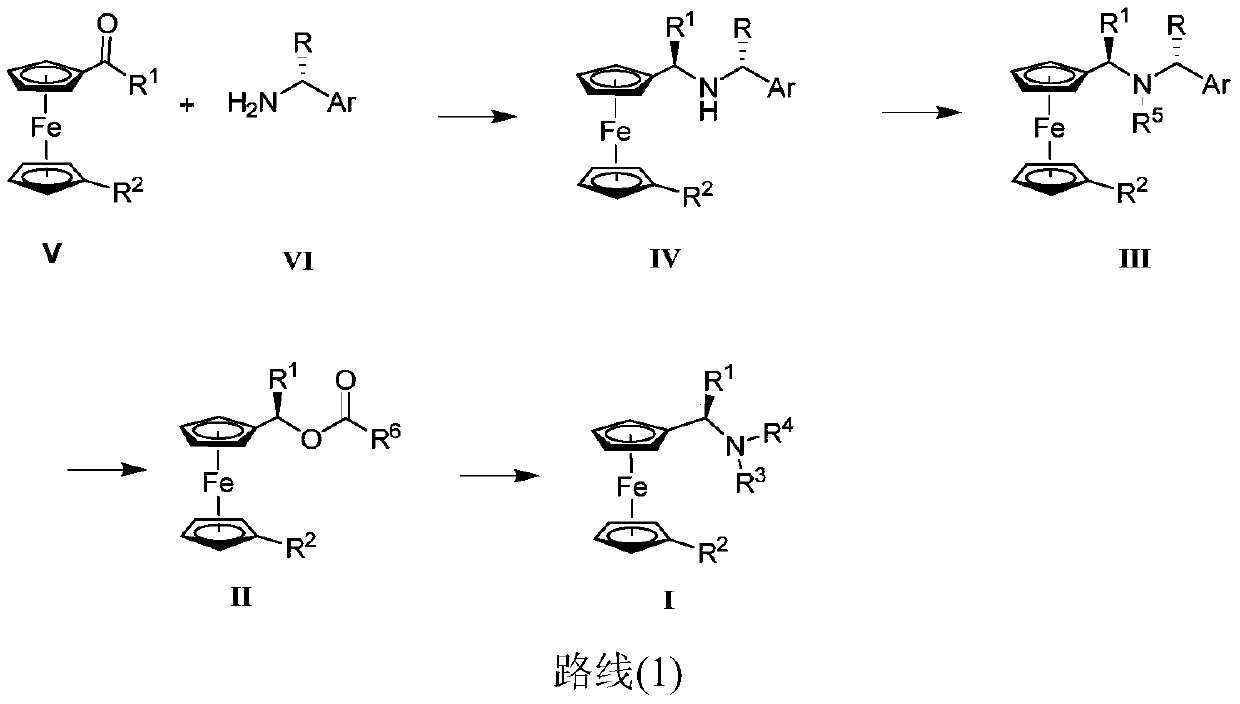
Preparation method and application of chiral ugi's amine and its derivatives and optical isomers
A technology of optical isomers and derivatives, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as lack of synthesis processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126]
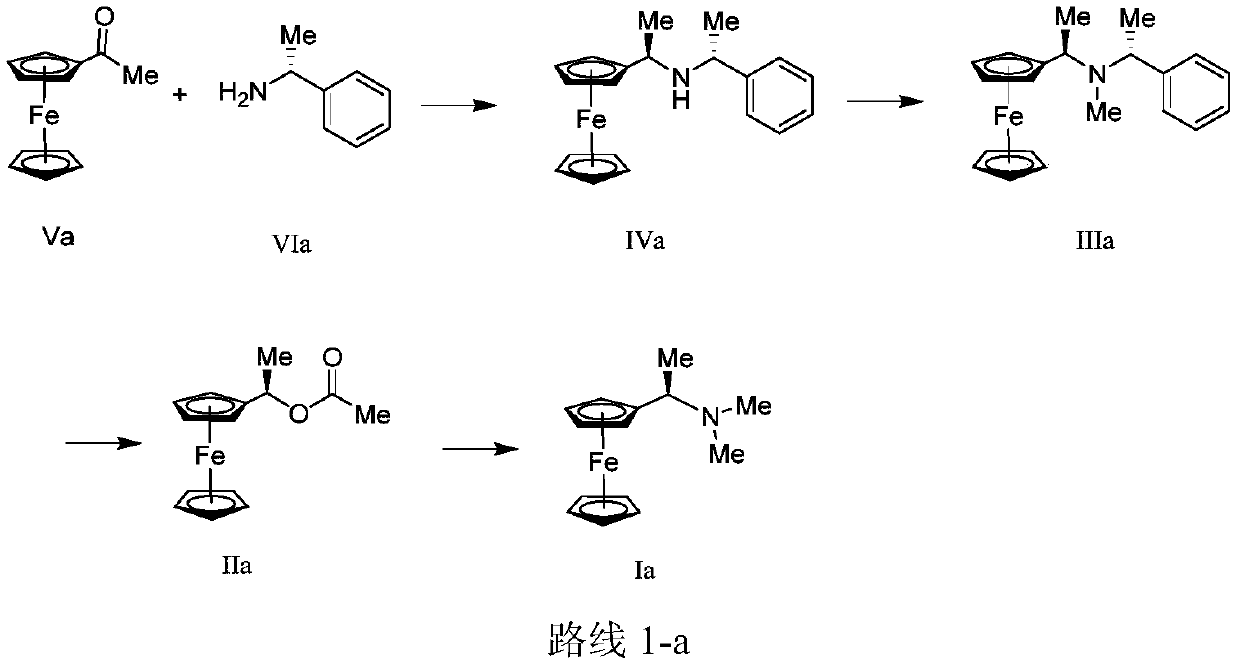
[0127] Weigh Va (100.0g, 438.4mmol), tetraisopropyl titanate [Ti(OiPr) 4 ] (263.0 mL, 876.9 mmol) and R-(+)-α-methylbenzylamine (113.1 mL, 876.9 mmol) were placed in a 2000 mL round bottom flask, and 500.0 mL of EtOH was added to dissolve it. After stirring and reacting for 14h, weigh the NaBH 4 (24.9g, 657.7mmol) was added to the reaction flask in batches, then stirred for another 2h, and the solvent was removed by rotary evaporation. Add 2400.0 mL of methyl tert-butyl ether (MTBE) to the residue to dissolve, then add 1200.0 mL of 2 N hydrochloric acid, stir for 2 h, filter to obtain a yellow solid, and use alkali to obtain 86.0 g of target compound IVa, yield: 58.9% , yellow solid. Mass Spectrometry MALDI-TOF-MS m / z:333(M + ).
[0128] 1 H-NMR (CDCl 3 / TMS,400MHz):δ(ppm)7.26-7.33(m,4H),7.19-7.23(m,1H),4.09(br.s.,1H),4.05(d,J=6.8Hz,2H), 4.01(s,5H),3.95(s,1H),3.72-3.79(m,1H),3.26(q,J=6.4Hz,1H),1.33(d,J=6.3Hz,3H),1.16(d , J=6.3Hz, 3H).
[0129] Route 1:
...
Embodiment 2
[0132]
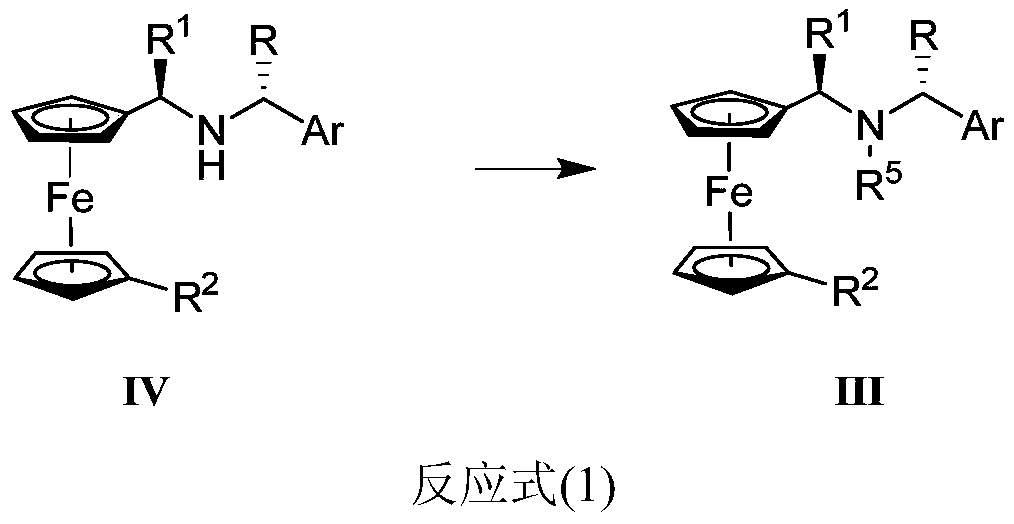
[0133] Weigh IVa (68.0g, 240.1mmol) and NaBH 3 CN (10.3g, 163.2mmol) was dissolved in a 1000mL round bottom flask by adding 340.0mL MeOH, and aqueous formaldehyde solution (58.0g, 714.2mmol, 37%) was weighed and added dropwise to the reaction flask while stirring. After 2 hours, the reaction was completed, quenched by adding 3400.0 mL of water, a large amount of yellow solid was precipitated, filtered to obtain 57.1 g of target compound IIIa, yield: 80.6%, yellow solid. Mass spectrometry MALDI-TOF-MS m / z: 347 (M + ).
[0134] 1 H-NMR (CDCl 3 / TMS,400MHz):δ(ppm)7.26-7.34(m,4H),7.18-7.22(m,1H),4.04(m,4H),3.95(s,5H),3.72(d,J=6.8Hz , 1H), 3.43(d, J=6.5Hz, 1H), 1.85(s, 3H), 1.31(d, J=6.8Hz, 3H), 1.17(d, J=6.5Hz, 3H).
Embodiment 3
[0136]
[0137] Weigh IIIa (25.0 g, 72.0 mmol) into a 250 mL round bottom flask, dissolve it with 125.0 mL of acetic anhydride, and heat to 65°C. After 16 hours of reaction, the acetic anhydride was removed by rotary evaporation, and the residue was dissolved in 140.0 mL of methanol, and an aqueous solution of dimethylamine (24.4 g, 216.0 mmol, 40%) was weighed and added dropwise to the reaction flask with stirring. After 16 hours, the reaction was completed. After removing MeOH by rotary evaporation, the residue was diluted with 400.0 mL of ethyl acetate, washed with water, dried, and ethyl acetate was removed by rotary evaporation to obtain 16.1 g of target compound Ia, yield: 87.0 %, yellow oil. Mass Spectrometry MALDI-TOF-MS m / z:257(M + ).
[0138] 1 H-NMR (CDCl3 / TMS, 400MHz): δ (ppm) 4.02-4.07 (m, 9H), 3.52 (d, J = 6.8Hz, 1H), 2.00 (s, 6H), 1.37 (d, J = 7.0 Hz, 3H).
[0139] Route 2:
[0140]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com