding agent. The method has the advantages of mild reaction condition, simple operation, high raw material conversion rate and products selectivity, convenient products separation and the like. The prepared 4-fluorine-N-isopropyl aniline is an important pesticide intermediate, and can synthesize an oxyacetamide herbicide flufenacet and the like.
Owner:SINOCHEM LANTIAN +1
Catalyst applied to preparation of isopropyl benzene by performing liquid-phase alkylation on propylene
ActiveCN102294257BImproved monocumene selectivityImprove technical effectMolecular sieve catalystsCatalyst activation/preparationAlkyl transferMolecular sieve
The invention relates to a catalyst applied to preparation of isopropyl benzene by performing liquid-phase alkylation on propylene, which is mainly used for solving the problem of low mono-isopropyl benzene selectivity in a liquid-phase alkylation reaction of benzene and the propylene in the prior art. The catalyst comprises the following components in parts by weight: 50-80 parts of at least onemolecular sieve selected from a Y molecular sieve, a beta molecular sieve or an MCM-22 molecular sieve and 20-50 parts of inorganic oxide, wherein the catalyst is modified with mixed liquor of benzene and isopropyl benzene at the treatment temperature 110-160 DEG C under the treatment pressure 2.0-2.8 MPa for 2-200 hours; the total liquid phase weight space velocity is 2-20 h<-1>; and the weight ratio of the benzene to the isopropyl benzene in the mixed liquor of the benzene and the isopropyl benzene is 15-80. By adopting the technical scheme, the problem is well solved; and the catalyst can be applied to industrial production of isopropyl benzene by performing liquid-phase alkylation on propylene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing strong alkali anion exchange resin with long spacer arm
Owner:CHANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com