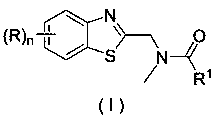
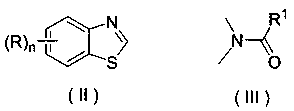
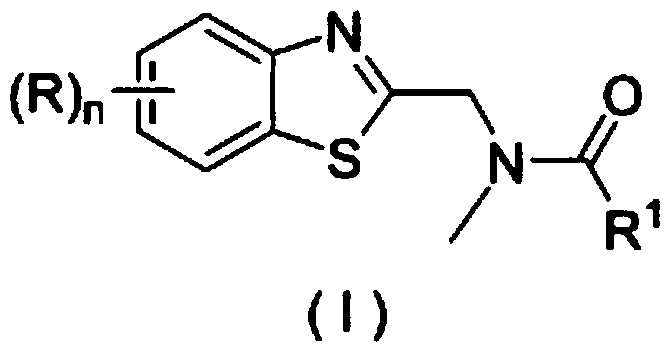
Application of a substituted benzothiazole C2 amide alkylation derivative as a fungicide
A technology of benzothiazole and amide alkyl, which is applied in the application field of substituted benzothiazole C2 amide alkylated derivatives as fungicides, can solve problems such as no literature reports in biological activity research, and achieve good inhibitory activity, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Derivative Ia ((R)n=H, R 1 =H) Synthesis
[0027] Weigh benzothiazole (0.5mmol, 67mg), K 2 S 2 o 8 (1mmol, 0.27g) and Eosin Y (0.0025mmol, 1.6mg) were placed in a 25mL Schlenk reaction tube, then N,N-dimethylformamide (87.5mmol, 6.4g) was added, and placed in a 15W LED white light Reaction under irradiation, stirring reaction at room temperature, tracking and monitoring the reaction process with TLC, after 20h, the reaction was completed, the reaction solution was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (eluent was sherwood oil / ethyl acetate with a volume ratio of 1:1 ester) to obtain a yellow oil, the derivative Ia. Yield 68%.
[0028] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0029] 1 H NMR (CDCl 3,500MHz)δ8.19(s,1H),7.99-8.03(t,J=7.5Hz,1H),7.85-7.90(m,1H),7.46-7.53(m,1H),7.37-7.44(m, 1H), 4.94(s, 2H), 3.07(s, 3H); 13 C NMR (CDCl 3 , 125MHz) δ166.2, ...
Embodiment 2
[0030] Example 2 Derivatives Ib ((R)n=5-chloro, R 1 =H) Synthesis
[0031] Weigh 5-chlorobenzothiazole (0.5mmol, 85mg), K 2 S 2 o 8 (1mmol, 0.27g) and Eosin Y (0.0035mmol, 2.3mg) were placed in a 25mL Schlenk reaction tube, then N,N-dimethylformamide (87.5mmol, 6.4g) was added, and placed in a 15W LED white light lamp Reaction under irradiation, stirring reaction at room temperature, tracking and monitoring the reaction process with TLC, after 20h, the reaction was completed, the reaction solution was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (eluent was sherwood oil / ethyl acetate with a volume ratio of 1:1 ester) to obtain a yellow oil, the derivative Ib. Yield 58%.
[0032] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0033] 1 H NMR (CDCl 3 ,500MHz)δ8.20(s,1H),8.01(dd,J=8.5,2.0Hz,1H),7.78-7.83(m,1H),7.38-7.44(m,1H),4.93(s,2H) ,3.10(s,3H); 13 C NMR (CDCl 3 , 125MHz) δ168.3...
Embodiment 3
[0034] Example 3 Derivatives Ic ((R)n=6-OMe, R 1 =H) Synthesis
[0035] Weigh 6-methoxybenzothiazole (0.5mmol, 83mg), K 2 S 2 o 8 (1mmol, 0.27g) and Eosin Y (0.0035mmol, 2.3mg) were placed in a 25mL Schlenk reaction tube, then N,N-dimethylformamide (87.5mmol, 6.4g) was added, and placed in a 15W LED white light lamp Reaction under irradiation, stirring reaction at room temperature, tracking and monitoring the reaction process with TLC, after 20h, the reaction was completed, the reaction solution was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (eluent was sherwood oil / ethyl acetate with a volume ratio of 1:1 ester) to obtain a yellow oil, the derivative Ic. Yield 71%.
[0036] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0037] 1 H NMR (CDCl 3 ,500MHz)δ8.16(s,1H),7.86(dd,J=9.0,6.5Hz,1H),7.30(dd,J=7.5,2.5Hz,1H),7.07(m,1H),4.87(s ,2H),3.85(s,3H),3.04(s,3H); 13 C NMR (CDCl 3 , 12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com