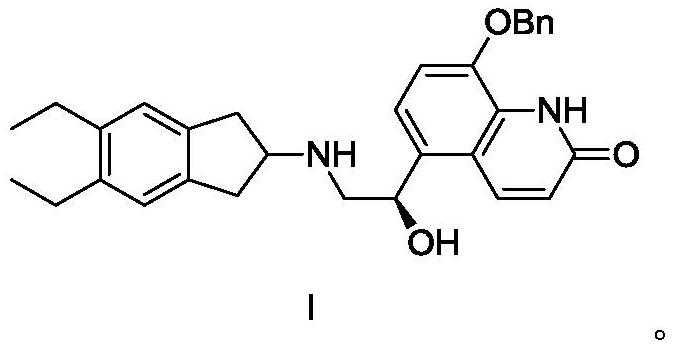
A kind of synthetic method and synthetic intermediate of indacaterol and its salt derivatives
A synthesis method and technology of derivatives, applied in the field of synthesis methods of indacaterol and its salt derivatives and intermediates for synthesis, can solve the problems of many by-products, difficult purification, low yield, etc., and avoid by-products , Improve the reaction yield and reduce the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
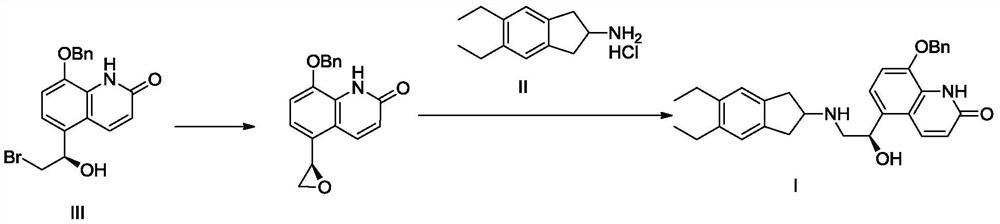
[0031] 8-benzyloxy-5-((R)-2-bromo-1-(tert-butyldimethylsilyl)oxy-ethyl)-1H-quinolin-2-one (compound of formula IV) synthesis
[0032] Add 50g of the compound of formula III and 600ml of dichloromethane into a 1000mL three-necked flask, stir and dissolve at room temperature, add 17g of triethylamine after dissolving, cool in an ice bath to 0°C, and add dropwise tert-butyldimethylsilyl chloride 24.8 g, after the addition is complete, return to room temperature and react for 2 to 4 hours. After the reaction, cool to 0°C, slowly add 1N HCl solution dropwise, separate layers, extract the aqueous phase with 200ml of dichloromethane, combine the organic phases, dry, and concentrate to obtain 78g of the target compound, with a yield of 99.3%.
Embodiment 2
[0034] Synthesis of 2-tert-butoxycarbonyl-amino-(5,6-diethyl)indan (compound of formula V)
[0035] Add 40g of the compound of formula II and 600ml of dichloromethane into a 1000ml three-neck flask, stir at room temperature, cool in an ice bath to 0-5°C, add 37.6g of triethylamine under stirring, and slowly dropwise add 39.4g of di-tert-butyl carbonate , about 0.5 hours after dropping, stirred and reacted at 0-5°C for 4 hours, poured the reaction solution into 500ml of ice water for layering, extracted the aqueous phase with 200ml of dichloromethane, combined the organic phases, dried and concentrated to obtain compound 49.1 of formula V g, yield 96%. h 1 NMR (300MHz, CD 3 OD)ppm: 7.20(s,2H), 4.15(t,1H), 2.90-3.15(m,4H), 2.83(q,4H), 1.41(s,9H), 1.28(t,6H); MS: 290.4 (M+).
Embodiment 3
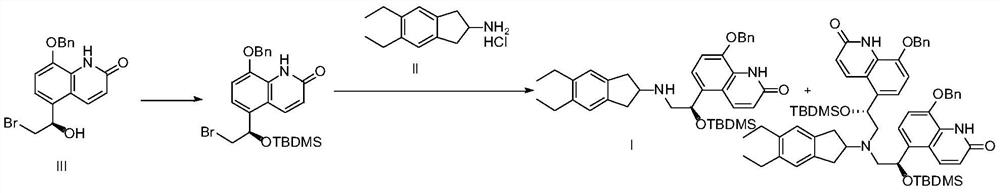
[0037] (R)-5-[2-(5,6-Diethylindan-2-ylamino)-1-hydroxyethyl]-8-benzyloxy-1H-quinolin-2-one hydrochloride Synthesis of salt (compound hydrochloride of formula I)
[0038] Add 48.8g (about 0.1mol) of the formula IV compound prepared in Example 1 and 29g (about 0.1mol) of the formula V compound prepared in Example 2 in a 1000ml three-necked flask, add 500ml DMF, and cool to -5 ~ in an ice-salt bath Under stirring at 0°C, slowly add 12.3 g of potassium tert-butoxide, keeping the temperature below 0°C. Slowly return the temperature to normal temperature after the addition, and after stirring for 4 hours, pour the reaction solution into 1000ml of ice water, extract twice with 300ml of ethyl acetate, combine the organic phases and concentrate to dryness, add 500mL of absolute ethanol, and add 40mL of concentrated hydrochloric acid , stirred at room temperature for 2 hours, and suction filtered to obtain 45.67 g of compound hydrochloride of formula I, with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com